Abstract
Previous research suggests that intrinsic efficacy of benzodiazepines is an important determinant of their behavioral effects. We evaluated the reinforcing effects of the benzodiazepine partial agonist bretazenil using behavioral economic models referred to as “consumer demand” and “labor supply”. Four rhesus monkeys were trained under a progressive ratio (PR) schedule of i.v. midazolam injection. A range of doses of bretazenil (0.001–0.03 mg/kg/injection and vehicle) was evaluated for self-administration with an initial response requirement of 40 that doubled to 640; significant self-administration was maintained at doses of 0.003 to 0.03 mg/kg/injection. Next, a dose of bretazenil that maintained peak injections/session was made available with initial response requirements doubling from 10 to 320 (maximum possible response requirements of 160 and 5120, respectively), and increasing response requirements decreased self-administration (mean number of injections/session) of a peak dose (0.01 mg/kg/injection). Analyses based on consumer demand revealed that a measure of reinforcing strength termed “essential value”, for bretazenil was similar to that previously obtained with midazolam (non-selective full agonist), but less than that observed for zolpidem (full agonist, selective for α1 subunit-containing GABAA receptors). According to labor supply analysis, the reinforcing effects of bretazenil were influenced by the economic concept referred to as a “price effect”, similar to our previous findings with midazolam but not zolpidem. In general, behavioral economic indicators of reinforcing effectiveness did not differentiate bretazenil from a non-selective full agonist. These findings raise the possibility that degree of intrinsic efficacy of a benzodiazepine agonist may not be predictive of relative reinforcing effectiveness.
Keywords: Bretazenil, Self-Administration, Behavioral Economics, Benzodiazepine, Rhesus Monkey
1. Introduction
Benzodiazepine (BZ) abuse is becoming an increasingly prevalent public health issue. For example, a recent review of Drug Abuse Warning Network (DAWN) data over a five-year period indicated that the number of emergency room visits associated with the non-medical use of BZs had increased 111% between 2004–2008 (Centers for Disease Control and Prevention, 2010). Moreover, a body of data suggests that abuse of BZs is becoming widespread among adolescents and young adults (for references see McCabe, 2005), and individuals who abuse these drugs tend to abuse other drugs, divert the drugs into the illicit market, and engage in risky behaviors.
As a result of this growing concern regarding the abuse potential of BZs, recent research has focused on discerning the mechanisms underlying the reinforcing effects of BZs (e.g., Tan et al. 2010). Knowledge of the existence of multiple subtypes of the GABAA receptor has led to the hypothesis that the addictive properties of BZs may be attributed to a specific subtype in much the same way that the sedative and motor effects of BZs have been associated with action at GABAA receptors containing an α1subunit (α1GABAA receptors), and their anxiolytic and myorelaxant effects have been associated with GABAA receptors containing α2 and/or α3 subunits (α2GABAA and α3GABAA receptors; see review by Möhler, 2006). To this end, studies examining the reinforcing effects of zolpidem, a non-BZ hypnotic with selectivity for α1GABAA receptors, have demonstrated robust intravenous self-administration greater than BZs and comparable to that observed with barbiturates and stimulants (Griffiths et al., 1992; Rowlett et al., 2005; Rowlett and Lelas, 2007). Recently, α1GABAA receptors located on ventral tegmental area neurons have been implicated in the abuse-related effects of BZs (Tan et al. 2010). While these results suggest that activity at α1GABAA receptors may play a critical role in mediating zolpidem’s reinforcing effects, other data demonstrated that the α1subunit is not necessary for self-administration (Rowlett et al., 2005). Thus, the reinforcing effects of BZ-type drugs may reflect a complex interaction among the different GABAA receptor subtypes.
Another potentially important factor to consider is the relationship between the intrinsic efficacy of a BZ-type drug at GABAA receptors and its reinforcing effects, since non-selective BZ-site partial agonists have been shown to differ from BZ-site full agonists with respect to behavior. For instance, the imidazobenzodiazepine bretazenil, which potentiates GABA-stimulated chloride flux sub-maximally (i.e., approximately 40% of the potentiation observed following application of triazolam, Facklam et al., 1992b), has potent anxiolytic and anticonvulsant effects at doses that do not produce the hallmark sedative or amnestic side effects of BZs (Facklam et al., 1992a; Jones et al., 1994; Martin et al., 1993). In contrast to full agonists, bretazenil also has exhibited reduced abuse-related effects compared with full agonists in laboratory animals (Martin et al., 1995; Mirza and Nielsen, 2006; Moreau et al., 1990; Schoch et al., 1993) as well as in human volunteers (Busto et al., 1994). Together, these studies illustrate that a low efficacy BZ such as bretazenil may have reduced abuse potential relative to higher efficacy BZs.
Previous studies on the abuse-related effects of bretazenil used essentially indirect measures of abuse-related properties, with none to our knowledge employing direct assessments of reinforcing effects such as self-administration procedures. The present study evaluated the reinforcing effects of bretazenil using progressive-ratio (PR) schedules of intravenous drug self-administration in rhesus monkeys, which have been shown to elucidate differences in apparent reinforcing effectiveness of drugs (e.g., Rowlett et al., 2002; Wilcox et al., 2000). PR schedules also can be useful for comparing quantitatively the relative reinforcing effectiveness of drugs when analyzed using behavioral economics approaches (e.g., Rodefer and Carroll, 1996; Rowlett and Lelas, 2007; for review, see Rowlett, 2000).
One of the most commonly-used behavioral economic approaches can be referred to as the consumer demand model, which assumes that the unit price of a commodity (e.g., the number of responses required for a particular drug dose) is a key factor determining consumption of the commodity (e.g., the total drug intake). A basic premise of the consumer demand model that has been observed under many different conditions and with different reinforcers, is that consumption decreases as a function of increasing unit price in a predictable fashion (Hursh et al., 1988; Hursh and Silberberg, 2008). This relationship has been used to determine a drug’s relative reinforcing effectiveness quantitatively (e.g., Bickel et al., 2000; Hursh et al., 2005). Recently, Hursh and Silberberg (2008) have introduced a new model that provides an exponential rate constant to determine the “essential value” (α) of a reinforcer, which provides a unique index of reinforcing effectiveness that is independent of scalar properties of a given reinforcer.
The consumer demand model addresses consumption as a function of the price of the commodity; however, consumption—as well as price—is determined in part by the responding that is emitted by the organism (i.e., labor) under the relevant contingencies (Allison, 1983). To evaluate the role of responding (labor) in determining consumption, Allison (1983) introduced the “labor supply model”. This model makes the basic assumption that consumption, or “income” (e.g., the total number of injections of a particular drug dose) declines as a function of increasing labor (e.g., the total number of responses during an experimental session). However, this model also postulates that at relatively high response requirements the income-labor relationship will reverse and labor will decrease as a function of decreasing income. These income-labor relationships are believed to be mediated differentially by “income” and “price” effects (Allison, 1983; 1993). Drug self-administration is mediated by an income effect when a negative correlation exists between income and labor. That is, income is maintained at the highest possible level in the face of increasing response requirements. A price effect occurs when a positive correlation exists between income and labor; that is, as response requirements (i.e., price) are increased, both income and labor decline. Income effects and price effects combine to form a backward-bending labor supply function (for further discussion, see Rowlett, 2000; Rowlett and Lelas, 2007). We propose that income and price effects provide behavioral mechanisms underlying reinforcement that can be used to determine the relative reinforcing effectiveness among different commodities (see also Rowlett and Lelas, 2007).
The present study sought to investigate the reinforcing effects of the partial agonist bretazenil using these two behavioral economic approaches. The results were compared with those obtained previously for midazolam and zolpidem (Rowlett and Lelas, 2007). Both midazolam and zolpidem are full agonists, with zolpidem having selectivity for α1GABAA receptors. Studies using in vitro measures of GABA-mediated chloride flux demonstrated that midazolam, similar to other full agonist BZs, tripled the potentiation of the control GABA response (Yakushiji et al., 1989), while zolpidem’s potentiation of the response at its preferred receptor subtype was comparable to that of triazolam (Wafford et al., 1993). We hypothesized that the low intrinsic efficacy of bretazenil would result in behavior indicative of having less reinforcing effectiveness; that is, bretazenil would lack reinforcing effects entirely (i.e., bretazenil would not support self-administration) or it would have reinforcing effects, but with lower effectiveness compared with full agonists. This research served two additional goals: (1) To use the most recent consumer demand model proposed by Hursh and Silberberg (2008) to determine the essential value of bretazenil, zolpidem, and midazolam as reinforcers, while also (2) providing additional data for establishing the validity of the labor supply model.
2. Method
2.1. Subjects
Two female and two male adult rhesus monkeys (Macaca mulatta), weighing 9.0 – 11.0 kg, were studied in daily experimental sessions (Mon – Fri). The monkeys had self-administered barbiturates and benzodiazepines previously under a PR schedule of i.v. drug delivery. All monkeys were housed in colony rooms with a 12 hr light/dark cycle (lights on at 0630 hr), had unrestricted access to water, and were fed Harlan Teklad Monkey Diet, supplemented by fresh fruit. Monkeys were anesthetized (ketamine, 10 mg/kg, i.v.) periodically to assess catheter patency and general health. All procedures were conducted with the approval and under the supervision of the Harvard University Institutional Animal Care and Use Committee. The monkeys were cared for according to the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Each monkey was prepared with a chronic indwelling venous catheter according to the general procedures described by Platt et al. (2005). A polyvinyl chloride catheter (Tygon®, inner diameter: 0.025 cm; outer diameter: 0.052 cm) was implanted in a jugular (internal or external), femoral, or brachial vein under isoflurane anesthesia and aseptic conditions. The proximal end of the catheter terminated above the right atrium, and the distal end was passed subcutaneously to exit in the mid-scapular region. Monkeys were treated postoperatively with antibiotics and analgesics and experimental sessions began seven days following surgery.
2.2. Apparatus
Monkeys were housed individually in stainless steel primate cages (Hartford Metal Products, Aberdeen, MD) that served as the experimental chambers. A removable panel was placed on the front of each cage and contained four stimulus lights (two red and two white; 3 cm, 1.1 W; Med Associates, Georgia, VT) and a response lever (Med Associates). Each monkey was fitted with a nylon-mesh jacket (Lomir Biomedical Inc., Malone, NY) that was connected to a 1-m stainless steel flexible tether (Lomir Biomedical). The monkey’s catheter was routed through the tether and attached to a fluid swivel (Lomir Biomedical) on top of the cage. The swivel was attached to an injection pump (Med Associates) located on top of the cage, which could infuse drug solutions at a rate of 0.2 ml/s. The stimulus lights, response levers, and infusion pump were connected to interfaces (Med Associates) and PC-compatible computers located in an adjacent room.
2.3. Procedure
Monkeys were trained to self-administer the short-acting benzodiazepine midazolam under a PR schedule of i.v. drug injection according to the methods described by Rowlett and Lelas (2007). Experimental sessions began daily at 1200 hr. At the beginning of the session, the white stimulus lights above the lever were illuminated to signal the start of a trial. Upon completion of the response requirement, the white lights were extinguished and the red stimulus lights were illuminated for 2 sec, coinciding with a 2-sec infusion of drug or saline. Each trial ended with either an injection or the expiration of a 30-min limited hold. Trials were separated by a 30-min timeout period, during which all lights were extinguished and responding had no programmed consequences.
Experimental sessions consisted of 5 components made up of 4 trials each, for a maximum possible of 20 trials per session. The response requirement remained constant for each of the 4 trials within a component, and doubled during each successive component. For example, a session with an initial response requirement (IRR) of 40 consisted of the following five components with increasing response requirements (4 trials each): 40, 80, 160, 320, and 640. This procedure allowed for the determination of injections/session and break point (BP) based on n=1 to 4 trials, rather than the more typical n=1 trial. The number of trials per response requirement was chosen so that the maximum number of injections could be completed within a day (maximum session time was approximately 9.5 hours). The session ended when a monkey self-administered a maximum of 20 injections or when the response requirement was not completed for two consecutive trials.
All self-administration studies were conducted under the PR schedule of reinforcement described above. Under the initial training conditions, midazolam (0.03 mg/kg/injection) or saline was available for injection on alternating days, seven days per week. Reliable self-administration was determined according to the following criteria: (1) the number of injections/session maintained by midazolam was greater than or equal to 11 for three sessions, and the number of injections/session maintained by saline was less than or equal to 5 for three sessions; and (2) no upward or downward trends were observed across consecutive sessions of midazolam availability and consecutive sessions of saline availability. Once self-administration was stable, test sessions (T) were added to the alternating sequence of midazolam (M) and saline (S) sessions according to the following sequence: STSMTMTMST, etc. Test sessions were identical to training sessions except that the dose, and/or IRR were manipulated. Bretazenil (0.001, 0.003, 0.01, and 0.03 mg/kg/injection) was tested initially under an IRR of 40. Once the four doses were determined twice in each monkey, the dose maintaining the highest number of injections/session (0.01 mg/kg/injection) was chosen for IRR manipulations. For these tests, six IRRs were evaluated, starting with IRR 10 and doubling to IRR 320. IRRs higher than 320 were not evaluated due to the lack of significant self-administration maintained at this IRR (see Results). IRR tests were conducted as described above for the dose-response tests, with baseline midazolam and saline sessions (IRR 40) occurring between IRR tests. Each IRR was tested twice, and the results were averaged to provide data points for each monkey. The different IRRs were tested in random order for each monkey.
2.4. Statistical and Economic Analysis
The number of injections/session and the BPs were determined for individual monkeys under each test condition. The injections/session data were analyzed by repeated measures analysis of variance (ANOVA) with dose and drug as the factors. A dose of drug was determined to be self-administered significantly above vehicle levels by comparing mean injections/session for each dose to the corresponding vehicle control value (Bonferroni t-test, alpha level equal to p< 0.05). BP, defined as the highest response requirement completed during a test session, was calculated under each test condition. The maximum BP (BPmax) was calculated as the highest mean BP, irrespective of dose.
For consumer demand analysis, the relationship of price and consumption was determined using the equation described recently by Hursh and Silberberg (2008):
| (1) |
In this equation, Q is consumption, Q0 is the consumption at zero price, i.e., maximum level of demand, k specifies the range of the dependent variable in logarithmic units, C is the cost of each reinforcer, and α is the rate of change in the exponential function. According to Hursh and Silberberg (2008), α scales the “essential value” for a reinforcer, and is an appropriate index for reinforcing strength with the advantage of being independent of the scalar dimensions of the reinforcer.
In order to compare bretazenil with midazolam and zolpidem using this new approach to analysis, we re-analyzed the midazolam and zolpidem self-administration data from Rowlett and Lelas (2007) in comparison with the present results with bretazenil. To make comparisons among the drugs with differing potencies, we used the “normalized price” and “normalized consumption” constructs (Hursh and Silberberg, 2008). Normalized price is the responses per 1 percent of the maximum (C × Q0/100), whereas normalized consumption is the percentage of reinforcers obtained out of the maximum (Q0).
A modification of the above procedures was necessitated by our use of a PR schedule of reinforcement. The analysis described by Hursh and Silberberg (2008) is based on fixed-ratio schedules, with price represented as the response requirement, which is not feasible with PR sequences consisting of multiple response requirements. We therefore substituted the more typical price used in our previous study (Rowlett and Lelas, 2007): Total responses/injection/dose. Conceptually, this is considered to be the unit price, and in the case of PR schedules, can be thought of as the unit price generated by the subject (in microeconomic terms, the amount of labor generated by the good at a particular price). For the normalized price calculation, unit price was used as the cost of each reinforcer (C). These data were analyzed using a Microsoft Excel spreadsheet developed by S.R. Hursh and provided via the Institutes for Behavior Resources website (http://www.ibrinc.org/ibr/centers/bec/BEC_demand.html).
For labor supply analysis, total income was defined as the number of injections/session and was plotted as a function of total responses/session, which represents total labor (Allison, 1993; Rowlett, 2000; Rowlett and Lelas, 2007). According to labor supply theory, as the response cost (response requirement) increases, total income is reduced initially according to a negative linear relationship between income and labor followed by a positive linear relationship at the highest response costs. This hypothesis was tested by regressing the mean injections/session and total responses/session according to the non-linear regression model:
| (2) |
In the equation, the variable “NC” is the injections/session, “B” is the y-intercept, “NR” is the total responses/session, and R/C is the response cost (total responses/session divided by injections/session). The slopes that determine the decrements based on NR and R/C are represented by the factors “a” and “b”. As can be seen in Eq. 2, as b approaches zero, the equation takes on the form of a linear function. On the other hand, as b approaches 1.0, i.e., as R/C increases, decreases in income are accompanied by decreases in labor (Allison and Boulter, 1982). Specifics of the analyses and model testing were conducted as described by Rowlett (2000; see Appendix in that article).
2.5. Drugs
Bretazenil base was provided by F. Hoffman LaRoche Laboratories (Nutley, NJ). Midazolam base was obtained from Sigma-RBI (Natick, MA). Both compounds were dissolved in propylene glycol and diluted in sterile saline (maximum concentration of propylene glycol= 50%) and sterilized by filtration. Bretazenil solutions were prepared the day of test sessions, and midazolam was prepared as a stock solution for dilution once per week. The drugs were administered as a 0.4 ml infusion over 2 sec.
3. Results
3.1. Self-administration of bretazenil
All monkeys maintained stable self-administration under the alternating schedule over the course of the studies: Midazolam self-administration averaged 13 ± 1.1 injections/session (mean ± SEM) and an average of 3.5 ± 0.5 injections/session (mean ± SEM) was maintained under conditions of saline availability. For bretazenil, the mean number of injections/session increased to asymptotic levels at 0.003 mg/kg/injection and above (Fig. 1A). Repeated measures ANOVA showed a significant effect of dose [F(4,12)= 27.3, P< 0.05] and multiple comparison tests showed that doses of 0.003 and above maintained self-administration significantly above vehicle levels (Bonferroni t-tests, P< 0.05). Analysis of BPmax (maximum BP values irrespective of dose) showed an average value of 200 ± 40 (Mean ± SEM). The studies designed for behavioral economic analyses were conducted with the bretazenil dose of 0.01 mg/kg/injection, which was the dose that maintained the highest levels of injections/session (see Fig. 1A). As shown in Fig. 1B, increasing the IRR resulted in a decrease in the mean number of injections/session [F(5,15)= 18.26, P< 0.05]. Fig. 1C shows that in contrast to injections/session, the mean BP values increased as a function of IRR up to IRR 40 [F(5,15)= 15.1, P< 0.05].
FIG. 1.
Self-administration of bretazenil by rhesus monkeys trained under a progressive-ratio schedule of intravenous midazolam delivery. The progressive-ratio schedule consisted of an initial response requirement (IRR) of 40, which doubled to a maximum of 640. Panel A: Dose-response effects of bretazenil, expressed as the mean number of injections/session ± SEM. Panels B and C: Self-administration of the peak reinforcing dose of bretazenil (0.01 mg/kg/injection) at different initial response requirements. Data for Panel B are the mean number of injections/session ± SEM. Data for Panel C are mean break point (maximum response requirement completed for a test session) ± SEM. All data are averages of two determinations per monkey, which were used to calculate means (± SEM) for N= 4 monkeys. F values are for repeated measures ANOVAs. *P < 0.05, Bonferroni t tests, comparison with vehicle. BPmax = Maximum break point irrespective of dose and “V” = vehicle.
3.2. Consumer demand
For the consumer demand analysis, data from the study in which the IRR was varied was combined with the data from the dose-response function study in order to provide a sufficient sample size for the demand curve calculation (total number of data points per drug = 10). The re-analysis of the data for zolpidem and midazolam from Rowlett and Lelas (2007) are shown in Figure 2, along with the analysis of the bretazenil data from the present study (Fig. 2, bottom panel). These data were fitted with Equation 1 using iterative curve-fitting procedures, with the k value (the range of data from Q0 to minimum) determined first for midazolam and held constant for zolpidem and bretazenil. Using this method, a reasonable “global” R2 value (i.e., the overall R2 value for all three curves) of 0.65 was obtained. As shown in Figure 2, the R2 values for the individual curves ranged from 0.60 to 0.79, suggesting that the majority of variance was accounted for by the Equation.
FIG. 2.
Analysis of bretazenil self-administration according to the consumer demand model, using exponential demand curves developed recently by Hursh and Silberberg (2008). Demand curves were determined by fitting Equation 1 to the data using iterative curve-fitting techniques (see Methods for complete details). Data sets for the dose-response analyses and the study in which initial response requirements (IRRs, ranging from 10 to 60) were varied were used for each drug. The data for zolpidem and midazolam were from Rowlett and Lelas (2007). Data are normalized consumption (mean injections/session divided by the maximum demand, Q0, and multiplied by 100) and normalized price (mean total responses/mg/session multiplied by Q0 and divided by 100). The α parameter represents the slope of the exponential curve, and is a measure of the “essential value” of each reinforcer.
The Hursh and Silberberg (2008) method provides a single variable, α, which represents the essential value of the reinforcer. Essential value is the exponential rate of change (slope) for the curve; therefore the essential value of the reinforcer is higher as α is a lower number (i.e., a shallower slope, indicating that demand is less affected by price). As can be seen in Figure 2, zolpidem had an approximately 3-fold lower α value than either midazolam or bretazenil, both of which had very similar α values. Thus, the compounds can be ranked as zolpidem > midazolam = bretazenil based on essential values. Also obtained were the maximum demand values, Q0, and the higher essential value for zolpidem presumably underlies the observation that Q0 is greater for this drug compared with midazolam and bretazenil.
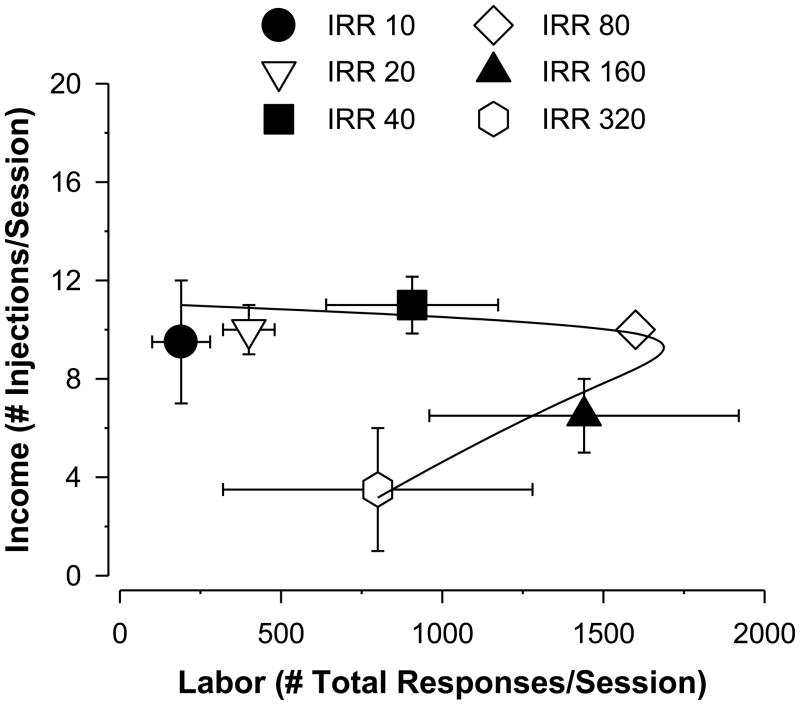
3.3. Labor supply
As can be seen in Figure 3, the plot of income (injections/session) as a function of labor (total responses/session) revealed that as the IRR increased, income was not affected appreciably until IRR 80 to IRR 320; however, labor both increased (IRR 10–80) and decreased (IRR 80–320). These findings suggest that bretazenil self-administration was mediated by both income and price effects over the range of IRRs studied.
FIG. 3.
Labor supply analysis of bretazenil (0.01 mg/kg/injection) self-administration by rhesus monkeys (N= 4). Data points represent the mean (± SEM) injections/session (income) as a function of the mean number of total responses/session (labor) for 6 different progressive-ratio response sequences. The individual sequences are identified by the initial response requirement (IRR), with the response requirements doubling over 5 trials (e.g., for IRR 10, the response requirements were 10, 20, 40, 80, 160). The labor supply curve was determined by Equation 2 (see Methods for details).
The non-linear regression analyses using Equation 2 revealed that this model provided an excellent fit of the data, with an R2= 0.89 and a significant model fit via ANOVA [F(2,3)= 21.52, P< 0.05]. The regression analyses showed that the value for the dimensional constant “a” was relatively small (−0.0022), consistent with the majority of points (IRR 10–80) on a straight line with essentially no slope. Accordingly; the “a” coefficient was not significantly different from zero. For the dimensional constant “b”, the value was 0.0279, suggesting the positive relationship between income and labor at higher labor requirements that is predicted by the labor supply model. The “b” coefficient was significantly different from zero (t= 6.51, P< 0.05), consistent with a significant price effect in the labor-income relationship associated with bretazenil over the IRRs evaluated in this study.
3.4. Comparison with midazolam and zolpidem
In our previous study (Rowlett and Lelas, 2007), analysis of BPmax (maximum BP values irrespective of dose) for zolpidem and midazolam revealed average values of 560 ± 80 (Mean ± SEM) and 280 ± 40 (Mean ± SEM), respectively. We compared these BPmax values to those obtained in the present study with bretazenil, and found a significant effect of drug [repeated measures ANOVA; F(2,6)= 11.8, P< 0.05]. Multiple comparison tests revealed that the mean BPmax value for zolpidem was higher than mean BPmax values for either bretazenil or midazolam; and the values for the latter two compounds did not differ (Bonferroni t-tests for all pair-wise comparisons).
The suggestion that the reinforcing effectiveness of bretazenil might be equal to midazolam’s under some conditions is supported further by consumer demand and labor supply analysis. Regarding the latter, although the “a” parameters were near zero for all three drugs, the “b” parameters, which indicate the relative influence of price effects, were significantly different from zero for bretazenil and midazolam. Based on this parameter, the rank order of reinforcing effectiveness was zolpidem (b value not significantly different from zero) > bretazenil (b = 0.0279, significantly different from zero) > midazolam (b = 0.041, significantly different from zero; zolpidem and midazolam data from Rowlett and Lelas, 2007).
4. Discussion
The present study employed a PR schedule of i.v. drug administration in rhesus monkeys in which the dose of drug and IRR were varied systematically in order to assess the reinforcing effects of bretazenil, a non-selective BZ partial agonist. Results were compared to those obtained in a previous study that examined midazolam, a non-selective BZ full agonist, and zolpidem, a BZ full agonist with selectivity at GABAA receptors containing α1 subunits (Rowlett and Lelas, 2007), to test the hypothesis that differences among these drugs in reinforcing effectiveness might be attributable to differences in their intrinsic efficacy. In order to explore further the potential differences in reinforcing effectiveness between bretazenil, midazolam, and zolpidem, a behavioral economic approach was employed which utilized concepts of consumer demand in addition to labor supply analysis.
Similar to other reports examining self-administration of BZ-type drugs under PR schedules (Rowlett et al. 2005; Rowlett and Lelas, 2007), bretazenil engendered dose-dependent increases in the mean number of injections/session, i.e., this partial agonist functioned as a reinforcer. The dose-response function was monophasic over the doses tested. Moreover, varying the IRRs with a peak dose of bretazenil resulted in a pattern of behavior similar to that observed with zolpidem and midazolam in our previous study. That is, as the IRR increased, there was a decline in the number of injections/session and an increase in BP.
Under the PR schedule used in this and previous studies, we calculated a BPmax value (i.e., maximum BP irrespective of dose) for each drug that allows for a determination of maximum BP not influenced by differences in potency across individual animals. For bretazenil, the BPmax was, as expected, lower than that of zolpidem, but surprisingly, this value was approximately comparable to that of midazolam.
The BPmax measure provides a reasonable single metric for evaluating relative reinforcing effectiveness; however, this discontinuous measure is limited in terms of its ability to provide information on behavioral mechanisms underlying differences in effectiveness among reinforcers (Rowlett, 2000; Hursh and Silberberg, 2008). To explore factors underlying possible differences in reinforcing effectiveness among the BZ-type drugs, we employed analytical techniques adapted from microeconomics. Behavioral economic analysis fundamentally relates the consumption of a commodity (i.e., i.v. drug delivery) to how it varies with price (i.e., response requirement) in different economies (e.g., closed economy, such as a controlled laboratory environment). Consumer demand models often use the unit dose, the number of injections taken during an experimental session, and total number of responses emitted in calculation of a “demand curve”, in which consumption is plotted as a function of price (Hursh et al., 1988; 2005; Hursh and Silberberg, 2008). The results presented here showed that consumption of bretazenil declined as the price increased. This observation is consistent with previous results examining the consumption of BZ-type drugs (Rowlett and Lelas, 2007) as well as many other drug reinforcers (for reviews, see DeGrandpre and Bickel, 1996; Hursh and Silberberg, 2008). It also is consistent with the economic principle referred to as the “law of demand”, which states that as the price of a commodity increases, consumer demand for the commodity will decrease.
The normalized price and consumption data for midazolam, zolpidem, and bretazenil fit well with the recently developed equation to describe exponential demand curves (Hursh and Silberberg, 2008); although the R2 values tended to be lower than those shown in the Hursh and Silberberg (2008) paper. The reason for this is unclear, although one very likely possibility is that by allowing the monkeys to “set their own prices” (rather than the experimenter doing so), the amount of variance associated with demand curves may be exacerbated simply due to individual differences associated with this type of behavioral measure. Another contributor to the increased variance might be the inclusion of data from both the dose-response study and the varying IRR study. We have shown previously with zolpidem and midazolam that variance increases for demand curves if these two different types of data are combined (Rowlett and Lelas, 2007), despite the fact that consumption of a drug is postulated to remain constant irrespective of how unit price is determined (Bickel et al., 1990; but see English et al. 1995; Rodefer and Carroll, 1996). Regardless of these issues, the exponential equation developed by Hursh and Silberberg (2008) provides a more straightforward approach to reinforcing strength than previous models (e.g., Hursh et al., 1988) by generating a single parameter, α, to provide a metric for reinforcing strength (referred to as “essential value”). Based on our analyses, the essential value for zolpidem was highest, followed by midazolam and bretazenil, which essentially were the same.
Labor supply models extend the consumer demand model by considering the quantitative relationship between the total labor emitted and the total amount of commodity obtained, or income (Allison, 1983; Rowlett, 2000). Labor supply makes specific predictions regarding the effects of increasing the response cost (i.e., IRR under PR schedules) on both income (i.e., injections/session) and labor (i.e., total responses/session). This model assumes that a negative linear relationship exists between income and labor such that, according to the law of demand, total income is reduced as response costs are increased, but labor is increased in order to maintain self-administration at the highest level possible. In other words, maintaining income is what drives the behavior. This is referred to as an “income effect” (Allison, 1983). “Price effects”, on the other hand, occur only when both income and labor decline as response costs increase (Allison, 1983). In this case, the price drives the behavior. The present findings suggest that self-administration of bretazenil under the IRR conditions used in this study was determined by a characteristic combination of income and price effects. Again, the results observed with bretazenil were similar to those effects obtained previously with midazolam but not zolpidem (Rowlett and Lelas, 2007). Thus, under similar conditions and constraints, the reinforcing effects of zolpidem were determined entirely by the income effect mechanism, whereas both income and price effects determined midazolam- and bretazenil-maintained behavior essentially to the same degree. While it is premature to identify pharmacological mechanisms that are linked to income and price effects, the observation that bretazenil’s reinforcing effects were similar to midazolam’s in terms of BPmax, essential value, and income/price effects suggests that the intrinsic efficacy of BZ-type drugs might be ruled out as an important pharmacological determinant of the reinforcing effects of these drugs.
Our observation that there is no significant relationship between intrinsic efficacy and reinforcing effectiveness of BZ-type drugs requires more studies with full and partial agonists. If confirmed, however, this lack of a relationship would clearly differentiate BZ-type drugs from other pharmacological classes. For example, studies with PR schedules have shown a positive relationship between intrinsic efficacy and reinforcing strength for dopamine D1 receptor agonists/partial agonists (Weed et al., 1997) and mu opioid receptor agonists/partial agonists (Hursh and Winger, 1995; Rowlett et al., 2002). The reasons such a positive relationship would be evident for some pharmacological classes and not others is unclear at present.
Our findings with bretazenil may shed some light on previous results in which similar procedures were used to evaluate the reinforcing effects of subtype-selective compounds. In this regard, L-838,417, a novel compound with zero intrinsic efficacy at α1GABAA receptors but partial agonist efficacy at α2, α3, and α5GABAA receptors, was self-administered to a lesser extent than non-selective full agonists, such as midazolam (Rowlett et al. 2005). We suggested that the relatively low level of self-administration may reflect L-838,417’s lack of efficacy at α1GABAA receptors and/or its partial agonist profile. The present results with bretazenil, however, do not support the explanation that the partial agonist profile of L-838,417 determined its reinforcing effectiveness. That is, if intrinsic efficacy is the sole predictor of the extent of self-administration, then bretazenil should have maintained considerably lower levels of self-administration relative to full agonists. Because of the robustness of bretazenil self-administration, it therefore seems likely that the α1GABAA-sparing properties of L-838,417 contributed primarily to this compound’s relatively low level of self-administration or reinforcing effectiveness (cf. Rowlett et al. 2005).
Bretazenil’s midazolam-like reinforcing effectiveness was unanticipated given previous studies demonstrating its reduced abuse-related effects compared with non-selective full agonists. Subjective measures in human volunteers suggested that bretazenil led to fewer abuse-like effects relative to diazepam or alprazolam (Busto et al., 1994), while treatment with bretazenil also failed to lead to physical dependence in mice (Moreau et al., 1990), rats (Bronson, 1994), and monkeys (Martin et al., 1988; Schoch et al., 1993). Drug discrimination studies have been mixed in that bretazenil engenders BZ-like discriminative stimulus effects under some conditions (Sannerud and Ator, 1995; Ator, 1999) but not in others (Rijnders et al., 1991; Sannerud and Ator, 1995; Lelas et al., 1999; McMahon and France, 2006; but see Mirza et al., 2006). It is worth noting however, that the presence or absence of abuse-related effects (e.g., discriminative stimulus effects) do not necessarily predict the extent to which a drug will be reinforcing (cf. Ator, 2002). Moreover, the present findings with bretazenil suggest clearly that the mechanism(s) of action underlying reinforcing effects may differ from those underlying other abuse-related effects.
These studies permitted the evaluation of the feasibility of using a newer consumer demand model to compare reinforcing strength of BZ-type drugs, while also providing additional validation of the traditional labor supply model employed in the past (Rowlett, 2000). The present results demonstrate the feasibility of using the new model, with the caveat that the amount of variance accounted for by the Hursh and Silberberg (2008) analysis was high compared with most of the demand curve analyses presented by these authors. However, when compared with the older analyses of demand curves, the R2 values for midazolam and zolpidem (0.87 and 0.57, respectively; Rowlett and Lelas, 2007) were not markedly different from the ones obtained in the present study (0.79 and 0.68, respectively). This observation suggests that the somewhat less robust fits reported here (typical R2 values reported by Hursh and Silberberg, 2008, were >0.90) may reflect the use of the PR procedure, or some other procedural factor unique to our studies. Regardless, the use of a single parameter (α) to compare the essential value of different reinforcers is both efficient and elegant conceptually; thus making interpretation more straightforward. Finally, the labor supply analysis of the data with bretazenil provides additional validation for this approach, and demonstrates that income and price effects can be assessed using our methods. A clear limitation of our labor supply methodology is the reliance on evaluating multiple IRRs at the peak dose—a next step for this method is to incorporate multiple doses and to test predictions for income and price effects based on dose-response relationships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison J. Behavioral Economics. Praeger Publishers; New York: 1983. [Google Scholar]
- Allison J. Response deprivation, reinforcement, and economics. J Exp Anal Behav. 1993;60:129–40. doi: 10.1901/jeab.1993.60-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J, Boulter P. Wage rate, nonlabor income, and labor supply in rats. Learn Motiv. 1982;13:324–42. [Google Scholar]
- Ator NA. High-dose discrimination training with midazolam: context determines generalization profile. Pharmacol Biochem Behav. 1999;64:237–43. doi: 10.1016/s0091-3057(99)00050-7. [DOI] [PubMed] [Google Scholar]
- Ator NA. Relation between discriminative and reinforcing effects of midazolam, pentobarbital, chlordiazepoxide, zolpidem, and imidazenil in baboons. Psychopharmacology. 2002;163:477–87. doi: 10.1007/s00213-002-1076-4. [DOI] [PubMed] [Google Scholar]
- Bickel WK, De Grandpre RJ, Higgins ST, Hughes JR. Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life Sci. 1990;47:1501–10. doi: 10.1016/0024-3205(90)90178-t. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Caroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: a theoretical perspective. Psychopharmacology. 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Bronson ME. Chlordiazepoxide, but not bretazenil, produces acute dependence, as evidenced by disruptions in schedule-controlled behavior. Pharmacol Biochem Behav. 1994;48:397–401. doi: 10.1016/0091-3057(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Busto U, Kaplan HL, Zawertailo L, Sellers EM. Pharmacologic effects and abuse liability of bretazenil, diazepam, and alprazolam in humans. Clin Pharmacol Ther. 1994;55:451–63. doi: 10.1038/clpt.1994.55. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Emergency department visits involving nonmedical use of selected prescription drugs-Unites States, 2004–2008. Morbidity and Mortality Weekly Report. 2010;59:705–736. [PubMed] [Google Scholar]
- DeGrandpre RJ, Bickel WK. Drug dependence as consumer demand. In: Green L, Kagel GH, editors. Advances in behavioral economics, vol 3, substance use and abuse. Norwood, NJ: Ablax Publishing Co; 1996. [Google Scholar]
- English JA, Rowlett JK, Woolverton WL. Unit-price analysis of opioid consumption by monkeys responding under a progressive-ratio schedule of drug injection. J Exp Anal Behav. 1995;64:361–371. doi: 10.1901/jeab.1995.64-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam M, Schoch P, Bonetti EP, Jenck F, Martin JR, Moreau JL, Haefely WE. Relationship between benzodiazepine receptor occupancy and functional effects in vivo of four ligands of differing intrinsic efficacies. J Pharmacol Exp Ther. 1992a;261:1113–21. [PubMed] [Google Scholar]
- Facklam M, Schoch P, Haefely WE. Relationship between benzodiazepine receptor occupancy and potentiation of gamma-aminobutyric acid-stimulated chloride flux in vitro of four ligands of differing intrinsic efficacies. J Pharmacol Exp Ther. 1992b;261:1106–12. [PubMed] [Google Scholar]
- Griffiths RR, Sannerud CA, Ator NA, Brady JV. Zolpidem behavioral pharmacology in baboons: self-injection, discrimination, tolerance and withdrawal. J Pharmacol Exp Ther. 1992;260:1199–1208. [PubMed] [Google Scholar]
- Hursh SR, Galuska CM, Winger G, Woods JH. The economics of drug abuse: a quantitative assessment of drug demand. Mol Interv. 2005;5:20–8. doi: 10.1124/mi.5.1.6. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Raslear TG, Shurtleff D, Bauman R, Simmons L. A cost-benefit analysis of demand for food. J Exp Anal Behav. 1988;50:419–40. doi: 10.1901/jeab.1988.50-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. J Exp Anal Behav. 1995;64:373–84. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. 7. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Jones GH, Schneider C, Schneider HH, Seidler J, Cole BJ, Stephens DN. Comparison of several benzodiazepine receptor ligands in two models of anxiolytic activity in the mouse: an analysis based on fractional receptor occupancies. Psychopharmacology. 1994;114:191–99. doi: 10.1007/BF02244836. [DOI] [PubMed] [Google Scholar]
- Lelas S, Gerak LR, France CP. Discriminative stimulus effects of triazolam and midazolam in rhesus monkeys. Behav Pharmacol. 1999;10:39–50. doi: 10.1097/00008877-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Martin JR, Jenck F, Moreau JL. Comparison of benzodiazepine receptor ligands with partial agonistic, antagonistic or partial inverse agonistic properties in precipitating withdrawal in squirrel monkeys. J Pharmacol Exp Ther. 1995;275:405–11. [PubMed] [Google Scholar]
- Martin JR, Pieri L, Bonetti EP, Schaffner R, Burkard WP, Cumin R, Haefely WE. Ro 16-6028: A novel anxiolytic acting as a partial agonist at the benzodiazepine receptor. Pharmacopsychiatry. 1988;21:360–2. doi: 10.1055/s-2007-1021947. [DOI] [PubMed] [Google Scholar]
- Martin JR, Schoch P, Jenck F, Moreau JL, Haefely WE. Pharmacological characterization of benzodiazepine receptor ligands with intrinsic efficacies ranging from high to zero. Psychopharmacology. 1993;111:415–22. doi: 10.1007/BF02253530. [DOI] [PubMed] [Google Scholar]
- McCabe SE. Correlates of nonmedical use of prescription benzodiazepine anxiolytics: results from a national survey of U.S. college students. Drug Alcohol Depend. 2005;79:53–62. doi: 10.1016/j.drugalcdep.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, France CP. Differential behavioral effects of low efficacy positive GABAA modulators in combination with benzodiazepines and a neuroactive steroid in rhesus monkeys. Br J Pharmacol. 2006;147:260–68. doi: 10.1038/sj.bjp.0706550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza NR, Nielsen EØ. Do subtype-selective γ-aminobutyric acidA receptor modulators have a reduced propensity to induce physical dependence in mice? J Pharmacol Exp Ther. 2006;316:1378–85. doi: 10.1124/jpet.105.094474. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Rodgers RJ, Mathiasen LS. Comparative cue generalization profiles of L-838,417, SL651498, zolpidem, CL218,872, ocinaplon, bretazenil, zopiclone, and various benzodiazepines in chlordiazepoxide and zolpidem drug discrimination. J Pharmacol Exp Ther. 2006;316:1291–99. doi: 10.1124/jpet.105.094003. [DOI] [PubMed] [Google Scholar]
- Möhler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Jenck F, Pieri L, Schoch P, Martin JR, Haefely WE. Physical dependence induced in DBA/2J mice by benzodiazepine receptor full agonists, but not by the partial agonist Ro 16-6028. Eur J Pharmacol. 1990;190:269–73. doi: 10.1016/0014-2999(90)94138-n. [DOI] [PubMed] [Google Scholar]
- Platt DM, Carey GJ, Spealman RD. Intravenous self-administration techniques in monkeys. In: Enna S, Williams M, Ferkany J, Kenakin T, Porsolt R, Sullivan J, editors. Current Protocols in Neuroscience. Unit 9.21. New York: Wiley; 2005. [DOI] [PubMed] [Google Scholar]
- Rijnders HJ, Järbe TU, Slangen JL. The pentylenetetrazole-cue antagonist actions of bretazenil (Ro 16-6028) as compared to midazolam. Pharmacol Biochem Behav. 1991;39:129–32. doi: 10.1016/0091-3057(91)90409-u. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Carroll ME. Progressive-ration and behavioral economic evaluation of the reinforcing efficacy of orally delivered phencyclidine and ethanol in monkeys: effects of feeding conditions. Psychopharmacology. 1996;128:265–73. doi: 10.1007/s002130050134. [DOI] [PubMed] [Google Scholar]
- Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: Antecedents, methodologies, and perspectives. Psychopharmacology. 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Lelas S. Comparison of zolpidem and midazolam self- administration under progressive-ratio schedules: consumer demand and labor supply analyses. Exp Clin Psychopharmacol. 2007;15:328–37. doi: 10.1037/1064-1297.15.4.328. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci USA. 2005;102:915–20. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Rodefer JS, Spealman RD. Self-administration of cocaine, alfentanil and nalbuphine under progressive-ratio schedules: Consumer demand and labor supply analyses of relative reinforcing effectiveness. Exp Clin Psychopharmacol. 2002;10:367–75. doi: 10.1037//1064-1297.10.4.367. [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Ator NA. Drug discrimination analysis of midazolam under a three-lever procedure. II: Differential effects of benzodiazepine receptor agonists. J Pharmacol Exp Ther. 1995;275:183–93. [PubMed] [Google Scholar]
- Schoch P, Moreau JL, Martin JR, Haefely WE. Aspects of benzodiazepine receptor structure and function with relevance to drug tolerance and dependence. Biochem Soc Symp. 1993;59:121–34. [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouèbe Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–775. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Whiting PJ, Kemp JA. Differences in affinity and efficacy of benzodiazepine receptor ligands at recombinant gamma-aminobutyric acidA receptor subtypes. Mol Pharmacol. 1993;43:240–244. [PubMed] [Google Scholar]
- Weed MR, Paul IA, Dwoskin LP, Moore SE, Woolverton WL. The relationship between reinforcing effects and in vitro effects of D1 agonists in monkeys. J Pharmacol Exp Ther. 1997;283:29–38. [PubMed] [Google Scholar]
- Wilcox KM, Rowlett JK, Paul IA, Ordway GA, Woolverton WL. On the relationship between dopamine transporter and the reinforcing effects of local anesthetics: Practical and theoretical concerns. Psychopharmacology. 2000;153:139–47. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]
- Yakushiji T, Fukuda T, Oyama Y, Akaike N. Effects of benzodiazepines and non-benzodiazepine compounds on the GABA-induced response in frog isolated sensory neurones. Br J Pharmacol. 1989;98:735–740. doi: 10.1111/j.1476-5381.1989.tb14600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]