Abstract
Laboratory studies show that individuals differ appreciably in the magnitude of their inflammatory responses to acute psychological stress. These individual differences are poorly understood, yet may contribute to variation in stress-associated disease vulnerability. The present study examined the possibility that affective responses to acute stress contribute to these differences. For this purpose, 102 relatively-healthy community volunteers (mean age 50 years; 60% female; 91.2% white) performed an acute stress protocol and measures of affective state and serum levels of the proinflammatory cytokine, interleukin (IL)-6 were collected at the end of a 30-min resting baseline, a 5-min evaluative public speaking task, and a 30-min recovery period. Results of regression analyses, controlling for age, race, gender, menopausal status, and body mass index, revealed a positive association of task-related increases in anger and anxiety with increases in IL-6 (R2 change = .08, p = .004; R2 change = .08, p = .005, respectively). Further examination showed that these affective responses to the task were independent predictors of change in IL-6. Cardiovascular reactivity to the task did not explain the association. These results suggest that individuals who exhibit angry or anxious responses to acute challenge are more vulnerable to stress-related increases in markers of systemic inflammation, possibly rendering them more susceptible to inflammatory disease.
Keywords: Inflammation, laboratory stress, interleukin-6, anger, anxiety, immune reactivity
Introduction
It is generally accepted that psychological stress contributes to susceptibility to immune-related disease (e.g., Black, 2003; Cohen & Hamrick, 2003; Rozanski et al., 1999; Schneiderman et al., 2008), yet not all similarly stressed individuals are equally likely to develop disease, suggesting that variability must exist either in psychological and/or biological vulnerability (Schneiderman et al., 2008). In this regard, laboratory studies show that individuals differ appreciably in the magnitude of their immune reactivity to acute stress, with some individuals exhibiting large inflammatory responses, as indexed by increases in circulating levels of inflammatory mediators, and others little or no response (Black, 2003; Cohen & Hamrick, 2003; Rozanski et al., 1999; Schneiderman et al., 2008; Steptoe et al., 2007). This interindividual variability is reproducible on retesting (Marsland et al., 1995 & 2002), raising the possibility that individual differences in inflammatory reactivity to stress provide a marker of increased vulnerability to immune-related disease.
Current models of stress and disease suggest that individuals differ in their emotional reactions to environmental situations and that it is this emotional reactivity that drives physiologic responses (Holmes et al., 2006). In support of these models, negative emotional reactions to laboratory stress have been shown to covary with (1) larger cardiovascular (heart rate and blood pressure) responses (Feldman et al., 1999), (2) increased expression of beta2-integrins on monocytes, a marker of inflammatory activity (Greeson et al., 2009), (3) increased levels of the inflammatory marker interleukin (IL)-6 in oral fluids (Moons et al., 2010), and (4) increased stimulated production of IL-6 among male participants with higher insulin resistance (Suarez et al., 2006). Furthermore, cardiovascular reactivity and other more direct indices of sympathetic nervous system activation parallel (and predict) interindividual variability in the magnitude of many immune responses to acute stress (Manuck et al., 1991). However, to date, no studies have examined whether variability of circulating levels of inflammatory mediators in response to acute stress reflects individual differences in task-induced emotional activation, possibly as a function of emotionally-induced activation of the sympathetic nervous system (Feldman et al., 1999; Suarez et al., 1998; Suarez & Williams, 1990). Thus, the purpose of the present study was to examine whether negative emotional responses (anger, anxiety) to a simulated public speaking task are associated with the magnitude of change in circulating levels of IL-6, and to explore whether cardiovascular responses to stress, an index of activation of the sympathetic nervous system, account for any observed associations.
Methods
Participants
Data for the present study were derived from the Vaccination and Immunity Project (VIP), a longitudinal study examining the association of psychosocial, physiologic and behavioral factors with antibody response to hepatitis B vaccination among a middle aged (40–60 years) community sample. Participants in the VIP project were recruited by mass mail solicitation in Allegheny County, Pennsylvania. Individuals were ineligible to participate if they smoked, had taken medications known to affect the endocrine, nervous, or immune systems in the past three months, or had a reported history or symptoms of systemic diseases known to affect these same systems. Data collection occurred over multiple laboratory sessions, and informed consent was obtained in accordance with approved protocol guidelines of the University of Pittsburgh Institutional Review Board.
For the present analyses, we used data collected at a laboratory session, which started between 7:00 and 9:00 AM, lasted approximately 1.5 hours, and took place prior to the first hepatitis B vaccination on a day when the participant reported being 2 weeks free of symptoms of infection or antibiotic use. At this session, measurements of affect and peripheral IL-6 were obtained before and after participants performed a 5-minute, videotaped public speaking task. The current analyses included data from 102 VIP participants (60% female; mean age = 50 years) on whom we had complete measures of affect and IL-6.
Procedures
Prior to the laboratory session, participants were asked to abstain from caffeine, beverages (other than water), and food for 12 hours, from strenuous physical activity and non-prescription medications for 24 hours, and from alcohol for 48 hours. On arrival, participants completed a medical history interview, filled out questionnaires, and had measurements of height and weight taken. Next, an intravenous catheter was inserted into the antecubital fossa of one arm for the collection of blood samples, and an occluding cuff was placed on the other arm for automated measurement of heart rate (HR) and systolic and diastolic blood pressure (SBP and DBP; Critikon Dinamap 8100 Vital Signs Monitor, Tampa, FL). Following instrumentation, participants were seated for a 30-minute resting period. Baseline recordings of blood pressure (BP) and HR were recorded every 90 seconds for the last 6 minutes of this rest period (4 readings) and 4ml of blood was drawn for the determination of IL-6 levels. Participants then completed a measure assessing mood state across the baseline period. Following the baseline, participants performed a simulated public speaking task, consisting of 2 minutes of preparation for a speech defending themselves against an alleged transgression (shoplifting or traffic violation), followed by 3 minutes of videotaped speech delivery. We have previously demonstrated that this task elicits reproducible cardiovascular and immune responses (Marsland et al., 1995, 2002). HR and BP were measured every 90 seconds during speech preparation and performance, and a second 4ml of blood was collected immediately after task completion. Following sample collection, mood was reassessed, focusing on feelings during speech delivery. Participants then rested quietly for a 30 minute period, with BP and HR recorded every 90 seconds during the final 6 minutes of this period (4 readings), a 4ml blood sample drawn in the final minute, and mood during this period assessed.
Measures
Serum IL-6
Serum samples were stored at −80°C until analysis in batches. IL-6 levels were determined using a high sensitivity quantitative sandwich enzyme immunoassay kit (R & D Systems). Briefly, standards, controls and samples were added to a 96-well microplate pre-coated with monoclonal anti-IL-6 antibodies. Unbound substances were removed by washing and an enzyme linked polyclonal anti-IL-6 antibody was then added. This was followed by washing, incubation with a substrate solution and then with the amplifier solution. The intensity of the color that resulted was measured at 490nm. The assay standard range is 0.156 to 10 pg/mL. IL-6 levels were extrapolated from a standard curve with linear regression from a log-linear curve. All samples were run in duplicate and the average inter- and intra- assay coefficients of variation were 7% and 5%, respectively. Natural log transformation was applied to normalize raw score distributions of the IL-6 values.
State Affect
Affective state was assessed with a mood adjective rating scale derived from the POMS (McNair et al., 1971), with item selection based on Usala and Hertzog's (1989) factor analysis of item loading. Items assessed anxiety (e.g., tense, nervous, uneasy, on edge), anger (e.g., angry, resentful, hostile), depression (e.g., sad, unhappy, depressed), calm (e.g., relaxed, comfortable, calm, at ease), fatigue (e.g., tired, sluggish, sleepy, fatigued, worn out), vigor (e.g., lively, energetic, full of pep), and well-being (e.g., cheerful, happy, pleased). Participants rated their mood on a scale ranging from 0 (not at all) to 4 (extremely).
Covariates
Participant's age, gender, and race were determined through self-report. Body mass index (BMI) was calculated by dividing weight (kg) by height2 (m2). Menopausal status was determined through self-report of menstruation, with classification of pre-menopausal when subject reported regular, normal menstrual cycles (n = 18), peri- or post-menopausal when reporting either irregular or no menstrual cycle (n = 42).
Statistical analyses
SPSS for windows (version 17.0) was used for all analyses. First, Spearman and Pearson product-moment correlations were conducted to assess associations of covariates (age, gender, race, and BMI) with baseline IL-6. Next, to evaluate any main effects of the speech task on cardiovascular, affective, and inflammatory parameters, repeated-measures analyses of variance (ANOVAs) were conducted on each dependent variable, followed by Least Significant Difference (LSD) pairwise comparisons when indicated. For these analyses, mean HR and BP values were calculated for baseline, task, and post-task periods. When sphericity could not be assumed, Greenhouse-Geisser correction for repeated-measure ANOVA was used. Next, to test our primary hypothesis, Pearson product-moment correlations were used to assess associations of task-induced affective responses with the magnitude of concomitant cardiovascular and immune changes. For this purpose, we calculated baseline-adjusted change scores by regressing task measurements onto corresponding baselines and saving the residuals. On analysis of IL-6 responses, we focused on changes in circulating levels of IL-6 from baseline to 30 minutes post-task given evidence that IL-6 responses to acute psychological challenge are delayed (Steptoe et al., 2007). We also ran a series of linear regression analyses entering covariates (age, gender, race, menopausal status, and BMI) in the first step, and baseline-to-task affective changes in the second step of a model predicting baseline-to 30 minute post-task changes in IL-6. We did not adjust p values for multiple analyses.
Results
Participants were 102 adults (59.8% female; 91.2% Caucasian) with a mean age of 50.3 (SD= 5.1) years and BMI of 26.3 (SD = 3.8). An initial examination of associations between demographic/health characteristics, baseline affect, and baseline IL-6 revealed a significant association of IL-6 with BMI (r = .50, p < .001), but not with demographic characteristics or baseline affective states. Being female was positively associated with baseline fatigue (r = .23, p = .02) and anxiety (r = .22, p = .03), and negatively associated with baseline vigor (r = −.23, p = .02).
Effects of the speech task on mean affect, BP, HR, and IL-6
Mean values of affect, BP, HR, and IL-6 levels at baseline, task, and 30 minutes post-task are presented in Table 1. Repeated measures ANOVAS reveal a significant period main effect on analysis of anger (F(1, 124) = 31.97, p < .001), anxiety (F(1,133) = 82.23, p < .001), depressive affect (F(2, 165) = 6.04, p = .006), and vigor (F(2,176) = 45.87, p < .001), with levels increasing from baseline to task, and returning to baseline levels 30 minutes post-task. Decreases in fatigue (F(2,193) = 72.78, p < .001), well-being (F(2, 156) = 21.19, p < .001), and calm (F(2, 168) = 111.17, p < .001) from baseline to task were also observed. In regard to cardiovascular parameters, significant period main effects were observed for SBP (F(1,135) = 193.23, p < .001), DBP (F(1, 157) = 231.69, p < .001), and HR (F(1, 129) = 109.53, p < .001), with task values being significantly higher than baseline and 30 minute post-task levels. Finally, there was a trend towards a period main effect on analysis of IL-6 levels (F(1,144) = 3.08, p = .066), with pairwise comparisons revealing a significant increase in IL-6 from task to 30 minutes post-task (p = .04), but no significant change from baseline to task, or from baseline to 30 minutes post-task.
Table 1.
Mean Blood Pressure, Heart Rate, Negative Affect Scores, and IL-6 Levels at Baseline, Task, and 30-Minute Post-Task Intervals
| Baseline | Task | 30 Min Post-task | |
|---|---|---|---|
| Systolic Blood Pressure (mm Hg)a,b | 112.0 (14.0) | 131.3(18.6) | 114.9(14.6) |
| Diastolic Blood Pressure (mm Hg)a,b | 71.1(9.3) | 80.6(10.6) | 71.9(8.9) |
| Heart Rate (bpm)a,b | 60.3(7.4) | 69.0(11.5) | 60.5(8.0) |
| Anxietya,b | 1.06(1.6) | 5.06(4.3) | 1.46(2.0) |
| Angera,b | .10(.5) | 1.36(2.1) | .23(.7) |
| Depressiona,b | .32(.9) | .64(1.1) | .35(.8) |
| IL-6 (pg/ml)b | 1.22(.61) | 1.20(.6) | 1.26(.6) |
Difference significant at p < .05 for Baseline to Task
Difference significant at p < .05 for Task to 30 Min. Post-task
Factors that may contribute to variability in the magnitude of IL-6 responses to stress
Consistent with the findings of others (Steptoe et al., 2007), we observed considerable variability in the magnitude of change in IL-6 from baseline to 30 minutes post-task (mean change score = .04 pg/ml, SD = .32 pg/ml), suggesting that individuals vary markedly in the magnitude of their inflammatory response to the task. To explore factors associated with this variability in IL-6 reactivity, we first examined associations of age, gender, race, and BMI with residualized changes in IL-6. Of these characteristics, only age was associated with IL-6 reactivity (r = .21, p < .05). Interestingly, age also predicted greater increases in depressive affect in response to the task (r = .24, p < .05). For women, we also examined the association of menopausal status with residualized changes in IL-6. Here, correlations revealed a significant association of greater IL-6 responses in peri-/post-menopausal women (r = .25, p = .05).
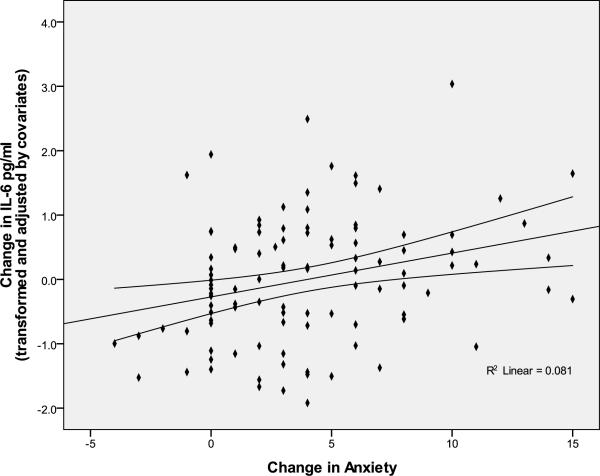
Preliminary bivariate analyses revealed expected associations of task-related increases in anger, anxiety, and depression with IL-6 reactivity (r = .27, p < .01; r = .25, p < .02; r = .21, p < .05, respectively) (See Table 2) . No significant correlations were found between the other dimensions of affective reactivity and IL-6 change. As shown in Figures 1 and 2, regression analyses showed that positive associations between angry and anxious responses to the task and IL-6 reactivity were retained after controlling for gender, age, race, BMI, and menopausal status (anger: β = .29; R2 change = .08; F(1,94) = 8.91, p = .004; anxiety: β = .28; R2 change = .08; F(1,94) = 8.46, p = .005), with a similar trend on analysis of depressive responses (β = .16; R2 change = .024, F(1,93) = 2.45, p = .12). In contrast, exploratory analyses revealed a tendency for calm responses to the task to be negatively associated with IL-6 responses (β = −.16; R2 change = .024, F(1,93) = 2.48, p = .12). No significant associations were observed between task-related changes in fatigue, vigor, or well-being and IL-6 reactivity.
Table 2.
Correlations of Baseline-to-Task Change in Negative Affect with Concomitant Change in Cardiovascular Measures and Baseline-to-30-Minute Post-Task Change in Serum IL-6.
| ΔIL-6 | ΔHR | ΔSBP | ΔDBP | |
|---|---|---|---|---|
| 1. Δ Anxiety | .25* | .23* | .32** | .09 |
| 2. Δ Anger | .27* | −.03 | .04 | .04 |
| 3. Δ Depression | .21* | −.04 | −.06 | .01 |
| 4. Δ Heart rate | −.05 | -- | .47** | .23* |
| 5. Δ Systolic BP | −.02 | .47** | -- | .65** |
| 6. Δ Diastolic BP | −.07 | .23* | .65** | -- |
All change scores were residuals adjusting for baseline levels
p < .05,
p<.001
Figure 1.
Association of anxious responses to the speech task with change in IL-6 from baseline to 30 minutes post-task with 95% mean prediction interval.
Figure 2.
Association of angry responses to the speech task with change in IL-6 from baseline to 30 minutes post-task with 95% mean prediction interval.
In light of a significant association between the magnitude of angry and anxious responses to the task (r = .45, p < .001), we next examined whether these moods contributed independently to IL-6 reactivity. Here, we entered task-related change in anger (or anxiety) along with covariates in the first step, and change in anxiety (or anger) in the second step of a regression model predicting IL-6 reactivity. Results showed a small decrease in the association of angry responses and IL-6 reactivity with anxiety in the model (beta changed from .29 to .21), with a trend for an independent effect of anger (p = .06). Similarly, although including angry responses in the model reduced the anxiety effect size (beta changed from .28 to .18), there was a trend for an independent relationship between anxiety and IL-6 reactivity (p = .08). Overall, these analyses suggest that the association of angry and anxious responses to the speech task and IL-6 reactivity are largely independent. Indeed, entering both mood responses into the model together accounted for greater variance in IL-6 reactivity than either alone (R2 change = .11, F(2,93) = 6.10, p = .003).
Next, we examined whether gender moderated the association of affective reactivity with IL-6 responses to the task. Here, we entered task-related change in anger (or anxiety) along with gender, age, race, menopausal status, and BMI in the first step, and the interaction of gender with change in anger (or anxiety) in the second step of a regression model predicting IL-6 reactivity. Results showed no significant gender X anxiety interaction in the prediction of IL-6 reactivity. However, the interaction of gender with anger was marginally associated with IL-6 response (R2 change = .028, p = .08). Further analyses broken down by gender revealed that angry responses to the task were positively associated with IL-6 reactivity for males (R2 change = = .16, p = .007), but not females (R2 change = .02, p = .30).
Cardiovascular reactivity
Finally, we examined whether cardiovascular responses to the task accounted for the variance in IL-6 reactivity associated with affective responses. After adjusting for covariates, anxious responses to the speech task were significantly associated with baseline-to-task increases in SBP (r = .30, p = .003) and HR reactivity (r = .23, p = .03), but not DBP. In contrast, there were no associations between anger and cardiovascular responses to the task. Moreover, cardiovascular responses were unrelated to IL-6 reactivity and entering SBP or HR reactivity into the regression model did not alter the magnitude of the association between anxious responses and IL-6 reactivity.
Discussion
Consistent with the findings of others, we observed widespread individual differences in the magnitude of change in circulating levels of IL-6 in response to acute psychological stress. The primary goal of the current study was to examine whether task-induced affective states contributed to this variability in inflammatory response. In our sample of relatively healthy, mid-life adults, angry and anxious responses to a simulated public speaking task were associated positively with changes in circulating levels of IL-6, as measured 30 minutes following the task. These associations were independent of age, race, BMI, menopausal status for women, and baseline levels of IL-6. In regard to gender, anxious reactions predicted increases in IL-6 for males and females; however, associations of task-induced anger with IL-6 were observed primarily among males. In sum, our findings provide initial evidence that affective responses to acute psychological stress contribute to heightened inflammatory responses. These findings are consistent with recent evidence that negative affective states result in activation of innate inflammatory pathways. For example, negative affective responses to an anger recall interview (ARI) are associated with increased B2-integrin expression (Greeson et al., 2009) and monocyte-stimulated proinflammatory cytokine production among insulin resistant men (Suarez et al., 2006). Similarly, negative affective responses to the Trier Social Stress Test (TSST) have been positively associated with magnitude of inflammatory response, with perceived stress predicting increases in circulating levels of IL-1β (Yamakawa and colleagues,2009) and fear predicting increases in IL-6 in oral fluids (Moons et al., 2010). In contrast to the current findings, Moons et al (2010) did not observe an association of anger in response to the TSST and inflammatory markers in oral fluids. Reasons for these discrepant findings are unclear, but may relate to the examination of oral versus circulating inflammatory markers or be a function of the timing of the assessment of affective state, with measures being taken immediately following the task in the current study and 30 minutes after the task in the Moons et al. (2010) study.
Interestingly, although we observed that angry and anxious responses to the speech task covaried, the positive associations of these activated negative emotions with inflammatory responses following acute challenge were largely independent. Stress-induced increases in depression and fatigue, which represent less physiologically activating negative moods than anxiety and anger, were not significantly associated with IL-6 reactivity after adjusting for covariates. Changes in positive affect (i.e., calm, vigor, and well-being) were also unrelated to the magnitude of IL-6 responses. These findings raise the possibility that activated negative emotional states contribute to the increase in circulating levels of IL-6 that follows acute psychological challenge. However, it is also possible that our failure to find more significant associations of depression, calm, vigor, fatigue, and well-being with IL-6 reflects the relatively small task-related changes in these affective states, when compared with anxiety and anger, limiting our ability to detect relationships.
Although there is growing evidence that circulating levels of IL-6 typically increase following acute psychological stress (see Steptoe et al 2007 for review), mean effect sizes are modest (Steptoe et al., 2007), with a number of studies finding no significant IL-6 response to laboratory stress (e.g., Miller et al., 2005; Lutgendorf et al., 2004). The current findings are generally consistent with this literature and show a trend toward a mean increase in IL-6 from pre- to 30 minutes post-task. Our findings extend this literature by providing the first evidence to show that individual differences in affective response to the task are associated with the magnitude of this inflammatory response in healthy mid-life adults. We also provide initial evidence to suggest that there may be gender differences in the impact of acute affective states on systemic inflammation, with both sexes showing similar inflammatory responses to anxiety, but males showing greater responses to anger than females. Thus, inconsistencies across studies may reflect gender differences and/or heterogeneity of affective responses, with results varying as a function of the type or intensity of emotions induced by the laboratory task. It is also likely that neurological (Slavich et al., 2010) or genetic (Cole et al., 2010) vulnerabilities contribute to individual differences in affective and/or inflammatory responses. The clinical significance of the increases in systemic inflammation that follow acute laboratory challenge remains to be determined. In this regard, Brydon & Steptoe (2005) showed a positive association of magnitude of IL-6 response to acute laboratory stress with increases in blood pressure over a 3 year follow-up period; an effect that was independent of initial blood pressure, baseline IL-6, age, BMI, gender and smoking status. This raises the possibility that individuals prone to stress-induced increases in inflammation, possibly by virtue of their affective responses to environmental challenges, may be at greater risk for cardiovascular disease.
Several potential mechanisms may underlie associations of activated negative emotions and inflammatory responses to acute stress. Based on existing literature, we expected that negative emotional states would activate the sympathetic division of the autonomic nervous system, resulting in increases in circulating levels of IL-6 as the consequence of (1) activation of peripheral blood mononuclear cells (Bierhaus et al., 2003), (2) the release of IL-6 from adipocytes (Burysek & Houstek, 1997), and (3) pro-coagulant/proinflammatory responses elicited from damaged vascular walls under sheer stress (Moshage, 1997; Thrall et al., 2007; von Kanel et al., 2001). However, in contrast to the findings of others (Owen & Steptoe, 2003), our analyses did not provide clear evidence for an association of cardiovascular responses to the stress task, an indirect measure of autonomic activity, with the magnitude of inflammatory response. Although anxious responses to the speech stressor were associated with concomitant increases in SBP and HR, this cardiovascular reactivity did not account for variability in IL-6 response associated with anxious mood. Moreover, there was no association of task-induced anger with cardiovascular reactivity in the current sample. Thus, our findings do not provide evidence that activation of the sympathetic nervous system drives the increase in peripheral inflammation associated with activated negative mood states. That said, measures of HR and BP provide an indirect measure of sympathetic activation, and it remains possible that a more direct measure of autonomic activation, such as circulating catecholamine levels, could yield a different pattern of results.
Given that stress-related increases in markers of peripheral inflammation are typically not detected until at least 30 minutes following the stressor, it is also possible that the kinetics of the autonomic response to stress are more influential than the peak stress response. For example, individuals who take longer to recover to baseline levels after a task may be at greater risk for inflammatory responses. In support of this possibility, delayed blood pressure recovery following acute stress has been positively associated with the magnitude of inflammatory response (Steptoe & Marmot, 2006). This delay in blood pressure recovery may be the result of prolonged sympathetic activity, decreased parasympathetic activity, or greater peripheral damage as a consequence of prolonged blood pressure. In regard to the parasympathetic division of the autonomic nervous system, lower heart rate variability, an indirect measure of parasympathetic (vagal) control over variations in heart rate, accompanies both negative emotional states (Brosschot & Thayer, 1998; Friedman & Thayer, 1998) and inflammation (Frasure-Smith et al., 2009; Marsland et al., 2007). Thus, it is possible that stress-induced decreases in parasympathetic activation may contribute to associations of negative emotional states with markers of inflammation. Finally, activation of the hypothalamic-pituitary adrenal (HPA) axis may also play a role, as cortisol shows a delayed response to acute psychological stress that parallels the timing of the inflammatory response. In this regard, it has been proposed that individuals who are emotionally sensitive to psychological stressors may exhibit blunted cortisol responses to stress and thus increased activation of inflammatory processes (Burke et al., 2005).
Several limitations of the current research should be noted. First, the sample was healthy, nonsmoking, and free of medications affecting immune, nervous, and endocrine systems; thus, results may not generalize to clinical or at-risk samples. Second, analyses were based on affect and IL-6 reactivity from only one laboratory session -- repeated assessments across time and situations would provide a more reliable measure of individual differences. In addition, our protocol did not include assessment of changes in IL-6 later than 30 minutes after the task. Given that increased inflammatory activity have been observed up to 2 hours after acute stress, future work would benefit from exploring whether affective response predicts more sustained elevations in IL-6 (Steptoe et al., 2007). Finally, although relatively small increases in circulating markers of inflammation (i.e. CRP, IL-6) have been shown to predict increased disease risk (Libby & Ridker, 2004; Libby et al., 2002; Ward et al., 2009), the clinical significance of the small, transient, stress-induced increase in IL-6 remains to be determined.
In conclusion, the present findings suggest that individual differences in affective responses to a mildly stressful speaking task are associated with the magnitude of change in circulating IL-6 levels, a marker of systemic inflammatory activity. Gender may play a role in this relationship, with males showing greater inflammatory responses to acute anger than females, but both genders showing similar inflammatory responses to anxiety. Otherwise, positive associations of negative affective states with IL-6 were independent of demographic characteristics and cardiovascular reactivity. These results suggest that activated negative affect in response to acute stress is associated with stress-related increases in inflammation. Long-term or repeat activation of inflammatory pathways as a consequence of heightened negative affective responses to stressful situations in daily life may contribute to risk for inflammatory diseases.
Acknowledgments
This study was supported by grant NR008237 from the National Institute of Nursing Research (ALM). The expert technical assistance of Kevin McDade, M.S., is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
References
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav. Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: a model of the link between hostility and cardiovascular disease. Ann. Behav. Med. 1998;20:326–332. doi: 10.1007/BF02886382. [DOI] [PubMed] [Google Scholar]
- Brydon L, Steptoe A. Stress-induced increases in interleukin-6 and fibrinogen predict ambulatory blood pressure at 3-year fllow-up. J. Hypertens. 2005;23:1001–1007. doi: 10.1097/01.hjh.0000166841.57474.d0. [DOI] [PubMed] [Google Scholar]
- Burysek L, Houstek J. Beta-Adrenergic stimulation of interleukin-1 alpha and interleukin-6 expression in mouse brown adipocytes. Febs Letters. 1997;411:83–86. doi: 10.1016/s0014-5793(97)00671-6. [DOI] [PubMed] [Google Scholar]
- Burke H, Davis M, Otte C, Mohr D. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick N. Stable individual differences in physiological response to stressors: implications for stress-elicited changes in immune related health. Brain Behav. Immun. 2003;17:407–414. doi: 10.1016/s0889-1591(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Cole S, Arevalo JMG, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene-social environment interaction at the human IL6 locus. PNAS. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman PJ, Cohen S, Lepore SJ, Matthews KA, Kamarck TW, Marsland AL. Negative emotions and acute physiological responses to stress. Ann. Behav. Med. 1999;21:216–22. doi: 10.1007/BF02884836. discussion 223–226. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Irwin M, Talajic M, Pollock B. The relationships among heart rate variability, inflammatory markers and depression in coronary heart disease patients. Brain Behav. Immun. 2009;23:1140–1147. doi: 10.1016/j.bbi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF. Autonomic balance revisited: Panic anxiety and heart rate variability. J. Psychosom. Res. 1998;44:133–151. doi: 10.1016/s0022-3999(97)00202-x. [DOI] [PubMed] [Google Scholar]
- Greeson JM, Lewis JG, Achanzar K, Zimmerman E, Young KH, Suarez EC. Stress-induced changes in the expression of monocytic beta2-integrins: the impact of arousal of negative affect and adrenergic responses to the Anger Recall Interview. Brain Behav. Immun. 2009;23:251–6. doi: 10.1016/j.bbi.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SD, Krantz DS, Rogers H, Gottdiener J, Contrada RJ. Mental stress and coronary artery disease: a multidisciplinary guide. Prog. Cardiovasc. Dis. 2006;49:106–22. doi: 10.1016/j.pcad.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am. J. Med. 2004;116:9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Logan H, Costanzo E, Lubaroff D. Effects of acute stress, relaxation, and a neurogenic inflammatory stimulus on interleukin-6 in humans. Brain Behav. Immunol. 2004;18:55–64. doi: 10.1016/s0889-1591(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Cohen S, Rabin BS, Muldoon MF, Bachen EA. Individual differences in cellular immune response to stress. Psychol. Sci. 1991;2:111–115. [Google Scholar]
- Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom. Med. 2007;69:709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Henderson BN, Chambers WH, Baum A. Stability of individual differences in cellular immune responses to two different laboratory tasks. Psychophysiology. 2002;39:865–868. doi: 10.1111/1469-8986.3960865. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Manuck SB, Fazzari TV, Stewart CJ, Rabin BS. Stability of Individual Differences in Cellular Immune Responses to Acute Psychological Stress. Psychosom. Med. 1995;57:295–298. doi: 10.1097/00006842-199505000-00012. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of Mood States (POMS) Educational and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom. Med. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity. 2010;24:215–219. doi: 10.1016/j.bbi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Moshage H. Cytokines and the hepatic acute-phase response. J. Pathol. 1997;181:257–266. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Owen N, Steptoe A. Natural killer cell and proinflammatory cytokine responses to mental stress: Associations with heart rate and heart rate variability. Biological Psychiatry. 2003;63:101–115. doi: 10.1016/s0301-0511(03)00023-1. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Kaplan J. Impact of Psychological Factors on the Pathogenesis of Cardiovascular Disease and Implications for Therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, Siegel SD. Stress and Health: Psychological, Behavioral, and Biological Determinants. Annu. Rev. Clin. Psychol. 2008;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. PNAS. 2010 doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. Psychosocial, hemostatic, and inflammatory correlates of delayed poststress blood pressure recovery. Psychosom. Med. 2006;68:531–537. doi: 10.1097/01.psy.0000227751.82103.65. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Boyle SH, Lewis JG, Hall RP, Young KH. Increases in stimulated secretion of proinflammatory cytokines by blood monocytes following arousal of negative affect: the role of insulin resistance as moderator. Brain, Behav. Immun. 2006;20:331–338. doi: 10.1016/j.bbi.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Kuhn CM, Schanberg SM, Williams RB, Zimmermann EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: the role of interpersonal challenge. Psychosom. Med. 1998;60:78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Williams RB. The relationships between dimensions of hostility and cardiovascular reactivity as a function of task characteristics. Psychosom. Med. 1990;52:558–570. doi: 10.1097/00006842-199009000-00008. [DOI] [PubMed] [Google Scholar]
- Thrall G, Lane D, Carroll D, Lip GY. A systematic review of the effects of acute psychological stress and physical activity on haemorheology, coagulation, fibrinolysis and platelet reactivity: Implications for the pathogenesis of acute coronary syndromes. Thromb. Res. 2007;120:819–847. doi: 10.1016/j.thromres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Usala PD, Hertzog C. Measurement of affective states in adults: Evalulation of an adjective rating scale instrument. Res. Aging. 1989;11:403–426. doi: 10.1177/0164027589114001. [DOI] [PubMed] [Google Scholar]
- von Känel R, Mills PJ, Fainman C, Dimsdale JE. Effects of psychological stress and psychiatric disorders on blood coagulation and fibrinolysis: a biobehavioral pathway to coronary artery disease? Psychosom. Med. 2001;63:531–544. doi: 10.1097/00006842-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Ward J, Wilson H, Francis S, Crossman D, Sabroe I. Translational Mini-Review Series on Immunology of Vascular Disease: Inflammation, infections and Toll-like receptors in cardiovascular disease. Clin. Exp. Immunol. 2009;156:386–394. doi: 10.1111/j.1365-2249.2009.03886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa K, Matsunaga M, Isowa T, Kimura K, Kasugai K, Yoneda M, Kaneko H, Ohira H. Transient responses of inflammatory cytokines in acute stress. Biol. Psychol. 2009;82:25–32. doi: 10.1016/j.biopsycho.2009.05.001. [DOI] [PubMed] [Google Scholar]