Abstract
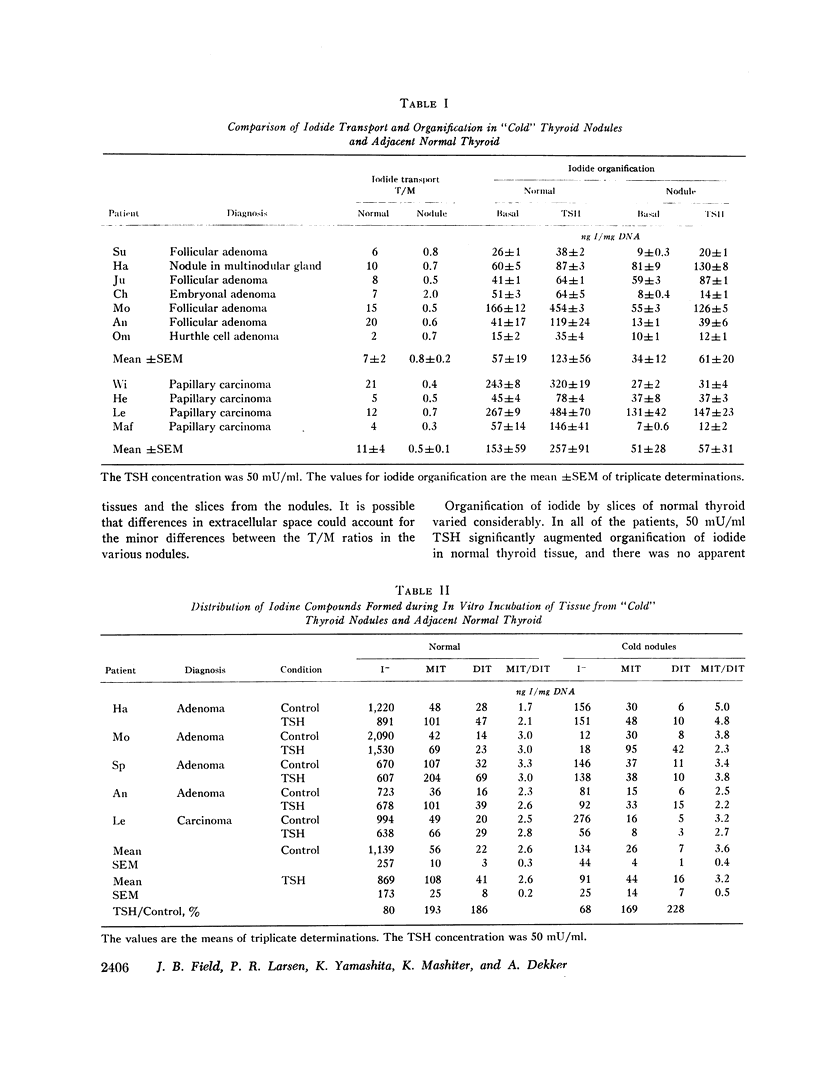
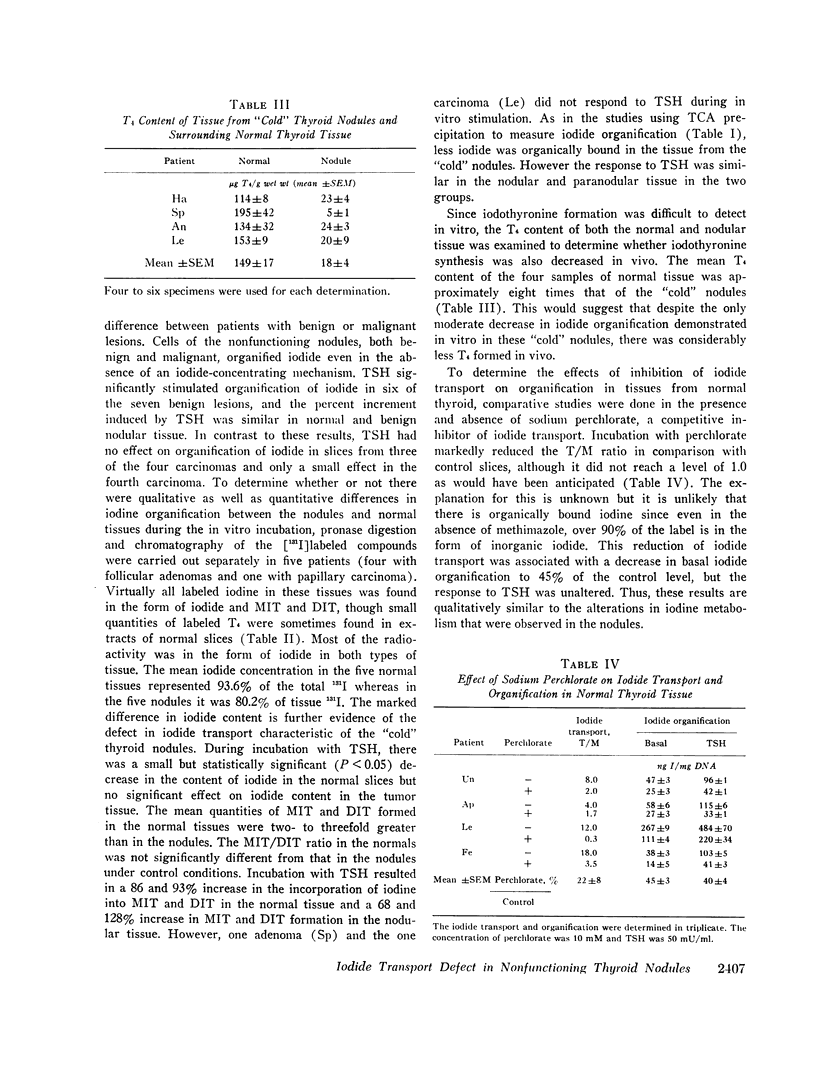
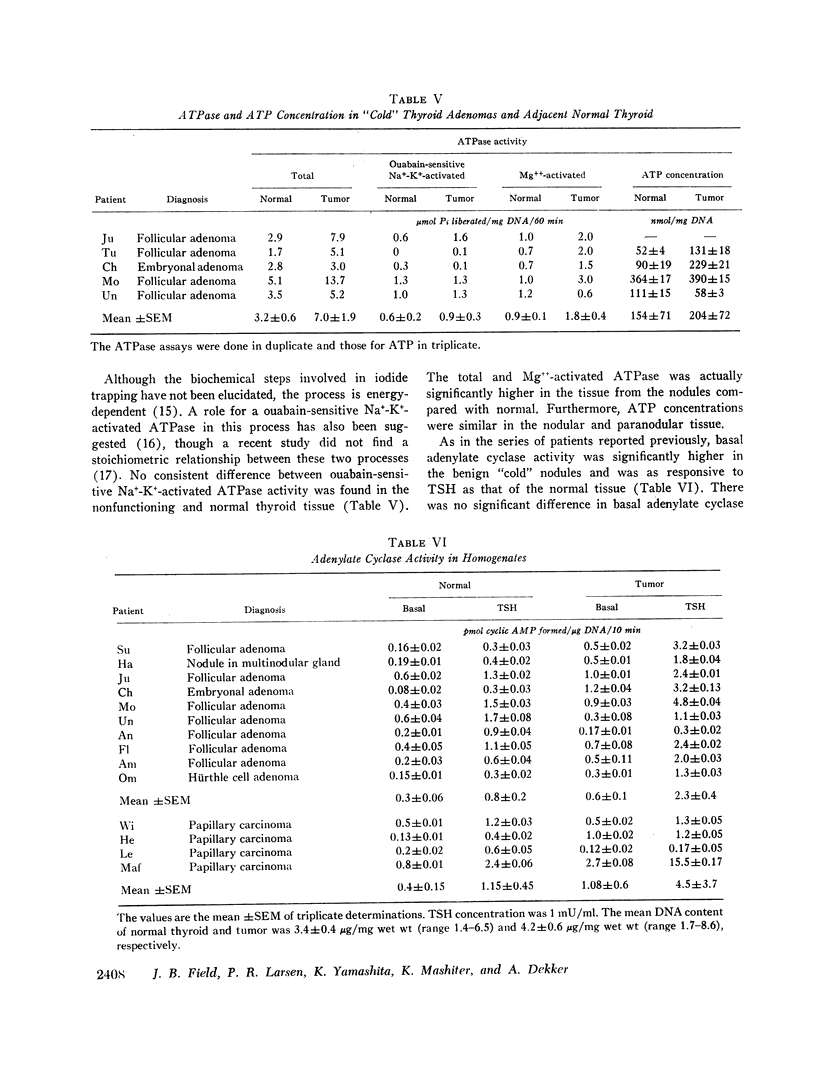
Benign and malignant nodules in human thyroid glands, which did not concentrate iodide in vivo, were also unable to accumulate iodide in vitro. The mean thyroid-to-medium ratio (T/M) in seven benign nodules was 0.8±0.2 compared with 7±2 in adjacent normal thyroid tissue. In four malignant thyroid nodules, the mean T/M was 0.5±0.1 compared with 11±4 in adjacent normal thyroid. Despite the inability of such nodules to concentrate iodide, iodide organification was present but was only one-half to one-third as active as in surrounding normal thyroid. Thyroid-stimulating hormone (TSH) increased iodide organification equally in both benign nodules and normal thyroid although it had no effect in three of the four malignant lesions. The reduction in organification is probably related to the absence of iodide transport, since incubation of normal thyroid slices with perchlorate caused similar diminution in iodide incorporation but no change in the response to TSH. Monoiodotyrosine (MIT) and di-iodotyrosine (DIT) accounted for most of the organic iodide in both the nodules and normal tissue. The MIT/DIT ratio was similar in normal and nodule tissue. The normal tissue contained much more inorganic iodide than the nodules, consistent with the absence of the iodide trap in the latter tissue. The thyroxine content of normal thyroid was 149±17 μg/g wet wt and 18±4 μg/g wet wt in the nodules. The transport defect in the nodules was not associated with any reduction in total, Na+-K+- or Mg++-activated ATPase activities or the concentration of ATP. Basal adenylate cyclase was higher in nodules than normal tissue. Although there was no difference between benign and malignant nodules, the response of adenylate cyclase to TSH was greater in the benign lesions.
These studies demonstrate that nonfunctioning thyroid nodules, both benign and malignant, have a specific defect in iodide transport that accounts for their failure to accumulate radioactive iodide in vivo. In benign nodules, iodide organification was increased by TSH while no such effect was found in three of four malignant lesions, suggesting additional biochemical defects in thyroid carcinomas.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn C. S., Rosenberg I. N. Iodine metabolism in thyroid slices: effects of TSH, dibutyryl cyclic 3',5'-AMP, NaF and prostaglandin E-1. Endocrinology. 1970 Feb;86(2):396–405. doi: 10.1210/endo-86-2-396. [DOI] [PubMed] [Google Scholar]
- Ahn C. S., Rosenberg I. N. Prompt stimulation of the organic binding of iodine in the thyroid by adenosine 3',5'-phosphate in vivo. Proc Natl Acad Sci U S A. 1968 Jul;60(3):830–835. doi: 10.1073/pnas.60.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. The relation between the synthesis of deoxyribonucleic acid and the synthesis of protein in the multiplication of bacteriophage T2. Biochem J. 1955 Nov;61(3):473–483. doi: 10.1042/bj0610473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunberg J. A., Halmi N. S. The role of ouabain-sensitive adenosine triphosphatase in the stimulating effect of thyrotropin on the iodide pump of the rat thyroid. Endocrinology. 1966 Oct;79(4):801–807. doi: 10.1210/endo-79-4-801. [DOI] [PubMed] [Google Scholar]
- DeRubertis F., Yamashita K., Dekker A., Larsen P. R., Field J. B. Effects of thyroid-stimulating hormone on adenyl cyclase activity and intermediary metabolism of "cold" thyroid nodules and normal human thyroid tissue. J Clin Invest. 1972 May;51(5):1109–1117. doi: 10.1172/JCI106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. E. The action of thyrotropin on thyroid metabolism. Vitam Horm. 1971;29:287–412. doi: 10.1016/s0083-6729(08)60051-5. [DOI] [PubMed] [Google Scholar]
- Inoue K., Taurog A. Digestion of 131I-labeled thyroid tissue with maximum recovery of 131I-iodothyronines. Endocrinology. 1967 Aug;81(2):319–332. doi: 10.1210/endo-81-2-319. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., DeRubertis F., Yamashita K., Dekker A., Field J. B. In vitro demonstration of iodide-trapping defect but normal thyrotropin (TSH) responsiveness in benign and malignant "cold" thyroid nodules. Trans Assoc Am Physicians. 1972;85:309–316. [PubMed] [Google Scholar]
- Larsen P. R., Yamashita K., Dekker A., Field J. B. Biochemical observations in functioning human thyroid adenomas. J Clin Endocrinol Metab. 1973 May;36(5):1009–1018. doi: 10.1210/jcem-36-5-1009. [DOI] [PubMed] [Google Scholar]
- Ohta M., Jarrett R. J., Field J. B. Measurement of ATP in tissues with the use of C14-O2 production from glucose-1-C14. J Lab Clin Med. 1966 Jun;67(6):1013–1024. [PubMed] [Google Scholar]
- Pochin E. E. Thyroid adenocarcinoma: a functioning tumour. Lancet. 1969 Jan 11;1(7585):94–98. doi: 10.1016/s0140-6736(69)91105-2. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER P. B., WOLFF J. THYROIDAL IODIDE TRANSPORT. VI. ON A POSSIBLE ROLE FOR IODIDE-BINDING PHOSPHOLIPIDS. Biochim Biophys Acta. 1965 Jan 25;94:114–123. doi: 10.1016/0926-6585(65)90014-2. [DOI] [PubMed] [Google Scholar]
- Schneider P. B. Effects of thyrotropin on thyroidal phospholipid and adenosine 5'-triphosphate metabolism. J Biol Chem. 1969 Aug 25;244(16):4490–4493. [PubMed] [Google Scholar]
- Steinberg M., Cavalieri R. R., Choy S. H. Uptake of technetium 99-pertechnetate in a primary thyroid carcinoma: need for caution in evaluating nodules. J Clin Endocrinol Metab. 1970 Jul;31(1):81–84. doi: 10.1210/jcem-31-1-81. [DOI] [PubMed] [Google Scholar]
- TONG W. THYROTROPIN STIMULATION OF THYROXINE SYNTHESIS IN ISOLATED THYROID CELLS TREATED WITH PERCHLORATE. Endocrinology. 1964 Dec;75:968–969. doi: 10.1210/endo-75-6-968. [DOI] [PubMed] [Google Scholar]
- Tyler D. D., Gonze J., Lamy F., Dumont J. E. Influence of mitochondrial inhibitors on the respiration and energy-dependent uptake of iodide by thyroid slices. Biochem J. 1968 Jan;106(1):123–133. doi: 10.1042/bj1060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher M. S., Arzoumanian A. Y. Thyroid nodule scans made with pertechnetate and iodine may give inconsistent results. J Nucl Med. 1971 Mar;12(3):136–137. [PubMed] [Google Scholar]
- Valenta L. Metastatic thyroid carcinoma in man concentrating iodine without organification. J Clin Endocrinol Metab. 1966 Dec;26(12):1317–1324. doi: 10.1210/jcem-26-12-1317. [DOI] [PubMed] [Google Scholar]
- WOLFF J., HALMI N. S. Thyroidal iodide transport. V. The role of Na-K-activated, ouabain-sensitive adenosinetriphosphatase activity. J Biol Chem. 1963 Feb;238:847–851. [PubMed] [Google Scholar]
- Weinhouse S. Glycolysis, respiration, and anomalous gene expression in experimental hepatomas: G.H.A. Clowes memorial lecture. Cancer Res. 1972 Oct;32(10):2007–2016. [PubMed] [Google Scholar]
- Wilson B., Raghupathy E., Tonoue T., Tong W. TSH-like actions of dibutyryl-cAMP on isolated bovine thyroid cells. Endocrinology. 1968 Oct;83(4):877–884. doi: 10.1210/endo-83-4-877. [DOI] [PubMed] [Google Scholar]
- Wolff J., Jones A. B. The purification of bovine thyroid plasma membranes and the properties of membrane-bound adenyl cyclase. J Biol Chem. 1971 Jun 25;246(12):3939–3947. [PubMed] [Google Scholar]
- Zor U., Kaneko T., Lowe I. P., Bloom G., Field J. B. Effect of thyroid-stimulating hormone and prostaglandins on thyroid adenyl cyclase activation and cyclic adenosine 3',5',-monophosphate. J Biol Chem. 1969 Oct 10;244(19):5189–5195. [PubMed] [Google Scholar]


