Abstract
Increased amounts of hyaluronic acid accumulate in fibroblasts cultured from patients with Marfan's disease, an autosomal dominant disorder. In the recessive Hurler's disease, the storage of glycosaminoglycan (GAG) is due to impaired degradation. This study examines the kinetics of GAG accumulation in Marfan's disease in order to determine whether the mechanism of accumulation differs from that in Hurler's disease.
Marfan-derived fibroblasts incorporated [14C]acetate or [14C]glucosamine into GAG to a level 4-6 times greater than control fibroblasts. Sugar analyses, electrophoretic mobility, and enzyme susceptibility studies showed that the isolated material was hyaluronic acid. There were no differences in activity of a variety of glycosidases between Marfan and control fibroblasts, nor were there differences in the ability to degrade prelabeled hyaluronate by cell-free extracts. Finally, chase experiments showed parallel rates of loss of labeled GAG from control fibroblasts and fibroblasts from Marfan patients.
It appears that hyaluronic acid was accumulating in greater amounts in the fibroblasts from patients with Marfan's disease because of a greater rate of synthesis as opposed to a decreased rate of breakdown.
Full text
PDF

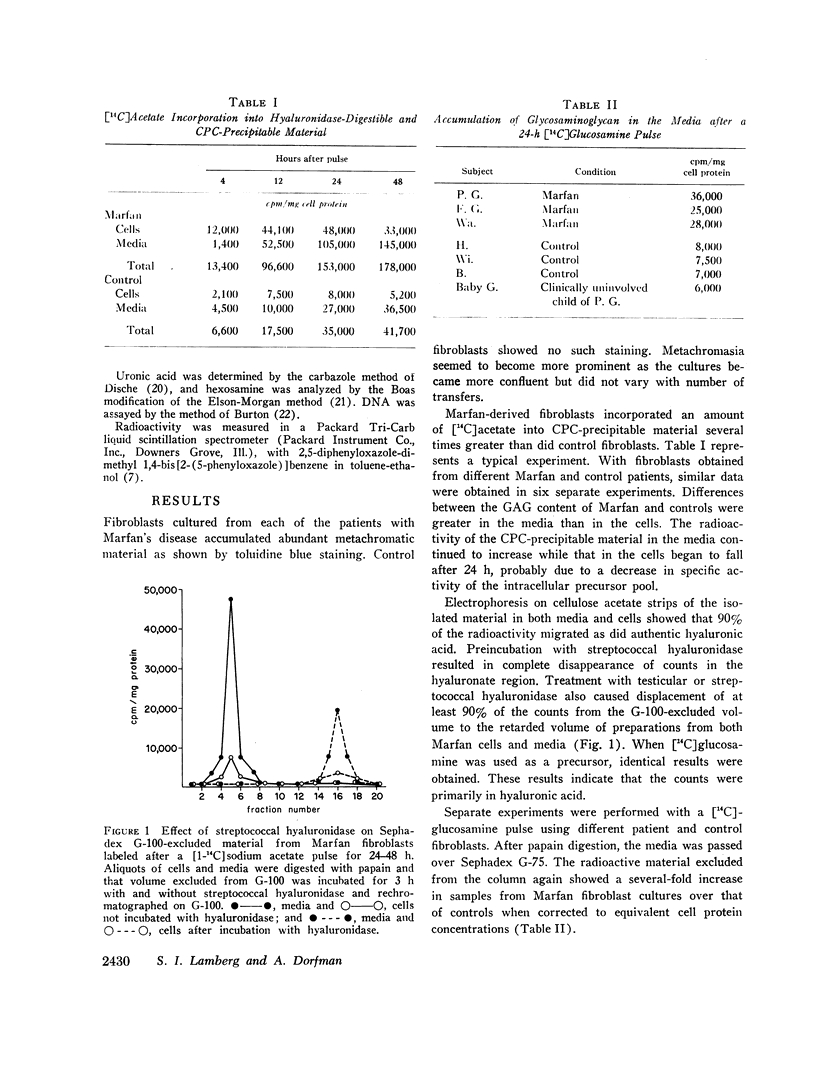
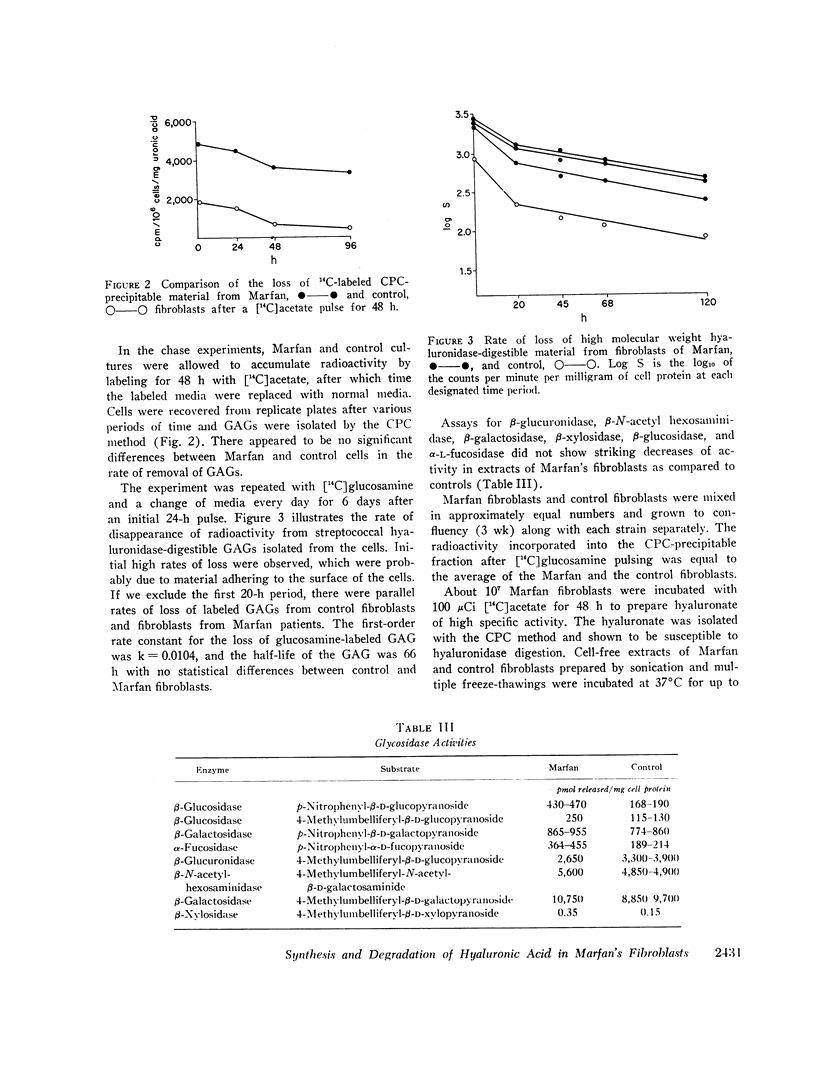


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- BOLANDE R. P. THE NATURE OF THE CONNECTIVE TISSUE ABIOTROPHY IN THE MARFAN SYNDROME. Lab Invest. 1963 Nov;12:1087–1093. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach G., Friedman R., Weissmann B., Neufeld E. F. The defect in the Hurler and Scheie syndromes: deficiency of -L-iduronidase. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2048–2051. doi: 10.1073/pnas.69.8.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson G. S., Dalferes E. R., Jr Urinary excretion of mucopolysaccharides in normal individuals and in the Marfan syndrome. Biochim Biophys Acta. 1965 Jul 1;101(2):183–192. doi: 10.1016/0926-6534(65)90049-7. [DOI] [PubMed] [Google Scholar]
- Conrad A. H., Ruddle F. H. Regulation of thymidylate synthetase activity in cultured mammalian cells. J Cell Sci. 1972 Mar;10(2):471–486. doi: 10.1242/jcs.10.2.471. [DOI] [PubMed] [Google Scholar]
- Danes B. S., Bearn A. G. Hurler's syndrome. A genetic study in cell culture. J Exp Med. 1966 Jan 1;123(1):1–16. doi: 10.1084/jem.123.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler's and Hunter's syndromes: faulty degradation of mucopolysaccharide. Proc Natl Acad Sci U S A. 1968 Jun;60(2):699–706. doi: 10.1073/pnas.60.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germinario R. J., Kahlenberg A., Pinsky L. The disorder of hyaluronic acid metabolism in cultured skin fibroblasts derived from a patient with the Hurler syndrome. Biochem J. 1973 Mar;132(3):403–408. doi: 10.1042/bj1320403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laitinen O., Uitto J., Iivanainen M., Hannuksela M., Kivirikko K. I. Collagen metabolism of the skin in Marfan's syndrome. Clin Chim Acta. 1968 Sep;21(3):321–326. doi: 10.1016/0009-8981(68)90062-4. [DOI] [PubMed] [Google Scholar]
- MATHEWS M. B., ROSEMAN S., DORFMAN A. Determination of the chondroitinase activity of bovine testicular preparations. J Biol Chem. 1951 Jan;188(1):327–334. [PubMed] [Google Scholar]
- Macek M., Hurych J., Chvapil M., Kadlecová V. Study of fibroblasts in Marfan's syndrome. Humangenetik. 1966;3(2):87–97. doi: 10.1007/BF00291289. [DOI] [PubMed] [Google Scholar]
- Matalon R., Dorfman A. Hurler's syndrome, an -L-iduronidase deficiency. Biochem Biophys Res Commun. 1972 May 26;47(4):959–964. doi: 10.1016/0006-291x(72)90586-4. [DOI] [PubMed] [Google Scholar]
- Matalon R., Dorfman A. Hurler's syndrome: biosynthesis of acid mucopolysaccharides in tissue culture. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1310–1316. doi: 10.1073/pnas.56.4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon R., Dorfman A. The accumulation of hyaluronic acid in cultured fibroblasts of the Marfan syndrome. Biochem Biophys Res Commun. 1968 Jul 26;32(2):150–154. doi: 10.1016/0006-291x(68)90361-6. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S. Sanfilippo syndrome: profound deficiency of alpha-acetylglucosaminidase activity in organs and skin fibroblasts from type-B patients. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1720–1722. doi: 10.1073/pnas.69.7.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrink B., Wasteson A. Nature of the interaction of chondroitin 4-sulphate and chondroitin sulphate-proteoglycan with collagen. Biochem J. 1971 Jan;121(2):227–233. doi: 10.1042/bj1210227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C., Hamerman D. Partial inhibition by cycloheximide of haluronate synthesis in cell culture. Proc Soc Exp Biol Med. 1968 Apr;127(4):988–991. doi: 10.3181/00379727-127-32851. [DOI] [PubMed] [Google Scholar]
- Van Hoof F., Hers H. G. The abnormalities of lysosomal enzymes in mucopolysacc- haridoses. Eur J Biochem. 1968 Dec;7(1):34–44. doi: 10.1111/j.1432-1033.1968.tb19570.x. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]
- von Figura K., Kresse H. The sanfilippo B corrective factor: a N-acetyl-alpha-D-glucosamindiase. Biochem Biophys Res Commun. 1972 Jul 25;48(2):262–269. doi: 10.1016/s0006-291x(72)80044-5. [DOI] [PubMed] [Google Scholar]


