Abstract
Background
Blood flow in the corpus luteum (CL) is closely related to luteal function. It is unclear how luteal blood flow is regulated. Standardized ovarian-stimulation protocol with a gonadotropin-releasing hormone agonist (GnRHa long protocol) causes luteal phase defect because it drastically suppresses serum LH levels. Examining luteal blood flow in the patient undergoing GnRHa long protocol may be useful to know whether luteal blood flow is regulated by LH.
Methods
Twenty-four infertile women undergoing GnRHa long protocol were divided into 3 groups dependent on luteal supports; 9 women were given ethinylestradiol plus norgestrel (Planovar) orally throughout the luteal phase (control group); 8 women were given HCG 2,000 IU on days 2 and 4 day after ovulation induction in addition to Planovar (HCG group); 7 women were given vitamin E (600 mg/day) orally throughout the luteal phase in addition to Planovar (vitamin E group). Blood flow impedance was measured in each CL during the mid-luteal phase by transvaginal color-pulsed-Doppler-ultrasonography and was expressed as a CL-resistance index (CL-RI).
Results
Serum LH levels were remarkably suppressed in all the groups. CL-RI in the control group was more than the cutoff value (0.51), and only 2 out of 9 women had CL-RI values < 0.51. Treatments with HCG or vitamin E significantly improved the CL-RI to less than 0.51. Seven of the 8 women in the HCG group and all of the women in the vitamin E group had CL-RI < 0.51.
Conclusion
Patients undergoing GnRHa long protocol had high luteal blood flow impedance with very low serum LH levels. HCG administration improved luteal blood flow impedance. This suggests that luteal blood flow is regulated by LH.
Background
During corpus luteum formation after the ovulatory LH surge, active angiogenesis occurs and the corpus luteum becomes one of the most highly vascularized organs in the body [1,2]. Blood flow in the corpus luteum is important for the development of the corpus luteum and maintenance of luteal function [3-5]. Adequate blood flow in the corpus luteum is necessary to provide luteal cells with the large amounts of cholesterol that are needed for progesterone synthesis and to deliver progesterone to the circulation [6].
Luteal phase defect has been implicated as a cause of infertility and spontaneous miscarriage. However, luteal phase defect has a complicated etiology and various causes. We recently reported a close relationship between luteal blood flow and luteal function [4]. Interestingly, luteal blood flow was significantly correlated with serum progesterone concentration during the mid-luteal phase, and luteal blood flow was significantly lower in women with luteal phase defect than in women with normal luteal function, suggesting that low blood flow of the corpus luteum is associated with luteal phase defect. Furthermore, we found that luteal phase defect can be improved by increasing luteal blood flow [5]. Therefore, a decrease in luteal blood flow is one of the causes of luteal phase defect.
However, it is still unclear how the decrease in blood flow is caused in patients with luteal phase defect, and how luteal blood flow is regulated in the ovary during the menstrual cycle. Luteal blood flow was increased by HCG administration during the luteal phase [5,7]. Luteal blood flow was also found to be related with serum HCG levels between 5 and 16 weeks of gestation [8]. These findings suggest that HCG or LH has a role in the regulation of luteal blood flow.
Gonadotropin-releasing hormone agonist (GnRHa) has been used to suppress endogenous gonadotropin secretion in standardized ovarian-stimulation protocol for IVF-ET, so called GnRHa long protocol. It is interesting to note that GnRHa long protocol causes luteal phase defect because of remarkable suppression of serum LH levels. Examining luteal blood flow in the patient undergoing GnRHa long protocol would be useful to know whether luteal blood flow is regulated by LH. Therefore, the present study was undertaken to examine luteal blood flow in the patient undergoing GnRHa long protocol.
Methods
The project was reviewed and approved by the Institutional Review Board of Yamaguchi University Graduate School of Medicine. Informed consent was obtained from all the patients in this study.
Ultrasonography
Blood flow in the corpus luteum was measured as reported previously [4] using a computerized ultrasonography with an integrated pulsed Doppler vaginal scanner [Aloka ProSound SSD-3500SV and Aloka UST-984-5 (5.0 MHz) vaginal transducer, Aloka Co. Ltd, Tokyo, Japan]. The high pass filter was set at 100 Hz, and the pulse repetition frequency was 2-12 kHz, for all Doppler spectral analyses. After the endovaginal probe was gently inserted into the vagina, adnexal regions were thoroughly scanned. The ovary was identified, and color signals were used to detect the area with the highest blood flow within the corpus luteum. Blood flow was identified in the peripheral area of the corpus luteum [4]. The pulsed Doppler gate was then placed on that area to obtain flow velocity waveforms. An acceptable angle was less than 60°, and the signal was updated until at least four consecutive flow velocity waveforms of good quality were obtained. Blood flow impedance was estimated by calculating the resistance index (RI), which is defined as the difference between maximal systolic blood flow (S) and minimal diastolic flow (D) divided by the peak systolic flow (S-D/S). Blood flow impedances were examined in the corpus luteum during the mid-luteal phase (6-8 days after ovulation). The day of ovulation was determined by urinary LH, transvaginal ultrasonography and basal body temperature records. The cutoff value of the RI of the corpus luteum (CL-RI) was previously determined by receiver operating characteristic curve (ROC) analysis [5]. A cutoff value of 0.51 provided the best combination with 84.3% sensitivity and 85.6% specificity to discriminate between normal luteal function and luteal phase defect [5]. Thus, when CL-RI was more than 0.51, the patient was diagnosed as having decreased luteal blood flow. Since the interobserver coefficient of variation for Doppler flow measurements in the present study was less than 10%, the Doppler flow measurements were judged to be reproducible.
Clinical studies
Twenty-four patients were enrolled in this study. The mean age was 36.6 years, with a range of 23-43 years. The patients were non-smokers and free from major medical illness including hypertension; they were excluded if they had myoma, adenomyosis, congenital uterine anomaly, or ovarian tumors or if they used estrogens, progesterone, androgens, or had chronic use of any medication, including nonsteroidal anti-inflammatory agents. The patients received artificial insemination with husband's semen (AIH) under the standardized ovarian-stimulation protocol (GnRHa long protocol), consisting of GnRHa (900 mg/day buserelin acetate, Suprecur; Mochida Pharmaceutical Co. Ltd., Tokyo, Japan) beginning in the mid-luteal phase of the previous cycle, followed by 225 IU follicle-stimulating hormone (FSH, Folyrmon-P; Fuji Pharmaceutical Co. Ltd., Tokyo, Japan) on the third day and days 4 and 5, and thereafter by 150 IU human menopausal gonadotropin (hMG, HMG-F; Fuji Pharmaceutical Co. Ltd., Tokyo, Japan). When follicles reached 18 mm or more in diameter by ultrasonography, 10,000 IU human chorionic gonadotropin (HCG, Gonatropin; Asuka Pharmaceutical Co. Ltd., Tokyo, Japan) was administered for ovulation induction. Since the GnRHa long protocol causes luteal phase defect because of low serum LH levels due to GnRHa-induced gonadotropin suppression, the patients received some treatments as a luteal support. Dependent on luteal supports, the patients were randomly divided into 3 groups; 9 women were given ethinylestradiol (0.05 mg) plus norgestrel (0.5 mg) (Planovar, Weis-Ezai Co Ltd., Tokyo, Japan) orally from the day after ovulation induction throughout the luteal phase (control group); 8 women were given HCG 2,000 IU on days 2 and 4 after ovulation induction in addition to Planovar (HCG group); 7 women were given vitamin E (600 mg/day, 3 times per day; Eisai Co., Ltd., Tokyo, Japan) orally throughout the luteal phase in addition to Planovar (vitamin E group). Planovar was used as a control in this study because it did not affect luteal blood flow in our preliminary study [CL-RI of the treatment group and the no treatment group: 0.515 ± 0.073 v.s. 0.505 ± 0.019 (mean ± SEM, n = 11), not significant]. Vitamin E was used to increase luteal blood flow as we reported previously [5]. CL-RI as blood flow impedances in the corpus luteum and serum concentrations of LH, FSH, and progesterone were measured during the mid-luteal phase (6-8 days after ovulation). For patients with multiple ovulations, CL-RI was examined in each corpus luteum, and the mean was used as a patient mean value.
Statistical analyses
Statistical analysis was carried out with SPSS for Windows 13.0. Kruskal-Wallis test followed by the Mann-Whitney U-test using the Bonferroni correction and chi-squared test were used as appropriate. A value of P < 0.05 was considered significant.
Results
Table 1 shows the patient profile of the treatment groups. The numbers of matured follicles and ovulated follicles and serum progesterone concentrations did not significantly differ among the groups (Table 1). Serum concentrations of LH and FSH were remarkably suppressed in all groups (Table 1).
Table 1.
Profiles of the treatment groups
| n | age | No. of preovulatory follicles (18 mm or greater) | No. of ovulation | serum concentrations | |||
|---|---|---|---|---|---|---|---|
| LH (mIU/ml) | FSH (mIU/ml) | P4 (ng/ml) | |||||
| Control | 9 | 36.4 ± 1.7 | 2.1 ± 0.4 | 1.9 ± 0.4 | 0.10 ± 0.03 | 1.20 ± 0.4 | 38.9 ± 7.8 |
| HCG | 8 | 37.8 ± 1.7 | 2.8 ± 0.7 | 2.6 ± 0.5 | 0.12 ± 0.01 | 0.94 ± 0.2 | 56.2 ± 22.9 |
| Vitamin E | 7 | 35.7 ± 1.4 | 2.3 ± 0.5 | 2.6 ± 0.8 | 0.22 ± 0.04 | 0.85 ± 0.1 | 32.3 ± 10.9 |
Twenty-four patients who underwent AIH under the standardized ovarian-stimulation protocol with GnRHa were recruited in this study. The numbers of follicles (18 mm or greater) were measured at the day of HCG injection for ovulation induction. The numbers of ovulated follicles were estimated 2 days after HCG injection. Dependent on luteal supports, the patients were divided into 3 groups; 9 women were given ethinylestradiol (0.05 mg) plus norgestrel (0.5 mg) (Planovar) orally throughout the luteal phase; 8 women were given hCG 2,000 IU on days 2 and 4 after ovulation induction in addition to Planovar (HCG group); 7 women were given vitamin E (600 mg/day, 3 times per day) orally throughout the luteal phase in addition to Planovar (vitamin E group). Planovar was used as a control in this study because it does not affect luteal blood flow. Vitamin E was used to increase luteal blood flow. Serum concentrations of LH, FSH, and progesterone were examined during the mid-luteal phase (6-8 days after ovulation). Values are mean ± SEM. There were no significant differences in any parameters between the three treatment groups.
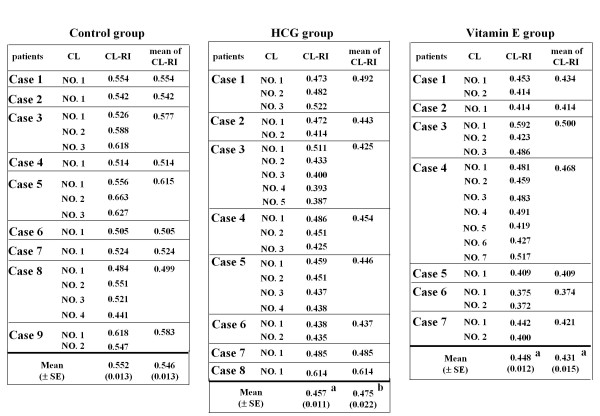
The mean CL-RI of the control group (0.564 ± 0.013) was more than the cutoff value (0.51); only 2 of the 9 patients in this group had CL-RI < 0.51 (Table 2 and Figure 1). Treatments with HCG or vitamin E significantly improved the CL-RI to less than 0.51; only 1 of the 8 patients in the HCG group and none of the 7 patients in the vitamin E group had CL-RI > 0.51 (Table 2 and Figure 1).
Table 2.
CL-RI of the treatment groups
| No. of patients | CL-RI | No. of patients with CL-RI < 0.51 | |
|---|---|---|---|
| Control | 9 | 0.546 ± 0.013 | 2/9 |
| HCG | 8 | 0.475 ± 0.022b | 7/8c |
| Vitamin E | 7 | 0.431 ± 0.015a | 7/7c |
The table summarizes the data in Figure 1. Corpus luteum-resistance index (CL-RI) was measured during the mid-luteal phase in the control group, HCG group, and vitamin E group. The value shows the mean ± SEM from the patient mean value. In this study, when CL-RI was more than 0.51, the patient was diagnosed as having decreased luteal blood flow.
a; p < 0.01 and b; p < 0.05 v.s. control (Kruskal-Wallis test followed by the Mann-Whitney U-test using the Bonferroni correction). c; p < 0.01 v.s. control (x2-test with Bonferroni correction).
Figure 1.
Corpus luteum-resistance index (CL-RI) of each corpus luteum of each patient in the treatment groups. Twenty-four patients who underwent AIH under the standardized ovarian-stimulation protocol with GnRHa were recruited in this study. Dependent on luteal supports, the patients were divided to 3 groups; 9 women were given ethinylestradiol plus norgestrel (Planovar) orally throughout the luteal phase; 8 women were given HCG 2,000 IU on days 2 and 4 after ovulation induction in addition to Planovar (HCG group); 7 women were given vitamin E (600 mg/day, 3 times per day) orally throughout the luteal phase in addition to Planovar (vitamin E group). Planovar was used as a control in this study because it does not affect luteal blood flow. Vitamin E was used to increase luteal blood flow. CL-RI was examined during the mid-luteal phase (6-8 days after ovulation). In case of patients with multiple ovulations, CL-RI was examined in each corpus luteum, and the mean was used as a patient mean value. a; p < 0.01 and b; p < 0.05 v.s. control group (Kruskal-Wallis test followed by the Mann-Whitney U-test using the Bonferroni correction).
We further focused on the CL-RI of each corpus luteum in case of the patients with multiple ovulations (Figure 1). In patients with multiple corpora lutea, CL-RI did not vary much among the corpora lutea (Figure 1). The mean CL-RI of corpora lutea in the control group (0.552 ± 0.013) was more than the cutoff value; only 3 of the 17 corpora lutea in this group had CL-RI < 0.51 (Table 3). Treatments with HCG or vitamin E significantly improved the CL-RI to less than 0.51, and the number of corpora lutea with CL-RI < 0.51 was 18 out of 21 corpora lutea in the HCG group and 16 out of 18 corpora lutea in the vitamin E group (Table 3).
Table 3.
Corpus luteum-related CL-RI in the treatment groups
| No. of CL | CL-RI | No. of CL with CL-RI < 0.51 | |
|---|---|---|---|
| Control | 17 | 0.552 ± 0.013 | 3/17 |
| HCG | 21 | 0.457 ± 0.011a | 18/21b |
| Vitamin E | 18 | 0.448 ± 0.012a | 16/18b |
Corpus luteum-resistance index (CL-RI) was measured in each corpus luteum of the patient during the mid-luteal phase in the control group, HCG group, and vitamin E group. The table summarizes the data in Figure 1. The value shows the mean ± SEM from the CL-RI of each corpus luteum. In this study, when CL-RI was more than 0.51, the corpus luteum was evaluated as having decreased blood flow.
a; p < 0.01 v.s. control (Kruskal-Wallis test followed by the Mann-Whitney U-test using the Bonferroni correction). b; p < 0.01 v.s. control (x2-test with Bonferroni correction).
Discussion
Our results show that patients undergoing the GnRHa long protocol have high blood flow impedance of the corpus luteum with very low serum LH levels, and that HCG treatment significantly improved blood flow impedance of the corpus luteum. Because high blood flow impedance of the corpus luteum in patients with luteal phase defect was improved by HCG administration [5], it is likely that LH is involved in the regulation of luteal blood flow.
Interestingly, in patients with multiple corpora lutea, CL-RI did not vary much among the individual corpora lutea, which suggests that CL-RI is influenced by endocrine factors.
Luteal phase defect has various causes. The GnRHa long protocol is known to cause luteal phase defect because it drastically suppresses serum LH levels. Luteal blood flow is closely related to luteal function [4,5]. The decrease in luteal blood flow is a critical factor in luteal phase defect [9-12]. Therefore, luteal phase defect caused by GnRHa long protocol is due not only to low serum LH levels but also to the decreased luteal blood flow.
The present study showed vitamin E has an ability to improve luteal blood flow impedance, in agreement with previous studies that showed vitamin E increases blood flow in a variety of organs including corpora lutea and endometrium [5,13-15].
Although HCG has an ability to improve luteal blood flow impedance, the mechanism is unclear. In the present study, HCG injection on days 2 and 4 after ovulation induction decreased luteal blood flow impedance. It is, therefore, likely that HCG influences luteal blood flow through some mediators rather than by its direct action [16]. One possible mediator is VEGF, which stimulates angiogenesis in the corpus luteum [17-19], and VEGF expression is increased by HCG [20-23]. HCG may, therefore, increase luteal blood flow by stimulating angiogenesis in the corpus luteum. HCG may also work through vasoactive substances such as nitric oxide (NO) or endothelin [24,25]. HCG increases NO synthase expression in the ovary of the rat and sheep [26,27], and increases rat ovarian blood flow via locally produced NO [28]. Endothelin-1, a vasoconstrictor, is produced by luteal cells [29], and HCG may affect luteal blood flow by regulating endothelin-1 [30]. However, further studies are needed to determine whether these factors have a role in the mechanism by which HCG increases luteal blood flow.
Conclusions
The present results show that the GnRHa long protocol causes high blood flow impedance of the corpus luteum and very low serum LH levels. Our result also showed that HCG administration decreases luteal blood flow impedance. Taken together, these results strongly suggested that luteal blood flow is regulated by LH.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AT conceived of the study, carried out the ultrasonographic studies, and performed the statistical analysis. IT, FK, RL, RM, HA, TT, HT, KS, and HM carried out the ultrasonographic studies. NS conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Contributor Information
Akihisa Takasaki, Email: a-takasaki@simo.saiseikai.or.jp.
Isao Tamura, Email: k006um@yamaguchi-u.ac.jp.
Fumie Kizuka, Email: m008um@yamaguchi-u.ac.jp.
Lifa Lee, Email: m014um@yamaguchi-u.ac.jp.
Ryo Maekawa, Email: ryomaekawa@gmail.com.
Hiromi Asada, Email: asapon@yamaguchi-u.ac.jp.
Toshiaki Taketani, Email: taketani@yamaguchi-u.ac.jp.
Hiroshi Tamura, Email: hitamura@yamaguchi-u.ac.jp.
Katsunori Shimamura, Email: k-shimamura@simo.saiseikai.or.jp.
Hitoshi Morioka, Email: h-morioka@simo.saiseikai.or.jp.
Norihiro Sugino, Email: sugino@yamaguchi-u.ac.jp.
Acknowledgements
This work was supported in part by Grants-in-Aid 20591918, 21592099, and 21791559 for Scientific Research from the Ministry of Education, Science, and Culture, Japan.
References
- Sugino N, Matsuoka A, Taniguchi K, Tamura H. Angiogenesis in the human corpus luteum. Reprod Med Biol. 2008;7:91–103. doi: 10.1111/j.1447-0578.2008.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino N, Suzuki T, Sakata A, Miwa I, Asada H, Taketani T, Yamagata Y, Tamura H. Angiogenesis in the human corpus luteum: changes in expression of angiopoietins in the corpus luteum throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. 2005;90:6141–6148. doi: 10.1210/jc.2005-0643. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Shirasuna K, Wijayagunawardane MP, Watanabe S, Hayashi M, Yamamoto D, Matsui M, Acosta TJ. Blood flow: a key regulatory component of corpus luteum function in the cow. Domest Anim Endocrinol. 2005;29:329–339. doi: 10.1016/j.domaniend.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Tamura H, Takasaki A, Taniguchi K, Matsuoka A, Shimamura K, Sugino N. Changes in blood flow impedance of the human corpus luteum throughout the luteal phase and during early pregnancy. Fertil Steril. 2008;90:2334–2339. doi: 10.1016/j.fertnstert.2007.10.056. [DOI] [PubMed] [Google Scholar]
- Takasaki A, Tamura H, Taniguchi K, Asada H, Taketani T, Matsuoka A, Yamagata T, Shimamura K, Morioka H, Sugino N. Luteal blood flow and luteal function. J Ovarian Res. 2009;2:1. doi: 10.1186/1757-2215-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka-Sakata A, Tamura H, Asada H, Miwa I, Taketani T, Yamagata Y, Sugino N. Changes in vascular leakage and expression of angiopoietins in the corpus luteum during pregnancy in rats. Reproduction. 2006;131:351–360. doi: 10.1530/rep.1.00947. [DOI] [PubMed] [Google Scholar]
- Beindorff N, Honnens A, Penno Y, Paul V, Bollwein H. Effects of human chorionic gonadotropin on luteal blood flow and progesterone secretion in cows and in vitro-microdialyzed corpora lutea. Theriogenology. 2009;72:528–534. doi: 10.1016/j.theriogenology.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Jurkovic D, Delogne-Desnoek J, Meuris S. Influence of human chorionic gonadotropin, oestradiol and progesterone on uteroplacental and corpus luteum blood flow in normal early pregnancy. Hum Reprod. 1992;7:1467–1473. doi: 10.1093/oxfordjournals.humrep.a137596. [DOI] [PubMed] [Google Scholar]
- Kupesic S, Kurjak A. The assessment of normal and abnormal luteal function by transvaginal color Doppler sonography. Eur J Obstet Gynecol Reprod Biol. 1997;72:83–87. doi: 10.1016/S0301-2115(96)02666-8. [DOI] [PubMed] [Google Scholar]
- Alcazar JL, Laparte C, Lopez-Garcia G. Corpus luteum blood flow in abnormal early pregnancy. J Ultrasound Med. 1996;15:645–649. doi: 10.7863/jum.1996.15.9.645. [DOI] [PubMed] [Google Scholar]
- Glock JL, Brumsted JR. Color flow pulsed Doppler ultrasound in diagnosing luteal phase defect. Fertil Steril. 1996;64:500–504. [PubMed] [Google Scholar]
- Kalogirou D, Antoniou G, Botsis D, Kontoravdis A, Vitoratos N, Giannikos L. Transvaginal Doppler ultrasound with color flow imaging in the diagnosis of luteal phase defect (LPD) Clin Exp Obstet Gynecol. 1997;24:95–97. [PubMed] [Google Scholar]
- Takasaki A, Tamura H, Taniguchi K, Miwa I, Taketani T, Shimamura K, Sugino N. Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil Steril. 2010;93:1851–1858. doi: 10.1016/j.fertnstert.2008.12.062. [DOI] [PubMed] [Google Scholar]
- Chung TW, Chen TZ, Yu JJ, Lin SY, Chen SC. Effects of α-tocopherol nicotinate on hemorheology and retinal capillary blood flow in female NIDDM with retinopathy. Clin Hemorheol. 1995;15:775–782. [Google Scholar]
- Chung TW, Yu JJ, Liu DZ. Reducing lipid peroxidation stress of erythrocyte membrane by α-tocopherol nicotinate plays an important role in improving blood rheological properties in type 2 diabetic patients with retinopathy. Diabetic Med. 1998;15:380–385. doi: 10.1002/(SICI)1096-9136(199805)15:5<380::AID-DIA592>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Norjavaara E, Olofsson J, Gafvels M, Selstam G. Redistribution of ovarian blood flow after injection of human chorionic gonadotropin and luteinizing hormone in the adult pseudopregnant rat. Endocrinology. 1987;120:107–114. doi: 10.1210/endo-120-1-107. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Chen H, Davis-Smyth T, Geber HP, Nguyen TN, Peers D, Chisholm V, Hillan K, Schwall R. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med. 1998;4:336–340. doi: 10.1038/nm0398-336. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Dickson SE, Lunn SF, Wulff C, Morris KD, Carroll VA, Bicknell R. Suppression of luteal angiogenesis in the primate after neutralization of vascular endothelial growth factor. Endocrinology. 2000;141:995–1000. doi: 10.1210/en.141.3.995. [DOI] [PubMed] [Google Scholar]
- Kashida S, Sugino N, Takiguchi S, Karube A, Takayama H, Yamagata Y, Nakamura Y, Kato H. Regulation and role of vascular endothelial growth factor in the corpus luteum during mid-pregnancy in rats. Biol Reprod. 2001;64:317–323. doi: 10.1095/biolreprod64.1.317. [DOI] [PubMed] [Google Scholar]
- Sugino N, Kashida S, Takiguchi S, Karube A, Kato H. Expression of vascular endothelial growth factor and its receptors in the human corpus luteum during the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. 2000;85:3919–3924. doi: 10.1210/jc.85.10.3919. [DOI] [PubMed] [Google Scholar]
- Wulff C, Dickson SE, Duncan WC, Fraser HM. Angiogenesis in the human corpus luteum simulated early pregnancy by HCG treatment is associated with both angiogenesis and vessel stabilization. Hum Reprod. 2001;16:2515–2524. doi: 10.1093/humrep/16.12.2515. [DOI] [PubMed] [Google Scholar]
- Neulen J, Yan Z, Raczek S, Weindel K, Keck C, Weich HA, Marmé D, Breckwoldt M. Human chorionic gonadotropin-dependent expression of vascular endothelial growth factor/vascular permeability factor in human granulosa cells: importance in ovarian hyperstimulation syndrome. J Clin Endocrinol Metab. 1995;80:1967–1971. doi: 10.1210/jc.80.6.1967. [DOI] [PubMed] [Google Scholar]
- Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Münstedt K, Rao CV, Lang U, Preissner KT. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290–5296. doi: 10.1210/jc.2002-020642. [DOI] [PubMed] [Google Scholar]
- Klipper E, Gilboa T, Levy N, Kisliouk T, Spanel-Borowski K, Meidan R. Characterization of endothelin-1 and nitric oxide generating systems in corpus luteum-derived endothelial cells. Reproduction. 2004;128:463–473. doi: 10.1530/rep.1.00271. [DOI] [PubMed] [Google Scholar]
- Rosiansky-Sultan M, Klipper E, Spanel-Borowski K, Meidan R. Inverse relationship between nitric oxide synthases and endothelin-1 synthesis in bovine corpus luteum: interactions at the level of luteal endothelial cell. Endocrinology. 2006;147:5228–5235. doi: 10.1210/en.2006-0795. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kashida S, Nakata M, Takiguchi S, Yamagata Y, Takayama H, Sugino N, Kato H. Changes in nitric oxide synthase activity in the ovary of gonadotropin treated rats: the role of nitric oxide during ovulation. Endocr J. 1999;46:529–538. doi: 10.1507/endocrj.46.529. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Navanukraw C, Johnson ML, Arnold DA, Reynolds LP, Redmer DA. Expression of endothelial nitric oxide synthase in the ovine ovary throughout the estrous cycle. Reproduction. 2006;132:579–587. doi: 10.1530/REP-06-0009. [DOI] [PubMed] [Google Scholar]
- Mitsube K, Zackrisson U, Brannstrom M. Niric oxide regulates ovarian blood flow in the rat during the periovulatory period. Hum Reprod. 2002;17:2509–2516. doi: 10.1093/humrep/17.10.2509. [DOI] [PubMed] [Google Scholar]
- Miceli F, Minici F, Garcia-Pardo M, Navarra P, Proto C, Mancuso S, Lanzone A, Apa R. Endothelins enhance prostaglandin (PGE(2) and PGF (2alpha)) biosynthesis and release by human luteal cells: evidence of a new paracrine/autocrine regulation of luteal function. J Clin Endocrinol Metab. 2001;86:811–817. doi: 10.1210/jc.86.2.811. [DOI] [PubMed] [Google Scholar]
- Chan YF, O WS, Tang F. Adrenomedullin in the rat testis. I: Its production, actions on testosterone secretion, regulation by human chorionic gonadotropin, and its interaction with endothelin 1 in the leydig cell. Biol Reprod. 2008;78:773–779. doi: 10.1095/biolreprod.107.060871. [DOI] [PubMed] [Google Scholar]