Abstract
Introduction
Patients with metastatic colorectal cancer who progress on standard chemotherapy have limited treatment options. New and effective drugs are needed for these patients. Depsipeptide is a histone deacetylase inhibitor that can alter chromatin structure and gene transcription leading to multiple changes in cellular protein production. This may result in cell cycle arrest and tumor growth inhibition. Depsipeptide has shown anti-proliferative activity in vitro against multiple mouse and human tumor cell lines and in vivo in human tumor xenograft models.
Patients and Methods
Patients were required to have pathologically verified, measurable, metastatic or locally advanced colorectal cancer that was surgically unresectable. They must have failed either one or two prior chemotherapy regimens, had performance status of 0–1, adequate bone marrow, renal and hepatic function, and no significant cardiac disease. Patients were treated with depsipeptide at a dose of 13 mg/m2 as a 4 hour iv infusion on days 1, 8, and 15 of a 28 day cycle. The study had a two stage design. The primary objective of the study was to determine the confirmed response probability in this group of patients treated with depsipeptide.
Results
Twenty-eight patients were registered to the study, two of whom were ineligible. One eligible patient refused all treatment and was not analyzed. For the 25 remaining patients, performance status was 0 in 16 patients and 1 in 9 patients. Ten patients had received one prior chemotherapy regimen and fifteen 2 prior regimens. Out of the 25 eligible and analyzable patients accrued in the first stage of the protocol, no objective responses were observed and the study was permanently closed. Four patients had stable disease as the best response. Twenty-five patients were assessed for toxicity. No grade 4 or greater toxicities were seen. Fourteen of the 25 patients experienced grade 3 toxicities the most common of which were fatigue or anorexia.
Conclusion
Depsipeptide at this dose and schedule is ineffective in the treatment of patients with metastatic colorectal cancer after prior chemotherapy. Future trials might evaluate combinations of depsipeptide with chemotherapeutic or other agents.
INTRODUCTION
The treatment of metastatic colorectal cancer has been improved with the addition of irinotecan or oxaliplatin to 5-FU and leucovorin, plus the biologic agents bevacizumab and cetuximab. Still, it cannot be cured with currently available drugs and biologic agents. New treatments are needed. Once patients have failed standard therapy, their options are limited. Many are still in good condition and are candidates for clinical trials. This multicenter Southwest Oncology Group phase II trial evaluated the effectiveness and toxicity of the agent depsipeptide in patients with metastatic colorectal cancer who had progressed on standard therapy.
Depsipeptide (FK 228, FR 901228) is a unique bicyclic peptide originally isolated from Chromobacterium violaceum strain 968 (1). It has a novel chemical structure composed of four amino acids (D-valine, D-cysteine, dehdrobutyrine, and L-valine) and a novel acid (3-hydroxy-7-mercapto-4heptenoic acid) configured in a cage-shaped bicyclic depsipeptide. Depsipeptide was discovered by Fujisawa Pharmaceuticals Co., Ltd., as part of a concerted search for novel compounds from natural products that would reverse the ras-transformed phenotype to normal.
Results of early studies showed that depsipeptide inhibited the growth of the Ha-rastransformed NIH3T3 clonal cell line, Ras-1, and induced reversion of the transformed morphology to normal within 1 day at a concentration of 2.5 ng/ml (2). mRNA expression of the c-myc oncogene in Ras-l cells was decreased in the presence of depsipeptide, but Ha-ras mRNA expression was not affected. Depsipeptide blocked cell cycle transition from G0/G1 to S phase. It was proposed that the growth inhibition and 5 G0/G1 arrest resulted from depsipeptide blocking the ras-mediated signaling transduction pathway (3). There is evidence that depsipeptide can inhibit signal transduction through MAP kinase and cause p53-independent G1 arrest (4).
Depsipeptide is also a potent inhibitor of the enzyme histone deacetylase. Deregulation of histone acetylation has been implicated in the development of several types of cancer. Genes that encode histone acetyltransferase enzymes are translocated, amplified, overexpressed and/or mutated in various cancers (5, 6). These findings suggest that deregulated acetylation of histones plays a role in the pathogenesis of hematological as well as solid tumors by changing the chromatin structure and transcription of genes involved in cell cycle control, differentiation or apoptosis. Consequently, there is considerable interest in histone deacetylase inhibition as a potential therapeutic modality in the treatment of hematologic malignancies and solid tumors.
Depsipeptide has been identified as a histone deacetylase (HDAC) inhibitor similar to trichostatin A based on its ability to cause arrest of the cell cycle at both G1 and G2/M phases, to induce internucleosomal breakdown of chromatin, and to inhibit intracellular HDAC activity resulting in an accumulation of marked amounts of acetylated histone species within M-8 cells (7). DNA methylation and histone deacetylase inhibition may act synergistically in the re-expression of genes silenced in cancer (8). Depsipeptide, either alone or in combination with hypomethylating agents, has been shown to induce a number of cellular proteins that may have critical effects on apoptosis, proliferation 6 and susceptibility to immunologic manipulation (9–15). The compound may also have antiangiogenic activity that contributes to antitumor efficacy (16).
Pre-Clinical Studies
Potent antitumor effects of depsipeptide have been demonstrated both in vitro and in vivo. In vitro, depsipeptide exerted antiproliferative activity against 12 human solid tumor cell lines including lung adenocarcinoma (A549, PC-9), lung squamous cell carcinoma (PC-1, PC-10), small cell lung carcinoma (ADH, LX-1), breast adenocarcinoma (MCF-7, ZR-75-1) stomach adenocarcinoma (MKN28, MKN74) and colon adenocarcinoma (Colo201, SW480), but was less potent against cultured normal cells (1). Similarly, it induced G1 arrest or apoptotic cell death in a study of 13 lymphoid cell lines with less sensitivity against normal mononuclear cells and bone marrow (17). In non-small cell lung cancer (NSCLC) cells expressing wild-type or mutant p53, depsipeptide inhibited growth and induced apoptosis (18). Depsipeptide induced apoptotic-like cell death in human breast cancer cell lines MCF 7 and MDA-MB231 (19).
In vivo, depsipeptide had moderate antitumor efficacy in various model systems, including murine Bl6 melanoma, M5076 sarcoma, Meth A fibrosarcoma, and colon 38 carcinoma plus several human tumor xenograft models, including stomach adenocarcinoma (SC-6), lung (Lu-65, LC-6, A549), and breast (MX-1) (20). Depsipeptide was much more active against the SC-6 stomach adenocarcinoma than either mitomycin C or cisplatin. It was also strongly active against mitomycin and 5-fluorouracil drug resistant P388 murine leukemia but much less active against doxorubicin resistant P388 cells (20). Results from animal studies indicated that an intermittent administration schedule (q4d × 3) of depsipeptide resulted in lower toxicity and greater efficacy than a daily schedule (qd × 5) (21). The greater antitumor activity observed with an intermittent schedule may be attributable to the higher individual doses that were administered because of the greater tolerance to depsipeptide.
PATIENTS AND METHODS
To be eligible for this trial, patients were required to have a pathologically verified diagnosis of colorectal cancer with metastatic disease or locally advanced disease that was not surgically resectable. Measurable disease as defined by the RECIST criteria was obligatory (22). Prior surgery or radiation therapy was allowed provided patients were at least 28 days beyond such treatment and had recovered from all side effects. Patients must have received treatment with either one or two prior chemotherapy regimens for advanced colorectal cancer which may have included either oxaliplatin or irinotecan, but they could not have received prior depsipeptide nor be receiving any other investigational agent or drugs known to have histone deacetylase inhibitor activity. Prior chemotherapy had to have been completed at least 28 days prior to entry on this trial and patients recovered from all effects of that treatment. Patients were required to have a Zubrod performance status of 0–1. Mandatory parameters demonstrating adequate organ function included WBC ≥ 3000/µl, ANC ≥ 1500/µl, and platelet count ≥ 100,000/µl, obtained within 14 days prior to registration; serum creatinine ≤ the institutional upper limit of normal (IULN), total bilirubin ≤ IULN, SGOT or SGPT ≤2.5 × IULN, serum potassium ≥ 4 mmol/L, and magnesium ≥ 2 mg/dL, these tests obtained within 28 days prior to registration. Patients could not be taking hydrochlorothiazide (HCTZ) and had to be switched to a potassium conserving combination such as Maxzide or Dyazide or other anti-hypertensive agent. Because preclinical toxicology data indicated depsipeptide caused cardiac injury in several animal species particularly dogs, and increased CPK, asymptomatic EKG changes, and supraventricular arrhythmias were seen in early trials, cardiac requirements were strict. Patients could not have significant cardiac disease including congestive heart failure meeting New York Heart Association (NYHA) class III or IV definitions, a history of myocardial infarction within one year of study entry, uncontrolled dysrhythmias, poorly controlled angina or left ventricular hypertrophy, a history of a serious ventricular arrhythmia (VT or VF, ≥ 3 beats in a row), QTc ≥ 500 msec or left ventricular hypertrophy by baseline EKG within 28 days prior to registration. Patients could not be co-medicated with an agent that caused QTc prolongation. No concurrent treatment for the patient’s cancer was allowed and no prior malignancy was allowed except for adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, or other cancer for which the patient had been disease-free for 5 years. There could be no history of allergic reactions attributed to compounds of similar chemical or biologic composition to depsipeptide. HIV-positive patients receiving combination anti-retroviral therapy were excluded from the study as were patients with known brain metastases. No pregnant or nursing women were allowed and use of contraception was required of both men and women. It was requested that patients have a hemoglobin value of ≥10 mg/dl. All patients were required to give written informed consent prior to treatment on the study.
TREATMENT DESIGN
Depsipeptide was administered at a dose of 13 mg/m2 as a 4 hour iv infusion on days 1, 8, and 15 of a 28 day cycle. Because depsipeptide is moderately emetogenic, patients were given prophylactic antiemetics such as a 5HT3 antagonist and dexamethasone before treatment. Magnesium and potassium levels were required to be checked before the administration of drug and replaced appropriately according to guidelines in the protocol to maintain high upper limits of normal levels. Patients were required to have a potassium level ≥ 4.0 mmol/L and a magnesium level ≥ 2.0 mg/dL before drug administration. Because of potential interaction between depsipeptide and warfarin, patients receiving that agent were required to be closely monitored. At the time of first drug administration, patients were required to have an ECG with rhythm strip performed 3 times, within 1 hour prior to drug administration, within 1 hour after the completion of administration, and within 24–72 hours after the completion of administration. If a patient required a dose reduction for cardiac toxicity, an ECG was required to be performed again within 1 hour prior to and within 1 hour following the completion of the first infusion of the reduced dose and as clinically indicated thereafter.
Patients were removed from protocol treatment for progression of disease, symptomatic deterioration, defined as a global deterioration of health status requiring discontinuation of treatment even though there was no objective evidence of progression, unacceptable toxicity, a delay of treatment for more than 4 weeks after the planned date of administration, or the patient could withdraw from the protocol treatment at any time for any reason.
Dose Modifications
Toxicity on this study was graded according to the NCI Common toxicity Criteria Version 3.0. Hematologic, hepatic, renal, and constitutional toxicities were to be evaluated prior to each infusion of depsipeptide and the dose modified and/or delayed based upon the results according to protocol guidelines. Once a dose was reduced, it was used for all subsequent depsipeptide infusions. Only one dose reduction was allowed. Patients requiring a second dose reduction were removed from protocol treatment. When multiple toxicities occurred, the dose modification was based on the toxicity that required the greatest dose reduction.
For an ANC ≤ 1000/µl and/or platelets ≤ 100,000/µl, treatment was delayed until recovery above those parameters and then dose was reduced to 10 mg/m2. Elevated SGOT > 3× ULN and/or bilirubin > 1.5 ULN or creatinine > 1.5 ULN required treatment delay until recovery and then a dose reduction to 10 mg/m2. Grade 4 vomiting or marked elevation in INR or bleeding required the patient to come off of the protocol treatment. For cardiac toxicities, sinus tachycardia > 140 beats/minute after recumbency, any new atrial dysrhythmia, SVT, fibrillation, or flutter, prolongation of QTc of ≥ 500 msec or an increase of ≥ 50 msec compared to baseline, T wave inversion of > 4mm, or ST-segment depression of ≥ 2 mm, required holding further treatment and appropriate treatment by a cardiologist. If the toxicity resolved, depsipeptide was restarted at a reduced dose of 10 mg/m2. If it did not resolve, the patient was removed from protocol treatment. For ventricular arrhythmia of tachycardia or fibrillation, appropriate treatment by a cardiologist was done and then the patient was removed from protocol treatment.
Definition of Response and Survival
In this trial, response was defined by the Response Evaluation Criteria in Solid tumors (RECIST) guidelines (22). To be considered a confirmed complete or partial response, there must have been documented 2 or more objective statuses meeting these criteria, separated by a minimum of at least 4 weeks. Survival was defined as the time from the date of registration to death due to any cause. The time to treatment failure was defined as the time from the date of registration to the date of the first observation of progressive disease, death due to any cause, symptomatic deterioration, or early discontinuation of treatment.
Statistical Design
The primary goal of the study was to evaluate the confirmed response probability (complete and partial) in patients with advanced colorectal cancer treated with depsipeptide. It was assumed that this therapy would be of no further interest if the true response probability was 5% or less, and of interest if the true response probability was 20% or more. The study had a two-stage design. If after the first 20 eligible patients at least one confirmed response was observed, an additional 20 patients would be accrued. Five or more responses out of the total 40 patients would be considered evidence that this drug was of interest in advanced colorectal cancer. This design had a power of 0.92 for a true response rate of 20%, and a significance level of 0.05. If forty patients were accrued, this would be sufficient to estimate the probability of a particular toxicity to within ± 16%. Any toxicity occurring with at least a 5% probability would be likely (87%) to be seen at least once.
Results
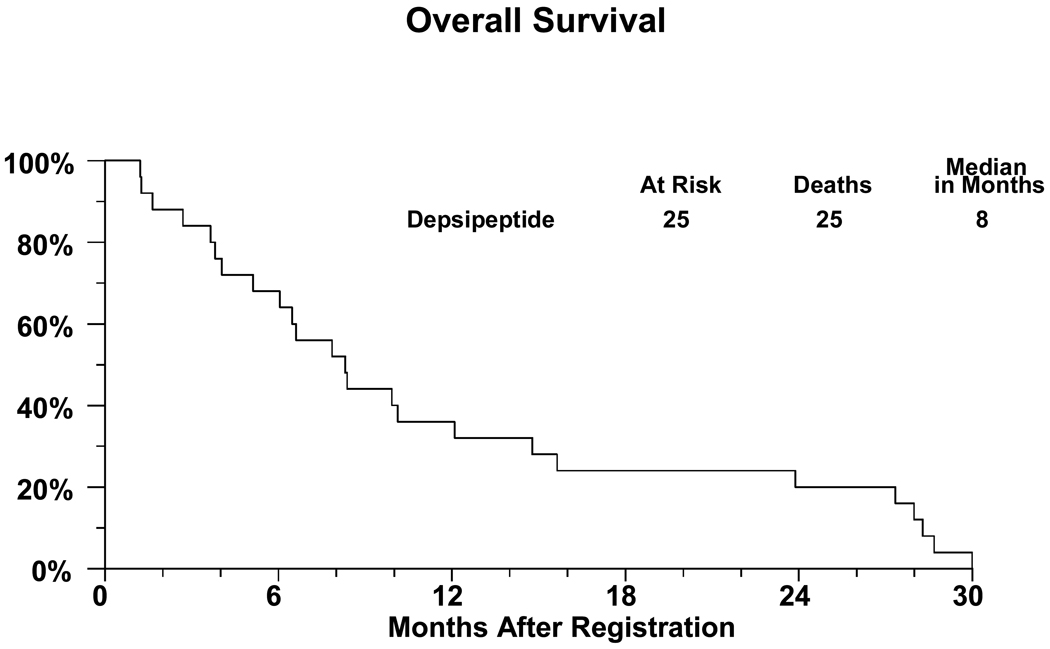
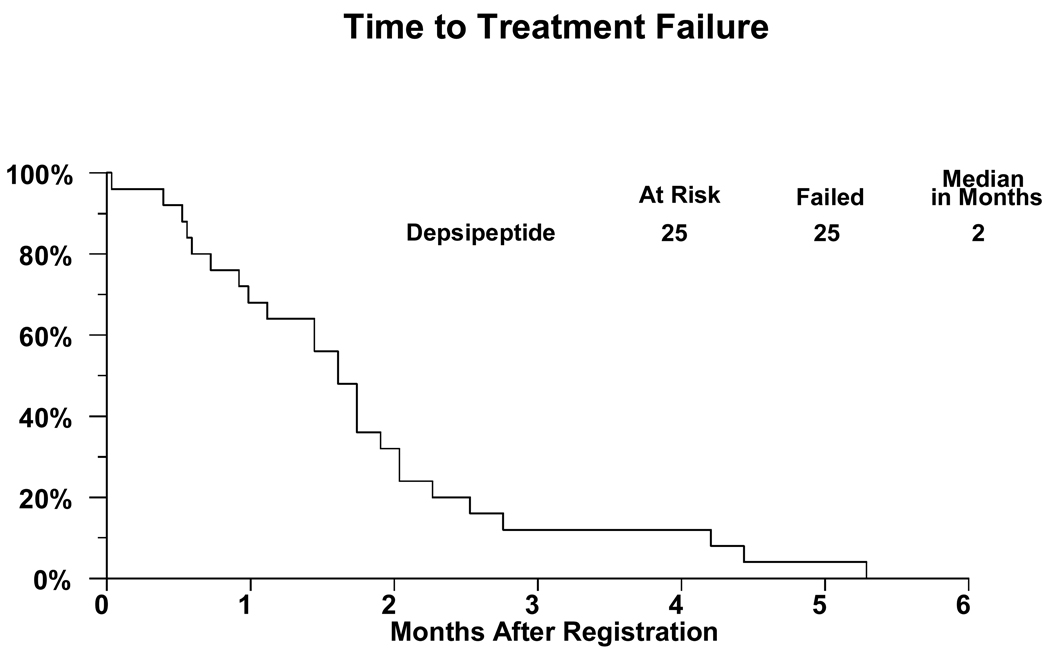
There were 28 patients registered to the study with 2 proving to be ineligible. One ineligible patient did not meet the magnesium level requirements and the other patient had baseline laboratories done more than 14 days prior to registration. One eligible patient refused all treatment due to lack of insurance coverage and is not analyzable for any endpoint. A major deviation is recorded for another patient who did not begin treatment until 19 days after registration. Thus there are 25 analyzable patients. For these patients, 15 (60%) are male and the median age was 58.3 years (range 17.9–84.2 years). Performance status of 0 was present in sixteen patients and nine had performance status of 1. Ten patients had received one prior chemotherapy regimen and fifteen two prior regimens. Additional patient characteristics including sites of disease are shown in Table 1. All patients are off treatment. A treatment summary giving reasons patients had stopped treatment is given in Table 2. Two patients refused further protocol treatment, one wanted to pursue symptom management only, and the other did not want to spend so much time in the clinic. Six patients were removed from treatment due to toxicities: thrombocytopenia and ST-T wave changes on EKG; nausea/vomiting, fatigue, and dehydration; weakness, anorexia and weight loss; persistent nausea; fevers; and, inability to tolerate study drug. Response data are detailed in Table 3. Out of the 25 eligible and analyzable patients accrued in the first stage of the protocol, no objective responses were observed and the study was permanently closed. Four patients (16%; 95% confidence interval 5%-36%) maintained stable disease as the best response. Twenty-five patients were assessed for toxicity. No grade 4 or greater toxicities were observed. Grade 3 Toxicity is given in Table 4. Fourteen of the 25 patients experienced grade 3 toxicities, primarily anorexia and fatigue. Curves for survival and failure-free survival are given as Figures 1 and 2.
Table 1.
Patient Characteristics (N = 25)
| Age | Median | 58.3 years | |
| Range | 17.9–84.2 years | ||
| Sex | Males | 15 (60%) | |
| Females | 10 (40%) | ||
| Performance Status | 0 | 16 (64%) | |
| 1 | 9 (36 %) | ||
| Number of Prior Chemotherapy Regimens | 1 | 10 patients (40%) | |
| 2 | 15 patients (60%) | ||
| Primary Site | Colon | 20 (80%) | |
| Rectum | 5 (20%) | ||
| Sites of Disease | Liver | 17 (68%) | |
| Lung | 14 (56%) | ||
| Distant Nodes | 5 (20%) | ||
| Regional Nodes | 4 (16%) | ||
| Other Abdominal Sites | 3 (12%) | ||
| Primary Site/Direct Extension | 2 (8%) | ||
| Bone | 2 (8%) | ||
| Other | 5 (20%) | ||
Table 2.
Reasons for Discontinuation of Treatment and Number of Protocol Deviations (N = 25)
| Adverse Events or Side Effects | 6 |
| Refusal Unrelated to Adverse Events | 2 |
| Progression/Relapse | 17 |
| Death | 0 |
| Other – Not Protocol Specified | 0 |
| Major Protocol Deviations | 1 |
Table 3.
Response (N = 25)
| Complete Response | 0 | 0% |
| Partial Response | 0 | 0% |
| Unconfirmed Complete Response | 0 | 0% |
| Unconfirmed Partial response | 0 | 0% |
| Stable/No Response | 4 | 16% |
| Increasing Disease | 17 | 68% |
| Symptomatic Deterioration | 3 | 12% |
| Assessment Inadequate | 1 | 4% |
| Total | 25 | 100% |
Table 4.
Toxicity Grade 3 (N = 25)
| Toxicity | Number of Patients with Grade 3 |
|---|---|
| 1. Anorexia | 5 |
| 2. Dehydration | 3 |
| 3. Fatigue | 6 |
| 4. Hemoglobin | 1 |
| 5. Hyperglycemia | 1 |
| 6. Hyponatremia | 1 |
| 7. Lung infection | 1 |
| 8. Nausea | 3 |
| 9. Headache | 2 |
| 10. Prolonged QTc | 1 |
| 11. Vomiting | 2 |
Figure 1.
Figure 2.
Discussion
Widely metastatic colorectal cancer is not curable with currently available chemotherapy agents and new treatments are needed. This trial evaluated depsipeptide, one of the new class of histone deacetylase inhibitors, in the treatment of patients with metastatic colorectal cancer who had failed one or two prior chemotherapy regimens. The rationale for this treatment is based on the finding that histone acetylation plays a role in chromatin structure and gene transcription which can effect cell division, differentiation, and apoptosis (23–26). Depsipeptide has shown antiproliferative activity against several human solid tumor cell lines and inhibition of stomach, lung, and breast cancer growth in human tumor xenograft models. Therefore it is disappointing that no activity was seen in this Phase II trial in colorectal cancer patients with advanced previously treated disease. This discrepancy between the model systems and patients may occur because of the lack of specificity of which genes are effected by the alterations in histone composition produced by the drug. The effect of histone deacetylase inhibitors on cellular function is complex (23–26). Whether an over all balance toward cellular proliferation or apoptosis may occur as a result of new protein production cannot be predicted in advance with our current state of knowledge. There may be both positive and negative effects on oncogenic and oncosuppressive mechanisms. Another factor to consider may be that resistance to depsipeptide may develop. Depsipeptide is reported to be a substrate for both P-glycoprotein and multidrug resistance-resistance associated protein (MRP 1) (20,26), and it can induce multidrug-resistance gene 1 expression in human colon cancer cell lines (21). All of these patients had failed one or two prior multidrug chemotherapy regimens and could reasonably be expected to have some degree of broad spectrum drug resistance. These types of patients were used for this trial because ethically, it was not considered proper to use a new investigational agent first line when proven effective, although non-curative, treatments existed for disseminated colorectal cancer.
Though single agent depsipeptide may lack clinical activity in this tumor type, there has been reported synergism between DNA hypomethylating agents and histone deacetylase inhibition in the re-expression of silenced genes. Similarly, there can be benefit from combining histone deacetylase inhibition and retinoic acid in resistant cases of acute promyelocytic leukemia (23,24,26). Histone deacetylase inhibitors may be synergistic with heat-shock protein 90 (HSP90) inhibitors, blocking the ability of HSP90 to chaperone proteins required for tumor cell survival (23,26). Histone deacetylase inhibitors may also prevent repair of DNA damaged by chemotherapy agents (23,24,26).
Histone deacetylase inhibitors have shown good activity in patients with T-cell cutaneous lymphoma (CTCL) and one agent in this class, vorinostat, has been FDA approved for treatment of this disease (24,26).
Future trials of these agents in colorectal cancer and other solid tumors might be attempted with close monitoring of specific gene activation or proteins produced as a result of treatment. Trials of depsipeptide in combination with DNA hypomethylating agents or chemotherapy drugs should also be considered with close monitoring of results. Multiple and complex changes occur in cells treated with the histone deacetylase inhibitors, and importantly, normal cells seem to be more resistant to histone deacetylase inhibition than tumor cells (1,23,25). It seems possible that with more definitive study, these interesting and complex agents will find additional roles in cancer treatment.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA58416, CA74647, CA58861, CA45807, CA35178, CA45560, CA46441, CA12644, CA20319, CA45808, CA35128, CA58723, CA22433, CA46113, CA105409, CA46113 CA20319
References
- 1.Ueda H, Nakajima H, Hori Y, Fujita T, Nishimura M, Goto T, Okuhara M. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J Antibiot. 1994;47:301–310. doi: 10.7164/antibiotics.47.301. [DOI] [PubMed] [Google Scholar]
- 2.Ueda H, Nakajima H, Hori Y, Goto T, Okuhara M. Action of FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968, on Ha-ras transformed NIH3T3 Cells. Biosci Biotech Biochem. 1994;58:1579–1583. doi: 10.1271/bbb.58.1579. [DOI] [PubMed] [Google Scholar]
- 3.Fecteau K, Mei J, Wang HC. Differential modulation of signaling pathways and apoptosis of ras-transformed lOTl/2 cells by the depsipeptide FR901228. J Pharmacol Exp Ther. 2002;300:890–899. doi: 10.1124/jpet.300.3.890. [DOI] [PubMed] [Google Scholar]
- 4.Sandor V, Senderowicz A, Mertins S, Sackett D, Sausville E, Blagoskionny MV, Bates SE. P21-dependent g(1)arrest with downregulation of cyclin D1 and upregulation of cyclin E by the histone deacetylase inhibitor FR901228. Br J Cancer. 2000;83:817–825. doi: 10.1054/bjoc.2000.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 6.Weidle UH, Grossman A. Inhibition of histone deacetylases: a new strategy to target epigenetic modifications for anticancer treatment. Anticancer Res. 2000;20:1471–1485. [PubMed] [Google Scholar]
- 7.Nakajima H, Kim YB, Terano H, Yoshida M, Horinouchi S. FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp Cell Res. 1998;241:126–133. doi: 10.1006/excr.1998.4027. [DOI] [PubMed] [Google Scholar]
- 8.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 9.Kitazono M, Robey R, Zhan Z, Sarlis NJ, Skarulis MC, Aikou T, Bates S, Fojo T. Low concentrations of the histone deacetylase inhibitor, depsipeptide ( FR901228), increase expression of the Na(+)/I(−) symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab. 2001;86:3430–3435. doi: 10.1210/jcem.86.7.7621. [DOI] [PubMed] [Google Scholar]
- 10.Kitazono M, Goldsmith ME, Aikou T, Bates S, Fojo T. Enhanced adenovirus transgene expression in malignant cells treated with the histone deacetylase inhibitor FR901228. Cancer Res. 2001;61:6328–6330. [PubMed] [Google Scholar]
- 11.Weiser TS, Ohnmacht GA, Guo ZS, Fischette MR, Chen GA, Hong JA, Nguyen DM, Schrump DS. Induction of MAGE-3 expression in lung and esophageal cancer cells. Ann Thorac Surg. 2001;71:295–301. doi: 10.1016/s0003-4975(00)02421-8. [DOI] [PubMed] [Google Scholar]
- 12.Weiser TS, Guo ZS, Ohnmacht GA, Parkhurst ML, Tong-On P, Marincola FM, Fischette MR, Yu X, Chen GA, Hong JA, Stewart JH, Nguyen DM, Rosenberg SA, Schrump DS. Sequential 5-Aza-2’-deoxycytidine-Depsipeptide FR901228 treatment induces apoptosis 23 preferentially in cancer cells and facilitates their recognition by cytolytic T lymphocytes specific for NY-ESO-l. J Immunother. 2001;24:151–161. doi: 10.1097/00002371-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Zhu WG, Dai Z, Ding H, Srinivasan K, Hall J, Duan W, Villalona-Calero MA, Plass C, Otterson GA. Increased expression of unmethylated CDKN2D by 5-aza-2’-deoxycytidine in human lung cancer cells. Oncogene. 2001;20:7787–7796. doi: 10.1038/sj.onc.1204970. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Lu S, Wu L, Chai G, Wang H, Chen Y, Sun J, Yu Y, Zhou W, Zheng Q, Wu M, Otterson GA, Zhu WG. Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21(Waf1/Cip1) Mol Cell Biol. 2006;26:2782–2790. doi: 10.1128/MCB.26.7.2782-2790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu WG, Otterson GA. The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells. Curr Med Chem Anticancer Agents. 2003;3:187–199. doi: 10.2174/1568011033482440. [DOI] [PubMed] [Google Scholar]
- 16.Kwon HJ, Kim MS, Kim MJ, Nakajima H, Kim KW. Histone deacetylase inhibitor FK228 inhibits tumor angiogenesis. Int J Cancer. 2002;97:290–296. doi: 10.1002/ijc.1602. [DOI] [PubMed] [Google Scholar]
- 17.Murata M, Towatari M, Kosugi H, Tanimoto M, Ueda R, Saito H, Naoe T. Apoptotic cytotoxic effects of a histone deacetylase inhibitor, FK228, on malignant lymphoid cells. Jpn J Cancer Res. 2000;91:1154–1160. doi: 10.1111/j.1349-7006.2000.tb00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X, Guo ZS, Marcu MG, Neckers L, Nguyen DM, Chen GA, Schrump DS. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 19.Rajgolikar G, Chan KK, Wang HC. Effects of a novel antitumor depsipeptide, FR901228, on human breast cancer cells. Breast Cancer Res Treat. 1998;51:29–38. doi: 10.1023/a:1006091014092. [DOI] [PubMed] [Google Scholar]
- 20.Ueda H, Manda T, Matsumoto S, Mukumoto S, Nishigaki F, Kawamura I, Shimomura K. FR9O1228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. III. Antitumor activities on experimental tumors in mice. J Antibiot. 1994;47:315–323. doi: 10.7164/antibiotics.47.315. [DOI] [PubMed] [Google Scholar]
- 21.Sandor V, Bakke S, Robey RW, Kang MH, Blagosklonny MV, Bender J, Brooks R, Piekarz RL, Tucker E, Figg WD, Chan KK, Goldspiel B, Fojo AT, Balcerzak SP, Bates SE. Phase I trial of the histone deacetylase inhibitor, depsipeptide ( FR901228, NSC 630176), in patients with refractory neoplasms. Clinical Cancer Research. 2002;8:718–728. [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New Guidelines to Evaluate Response to Treatrment in Solid Tumors. Journal of the National Cancer Institute. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Minucci S, Pelicci PG. Histone Deacetylase Inhibitors and the Promise of Epigenetic (and more) Treatments for Cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 24.Marchion D, Munster P. Development of histone deacetylase inhibitors for cancer treatment. Expert Rev. Anticancer Ther. 2007;7:583–598. doi: 10.1586/14737140.7.4.583. [DOI] [PubMed] [Google Scholar]
- 25.Piekarz RL, Frye AR, Wright JJ, Steinberg SM, Liewehr DJ, Rosing DR, Sachdev V, Fojo T, Bates SE. Cardiac studies in Patients Treated with Depsipeptide, FK228, in a Phase II Trial for T-Cell Lymphoma. Clin Cancer Res. 2006;12 doi: 10.1158/1078-0432.CCR-05-2095. 3762- [DOI] [PubMed] [Google Scholar]
- 26.Glaser KB. HDAC inhibitors: Clinical update and mechanism-based potential. Biochemical Pharmacology. 2007;74:659–671. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]