SUMMARY
Circadian systems are entrained and phase shifted by light. In Drosophila, the model of light-mediated phase shifting begins with photon capture by cryptochrome (CRY) followed by rapid timeless (TIM) degradation. In this study, we focused on phase delays and assayed TIM degradation within individual brain clock neurons in response to light pulses in the early night. Surprisingly, there was no detectable change in TIM staining intensity within the eight pacemaker s-LNvs. This indicates that TIM degradation within s-LNvs is not necessary for phase delays, and similar assays in other genotypes indicate that it is also not sufficient. In contrast, more dorsal circadian neurons appear light-sensitive in the early night. Because CRY is still necessary within the s-LNvs for phase shifting, the results challenge the canonical cell-autonomous molecular model and raise the question of how the pacemaker neuron transcription-translation clock is reset by light in the early night.
INTRODUCTION
Most eukaryotes possess endogenous and self-sustained circadian pacemakers, which control physiology and behavior with ~24 h periodicity. Both Drosophila and mammals exploit a similar intracellular circadian mechanism, which includes interlocked transcriptional feedback loops and several orthologous proteins. The Drosophila loops lead to a coordinated circadian oscillation of multiple gene products, including the core clock proteins period (PER) and timeless (TIM). There are about 150 neurons in the Drosophila brain network, which express the canonical clock machinery. Based on anatomical location, these clock neurons are divided into seven different groups (Nitabach and Taghert, 2008). Four are in the lateral brain and include large ventrolateral neurons (l-LNvs), small ventrolateral neurons (s-LNvs), dorsolateral neurons (LNds), and lateral posterior neurons (LPNs). Almost all of the l-LNvs and s-LNvs express the neuropeptide PDF, and they are the only PDF-expressing neurons in the fly brain; there is one exceptional small ventrolateral neuron with no PDF expression (5th s-LNv). The dorsal brain contains 3 different neuronal groups (DN1s, DN2s, and DN3s). The eight s-LNvs anticipate lights-on in light-dark cycles and also keep circadian time in constant darkness (DD) (Blanchardon et al., 2001; Helfrich-Forster, 1998; Lin et al., 2004; Nitabach et al., 2002; Park et al., 2000; Peng et al., 2003; Renn et al., 1999; Stoleru et al., 2004; Stoleru et al., 2005).
Drosophila rhythms are highly sensitive to light: a short light pulse in the early night causes a phase delay and mimics delayed dusk, whereas a late night light pulse causes a phase advance and mimics advanced dawn. This general feature of the phase response curve (PRC) applies to many circadian systems including mammals. In the Drosophila system, heat pulses cause modest phase shifts only in the early night (Edery et al., 1994; Kaushik et al., 2007). Note that the heat pulse-mediated phase delay protocol is distinct from true temperature-mediated entrainment (Miyasako et al., 2007; Picot et al., 2009; Sehadova et al., 2009; Yoshii et al., 2009).
The effect of light on Drosophila phase shifting is currently interpreted by a well-accepted molecular model: phase-resetting and even light entrainment occur principally via photon capture by the blue light photoreceptor cryptochrome (CRY) within circadian neurons (Emery et al., 1998; Helfrich-Forster et al., 2001; Rieger et al., 2003; Stanewsky et al., 1998; Veleri et al., 2007). This leads to a CRY-TIM interaction and then rapid TIM degradation, which causes a delay in the requisite early night accumulation of PER and TIM, whereas TIM degradation leads to an advance in the requisite early morning (dawn-mediated) degradation of PER and TIM.
Indeed, there is good evidence that photon capture causes a conformational change in CRY, which then associates with TIM and mediates TIM degradation via a proteosomal pathway (Busza et al., 2004; Ceriani et al., 1999; Hunter-Ensor et al., 1996; Koh et al., 2006; Lee et al., 1996; Lin et al., 2001; Myers et al., 1996; Zeng et al., 1996). There is also genetic evidence that TIM degradation is important for light-mediated phase resetting (Suri et al., 1998; Yang et al., 1998). The protein JETLAG (JET) is important for phase-shifting and is an E3 ligase, which collaborates with CRY in light-mediated TIM degradation (Koh et al., 2006; Peschel et al., 2009). Notable is that CRY is also necessary for heat pulse-mediated phase shifts (Kaushik et al., 2007).
In contrast, there are recent data supporting a more systems view of phase shifting in the Drosophila circadian system. For example, two new CRY antibodies detect no or very low expression of CRY in many clock neurons including some DN1s and most DN3s (Benito et al., 2008; Yoshii et al., 2008). We have recently shown that l-LNvs are necessary for early morning phase shifts, consistent with a network view of phase shifting in the advance zone and suggesting that some clock neurons, in this case l-LNvs, play a different role in the delay and the advance zones (Shang et al., 2008). This view may also apply to heat, as separate clock neurons are differentially responsive to temperature and to light when these cues are presented simultaneously (Miyasako et al., 2007). Recent studies also showed that yet another set of clock neurons, the DN2s, plays an important role in temperature entrainment of the larval brain (Picot et al., 2009).
The role of TIM degradation in light-mediated phase resetting has only been carefully examined in the late night-advance zone within circadian neurons (Hunter-Ensor et al., 1996; Koh et al., 2006; Naidoo et al., 1999; Yuan et al., 2005). This is because TIM as well as CRY levels are maximal in the late night, which is optimal for immunohistochemistry (Benito et al., 2008; Yoshii et al., 2008). Although western blotting data from heads indicate that there is also considerable TIM degradation after a light pulse in the early night-delay zone (Hunter-Ensor et al., 1996; Myers et al., 1996; Suri et al., 1998), there is no systematic study of the entire circadian brain network in response to light or heat at this time. As a consequence, it is uncertain whether TIM degradation takes place uniformly in all clock neurons and tissues, in response to light and/or heat in the early night.
To address this issue of TIM degradation within the brain circadian system at this time, we assayed TIM by confocal microscopy after a saturating 10 min light pulse. The study was aided by a new anti-TIM antibody, with somewhat better sensitivity than the previous one. Surprisingly, a ZT15 light pulse causes little or no change in TIM staining intensity in the pacemaker s-LNvs. These neurons run the behavioral program in constant darkness and ultimately must experience the ca. 4 h phase delay caused by the light pulse at this time. This indicates that TIM degradation within these neurons is not necessary for light-mediated phase delays. Overexpression of JET in the PDF cells indicates that TIM degradation within the PDF cells is also not sufficient for phase delays. In contrast, TIM staining disappears rapidly and essentially completely from many E-cells in response to the delay zone light pulse. A heat pulse and a lower intensity light pulse generate comparable ca. 2 hour (half-maximal) phase delays and cause complete and uniform TIM degradation only in a subset of the light-sensitive neurons. Also based on previous results (Benito et al., 2008; Kaushik et al., 2007; Yoshii et al., 2008), we suggest that these are the are the most light-sensitive neurons in the early night. Our data also suggest that the extent of phase shift is determined by the number of E-cells that respond to the heat or light stimulus, either directly or indirectly. These cells then signal light information to the s-LNvs, the cells that keep time in constant darkness. Because these cells still require CRY for delay zone phase shifts, the data call into question the classical cell-autonomous transcription-translation model of phase-shifting (Busza et al., 2004; Ceriani et al., 1999; Hunter-Ensor et al., 1996; Koh et al., 2006; Lee et al., 1996; Lin et al., 2001; Myers et al., 1996; Zeng et al., 1996).
RESULTS
Light-induced TIM degradation within the s-LNvs is not necessary for delay zone phase shifts
To address the response of Drosophila circadian brain neurons to light, we subjected flies to standard phase-shifting light pulses in the night and then assayed the short-term response of different circadian clock neuronal groups as well as individual neurons within each group by TIM staining. The goal was to take a snapshot of individual clock neuron responses, before the entire circadian system adjusts to a new light-mediated steady state period or phase. We focused on ZT15, three hours after lights off in the early night, because this is the least studied part of the circadian light response (see above) and because ZT15 is the time of maximal phase delays. Although the light pulse was delivered during the night of an LD cycle, the lights did not come on at the subsequent ZT0-dawn. Rather, the incubation was extended in constant darkness so that a phase shift could be measured.
During a standard LD cycle, TIM levels and the corresponding staining intensity decrease rapidly at dawn (ZT0) and then remain low for the rest of the day. Levels then increase during the end of the day and continue increasing through most of the night, reaching a maximum shortly before dawn (Marrus et al., 1996; Sehgal et al., 1994). Although TIM levels are therefore much lower at ZT15 than the peak achieved near the end of the night, TIM can still be easily detected at ZT15 within all clock neurons as well as within the optic lobes, with our previous anti-TIM antibody and even better with the newer TIM antibody used in this study (Figure 1A, top; 1C, blue bars; see Experimental Procedures).
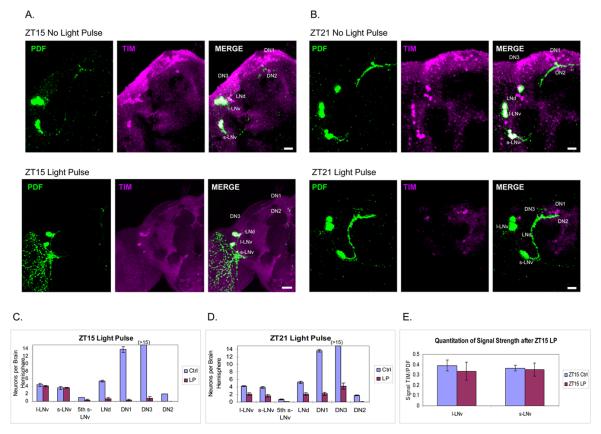
Figure 1.
Light-induced TIM degradation in clock neurons is different for LNvs in the delay zone (ZT15) and advance zone (ZT21). (A and B) The TIM staining pattern (magenta) with or without a light pulse at ZT15 (A) or ZT21 (B). LNvs are also stained with PDF (green). TIM can still be easily detected in the LNvs after a light pulse at ZT15. (C and D) Quantification of TIM-stained cells in each group of clock neurons with or without a light pulse at ZT15 (±SEM) (C) and ZT21 (±SEM) (D). (E) Quantification of TIM staining intensity in l-LNvs and s-LNvs after light pulse at ZT15 shows no statistic difference. Scale bar = 20 μm.
After a saturating 10 min light pulse at ZT15 and a subsequent 1 h in darkness, TIM was undetectable in almost all dorsal clock neurons and LNds. This reflects the expected light-mediated TIM degradation. In striking contrast, however, TIM remained qualitatively unaffected within all l-LNvs and s-LNvs. TIM staining intensity was also quantified and compared to the same neurons in control brains; no light effect was apparent (Figure 1A bottom, 1C, and 1E). The comparison between neuronal groups indicates that light-induced TIM degradation in the early night occurs preferentially within certain circadian neurons (DNs and LNds) rather than others (l-LNvs and s-LNvs). As the s-LNvs direct the circadian program in darkness and this is how phase shifts are measured, the light pulse must shift these pacemaker neurons eventually. However, rapid light-mediated TIM degradation within them is apparently unnecessary for a maximal light induced phase delay at ZT15.
To verify that this lack of TIM degradation in LNvs does not reflect a technical problem with the light pulse or the immunohistochemical assay, we performed the identical experiment but administered the light pulse in the advance zone in the late night, at ZT21. This is a time commonly used for TIM staining because levels are at or near a maximum.
A more uniform decrease in TIM staining is apparent across all brain circadian neurons at ZT21 (Figure 1B, Bottom; 1D). In addition to the fact that most of the DN and LNd population disappears like at ZT15 (Figure 1A and 1C), approximately half of the LNvs also disappear (Figure 1D). The increased number of apparently TIM-negative circadian neurons is despite the fact that there is considerably more TIM present in the late night at ZT21 than in the early night at ZT15 (Figure 1B). We conclude that there is no systematic technical issue and suggest that rapid TIM degradation within the pacemaker s-LNvs is not necessary for phase-delays.
Why does TIM not degrade within pacemaker s-LNvs after a ZT15 light pulse?
Although there are no indications that LNvs have particularly low CRY levels (Benito et al., 2008; Yoshii et al., 2008), most immunohistochemical assays of CRY are done after several days in DD. This is because CRY as well as TIM is degraded in response to light (Lin et al., 2001; Peschel et al., 2009), so staining intensity is much higher after some time in DD. It is therefore possible that the s-LNvs have low CRY levels relative to other circadian neurons three hours after lights off at ZT15 and that these low levels are responsible for the failure of TIM to degrade within the LNvs in response to a short light pulse. This suggested that overexpression of CRY within the LNvs might allow light-mediated TIM degradation at ZT15. To test this prediction, we performed a standard TIM degradation ZT15 full light pulse assay on yw;pdf-GAL4:UAS-cry flies (Figure 2C and 2D) and used sibling yw;UAS-cry flies as controls (Figure 2A and 2B).
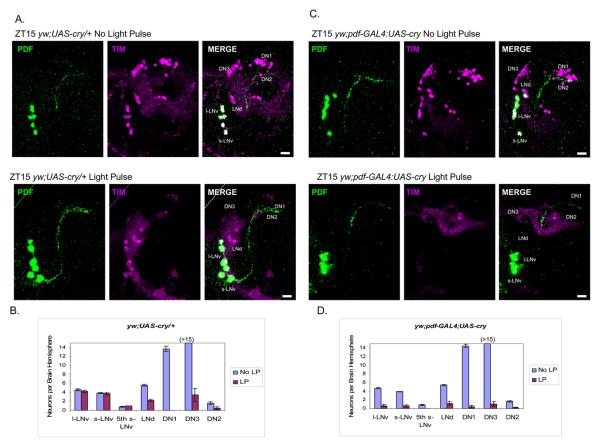
Figure 2.
Overexpression of CRY in LNvs promotes light-induced TIM degradation at ZT15.
(A) The TIM staining pattern in control flies (yw;UAS-cry/+) with or without a light pulse at ZT15. The results were identical to those from Canton-S flies in Figure 1A. LNvs are stained with PDF (green) (B) Quantification of TIM-positive cells in each group of clock neurons in control flies (yw;UAS-cry/+) treated with or without light pulse at ZT15 (±SEM). (C) CRY was overexpressed in the LNvs (yw;pdf-GAL4:UAS-cry), causing TIM degradation in the LNvs. The TIM staining pattern (magenta) and LNvs (green) with or without a light pulse at ZT15 were shown. (D) Quantification of TIM-positive neurons in each groups in yw;pdf-GAL4:UAS-cry flies with or without light pulse at ZT15 (±SEM). Scale bar = 20 μm.
In the yw;pdf-GAL4:UAS-cry strain, TIM disappearance in many clock neurons as well as from the optic lobes in response to a standard ZT15 full light pulse was similar to that in the control strain. Most importantly, this now included the pacemaker s-LNvs, (Figure 2C and 2D; compare with Figure 1A and 1C), consistent with the notion that the LNvs are normally CRY-deficient in the early night. The same TIM degradation in LNvs result occurred when CRY was overexpressed in a cryb background (Figure S1). As CRY overexpression rescues delay as well as advance zone phase shifts of the CRY-deficient cryb host strain (Emery et al., 2000), a somewhat more elaborate interpretation is that the transgenic CRY expression converts the circadian system to a more cell-autonomous organization.
TIM degradation within the s-LNvs is not sufficient for delay zone phase shifts
JETLAG is an F-box protein and a component of an E3 ubiquitin ligase complex that functions in light-mediated TIM degradation. Indeed, jet mutant strains have weak phase-shifting responses to light in both the delay and advance zones as well as impaired TIM degradation within circadian neurons (See Discussion). Successful JET rescue experiments had been carried out with broad circadian drivers (tim-GAL4, cry-GAL4), but there is no published JET rescue restricted to the LNvs of a jet mutant strain. To this end, we expressed JET in the jetc null mutant background with a pdf-GAL4 driver (Figure 3B and 3C).
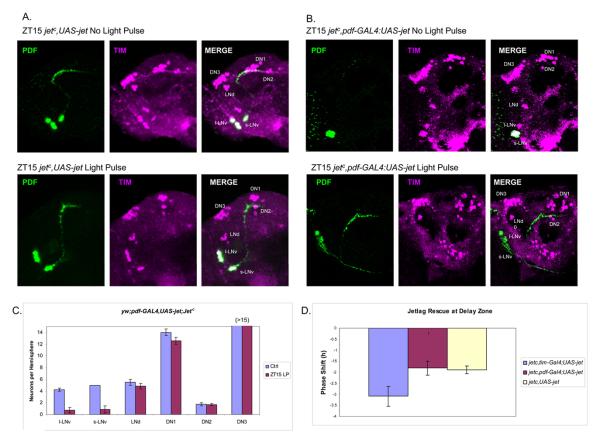
Figure 3.
Restricted expression of JET in LNvs causes TIM degradation in LNvs, but does not rescue phase shift behavior after a light pulse at ZT15.
(A) The TIM staining pattern (magenta) of jetc mutants (jetc,UAS-jet) with or without a light pulse at ZT15. LNvs are also stained with PDF (green). There is no detectable TIM degradation in all clock neurons after a light pulse at ZT15. (B) Restricted expression of JET in LNvs (jetc,pdf-GAL4:UAS-jet) with or without a light pulse at ZT15. TIM degradation is found in LNvs after light pulse. TIM positive neurons were stained in magenta and PDF in green. (C) Quantification of TIM-positive cells in clock neurons of jetc,pdf-GAL4:UAS-jet flies treated with or without light pulse at ZT15 (±SEM). (D) Behavioral rescue of jet mutants with different GAL4 drivers. UAS-jet can rescue the phase shift defects when driven by tim-GAL4, but not by pdf-GAL4 (±SEM). Scale bar = 20 μm.
Overexpression of JET in the LNvs caused a dramatic enhancement of light-mediated TIM degradation after a light pulse at ZT15 relative to control flies (Figure 3A, 3B and 3C). This was very similar to the response to CRY overexpression (Figure 2), indicating that the ZT15 defect in light-mediated TIM degradation can be overcome in multiple ways. Surprisingly however, these flies showed no behavioral rescue of the ZT15 phase shift deficit of the jetc mutant background (Figure 3D). The control was successful, i.e., use of the much broader tim-GAL4 driver rescued TIM degradation within all clock neurons as well as the ZT15 phase shift (Figure 3D), exactly as reported (Koh et al., 2006). Therefore, TIM degradation in LNvs is not sufficient for a successful delay zone phase shift. Moreover, the LNv rescue is not sufficient to induce TIM degradation in dorsal clock neurons and LNds. The data therefore suggest that light-mediated TIM degradation in clock neurons is cell-autonomous and CRY-JET dependent but that the pacemaker LNvs are still dependent on light input from elsewhere within the brain-circadian network, at least in the early night.
Heat shock and TIM degradation
A 30 min heat pulse at 37°C in the early night causes phase delays. Intriguingly, these are CRY-dependent, like light-mediated phase shifts (Kaushik et al., 2007). Although the complete mechanism and significance of heat-mediated phase delays are unknown, the similarities to light-mediated phase shifts suggest that a TIM degradation comparison between heat and light might be illuminating. In other words: 1) does TIM degradation in the LNvs fail to respond to heat like to light? 2) do heat-sensitive circadian neurons resemble or overlap with those that are most light-sensitive? To this end, we subjected entrained flies to a 30 min 37°C heat pulse at ZT15 after which brains were immediately dissected for whole mount immunohistochemistry with anti-TIM antibodies (Figure 4A and 4B).
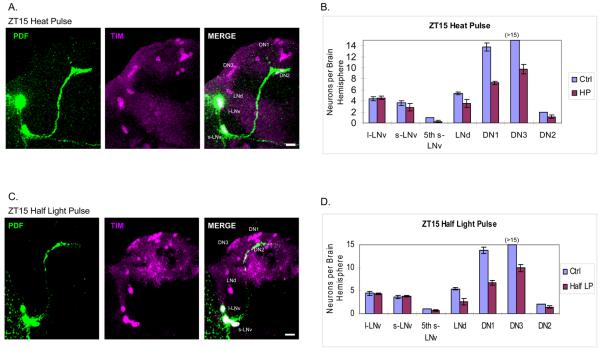
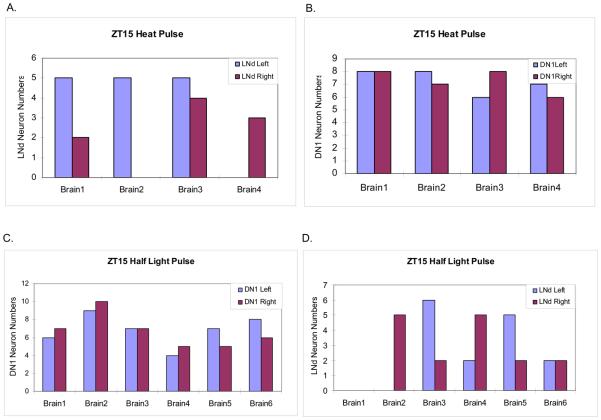
Figure 4.
Heat pulse and half light pulse cause similar TIM degradation patterns at ZT15. (A) The TIM staining pattern (magenta) after a 30 min heat pulse at ZT15. LNvs are also stained with PDF (green). (B) Quantification of TIM-stained cells in each group of clock neurons pulsed with heat at ZT15 (±SEM). We typically observed more than 15 TIM-positive DN1s in the control flies, but this number decreases to about 10 after the heat pulse. (C) The TIM staining pattern (magenta) with a half light pulse at ZT15 (see Experimental Procedures). LNvs are stained with PDF (green). (D) Quantification of TIM-stained cells in each group of clock neurons pulsed with half light pulse at ZT15 (±SEM). Scale bar = 20 μm.
The results were qualitatively similar to the ZT15 light pulse results (Figure 1A), i.e., TIM staining was unaffected in all PDF-containing LNvs but disappeared from many of the DNs and some of the LNds (Figure 4A and 4B). This indicates that TIM levels within the LNvs are resistant to the immediate effects of heat as well as light, whereas TIM disappearance from some of the DN and LNd clock cells correlates with the phase delays.
To address possible differences between neuronal groups as well as between different cells within the sensitive groups, we compared the number of stained LNds and DN1s between flies and between hemispheres after the ZT15 heat pulse (Figure 5). The number of stained LNds was highly variable, not only from fly to fly but also between hemispheres. In contrast, the number of TIM-stained DN1s was quite regular and reproducible. TIM staining always remained prominent in half (7-8) of the 15 DN1s on each side of the brain, i.e., TIM disappeared from almost precisely half of the DN1s on each side of the brain. This is entirely consistent with the reported number of CRY positive DN1s (Benito et al., 2008; Yoshii et al., 2008), and suggests that these cells undergo heat-mediated TIM degradation in a CRY-dependent manner, as previously indicated (Kaushik et al., 2007).
Figure 5.
Quantification of TIM stained neurons in DN1 and LNd of each brain hemisphere after a heat pulse or a half light pulse at ZT15. (A and B) TIM-positive cells in DN1s (A) and LNds (B). Eight hemispheres from 4 fly brains. Numbers of TIM-positive LNds are highly variable between hemispheres. Half of the 15 DN1s (+/−) are consistently TIM-positive within each hemisphere. (C and D) TIM-positive cells in DN1s (C) and LNds (D) of each hemisphere from 6 brains. Whereas TIM-positive cells in the LNds were highly variable between hemispheres, approximately half of the 15 DN1s (+/−1) are TIM-positive in every hemisphere. These results are similar to those from the heat pulses.
A half light pulse response resembles a heat pulse response
There was, however, a notable, quantitative difference between heat and light: TIM staining effectively disappeared from only about 50% of the DNs and LNds in response to heat (Figure 4B), whereas a much larger fraction of these neurons responded to light (Figure 1C). Consistent with this difference, the phase delay caused by the 37°C heat pulse is about half that of the 10 min light pulse, 2 h vs. 4 h, respectively (Edery et al., 1994; Kaushik et al., 2007).
To test this “strength of zeitgeber” hypothesis, we exploited the observation that lower light intensities result in reduced phase shifts at all times of day and night, including the peak delay zone time of ZT15 (Suri et al., 1998). This study showed that reduced light intensities also result in a reduced TIM degradation response as defined by Western blotting. A variable light intensity system was therefore calibrated by both assays, to generate a light-induced phase shift of 2 h (Figure S2). After this “half light pulse”, brains were dissected and double-stained with anti-TIM and anti-PDF antibodies as described above.
The results were essentially indistinguishable from those obtained after the heat pulse, namely, TIM staining of all s-LNvs and l-LNvs was identical to control brains, whereas approximately half of the LNds and DN1s disappeared (Figure 4C and 4D). As described above, this indicates that the half light pulse causes TIM to degrade in about half of the light-sensitive cells, whereas the other half appears unaffected. Also identical to the heat pulse was the fact that the reduced light pulse gives rise to a remarkably constant number of TIM-positive DN1s in every fly brain hemisphere, 7-8/15 DN1s, and therefore an equal number of TIM-negative DN1s (Figure 5C). LNd numbers were once again more variable, both between individual flies and between the left and right hemispheres (Figure 5D).
To try and further subdivide the sensitive and insensitive neuron populations, we assayed TIM staining as a function of time after half light pulse. In other words, flies were returned to darkness for 10, 20, 30, and 60 min rather than just the standard 60 min before immunostaining. The results indicated that there is no apparent “most sensitive subset” of these light-sensitive neurons as revealed by this assay, as TIM staining disappeared apparently uniformly between 30 and 60 min within each set of sensitive cells (Figure S3). This fits well with the time course of TIM disappearance as previously defined with a full light pulse (Busza et al., 2004). We also assayed TIM staining by waiting 3 h in darkness prior to fixation. The results were identical, i.e., no detectable change in TIM staining in the LNvs (Figure S4), confirming that the light pulse does not induce substantial TIM degradation in these cells at ZT15.
CRY is still necessary in the s-LNvs for phase shifting
Because there is no detectable TIM degradation in s-LNvs at ZT15, we used the validated R3 UAS-cryRNAi line (Picot et al., 2007) and the s-LNv-specific R6-GAL4 driver line (Helfrich-Forster et al., 2007) to test whether CRY is necessary in these neurons for delay zone phase shifts. In the early night (ZT15) as well in the late night (ZT21), there is a strong effect of cry knockdown within pacemaker neurons (Figure S5). We conclude that CRY is necessary within s-LNvs and therefore has a function in delay zone phase-shifting other than as a mediator of TIM degradation.
DISCUSSION
This study is the first to make a quantitative assessment of the Drosophila brain clock neuron response to phase-shifting light pulses. We focused on the early night at ZT15, when maximal phase delays result. Surprisingly, there was no detectable light pulse-mediated change in TIM staining intensity within the s-LNvs, indicating that TIM degradation within these pacemaker cells is not necessary for phase shifting in the delay zone. Experiments that overexpress the F-box protein JET indicated that TIM degradation within these neurons is also not sufficient for phase-shifting. In contrast, TIM staining disappeared rapidly and essentially completely from many other circadian neurons. Experiments with heat-mediated phase shifts and lower intensity light pulses suggest that about half of the CRY+ DN1s are among the most light-sensitive neurons in the early night, which signal to the s-LNv pacemaker cells (Figure 5). Because CRY is still necessary within these neurons for delay zone phase shifts, the results challenge the widely accepted cell-autonomous paradigm (Busza et al., 2004; Ceriani et al., 1999; Hunter-Ensor et al., 1996; Koh et al., 2006; Lee et al., 1996; Lin et al., 2001; Myers et al., 1996; Zeng et al., 1996).
This molecular model posits that light-mediated TIM degradation within the key timekeeping clock cells causes phase advances in the late night, when TIM levels are already decreasing or poised to decrease, but delays the clock when the degradation is “opposed” to the increase that TIM levels are normally programmed to undergo in the early night. The model is based in part on western blotting experiments from fly head extracts, which show potent and rapid light pulse-mediated TIM degradation in both the early night as well as the late night. The blotting assays mostly measure eye and fat body TIM degradation shortly after the light pulse, whereas behavioral phase shifts are measured over several days in darkness and therefore reflect the steady state response of the dark timekeepers, the 8 PDF-containing s-LNvs, to light pulses.
Neuronal TIM has also been assayed by immunohistochemistry, and the data confirm the potent light-mediated TIM degradation observed by western blotting. However, almost all of the literature focuses on the late night (Busza et al., 2004; Hunter-Ensor et al., 1996; Myers et al., 1996; Naidoo et al., 1999; Sidote et al., 1998; Suri et al., 1998). This is when TIM as well as CRY levels are highest during an LD cycle, so a stronger response to light is easier to observe and may even be easier to achieve (Figure 1). There is one report of light-mediated TIM degradation at ZT15 in LNvs, but the effect was modest and no comparisons with other circadian cells were presented (Koh et al., 2006).
Although we cannot rule out a very small difference, TIM levels appear quantitatively as well as qualitatively unaffected within the PDF-containing LNvs even by a saturating ZT15 light pulse. Moreover there is no apparent decrease in TIM staining intensity even 3 h after the light pulse (Figure S4). The lack of a detectable TIM light-response in the pacemaker LNvs suggests that they require signaling from light-sensitive circadian neurons.
Why do the s-LNv pacemaker neurons not degrade TIM in response to a light pulse at ZT15 like many other cells and tissues? As CRY is degraded in the day and accumulates throughout the night, perhaps there are major differences between different circadian neurons so that LNvs have not accumulated sufficient CRY in the three hours between lights off and ZT15 to be directly light-responsive. Light-mediated TIM degradation is CRY-dependent, as assayed by western blotting from fly heads (Stanewsky et al., 1998), and no light-dependent TIM degradation was detected in clock neurons of cryb flies (Figure S6). Moreover, early night CRY levels in LNvs have been reported to be relatively low compared to LNds (Benito et al., 2008; Yoshii et al., 2008). The fact that CRY overexpression with the pdf-GAL4 driver causes LNv TIM degradation to be light-sensitive at ZT15 is consistent with this interpretation.
However, JET overexpression also caused potent light-mediated TIM degradation, but it could not rescue the behavioral rhythm deficit of the jet mutant background (Figure 3B, 3C, and 3D). This suggests that a somewhat modified version of the “insufficient CRY levels” explanation might be more correct, namely, some aspect of the machinery responsible for light-mediated TIM degradation is insufficient at ZT15 in LNvs, quantitatively or qualitatively. An overlapping role of CRY and JET is consistent with recent experiments in S2 cells indicating that the two proteins function in concert (Peschel et al., 2009). Additionally, the failure to rescue behavioral phase-shifting with JET expression, taken together with successful CRY rescue (Emery et al., 2000) and CRY necessity within s-LNvs (Figure S5), suggests that CRY has a role in these pacemaker neurons other than light-mediated TIM degradation. This function may be relevant to the muted behavioral response to light pulses in jetc flies but the lack of a response in cryb flies (Koh et al., 2006; Stanewsky et al., 1998).
Yet our results as well as the well characterized role of JET in light-mediated TIM degradation and phase shifting (Koh et al., 2006; Stanewsky et al., 1998) indicate that TIM degradation is relevant for phase shifting in the early night but elsewhere within the circadian circuit. This agrees with previous experiments using less direct assays, which indicate that more dorsal neurons are especially important in the late day-early night phase of an LD cycle or in constant light, even for keeping time (Murad et al., 2007; Picot et al., 2007; Stoleru et al., 2007).
Only half of the DN1s are reported to stain positively with anti-CRY antibodies on each side of the brain after 3 days in constant darkness (DD) (Benito et al., 2008; Yoshii et al., 2008). (In our hands, staining is too weak at ZT15 to reliably count cells.) This fits well with the comparison between the full light pulse vs half light pulse and heat pulse data, i.e., it is presumably the CRY positive DN1s that degrade TIM in response to heat or reduced light intensity at ZT15 (Figure 4A and 4C). This comparison also indicates that the magnitude of the behavioral phase shift is summed from the number of TIM-degrading cells. However, all classes of non-LNv neurons respond similarly to the reduction in light intensity (Figure 4 vs Figure 1), indicating that we do not know which are most important for phase-shifting.
The simple picture of DN1 TIM degradation contrasts with the LNds, which respond in a less coherent way to light at ZT15. Despite the fact that half of the LNds are reported to contain CRY at DD3 just like the DN1s, the number of residual TIM-positive LNds is variable in response to a heat pulse as well as a half-light pulse, not only between different animals but also between brain hemispheres; sometimes more than half of the LNds degrade TIM and sometimes less. This LNd response as well as the nearly complete response of DN1s to a full light pulse suggests there is some non-cell autonomous TIM degradation, as previously concluded based on a ZT1 assay (Yoshii et al., 2008). Alternatively or in addition, apparently CRY-negative circadian neurons at DD3 may contain sufficient CRY at ZT15 to degrade TIM. In response to a half-light pulse or heat at this time, LNds may then respond in a stochastic manner but with nearly complete TIM degradation. An all-or-none response is consistent with results of a previous study on CRY-mediated TIM degradation (Busza et al., 2004).
It is notable that TIM appears not to degrade in some E-cells in response to a late night light pulse at ZT21. Indeed, TIM degradation is less complete at ZT21 despite what should be higher CRY levels everywhere at ZT21 than at ZT15 (Figure 2C and 2D). Similarly, heat shock is ineffective at eliciting TIM degradation as well as a phase shift at ZT21 in the late night (Figure S7) (Edery et al., 1994; Kaushik et al., 2007). Both observations suggest temporal gating of TIM degradation based on features other than just CRY levels.
Signaling from primary light-sensing circadian neurons to pacemaker s-LNvs in the early night is consistent with previous experiments that addressed CRY function and the role of the clock kinase gene shaggy (Stoleru et al., 2007). A systems view of light-mediated phase shifting is also supported by recent experiments indicating that the lLNvs may signal to the pacemaker s-LNvs to effect maximal phase shifts in the late night-advance zone (Shang et al., 2008). Yet, the observations made here now raise a different challenge: if TIM degradation does not occur and yet CRY is still necessary within pacemaker s-LNvs (Figure S5), how is the transcription-translation clock within these neurons reset in response to an early night light signal from more dorsal neurons?
EXPERIMENTAL PROCEDURES
Please see Supplemental Data.
Supplementary Material
Acknowledgements
We thank Patrick Emery, Charlotte Helfrich-Förster, François Rouyer, Amita Sehgal and Corinna Wülbeck for comments on the manuscript and members of the Rosbash lab for helpful discussions. We also thank Amita Sehgal for providing jetlag mutant strains, Paul Taghert for R6-GAL4 strain, François Rouyer for UAS-cryRNAi strain and Shawn Jennings for generating the anti-TIM antiserum. We especially thank Charlotte Helfrich-Förster for her generous support of the anti-cry antibody. We are grateful to Kristyna Palm Danish for administrative assistance. The work was supported in part by NIH grants P01 NS44232, P30 NS45713 to MR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Benito J, Houl JH, Roman GW, Hardin PE. The blue-light photoreceptor CRYPTOCHROME is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J Biol Rhythms. 2008;23:296–307. doi: 10.1177/0748730408318588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchardon E, Grima B, Klarsfeld A, Chelot E, Hardin PE, Preat T, Rouyer F. Defining the role of Drosophila lateral neurons in the control of circadian rhythms in motor activity and eclosion by targeted genetic ablation and PERIOD protein overexpression. Eur J Neurosci. 2001;13:871–888. doi: 10.1046/j.0953-816x.2000.01450.x. [DOI] [PubMed] [Google Scholar]
- Busza A, Emery-Le M, Rosbash M, Emery P. Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science. 2004;304:1503–1506. doi: 10.1126/science.1096973. [DOI] [PubMed] [Google Scholar]
- Ceriani MF, Darlington TK, Staknis D, Mas P, Petti AA, Weitz CJ, Kay SA. Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science. 1999;285:553–556. doi: 10.1126/science.285.5427.553. [DOI] [PubMed] [Google Scholar]
- Edery I, Rutila JE, Rosbash M. Phase shifting of the circadian clock by induction of the Drosophila period protein. Science. 1994;263:237–240. doi: 10.1126/science.8284676. [DOI] [PubMed] [Google Scholar]
- Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- Emery P, Stanewsky R, Helfrich-Förster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY Is a Deep Brain Circadian Photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C. Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J Comp Physiol [A] 1998;182:435–453. doi: 10.1007/s003590050192. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C, Shafer OT, Wulbeck C, Grieshaber E, Rieger D, Taghert P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol. 2007;500:47–70. doi: 10.1002/cne.21146. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30:249–261. doi: 10.1016/s0896-6273(01)00277-x. [DOI] [PubMed] [Google Scholar]
- Hunter-Ensor M, Ousley A, Sehgal A. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell. 1996;84:677–685. doi: 10.1016/s0092-8674(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Kaushik R, Nawathean P, Busza A, Murad A, Emery P, Rosbash M. PER-TIM interactions with the photoreceptor cryptochrome mediate circadian temperature responses in Drosophila. PLoS Biol. 2007;5:e146. doi: 10.1371/journal.pbio.0050146. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Koh K, Zheng X, Sehgal A. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science. 2006;312:1809–1812. doi: 10.1126/science.1124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Parikh V, Itsukaichi T, Bae K, Edery I. Resetting the Drosophila clock by photic regulation of PER and a PER-TIM complex. Science. 1996;271:1740–1744. doi: 10.1126/science.271.5256.1740. [DOI] [PubMed] [Google Scholar]
- Lin FJ, Song W, Meyer-Bernstein E, Naidoo N, Sehgal A. Photic signaling by cryptochrome in the Drosophila circadian system. Mol Cell Biol. 2001;21:7287–7294. doi: 10.1128/MCB.21.21.7287-7294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus S, Zeng H, Rosbash M. Effect of constant light and circadian entrainment of perS flies: evidence for light-mediated delay of the negative feedback loop in Drosophila. EMBO J. 1996;15:6877–6886. [PMC free article] [PubMed] [Google Scholar]
- Miyasako Y, Umezaki Y, Tomioka K. Separate sets of cerebral clock neurons are responsible for light and temperature entrainment of Drosophila circadian locomotor rhythms. J Biol Rhythms. 2007;22:115–126. doi: 10.1177/0748730407299344. [DOI] [PubMed] [Google Scholar]
- Murad A, Emery-Le M, Emery P. A subset of dorsal neurons modulates circadian behavior and light responses in Drosophila. Neuron. 2007;53:689–701. doi: 10.1016/j.neuron.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MP, Wager-Smith K, Rothenfluh-Hilfiker A, Young MW. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- Naidoo N, Song W, Hunter-Ensor M, Sehgal A. A role for the proteasome in the light response of the timeless clock protein. Science. 1999;285:1737–1741. doi: 10.1126/science.285.5434.1737. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Park JH, Helfrich-Forster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc.Natl.Acad.Sci.U.S.A. 2000;97:3608–3613. doi: 10.1073/pnas.070036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila Free-Running Rhythms Require Intercellular Communication. PLoS Biol. 2003;1:E13. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel N, Chen KF, Szabo G, Stanewsky R. Light-dependent interactions between the Drosophila circadian clock factors cryptochrome, jetlag, and timeless. Curr Biol. 2009;19:241–247. doi: 10.1016/j.cub.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Picot M, Cusumano P, Klarsfeld A, Ueda R, Rouyer F. Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock. PLoS Biol. 2007;5:e315. doi: 10.1371/journal.pbio.0050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M, Klarsfeld A, Chelot E, Malpel S, Rouyer F. A role for blind DN2 clock neurons in temperature entrainment of the Drosophila larval brain. J Neurosci. 2009;29:8312–8320. doi: 10.1523/JNEUROSCI.0279-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Rieger D, Stanewsky R, Helfrich-Forster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J Biol Rhythms. 2003;18:377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- Sehadova H, Glaser FT, Gentile C, Simoni A, Giesecke A, Albert JT, Stanewsky R. Temperature entrainment of Drosophila's circadian clock involves the gene nocte and signaling from peripheral sensory tissues to the brain. Neuron. 2009;64:251–266. doi: 10.1016/j.neuron.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Price J, Man B, Young M. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994;263:1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidote D, Majercak J, Parikh V, Edery I. Differential effects of light and heat on the Drosophila circadian clock proteins PER and TIM. Mol.Cell.Biol. 1998;18:2004–2013. doi: 10.1128/mcb.18.4.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta M, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Nawathean P, Fernandez Mde L, Menet JS, Ceriani MF, Rosbash M. The Drosophila circadian network is a seasonal timer. Cell. 2007;129:207–219. doi: 10.1016/j.cell.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng. Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- Suri V, Qian Z, Hall JC, Rosbash M. Evidence that the TIM light response is relevant to light-induced phase shifts in Drosophila melanogaster. Neuron. 1998;21:225–234. doi: 10.1016/s0896-6273(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Veleri S, Rieger D, Helfrich-Forster C, Stanewsky R. Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates TIM and PER expression in Drosophila clock neurons. J Biol Rhythms. 2007;22:29–42. doi: 10.1177/0748730406295754. [DOI] [PubMed] [Google Scholar]
- Yang Z, Emerson M, Su HS, Sehgal A. Response of the timeless protein to light correlates with behavioral entrainment and suggests a nonvisual pathway for circadian photoreception. Neuron. 1998;21:215–223. doi: 10.1016/s0896-6273(00)80528-0. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Todo T, Wulbeck C, Stanewsky R, Helfrich-Forster C. Cryptochrome is present in the compound eyes and a subset of Drosophila's clock neurons. J Comp Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Vanin S, Costa R, Helfrich-Forster C. Synergic entrainment of Drosophila's circadian clock by light and temperature. J Biol Rhythms. 2009;24:452–464. doi: 10.1177/0748730409348551. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Zeng H, Qian Z, Myers MP, Rosbash M. A light-entrainment mechanism for the Drosophila circadian clock. Nature. 1996;380:129–135. doi: 10.1038/380129a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.