Abstract
Sensitivity to the euphoric and locomotor-activating effects of drugs of abuse may contribute to risk for excessive use and addiction. Repeated administration of psychostimulants such as methamphetamine can result in neuroadaptive consequences that manifest behaviorally as a progressive escalation of locomotor activation, termed psychomotor sensitization. The present studies addressed the involvement of specific components of the corticotropin-releasing factor (CRF) system in locomotor activation and psychomotor sensitization induced by methamphetamine (1, 2 mg/kg) by utilizing pharmacological approaches, as well as a series of genetic knockout mice, each deficient for a single component of the CRF system: CRF-R1, CRF-R2, CRF, or the CRF-related peptide Urocortin 1 (Ucn1). CRF-R1 knockout mice did not differ from wild-type mice in sensitization to methamphetamine, and pharmacological blockade of CRF-R1 with CP-154,526 (15, 30 mg/kg) in DBA/2J mice did not selectively attenuate either the acquisition or expression of methamphetamine-induced sensitization. Deletion of either of the endogenous ligands of CRF-R1 (CRF, Ucn1) either enhanced, or had no effect, on methamphetamine-induced sensitization, providing further evidence against a role for CRF-R1 signaling. Interestingly, deletion of CRF-R2 attenuated methamphetamine-induced locomotor activation, elucidating a novel contribution of the CRF system to methamphetamine sensitivity, and suggesting the participation of the endogenous urocortin peptides Ucn2 and Ucn3. Immunohistochemistry for Fos was used to visualize neural activation underlying CRF-R2-dependent sensitivity to methamphetamine, identifying the basolateral and central nuclei of the amygdala as neural substrates involved in this response. Our results support further examination of CRF-R2 involvement in neural processes associated with methamphetamine addiction.
Keywords: amphetamine, corticotropin-releasing hormone, urocortin, addiction, amygdala
INTRODUCTION
Repeated administration of psychostimulants can result in an enhancement of their locomotor-activating properties, a phenomenon associated with persistent neuroadaptations underlying the chronic relapsing state characteristic of addiction (Robinson & Berridge, 1993). This “psychomotor sensitization” is thought to reflect changes within neurotransmitter systems implicated in both the positive-motivational and negative-reinforcement processes that perpetuate uncontrollable drug use (Koob, 2008, Koob & Le Moal, 2008, Vezina et al., 2002). Of particular importance in the transition from controlled to compulsive drug use is the corticotropin-releasing factor (CRF) neuropeptide system. Manipulations of the CRF system have revealed its involvement in multiple endophenotypes of psychostimulant addiction, including psychomotor sensitization (Cador et al., 1992, Erb & Brown, 2006), withdrawal (Richter & Weiss, 1999, Sarnyai et al., 1995, Vuong et al., 2009, Zorrilla et al., 2001), and reinstatement of drug-seeking (Erb et al., 2001, Moffett & Goeders, 2007, Wang et al., 2007).
Comprised of four endogenous ligands (CRF, Urocortins 1, 2 and 3) and two receptor subtypes (CRF-R1, CRF-R2), the CRF system initiates the neuroendocrine stress response via the hypothalamic-pituitary-adrenal (HPA) axis, and coordinates diverse behaviors via actions on extra-HPA loci. HPA-axis activation is controlled by release of CRF from the paraventricular nucleus of the hypothalamus (PVN) onto CRF-R1 in the pituitary, resulting in secretion of adrenocorticotropic hormone and glucocorticoids. Stress is a key factor in initiating relapse (Sinha, 2001), and the importance of the HPA-axis in predisposition to psychostimulant self-administration has been explored (Piazza et al., 1991). However, extra-HPA actions of CRF and urocortin peptides on the bed nucleus of the stria terminalis (Kash et al., 2008), amygdala (Fu et al., 2007, Krishnan et al., Orozco-Cabal et al., 2008, Pollandt et al., 2006), lateral septum (Liu et al., 2005), ventral tegmental area (Hahn et al., 2009, Wang et al., 2007), and dorsal raphé nucleus (Pringle et al., 2008, Vuong et al., 2009) also contribute to psychostimulant-induced neuroplasticity.
Anatomical organization of the CRF system is complex, as each component exhibits a distinct, yet partially overlapping, pattern of brain expression (Chalmers et al., 1995, Kozicz et al., 1998, Li et al., 2002, Merchenthaler et al., 1982, Reyes et al., 2001). Furthermore, CRF and Urocortin 1 (Ucn1) bind both receptor subtypes, while Urocortin 2 and 3 (Ucn2, Ucn3) are selective for CRF-R2 (Lewis et al., 2001, Reyes et al., 2001, Vaughan et al., 1995). Due to this complexity, elucidation of the involvement of specific components of the CRF system in the behavioral response to psychostimulants has remained incomplete. Thus, the present studies assessed locomotor activation and psychomotor sensitization to methamphetamine (MA) in four lines of mice, each containing a deletion of a single component of the CRF system (CRF-R1, CRF-R2, CRF, or Ucn1). Given previous reports implicating the HPA-axis in psychostimulant-induced sensitization, and to avoid interpretational issues associated with the use of genetic mutant mice, additional experiments evaluated the effects of the CRF-R1-selective antagonist CP-154,526 on the acquisition and expression of MA-induced sensitization. Finally, the neural substrates underlying CRF-R2-dependent acute stimulation to MA were examined using Fos immunohistochemistry.
MATERIALS AND METHODS
Animals
For experiments in knockout (KO) and wild-type (WT) littermates, we used single gene mutant mice created from embryonic stem cells that had undergone targeted gene inactivation. CRF-R1 KO mice generated on a 129P2/OlaHsd × CD1 background contained a deletion of exons 4-7 of the Crhr1 gene (Timpl et al., 1998), CRF-R2 KO mice generated on a 129X1/SvJ × C57BL/6 (B6) background contained a deletion of exons 3–4 of the Crhr2 gene (Coste et al., 2000), CRF KO mice generated on a 129S2/SvPas × B6 background contained a deletion of exon 2 of the Crh gene (Muglia et al., 1995), and Ucn1 KO mice generated on a 129X1/SvJ × B6 background contained a deletion of exon 2 of the Ucn gene (Vetter et al., 2002). Each KO was backcrossed onto a B6 genetic background for 8–10 generations. KO and WT mice were littermates, generated by heterozygous matings. B6 mice exhibit reliable, gradual MA-induced sensitization (Phillips et al., 1994), and a proportion of these mutant mice had already been backcrossed to B6 for a number of generations prior to the initiation of these studies, providing a rationale for the use of a B6 background for all KO and WT mice examined here. These mice were weaned at 28–32 days of age, isosexually housed, and tested at 8–13 weeks of age. Separate groups of 8–9 week-old female DBA/2J (D2) mice (The Jackson Laboratory; Sacramento, CA) were used for pharmacological studies with the CRF-R1 antagonist, CP-154,526. D2 mice were chosen for these studies because they have been used in similar previous work examining the role of CRF-R1 and the HPA-axis in drug-induced sensitization (Pastor et al., 2008, Roberts et al., 1995). All protocols were approved by the Oregon Health & Science University (OHSU) or Portland Department of Veterans Affairs (VA) animal care and use committee, and performed within the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Adequate measures were taken to minimize any pain or discomfort experienced by the mice.
Drugs
Methamphetamine HCl (Sigma; St. Louis, MO) was dissolved in 0.9% saline at 0.1 or 0.2 mg/ml, and CP-154,526 (a generous gift from Pfizer) was dissolved in 5.0% emulphor (Cremophor EL, Sigma) in 0.9% NaCl at 1.5 or 3.0 mg/ml. All injections were given intraperitoneally (i.p.) at 10 ml/kg.
MA-induced Psychomotor Sensitization in KO and WT Littermates
Male and female KO and WT mice from each of the lines were assessed for locomotor activation in a previously-established protocol in which repeated administration of 1 mg/kg MA induces reliable, progressive sensitization in B6 and other genotypes of mice (Kamens et al., 2005, Phillips et al., 1994). Following two days of saline administration to allow habituation (Day 1) and measure baseline activity levels (Day 2), locomotor activity was measured following an injection of MA (1 mg/kg) on Days 3, 5, 7, 9 and 11, and a saline injection on Day 12. The protocol included a final MA challenge (1 mg/kg) on Day 27, after a two week-long drug-free period during which additional neural changes associated with repeated MA exposure may become established (Yamamoto et al., 2006). On test days, mice were moved from the colony room, allowed one hour to acclimate to the room in which testing would occur, injected, and placed into activity chambers for 15 minutes. Details of the locomotor apparatus can be found in the Supplementary Materials.
CRF-R1 Antagonism and MA-induced Sensitization in DBA/2J Mice
Female D2 mice underwent a previously-established protocol which monitors the effects of pharmacological manipulations on either the acquisition or expression of drug-induced sensitization (Pastor et al., 2008, Roberts et al., 1995). For these experiments, mice received two injections per day, separated by thirty minutes. Doses of CP-154,526 (15 and 30 mg/kg) were chosen from previous work in which they were capable of attenuating ethanol-induced sensitization (Pastor et al., 2008), and the dose of MA (2 mg/kg) was determined by pilot studies (data not shown). For acquisition, mice were treated on Days 1–10 with CP-154,526 or vehicle, prior to MA or saline. On Day 11, mice received vehicle prior to MA. On Day 12, mice received vehicle prior to saline. For expression, mice were treated on Days 1–10 with vehicle, prior to MA or saline. On Day 11, mice received CP-154,526 or vehicle, prior to MA. On Day 12, mice received vehicle, prior to saline. For both experiments, horizontal locomotor activity was measured for 15 minutes immediately following the second injection on Days 11 and 12. Finally, due to apparent non-specific effects of CP-154,526 on locomotor behavior, activity levels of saline-treated mice were assessed in an additional control experiment. Mice were treated on Days 1–10 with vehicle followed by saline. On Day 11, mice received CP-154,526 or vehicle, followed by saline, and locomotor activity was assessed for 15 minutes following the second injection. Details of the locomotor apparatus can be found in the Supplementary Materials.
Acute Stimulation in CRF-R2 KO and WT Littermates
Additional studies in male and female CRF-R2 KO and WT littermates were performed to verify our results demonstrating CRF-R2-dependent MA sensitivity. Procedures were identical to the first three days of the protocol used for KO and WT littermates described above. Following two days of activity measurements following saline administration (Day 1, Day 2), mice were tested on Day 3 following either saline or 1 mg/kg MA.
Immunohistochemistry
Mice from the acute stimulation experiment described above were euthanized by CO2 120 minutes after Day 3 injection, and brains were extracted. Brains from male mice were processed for Fos immunohistochemistry, because we did not observe a significant main or interacting effect involving sex in the analysis of behavioral data. (Immunohistochemical protocol in Supplementary Materials).
Statistical Analyses
For sensitization experiments in KO and WT mice, repeated measures ANOVAs assessed differences in baseline activity levels across repeated saline trials (Days 1, 2) and differences in MA-induced activity levels across repeated MA trials (Days 3, 5, 7, 9, 11), with genotype and sex as the between-subjects variables, and day as the repeated measure. For analysis of Day 11 and Day 12 locomotor activity data from experiments assessing the effects of the CRF-R1 antagonist on acquisition of sensitization, MA treatment on Days 1–10 (0 or 2 mg/kg) and CP-154,526 dose on Days 1–10 (0, 15, or 30 mg/kg) served as the factors for a two-way ANOVA. For analysis of Day 11 and Day 12 locomotor activity data from experiments assessing the effects of the CRF-R1 antagonist on expression of sensitization, MA treatment on Days 1–10 and CP-154,526 dose on Day 11 served as the factors for a two-way ANOVA. For analysis of Day 11 locomotor activity data from saline control experiment, CP-154,526 dose on Day 11 served as the factor for a one-way ANOVA, followed by Tukey’s post-hoc comparisons. For the acute stimulation experiment in CRF-R2 KO and WT mice, Day 3 locomotor activity levels were analyzed by three-way ANOVA (factors of sex, genotype, and MA treatment), and baseline activity levels across repeated saline trials were analyzed as described above. For Fos immunohistochemistry in male CRF-R2 KO and WT mice, analysis was performed by two-way ANOVA (factors of genotype and MA treatment). Significant interactions were followed by simple main effect analyses. Additional control analyses were also performed (Supplementary Materials).
RESULTS
Deletion of CRF-R1 has no effect on methamphetamine-induced sensitization
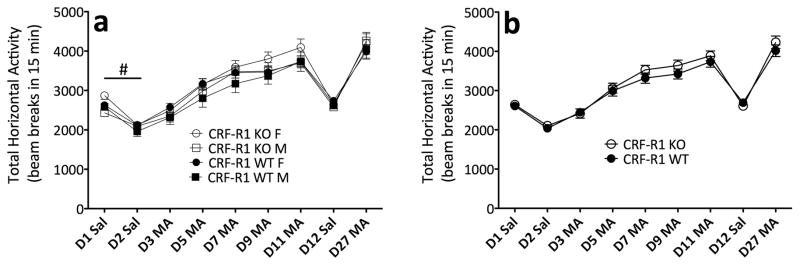
Figure 1 shows an increase in locomotor activation across repeated MA trials (Day 3 to Day 11), indicating that sensitization occurred in CRF-R1 KO and WT mice (F(4,268) = 107.7; p < .0001). However, analysis of repeated MA trials revealed no other significant effects or interactions, confirming that CRF-R1 KO and WT mice did not differ in MA-induced locomotor activation. In the analysis of repeated saline trials, there was no significant effect or interaction with genotype. However, female mice, regardless of genotype, displayed greater levels of baseline activity (F(1,67) = 4.4; p < .05). These data demonstrate that genetic deletion of CRF-R1 has no effect on the locomotor-activating properties of MA.
Figure 1.
CRF-R1 KO and WT mice do not differ in MA-induced sensitization. (a) Locomotor activity counts (mean ± SEM) in female and male CRF-R1 KO and WT mice from a 15-minute session following administration of saline (D1, D2, D12) or 1 mg/kg MA (D3, D5, D7, D9, D11, D27). (b) Sexes collapsed for clarity. #: main effect of sex across repeated saline days (p < .05). n = 15–20 per genotype, per sex. The same animals contributed to all scores in panels a and b.
CRF-R1 blockade has no effect on the acquisition of methamphetamine-induced sensitization
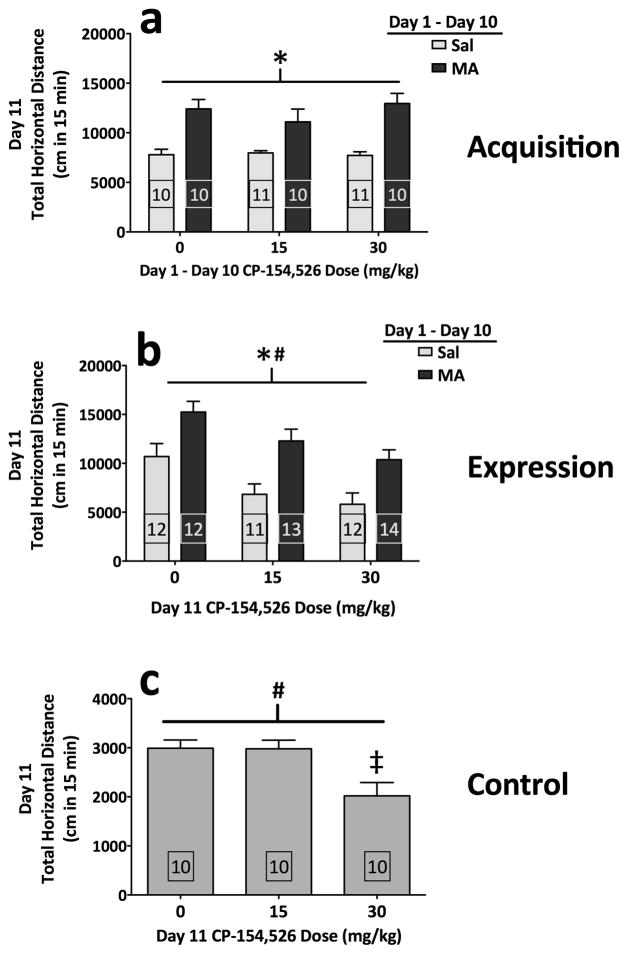
A possible role for CRF-R1 in MA-induced sensitization was further examined by administering CP-154,526 during the acquisition of sensitization in DBA/2J mice. Sensitization to MA was apparent (F(1,56) = 43.6; p < .0001), and CP-154,526 did not prevent MA-induced sensitization (Figure 2a). Challenge with saline on Day 12 revealed no effects of residual drug treatment on baseline activity levels (data not shown). These results demonstrate that the acquisition of MA-induced sensitization does not critically depend on CRF-R1 signaling.
Figure 2.
Selective blockade of CRF-R1 in DBA/2J mice does not selectively attenuate MA-induced sensitization. (a) Acquisition experiment. Locomotor activity scores (distance traveled in cm; mean + SEM) from Day 11 (see Methods). (b) Expression experiment. Locomotor activity scores (distance traveled in cm; mean + SEM) from Day 11 (see Methods). (c) Control experiment. Locomotor activity scores (distance traveled in cm; mean + SEM) from Day 11 (see Methods). *: main effect of MA (both p < .001); #: main effect of CP-154,526 (both p < .0005); ‡: 0 mg/kg vs. 30 mg/kg (p < .05) (Tukey’s HSD). Boxed numbers indicate number of animals per group; different animals contributed to separate experiments for panels a, b, and c.
CRF-R1 blockade inhibits locomotor activity in both methamphetamine- and saline-treated mice
When mice were tested for the effects of CRF-R1 blockade on the expression of MA-induced sensitization, significant sensitization was still apparent (F(1,69) = 27.8; p < .0001). Although CP-154,526 decreased the expression of MA-induced activity (F(2,69) = 10.1; p = .0001), this occurred independently of prior MA treatment (MA × CP-154,526 interaction; p = .904), indicating that CRF-R1 blockade decreased activity levels of both MA-sensitized mice and mice receiving MA for the first time (Figure 2b). Thus, the effects of CP-154,526 were non-specific to MA-induced neural adaptations. Challenge with saline on Day 12 revealed no effects of residual drug treatment on baseline activity levels (data not shown). A separate control experiment revealed that administration of CP-154,526 decreased activity levels of saline-treated mice (F(2,27) = 7.0; p = .004) (Figure 2c). These data support a non-specific effect of CP-154,526 on locomotor activation, rather than a role for CRF-R1 in MA-induced sensitization.
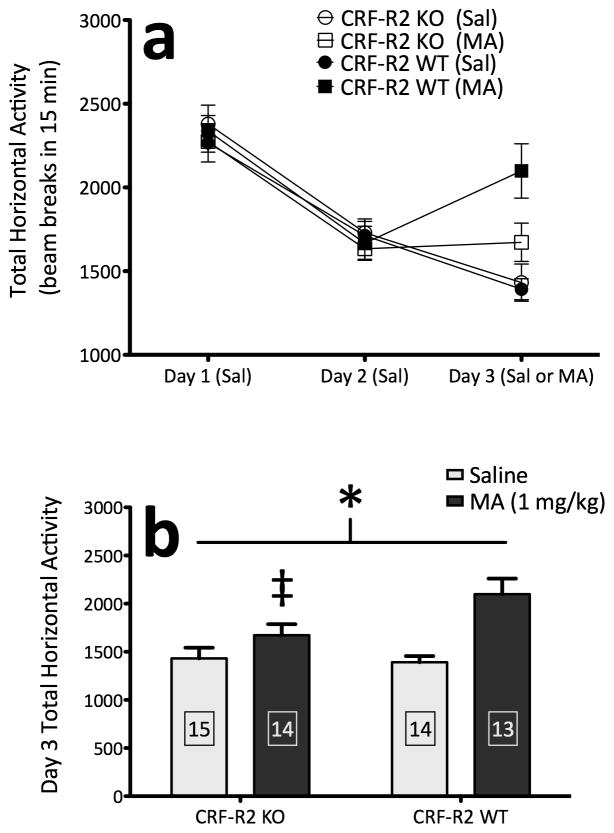
Deletion of CRF-R2 attenuates methamphetamine-induced locomotor activation
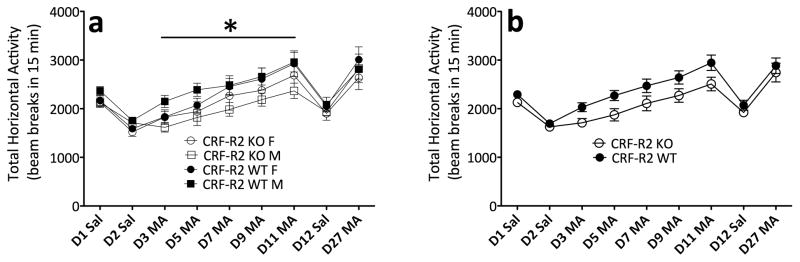
In the analysis of data from repeated MA trials in CRF-R2 KO and WT mice, there was a significant increase in locomotor activation across days, indicating that sensitization occurred (F(4,232) = 49.4; p < .0001). Interestingly, CRF-R2 KO mice displayed decreased locomotor activation across repeated MA trials, compared to WT mice (F(1,58) = 4.2; p < .05) (Figure 3). Analysis of repeated saline trials did not identify any significant effects or interactions, indicating that the lower activity levels in CRF-R2 KO mice across MA trials could not be attributed to lower levels of baseline activity. These data indicate that CRF-R2 signaling contributes to MA-induced locomotor activation.
Figure 3.
CRF-R2 KO mice display a decreased locomotor response to MA. (a) Locomotor activity counts (mean ± SEM) in female and male CRF-R2 KO and WT mice following administration of saline or 1 mg/kg MA. (b) Sexes collapsed for clarity. *: main effect of genotype across repeated MA trials (p < .05). n = 12–22 per genotype, per sex. The same animals contributed to all scores in panels a and b.
Deletion of CRF enhances methamphetamine-induced sensitization
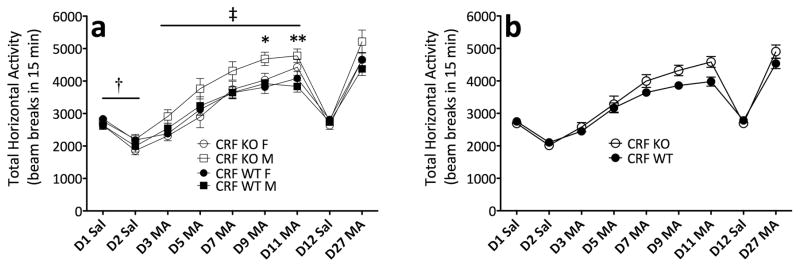
Next , we examined MA-induced locomotor activation in CRF KO mice. Analysis of repeated MA trials demonstrated that sensitization occurred (F(4,160) = 117.9; p < .0001), and that sensitization was enhanced in CRF KO mice (day × genotype interaction; F(4,160) = 2.7; p < .05) (Figure 4). Follow-up analysis of the significant interaction across MA trials revealed enhanced MA-induced activation in CRF KO mice on Days 9 and 11 (F(1,40) = 6.4; p < .05, F(1,40) = 8.6; p < .01, respectively). Analysis of repeated saline trials revealed genotypic differences in baseline activity levels that varied by sex (F(1,40) = 4.6; p < .05). Simple main effect analyses confirmed that, while female KO mice displayed decreased baseline locomotor activity levels, compared to female WT mice (F(1,23) = 5.4; p < .05), this genotypic difference was not apparent in male mice. In sum, the observation that CRF KO mice showed enhanced sensitization to MA makes it unlikely that CRF acting at CRF-R2 could be the ligand/receptor combination that underlies MA-induced hyperactivity.
Figure 4.
MA-induced sensitization is enhanced in CRF KO mice. (a) Locomotor activity counts (mean ± SEM) in female and male CRF KO and WT mice following administration of saline or 1 mg/kg MA. (b) Sexes collapsed for clarity. †: genotype × sex interaction (p < .05); ‡: day × genotype interaction (p < .05), *: simple main effect of genotype (p < .05), **: simple main effect of genotype (p < .01). n = 8–15, per genotype, per sex. The same animals contributed to all scores in panels a and b.
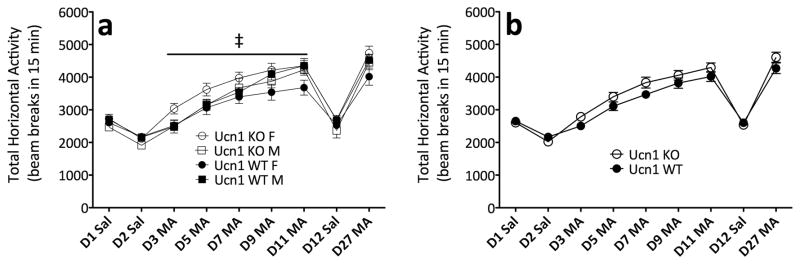
Deletion of Urocortin 1 has no effect on methamphetamine-induced sensitization
We next hypothesized that deletion of Ucn1 might attenuate MA-induced activation. Analysis of data from repeated MA trials demonstrated that sensitization occurred (F(4,272) = 136.0; p < .0001). Furthermore, male mice, regardless of genotype, showed a greater magnitude of sensitization to MA (sex × day interaction; F(4,272) = 4.8; p = .001) (Figure 5). Analysis of repeated saline trials yielded no significant differences based on genotype or sex. The observation that Ucn1 KO mice showed equivalent levels of MA-induced activation, compared to WT, makes it unlikely that Ucn1 acting at CRF-R2 could be the ligand/receptor combination that underlies MA-induced hyperactivity.
Figure 5.
Ucn1 KO and WT mice do not differ in MA-induced sensitization. (a) Locomotor activity counts (mean ± SEM) in female and male Ucn1 KO and WT mice following administration of saline or 1 mg/kg MA. (b) Sexes collapsed for clarity. ‡: day × sex interaction (p = .001). n = 16–19 per genotype, per sex. The same animals contributed to all scores in panels a and b.
Confirmation of CRF-R2-dependent sensitivity to methamphetamine
In this experiment, we replicated our finding that CRF-R2 KO mice are deficient in MA-induced locomotor activation. Baseline activity levels were equivalent between genotypes across two consecutive days of saline administration, and did not differ by sex (Figure 6a). On Day 3, the response to saline was similar between genotypes, yet the response to MA treatment was completely abolished in CRF-R2 KO mice, relative to WT mice (genotype × treatment interaction, F(1,48) = 4.2; p < .05) (Figure 6b). Simple main effect analyses confirmed that deletion of CRF-R2 significantly decreased MA-induced stimulation (F(1,23) = 4.9; p < .05). No interactions with sex were detected. These results verify the involvement of CRF-R2 in locomotor sensitivity to MA.
Figure 6.
Deletion of CRF-R2 abolishes acute stimulation to MA. (a) Locomotor activity counts (mean ± SEM) in female and male CRF-R2 KO and WT mice from a 15-minute session on Days 1 and 2 following administration of saline, and on Day 3 following administration of either saline or MA (1 mg/kg) (sexes collapsed). (b) Locomotor activity counts from Day 3 (mean + SEM) for mice treated with either saline or MA on Day 3, demonstrating a blunted acute stimulant response to MA in CRF-R2 KO mice (sexes collapsed). *: genotype × treatment interaction (p < .05); ‡: simple main effect of genotype within MA-treated groups (p < .05). Boxed numbers indicate number of animals per group; the same animals contributed to all scores in panels a and b.
Mapping the neural substrates of CRF-R2-dependent sensitivity to methamphetamine
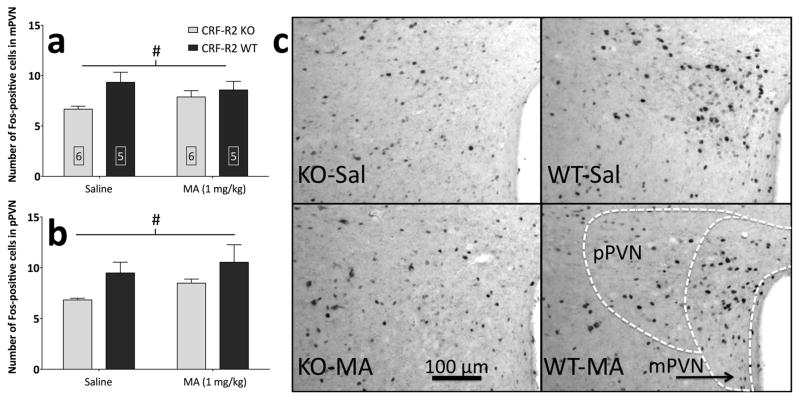
Following the behavioral testing described above, tissue from CRF-R2 KO and WT mice underwent immunohistochemistry for Fos, a marker of post-synaptic transcriptional activity within neurons (Morgan et al., 1987). Results are summarized in Table 1. MA increased Fos immunoreactivity (Fos-IR) in a number of brain regions, regardless of CRF-R2 genotype (Figure 7 and Table 1). Deletion of CRF-R2 decreased Fos-IR in the PVN (Figure 8), ventral lateral septum (vLS), and median preoptic nucleus (MnPO), regardless of MA treatment.
Table 1.
Statistical results for the effects of CRF-R2 genotype (KO or WT) and MA treatment (saline or 1 mg/kg MA) on neural activation (Fos-IR) in multiple brain regions.
| Region | MA Treatment | CRF-R2 Genotype | Interaction |
|---|---|---|---|
| NAcc core | F(1,18) = 32.59 p < .0001 | F(1,18) = 0.01 p = .935 | F(1,18) = 1.21 p = .286 |
| NAcc shell | F(1,18) = 32.50 p < .0001 | F(1,18) = 0.51 p = .486 | F(1,18) = 2.68 p = .119 |
| dorsolateral BNST | F(1,18) = 16.02 p = .001 | F(1,18) = 0.00 p = .975 | F(1,18) = 0.22 p = .646 |
| posterolateral BNST | F(1,18) = 8.83 p = .008 | F(1,18) = 0.13 p = .721 | F(1,18) = 0.21 p = .650 |
| ventral LS | F(1,17) = 0.44 p = .515 | F(1,17) = 5.90 p = .027 | F(1,17) = 3.52 p = .078 |
| intermediate LS | F(1,17) = 8.77 p = .009 | F(1,17) = 0.00 p = .979 | F(1,17) = 0.38 p = .545 |
| dorsal LS | F(1,17) = 14.29 p = .002 | F(1,17) = 0.00 p = .979 | F(1,17) = 0.35 p = .561 |
| MnPO | F(1,17) = 0.00 p = .977 | F(1,17) = 4.52 p = .048 | F(1,17) = 2.56 p = .128 |
| mPVN | F(1,18) = 0.10 p = .751 | F(1,18) = 5.70 p = .028 | F(1,18) = 1.97 p = .177 |
| pPVN | F(1,18) = 2.06 p = .167 | F(1,18) = 6.25 p = .022 | F(1,18) = 0.10 p = .753 |
| LA | F(1,17) = 8.44 p = .010 | F(1,17) = 0.44 p = .517 | F(1,17) = 1.85 p = .192 |
| BLA | F(1,17) = 13.72 p = .002 | F(1,17) = 3.26 p = .089 | F(1,17) = 5.91 p = .027 |
| CeA | F(1,17) = 3.88 p = .066 | F(1,17) = 2.90 p = .107 | F(1,17) = 5.44 p = .032 |
| BMA | F(1,19) = 2.21 p = .154 | F(1,19) = 0.00 p = .947 | F(1,19) = 0.01 p = .924 |
| dorsal MeA | F(1,19) = 8.46 p = .009 | F(1,19) = 0.00 p = .964 | F(1,19) = 0.01 p = .933 |
| ventral MeA | F(1,19) = 9.35 p = .007 | F(1,19) = 0.13 p = .724 | F(1,19) = 0.09 p = .769 |
| VMH | F(1,19) = 0.13 p = .727 | F(1,19) = 0.33 p = .571 | F(1,19) = 1.39 p = .254 |
| SN lateral/compacta | F(1,18) = 6.42 p = .021 | F(1,18) = 0.02 p = .903 | F(1,18) = 1.15 p = .298 |
| SN reticularis | F(1,18) = 3.21 p = .090 | F(1,18) = 0.41 p = .528 | F(1,18) = 0.99 p = .322 |
| VTA | F(1,18) = 6.46 p = .021 | F(1,18) = 0.07 p = .793 | F(1,18) = 0.35 p = .561 |
| pIIIU | F(1,18) = 8.20 p = .010 | F(1,18) = 0.09 p = .767 | F(1,18) = 0.06 p = .808 |
| MRN | F(1,19) = 4.42 p = .049 | F(1,19) = 0.02 p = .894 | F(1,19) = 0.32 p = .577 |
| lateral DRN | F(1,18) = 3.10 p = .095 | F(1,18) = 0.00 p = .954 | F(1,18) = 0.04 p = .836 |
| dorsal DRN | F(1,18) = 9.09 p = .007 | F(1,18) = 0.31 p = .587 | F(1,18) = 0.40 p = .537 |
| ventral DRN | F(1,18) = 9.48 p = .007 | F(1,18) = 0.03 p = .859 | F(1,18) = 0.23 p = .638 |
| intrafascicular DRN | F(1,18) = 4.95 p = .039 | F(1,18) = 0.04 p = .835 | F(1,18) = 0.74 p = .402 |
MA Treatment effect (left column): MA increased c-Fos expression in a number of brain regions, regardless of CRF-R2 genotype (indicated by bold type). CRF-R2 Genotype effect (center column): Deletion of CRF-R2 decreased c-Fos expression in ventral lateral septum (LS), median preoptic nucleus (MnPO) and both the magnocellular and parvocellular divisions of the paraventricular nucleus of the hypothalamus (mPVN, pPVN), regardless of MA treatment. Interaction effect (right column): Deletion of CRF-R2 attenuated the c-Fos response to MA, but had no effect of c-Fos expression following saline treatment, in the basolateral and central nuclei of the amygdala (BLA, CeA). NAcc, nucleus accumbens; BNST, bed nucleus of the stria terminalis; LA, lateral amygdala; BMA, basomedial amygdala; MeA, medial amygdala; VMH, ventromedial nucleus of the hypothalamus; SN, substantia nigra; VTA, ventral tegmental area; pIIIU, perioculomotor urocortin-containing neurons (non-preganglionic Edinger-Westphal nucleus); MRN, median raphé nucleus; DRN, dorsal raphé nucleus.
Figure 7.
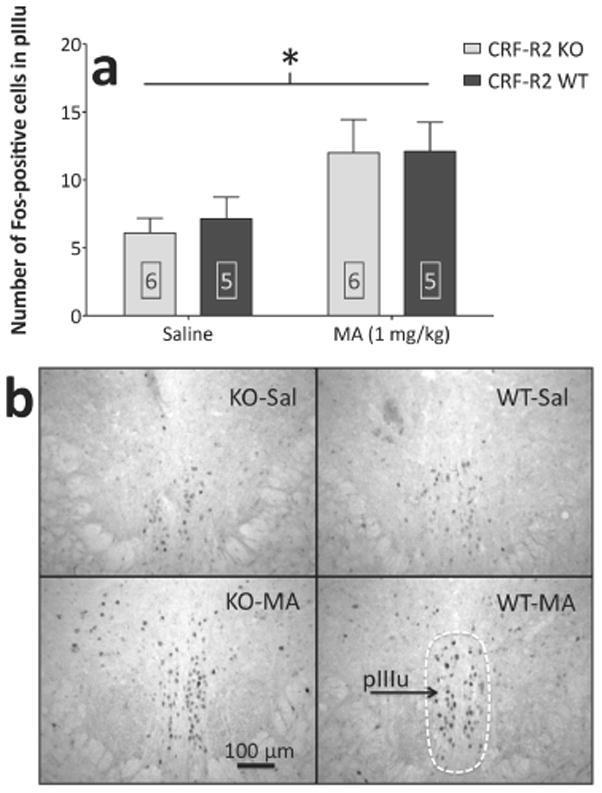
MA upregulates Fos-IR within the perioculomotor urocortin-containing neurons (pIIIu) of both CRF-R2 KO and WT mice. (a) Results from cell counts (mean + SEM) in the pIIIu. (b) Representative photomicrographs showing Fos-positive cells within the pIIIu; schematic shows the area delineated for counting. *: main effect of MA treatment (p < .05). Boxed numbers indicate number of animals per group.
Figure 8.
Deletion of CRF-R2 results in an overall decrease in Fos-IR within the paraventricular nucleus of the hypothalamus (PVN). Results from cell counts (mean + SEM) in (a) the magnocellular division of the PVN (mPVN) and (b) the parvocellular division of the PVN (pPVN). (c) Representative photomicrographs showing Fos-positive cells in the mPVN and pPVN; the schematic shows areas delineated for counting. #: main effect of CRF-R2 genotype (p < .05). Boxed numbers indicate number of animals per group; the same animals contributed to all values in panels a and b.
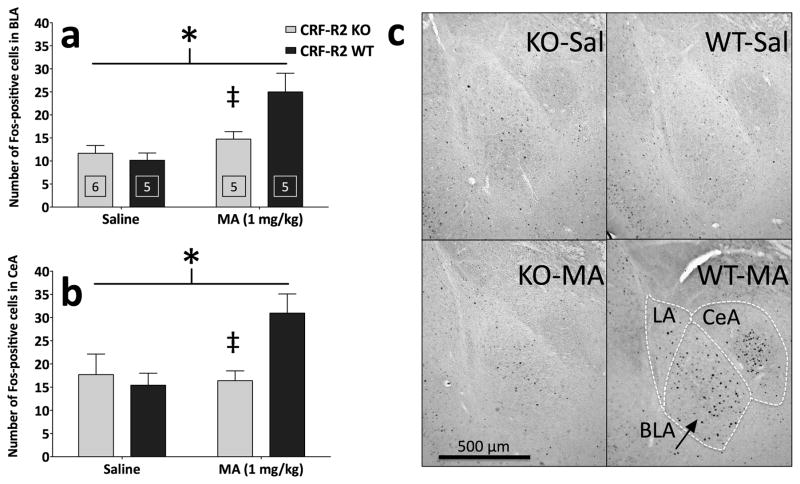
More importantly, significant interactions between CRF-R2 genotype and MA treatment were observed in the basolateral amygdala (BLA) (F(1,17) = 5.91; p < .05) and the central nucleus of the amygdala (CeA) (F(1,17) = 5.44; p < .05). In these areas, the Fos response to saline was similar between genotypes, yet CRF-R2 KO mice displayed a relatively decreased Fos response to MA, compared to WT mice (Figure 9). Simple main effect analyses confirmed that deletion of CRF-R2 significantly decreased MA-induced Fos-IR within the BLA (F(1,8) = 5.50; p < .05) and CeA (F(1,8) = 10.80; p < .05).
Figure 9.
Deletion of CRF-R2 attenuates acute MA-induced Fos-IR within the basolateral amygdala (BLA) and the central nucleus of the amygdala (CeA). Shown are the results from cell counts (mean + SEM) in the (a) basolateral and (b) central nuclei of the amygdala. (c) Representative photomicrographs showing Fos-positive cells in the lateral amygdala (LA), BLA and CeA; the schematic shows areas delineated for counting (for CeA, both the capsular and lateral divisions of the nucleus were included). *: CRF-R2 genotype × MA treatment interaction (both p < .05); ‡: simple main effect of genotype within MA-treated groups (both p < .05). Boxed numbers indicate number of animals per group; the same animals contributed to all values in panels a and b.
DISCUSSION
The principal findings of this study were that genetic deletion of CRF-R2 attenuated the locomotor response to MA, deletion of CRF-R1 or Ucn1 did not affect MA-induced sensitization, and deletion of CRF enhanced MA-induced sensitization. Furthermore, the CRF-R1 antagonist CP-154,526 had no effect on the acquisition, and non-specific effects on the expression, of MA-induced sensitization. Finally, we replicated our finding of CRF-R2-dependent locomotor sensitivity to MA, and identified the BLA and CeA as potential sites of involvement in this response.
These results show, through pharmacological and genetic manipulations, that psychomotor sensitization to a psychostimulant drug of abuse is unaffected by the absence of CRF-R1 signaling. Though these data appear contradictory to previous reports that CRF/CRF-R1 signaling and HPA-axis activation are required for psychostimulant-induced activation and sensitization (Cador et al., 1993a, Cador et al., 1992, Cole et al., 1990a, Erb & Brown, 2006, Koob & Cador, 1993, Sarnyai et al., 1992), many of these findings were established prior to the discovery of CRF-R2 (Lovenberg et al., 1995) and the urocortin peptides (Lewis et al., 2001, Reyes et al., 2001, Vaughan et al., 1995). Thus, these newer components of the CRF system must be considered when interpreting previous results.
Early evidence for HPA-axis involvement in psychostimulant-induced behavior was provided by data in which CRF antisera blocked cocaine-induced locomotor activation (Sarnyai et al., 1992), adrenalectomy prevented amphetamine-induced sensitization (Rivet et al., 1989), and glucocorticoid receptor agonists restored amphetamine-induced sensitization in adrenalectomized rats (Cador et al., 1993b, Deroche et al., 1992). Though these findings illustrate a role for glucocorticoids, the link with CRF-R1 is tenuous. The non-selective CRF-R antagonist α-helical CRF(9-41) prevented cocaine-induced locomotor activation (Sarnyai et al., 1992) and amphetamine-induced cross-sensitization to repeated stress (Cole et al., 1990b). However, while α-helical CRF(9-41) binds both CRF-Rs, it exhibits higher affinity for CRF-R2 (Perrin et al.,1999) and partial agonist activity at CRF-R1 (Smart et al., 1999). Thus, the effects of α-helical CRF(9-41) on psychostimulant-induced activation may be attributed to its affinity for CRF-R2 rather than CRF-R1.
In addition, intracerebroventricular (i.c.v.) administration of CRF induced cross-sensitization to amphetamine (Cador et al., 1993a), cocaine induced cross-sensitization to i.c.v. CRF (Erb et al., 2003), and i.c.v. infusion of the non-selective CRF-R antagonist D-Phe CRF(12-41) prevented the expression of cocaine-induced sensitization (Erb & Brown, 2006). While i.c.v. administration affords central distribution of peptides, organization of the endogenous CRF system complicates interpretation of these results. For instance, exogenous infusion of non-specific CRF-R ligands may control behavior via supra-physiological access to either CRF-R subtype.
Thus, psychostimulant-induced activation has been enhanced by exogenous CRF, and inhibited by immunoneutralization of CRF, interruption of glucocorticoids, and non-selective CRF-R blockade. Additional results also implicate glucocorticoids in psychostimulant-induced activation (De Vries et al., 1996, Deroche-Gamonet et al., 2003, Marinelli et al., 1997, Piazza et al., 1994, Wei et al., 2004). However, none of the above studies provide direct evidence for the involvement of CRF-R1, a limitation that must be considered when attempting to extract a putative mechanism. In this light, it is tempting to speculate that MA augments HPA-axis activity in a vasopressin-dependent manner, allowing glucocorticoid release and locomotor activation to occur independently of CRF-R1 signaling. This possibility is supported by the observation that the CRF-R2-selective agonist Ucn3 increases vasopressin (but not CRF) mRNA in the rat PVN (Jamieson et al., 2006). On the other hand, the contribution of the PVN to MA sensitivity would likely be accompanied by a modified Fos response in this region of CRF-R2 KO mice, which was not observed, casting doubt on this possibility.
Intriguingly, the difference between present experiments and previous findings could be attributed to the pharmacological profile of MA, because different psychostimulants vary in the precise mechanism by which they cause monoamine release and HPA-axis activation (Borowsky & Kuhn, 1991, Sarnyai et al., 1993, Scholl et al., 2009). Indeed, inhibition of glucocorticoid synthesis prevented reinstatement of lever-pressing for cocaine, but not MA (Goeders & Clampitt, 2002, Moffett & Goeders, 2007). Furthermore, while CP-154,526 prevented cocaine-induced activation (Lu et al., 2003, Przegalinski et al., 2005), the CRF-R1 antagonist antalarmin had non-specific effects on amphetamine-induced activation (Zorrilla et al., 2002), suggesting differential CRF system involvement in the behavioral effects of psychostimulants. Additionally, involvement of the CRF system in sensitization induced by ethanol and MA differs significantly. Whereas CRF-R2 KO and Ucn1 KO mice exhibit normal ethanol-induced sensitization, this is abolished in CRF-R1 KO mice (Pastor et al., 2008), and diminished in CRF KO mice (R. Pastor, T.J. Phillips, A.E. Ryabinin, unpublished data).
Analysis of brain regions in the present study revealed that deletion of CRF-R2 decreased Fos-IR in the mPVN, pPVN, vLS, and MnPO, regardless of MA treatment. The PVN and vLS show CRF-R2 expression (Chalmers et al., 1995, Van Pett et al., 2000), and the MnPO shows dense Ucn3 expression (Li et al., 2002). Thus, while CRF-R2 signaling and Ucn3 activity in these regions may be downregulated (or eliminated) in CRF-R2 KO mice, the absence of an interaction with MA treatment suggests that these populations may not be involved in CRF-R2-dependent sensitivity to MA.
In the BLA and CeA, CRF-R2 KO mice displayed a relatively decreased Fos response to MA, but not saline, which mirrored the locomotor activity levels (Figure 6, Figure 9). An immediate interpretation would be that CRF-R2 signaling in BLA/CeA underlies MA sensitivity. However, future experiments should clarify whether amygdalar CRF-R2 is truly critical, or whether these loci play an indirect role. While high levels of CRF-R2 mRNA have not been detected within the BLA and CeA, changes in BLA CRF-R2 mRNA have been detected following stress and alcohol consumption (Herringa et al., 2004, Sommer et al., 2008). Furthermore, CRF-R2-specific pharmacological manipulations within BLA and CeA have been shown to have profound electrophysiological and behavioral effects (Funk & Koob, 2007, Krishnan et al.), indicating the presence of functionally-relevant CRF-R2 expression in these regions.
Using KO mice, we found no evidence for the participation of CRF-R1 in the development (Figure 1) or long-term expression (Supplementary Materials) of MA-induced sensitization. While mutant mice may be prone to developmental compensations leading to altered behavioral phenotypes, this is an inadequate explanation for our results in CRF-R1 KO mice. Our inability to selectively alter the acquisition and expression of MA-induced sensitization with CP-154,526 provides further support for the conclusion that CRF-R1 is not involved in MA-induced activation. In addition, the locomotor activity observed here was unlikely to be influenced by stereotypy, because the doses of MA used are insufficient to induce stereotypy in mice (Atkins et al., 2001).
We predicted that deletion of CRF or Ucn1 would attenuate MA-induced locomotor activation, because exogenous CRF induces locomotor activation (Swerdlow et al., 1986), and because psychostimulants activate a population of Ucn1-containing neurons (Spangler et al., 2009) in which Ucn1 is co-localized with the cocaine- and amphetamine-regulated transcript (CART) peptide (Kozicz, 2003). However, results were not as predicted. Perhaps CRF KO mice demonstrated enhanced MA-induced sensitization because of compensatory changes in other CRF system components. CRF KO mice display decreased glucocorticoid levels, comparable to those of adrenalectomized animals (Muglia et al., 1995). Because adrenalectomy upregulates Ucn3 and CRF-R2 mRNA (Chen et al., 2005, Jamieson et al., 2006), it is plausible that the exaggerated response to MA in CRF KO mice is mediated via enhanced Ucn3/CRF-R2 signaling. In addition, CRF KO mice display increased levels of Ucn1 within the perioculomotor urocortin-containing neurons (pIIIu) (Weninger et al., 2000). Enhanced innervation of amygdalar CRF-R2 by pIIIu Ucn1 therefore represents a potential mechanism in line with our Fos mapping results that could explain the exaggerated response to MA in CRF KO mice. However, the lack of any difference in MA-induced sensitization between Ucn1 KO and WT mice minimizes the likelihood that a Ucn1-mediated mechanism is sufficient to explain our result.
We have used genetic tools in this study to demonstrate a novel role for CRF-R2 in the response to a psychostimulant drug of abuse. Future studies should confirm this role using pharmacological agents. In humans, the euphoric properties of MA are accompanied by states of intense vigilance, and the behavioral output associated with this hyperactivity (improved attention, lack of fatigue, hypophagia) contributes to the reinforcing effects of MA (Nida, 2006). The purpose of these studies was to assess the participation of specific components of the CRF system with regard to both MA-induced psychomotor sensitization and generalized hyperactivity. Thus, although we did not obtain data implicating CRF-R2 in the neural adaptations occurring during sensitization per se, the participation of CRF-R2 in MA-induced activation remains relevant to the etiology of addiction in human populations.
It is reasonable to consider the involvement of urocortin peptides, rather than CRF, in CRF-R2-dependent MA sensitivity. In general, evidence is accumulating that central urocortins participate in anxiety- and depression-like behaviors that may be associated with neural processes underlying addiction (Kozicz et al., 2008, Kuperman et al., Neufeld-Cohen et al.). While we are intrigued by the observation that the Ucn2 gene is located within a region of mouse chromosome 9 that has been associated with MA sensitivity (Palmer et al., 2005), the more precise contribution of Ucn1, Ucn2, and Ucn3 will need to be addressed in future studies. Within the context of our Fos mapping results, it is interesting that Ucn1 is the only urocortin known to innervate the CeA (Bittencourt et al., 1999), whereas urocortinergic innervation of the BLA has not been documented. Future studies should continue to focus on elucidating site-specific regulation of the behavioral response to MA by CRF-R2-dependent mechanisms, as newly-identified targets may lead to potential treatments for MA addiction.
Supplementary Material
Acknowledgments
This research was supported by NIH grants P50DA018165 (AER, TJP), RO3DA02854 (AER) and T32DA007262 (WJG), and resources were also provided by the Department of Veterans Affairs (TJP). We are grateful to Pfizer for their donation of their drug, CP-154,526, for use in our work.
References
- Atkins AL, Helms ML, O'Toole LA, Belknap JK. Stereotypic behaviors in mice selectively bred for high and low methamphetamine-induced stereotypic chewing. Psychopharmacology (Berl) 2001;157:96–104. doi: 10.1007/s002130100774. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- Borowsky B, Kuhn CM. Monoamine mediation of cocaine-induced hypothalamo-pituitary-adrenal activation. J Pharmacol Exp Ther. 1991;256:204–210. [PubMed] [Google Scholar]
- Cador M, Cole BJ, Koob GF, Stinus L, Le Moal M. Central administration of corticotropin releasing factor induces long-term sensitization to D-amphetamine. Brain Res. 1993a;606:181–186. doi: 10.1016/0006-8993(93)90982-s. [DOI] [PubMed] [Google Scholar]
- Cador M, Dulluc J, Mormede P. Modulation of the locomotor response to amphetamine by corticosterone. Neuroscience. 1993b;56:981–988. doi: 10.1016/0306-4522(93)90144-5. [DOI] [PubMed] [Google Scholar]
- Cador M, Dumas S, Cole BJ, Mallet J, Koob GF, Le Moal M, Stinus L. Behavioral sensitization induced by psychostimulants or stress: search for a molecular basis and evidence for a CRF-dependent phenomenon. Ann N Y Acad Sci. 1992;654:416–420. doi: 10.1111/j.1749-6632.1992.tb25985.x. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Perrin M, Brar B, Li C, Jamieson P, Digruccio M, Lewis K, Vale W. Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol. 2005;19:441–458. doi: 10.1210/me.2004-0300. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Cador M, Stinus L, Rivier C, Rivier J, Vale W, Le Moal M, Koob GF. Critical role of the hypothalamic pituitary adrenal axis in amphetamine-induced sensitization of behavior. Life Sci. 1990a;47:1715–1720. doi: 10.1016/0024-3205(90)90344-q. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Cador M, Stinus L, Rivier J, Vale W, Koob GF, Le Moal M. Central administration of a CRF antagonist blocks the development of stress-induced behavioral sensitization. Brain Res. 1990b;512:343–346. doi: 10.1016/0006-8993(90)90646-S. [DOI] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Tjon GH, Nestby P, Mulder AH, Vanderschuren LJ. Mifepristone prevents the expression of long-term behavioural sensitization to amphetamine. Eur J Pharmacol. 1996;307:R3–4. doi: 10.1016/0014-2999(96)00308-1. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Maccari S, Le Moal M, Simon H. Repeated corticosterone administration sensitizes the locomotor response to amphetamine. Brain Res. 1992;584:309–313. doi: 10.1016/0006-8993(92)90911-r. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, Kellendonk C, Le Moal M, Spanagel R, Schutz G, Tronche F, Piazza PV. The glucocorticoid receptor as a potential target to reduce cocaine abuse. J Neurosci. 2003;23:4785–4790. doi: 10.1523/JNEUROSCI.23-11-04785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Brown ZJ. A role for corticotropin-releasing factor in the long-term expression of behavioral sensitization to cocaine. Behav Brain Res. 2006;172:360–364. doi: 10.1016/j.bbr.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Erb S, Funk D, Le AD. Prior, repeated exposure to cocaine potentiates locomotor responsivity to central injections of corticotropin-releasing factor (CRF) in rats. Psychopharmacology (Berl) 2003;170:383–389. doi: 10.1007/s00213-003-1556-1. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Fu Y, Pollandt S, Liu J, Krishnan B, Genzer K, Orozco-Cabal L, Gallagher JP, Shinnick-Gallagher P. Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF1 receptors. J Neurophysiol. 2007;97:937–941. doi: 10.1152/jn.00349.2006. [DOI] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci. 2009;29:6535–6544. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Nanda SA, Hsu DT, Roseboom PH, Kalin NH. The effects of acute stress on the regulation of central and basolateral amygdala CRF-binding protein gene expression. Brain Res Mol Brain Res. 2004;131:17–25. doi: 10.1016/j.molbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Jamieson PM, Li C, Kukura C, Vaughan J, Vale W. Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology. 2006;147:4578–4588. doi: 10.1210/en.2006-0545. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–125. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, neuroplasticity (sensitization), and alcoholism. Proc Natl Acad Sci U S A. 2008;105:8809–8810. doi: 10.1073/pnas.0804354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Cador M. Psychomotor stimulant sensitization: the corticotropin-releasing factor-steroid connection. Commentary on Wise and Leeb "Psychomotor-stimulant sensitization: a unitary phenomenon?". Behav Pharmacol. 1993;4:351–354. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kozicz T. Neurons colocalizing urocortin and cocaine and amphetamine-regulated transcript immunoreactivities are induced by acute lipopolysaccharide stress in the Edinger-Westphal nucleus in the rat. Neuroscience. 2003;116:315–320. doi: 10.1016/s0306-4522(02)00772-8. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Tilburg-Ouwens D, Faludi G, Palkovits M, Roubos E. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience. 2008;152:1015–1023. doi: 10.1016/j.neuroscience.2007.12.050. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Centeno M, Pollandt S, Fu Y, Genzer K, Liu J, Gallagher JP, Shinnick-Gallagher P. Dopamine receptor mechanisms mediate corticotropin-releasing factor-induced long-term potentiation in the rat amygdala following cocaine withdrawal. Eur J Neurosci. 31:1027–1042. doi: 10.1111/j.1460-9568.2010.07148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman Y, Issler O, Regev L, Musseri I, Navon I, Neufeld-Cohen A, Gil S, Chen A. Perifornical Urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1003969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci. 2005;25:577–583. doi: 10.1523/JNEUROSCI.4196-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Liu Z, Huang M, Zhang Z. Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. J Neurochem. 2003;84:1378–1386. doi: 10.1046/j.1471-4159.2003.01635.x. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rouge-Pont F, De Jesus-Oliveira C, Le Moal M, Piazza PV. Acute blockade of corticosterone secretion decreases the psychomotor stimulant effects of cocaine. Neuropsychopharmacology. 1997;16:156–161. doi: 10.1016/S0893-133X(96)00169-8. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Vigh S, Petrusz P, Schally AV. Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. Am J Anat. 1982;165:385–396. doi: 10.1002/aja.1001650404. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007;190:171–180. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- Neufeld-Cohen A, Evans AK, Getselter D, Spyroglou A, Hill A, Gil S, Tsoory M, Beuschlein F, Lowry CA, Vale W, Chen A. Urocortin-1 and -2 double-deficient mice show robust anxiolytic phenotype and modified serotonergic activity in anxiety circuits. Mol Psychiatry. 15:426–441. 339. doi: 10.1038/mp.2009.115. [DOI] [PubMed] [Google Scholar]
- NIDA. Methamphetamine Abuse and Addiction. NIDA Research Report Series. 2006:1–8. [Google Scholar]
- Orozco-Cabal L, Liu J, Pollandt S, Schmidt K, Shinnick-Gallagher P, Gallagher JP. Dopamine and corticotropin-releasing factor synergistically alter basolateral amygdala-to-medial prefrontal cortex synaptic transmission: functional switch after chronic cocaine administration. J Neurosci. 2008;28:529–542. doi: 10.1523/JNEUROSCI.2666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ. Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm Genome. 2005;16:291–305. doi: 10.1007/s00335-004-2451-8. [DOI] [PubMed] [Google Scholar]
- Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci U S A. 2008;105:9070–9075. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin MH, Sutton SW, Cervini LA, Rivier JE, Vale WW. Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of corticotropin-releasing factor receptors. J Pharmacol Exp Ther. 1999;288:729–734. [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A. 1991;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Marinelli M, Jodogne C, Deroche V, Rouge-Pont F, Maccari S, Le Moal M, Simon H. Inhibition of corticosterone synthesis by Metyrapone decreases cocaine-induced locomotion and relapse of cocaine self-administration. Brain Res. 1994;658:259–264. doi: 10.1016/s0006-8993(09)90034-8. [DOI] [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- Pringle RB, Mouw NJ, Lukkes JL, Forster GL. Amphetamine treatment increases corticotropin-releasing factor receptors in the dorsal raphe nucleus. Neurosci Res. 2008;62:62–65. doi: 10.1016/j.neures.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przegalinski E, Filip M, Frankowska M, Zaniewska M, Papla I. Effects of CP 154,526, a CRF1 receptor antagonist, on behavioral responses to cocaine in rats. Neuropeptides. 2005;39:525–533. doi: 10.1016/j.npep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rivet JM, Stinus L, LeMoal M, Mormede P. Behavioral sensitization to amphetamine is dependent on corticosteroid receptor activation. Brain Res. 1989;498:149–153. doi: 10.1016/0006-8993(89)90411-3. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Lessov CN, Phillips TJ. Critical role for glucocorticoid receptors in stress- and ethanol-induced locomotor sensitization. J Pharmacol Exp Ther. 1995;275:790–797. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates 'anxiety-like' behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Telegdy G. Cocaine-induced elevation of plasma corticosterone is mediated by different neurotransmitter systems in rats. Pharmacol Biochem Behav. 1993;45:209–214. doi: 10.1016/0091-3057(93)90106-4. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Hohn J, Szabo G, Penke B. Critical role of endogenous corticotropin-releasing factor (CRF) in the mediation of the behavioral action of cocaine in rats. Life Sci. 1992;51:2019–2024. doi: 10.1016/0024-3205(92)90151-e. [DOI] [PubMed] [Google Scholar]
- Scholl JL, Feng N, Watt MJ, Renner KJ, Forster GL. Individual differences in amphetamine sensitization, behavior and central monoamines. Physiol Behav. 2009;96:493–504. doi: 10.1016/j.physbeh.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Smart D, Coppell A, Rossant C, Hall M, McKnight AT. Characterisation using microphysiometry of CRF receptor pharmacology. Eur J Pharmacol. 1999;379:229–235. doi: 10.1016/s0014-2999(99)00506-3. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spangler E, Cote DM, Anacker AM, Mark GP, Ryabinin AE. Differential sensitivity of the perioculomotor urocortin-containing neurons to ethanol, psychostimulants and stress in mice and rats. Neuroscience. 2009;160:115–125. doi: 10.1016/j.neuroscience.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Vaccarino FJ, Amalric M, Koob GF. The neural substrates for the motor-activating properties of psychostimulants: a review of recent findings. Pharmacol Biochem Behav. 1986;25:233–248. doi: 10.1016/0091-3057(86)90261-3. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Li C, Zhao L, Contarino A, Liberman MC, Smith GW, Marchuk Y, Koob GF, Heinemann SF, Vale W, Lee KF. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet. 2002;31:363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong SM, Oliver HA, Scholl JL, Oliver KM, Forster GL. Increased anxiety-like behavior of rats during amphetamine withdrawal is reversed by CRF(2) receptor antagonism. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci U S A. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weninger SC, Peters LL, Majzoub JA. Urocortin expression in the Edinger-Westphal nucleus is up-regulated by stress and corticotropin-releasing hormone deficiency. Endocrinology. 2000;141:256–263. doi: 10.1210/endo.141.1.7277. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Imai K, Kamegaya E, Takamatsu Y, Irago M, Hagino Y, Kasai S, Shimada K, Yamamoto T, Sora I, Koga H, Ikeda K. Repeated methamphetamine administration alters expression of the NMDA receptor channel epsilon2 subunit and kinesins in the mouse brain. Ann N Y Acad Sci. 2006;1074:97–103. doi: 10.1196/annals.1369.009. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.