Abstract
Hexokinases from the African trypanosome, Trypanosoma brucei, are attractive targets for the development of anti-parasitic drugs, in part because the parasite utilizes glycolysis exclusively for ATP production during the mammalian infection. Here, we have demonstrated that the bioflavanoid quercetin (QCN), a known trypanocide, is a mixed inhibitor of Trypanosoma brucei hexokinase 1 (TbHK1) (IC50 = 4.1 ± 0.8 μM). Spectroscopic analysis of QCN binding to TbHK1, taking advantage of the intrinsically fluorescent single tryptophan (Trp177) in TbHK1, revealed that QCN quenches emission of Trp177, which is located near the hinge region of the enzyme. ATP similarly quenched Trp177 emission, while glucose had no impact on fluorescence.
Supporting the possibility that QCN toxicity is a consequence of inhibition of the essential hexokinase, in live parasites QCN fluorescence localizes to glycosomes, the subcellular home of TbHK1. Additionally, RNAi-mediated silencing of TbHK1 expression expedited QCN induced death, while over-expressing TbHK1 protected trypanosomes from the compound. In summary, these observations support the suggestion that QCN toxicity is in part attributable to inhibition of the essential TbHK1.
Keywords: Trypanosoma brucei, hexokinase, quercetin, glycolysis
1. Introduction
Trypanosoma brucei is the causative agent of human African trypanosomiasis and nagana, a wasting disease, in livestock. The World Health Organization classifies T. brucei as a re-emerging/uncontrollable human pathogen, partly due to a lack of a vaccine and suitable treatments for the disease. Current therapeutics for human African trypanosomiasis (HAT) may have serious side effects, including blindness and death (Barrett, et al., 2003).
T. brucei relies exclusively on glycolysis for ATP generation in the mammalian bloodstream. Hexokinases (HK1) catalyze the first step in glycolysis facilitating the transfer of the γ-phosphoryl group of ATP to the C6 of glucose. The parasite expresses two HKs, TbHK1 and TbHK2, with proteomic studies revealing that both are found in the mammalian bloodstream (BSF) and insect (PF) forms of the parasites (Colasante, et al., 2006). Both the proteins reside in an unusual organelle called the glycosome that houses the majority of the enzymes that participate in glycolysis.
TbHK1 and TbHK2 are 98% identical at the amino acid level (Morris, et al., 2006). RNA interference (RNAi) has been used to demonstrate that both enzymes are essential to the BSF parasites, as silencing of either TbHK1 or TbHK2 results in the loss of HK activity and cell death (Albert, et al., 2005, Chambers, et al., 2008). In addition to this genetic evidence validating TbHKs as potential therapeutic targets, we have found that chemicals that inhibit HKs from other systems also inhibit TbHKs and are toxic to the trypanosome. For example, the anticancer drug lonidamine (LND), which functions in part by inhibiting human HK (Floridi and Lehninger, 1983, Paggi, et al., 1988), inhibits both recombinant TbHK1 and HKs from parasite lysate. Additionally, LND is toxic to BSF and PF parasites (Chambers, et al., 2008), likely as a result (at least in part) of inhibition of TbHKs. Supporting this, parasites were partially protected from LND-induced cell death by ectopic over-expression of TbHK1.
Quercetin (3,5,7,3′,4′ pentahydroxyflavone, QCN) is an abundant naturally occurring flavanol found in plants such as apples, onions, and capers. QCN and related flavanols are of interest as potential anti-cancer therapies, because they inhibit the growth of several types of cancer cell lines (Molnar, et al., 1981, Suolinna, et al., 1975). Potential in vivo QCN targets include a number of enzymes that are inhibited in vitro, ranging from the Src protein kinase (pp60v-src) to HKs (Graziani, 1977, Graziani, et al., 1983).
Biophysically, QCN has several unusual fluorescence properties, including intramolecular excited-state proton transfer and dual fluorescence behavior that have been exploited in the use of flavanols as environmental probes (Guharay, et al., 2001). Additionally, QCN binding to bovine serum albumin has been studied using these spectral properties (Sengupta and Sengupta, 2002).
Here, we have characterized the impact of QCN on recombinant TbHK1 and transgenic parasites. Our work builds on the observation that QCN is toxic to trypanosomes (Mamani-Matsuda, et al., 2004), revealing that TbHK1 may be a molecular target of the flavanoid. We have found that over-expression of TbHK1 provides protection from QCN, while RNAi depletion of TbHKs expedites parasite death. Additional spectroscopic investigations taking advantage of the intrinsic fluorescence of QCN suggest that QCN toxicity may be due in part to binding near the TbHK1 active site, causing enzyme inactivation.
2. Materials and Methods
2.1 Reagents
QCN (3,3′,4′,5,7-pentahydroxyflavone) was purchased from Spectrum Chemical Manufacturing Corporation (Gardena, CA).
2.2 Assays of recombinant and lysate-derived TbHK
Recombinant TbHK1 was expressed and purified as described previously (Morris, et al., 2006). Parasite lysates were prepared by hypotonic lysis of 1 × 107 cells in the presence of 1 mM PMSF, 20 μg/ml leupeptin, and 100 μg/ml TLCK. The mixture was added to lysis buffer (for a final concentration of 0.1 M triethanolamine (TEA) pH 7.4 and 0.1% Triton X-100) and lysates used in HK assays.
HK assays were performed in triplicate using a coupled reaction. Briefly, assays used glucose 6-phosphate dehydrogenase (1 unit/assay, EMD Biosciences, Inc, Sand Diego, CA) as a coupling enzyme to reduce NAD+ to NADH during the oxidation of glucose-6-P to 6-phosphogluconic acid (Morris, et al., 2006), a reaction that can be monitored spectrophotometrically. Final conditions were 0.1M TEA, pH 7.9 containing 1.0 mM ATP, 33 mM MgCl2, 20 mM glucose, and 0.75 mM NAD+. Assays were performed in 96-well microtiter plate format in a GENios spectrophotometer (Tecan Group Ltd., Switzerland).
2.3 Tryptophan Quenching Assay of TbHK1
Glucose (20 mM), QCN (50 μM) and ATP (varying concentrations) were added individually and in combination to a solution (3 ml) of 0.1M TEA, pH 7.4. A scanning spectrofluorometer (QM-Y, Photon Technology International, Birmingham, NJ) was used to monitor emission from 300-550 nm after excitation of the lone Trp on TbHK1 (W177) at 280 nm. After acquiring background emission, TbHK1 (~1 μg) was added to the cuvette, mixed by inversion, and an emission scan performed. Using the PTI software, the area under the emission curves from 370-380 nm was integrated. Values were converted into the percent of Trp emission lost and plotted versus concentration of substrate/inhibitor using KaliedaGraph software version 4.03. Sigmoidal curves were fit to the plots and IC50 values were determined for the substrate/inhibitor by setting y equal to 50 and solving for x using the sigmoidal equation.
2.4 QCN Localization in T. brucei by Fluorescence Microscopy
PF parasites (29-13, a 427 strain) were grown in SDM-79 with the T7 RNA polymerase and the tetracycline repressor constructs maintained by the addition of 2.5 μg/ml G418 and 5 μg/ml hygromycin to the medium. BSF parasites (cell line 90-13, a 427 strain) were grown in HMI-9 supplemented with 10% fetal bovine serum and 10% Serum Plus (Sigma-Aldrich, St. Louis, MO).
For microscopic examination of QCN localization, T. brucei were grown to 1 × 107/ml (PF 29-13) or 1 × 106/ml (BSF 90-13), harvested (800 x g, 10 min), and washed twice in modified PBS (5 mM KCl, 8 mM NaCl, 1 mM MgSO4, 20 mM Na2HPO4, 2 mM NaH2PO4, 20 mM glucose). QCN (100 μM) was then added to cells in the modified PBS. After incubation (15 min, at growth conditions), cells were pelleted, washed twice, and applied to slides after the addition of VectaShield mounting medium with DAPI (Vector Laboratories, Inc., Burlingame, CA). Images were captured by epifluorescence microscopy (Axiovert 200M, Carl Zeiss MicroImaging, Inc., Thornwood, NY).
For glycosome labeling, the aldolase peroxisomal targeting sequence (PTS2) (Blattner, et al., 1995) was introduced into a red fluorescent protein (mCherry) modified pXS (Marchetti, et al., 2000) expression vector to yield an N-terminal fusion with the mCherry. Briefly, FPTS2 (5′AGCTTATGAGTAAGCGTGTGGAGGTGCTTCTTACACAGCTTG 3′) and RPTS2 (5′CTAGCAAGCTGTGTAAGAAGCACCTCCACACGCTTACT CATA 3′) were annealed and the resulting product cloned into pXS. PF parasites were then transiently transfected with 10μg of the pXSAldoPTSmCherry construct and cultured 24 hr prior to examination. Live cells were visualized after resuspension in mounting medium (with DAPI) diluted 1:1 in PBS.
For RNAi studies, PF parasites were transfected and stable transformants selected as described (Wang, et al., 2000). TbHK1 was targeted specifically using an RNAi construct that targeted the unique 3′UTR of the transcript. Briefly, RNAi of TbHK1 was achieved using pZJM harboring a 341 bp fragment previously identified as a 3′ untranslated region sequence (Morris, et al., 2002). Parasite growth was monitored on a Becton-Dickinson FACScan flow cytometer.
For studies exploring the impact of over-expression of TbHK1 in PF cells, parental cells (PF 29-13) were transformed with linearized pLew111(2T7)GFPβ (Motyka, et al., 2006) harboring the TbHK1 gene in the multicloning site. This vector fuses the green fluorescent protein (GFP) to the carboxyl termini of expressed proteins. After selection for stable transformants, TbHK1 expression was induced by addition of tetracycline (1 μg/ml) to the media.
3. Results
Glycolysis is essential to the parasitic protozoan T. brucei, suggesting that inhibitors of enzymes in the pathway may be suitable targets for therapeutic development. TbHK1, which mediates the first step in this metabolic pathway, is an enzyme that has previously demonstrated to be essential for BSF parasites (Chambers et al. 2008a). As part of the validation of a high throughput screening campaign, we completed a pilot screen of a library of 1280 pharmaceutically active compounds (LOPAC, Sigma) (Sharlow, et al.). This screen yielded 12 primary hits including myricetin (IC50 of 48.9 ± 0.7 μM), a bioflavonoid that shares structural similarity with a known anti-trypanosomal compound, QCN (Fig. 1A) (Mamani-Matsuda, et al., 2004). Further, the observation that QCN inhibited mammalian HKs (Graziani, 1977) suggested that the trypanosome TbHK1 could be a target of QCN. These observations led us to further explore QCN as an inhibitor of TbHK1 while considering the possible connection between TbHK1 inhibition and the reported anti-parasitic activity of QCN.
Fig. 1.
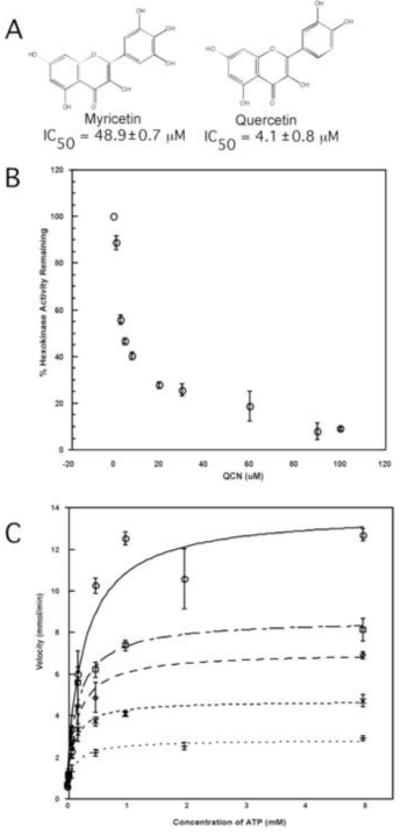
QCN is a potent inhibitor of TbHK1. (A) Structures of myricetin and QCN. (B) QCN inhibits rTbHK1. Increasing amounts (0-100 μM) of QCN were incubated with rTbHK1 (160 ng/assay) for 10 min at RT and HK assays were performed as described in the Materials and Methods. Please note that due to the complex nature of the mode of inhibition, IC50 values were estimated assuming single site binding. (C) QCN is a mixed inhibitor of TbHK1 with respect to ATP. Michaelis-Menten plots of inhibition with different QCN concentrations in assays containing varied ATP amounts.
3.1 QCN inhibits TbHK1 through mixed inhibition with ATP
Incubation of recombinant TbHK1 with QCN followed by a coupled enzyme assay for HK activity revealed that the compound inhibited the enzyme (IC50 = 4.1 ± 0.8 μM) (Fig. 1B). Inhibition was not as a result of dissociation of TbHK1 oligomers (a previously characterized mechanism for regulation of activity (Chambers, et al., 2008)), as QCN did not cause dissociation of TbHK hexamers (data not shown).
Many different kinases are inhibited by QCN, indicating that the molecule interacts with a structural feature common to all of the proteins, with the ATP binding site being a likely candidate binding site (Matter, et al., 1992, Srivastava, 1985) (Granot, 2002). Analysis of the nature of TbHK1 inhibition revealed that QCN was a mixed inhibitor with respect to ATP with a Ki value of 2.9 ± 0.9 μM (Fig. 1C).
To determine if endogenous HK activity from parasite lysates was also sensitive to QCN inhibition, cell lysates from BSF or PF parasites were incubated with QCN and then assayed for HK activity. QCN inhibited both BSF and PF HK activity similarly, with IC50s = 24 μM and 30 μM, respectively.
3.2 TbHK1 inhibition coincides with changes in Trp177 fluorescence
TbHK1 harbors a single Trp (residue 177) that is modeled to lie on the face of the enzyme near the hinge region and catalytic base (Asp214) (Fig. 2A) (Morris, et al., 2006). Excitation of the single Trp177 in TbHK1 at 280 nm yielded a characteristic Trp emission band at ~370 nm (Fig. 2B, inset). While QCN is intrinsically fluorescent with a maximum emission wavelength of 550 nm when excited at 280 nm, it alone yielded little fluorescence at 370 nm (not shown). Addition of increasing amounts of QCN to TbHK1, however, quenched the Tryp177 emission, yielding an IC50 for quenching of ~ 35 μM (Fig. 2B).
Fig. 2.
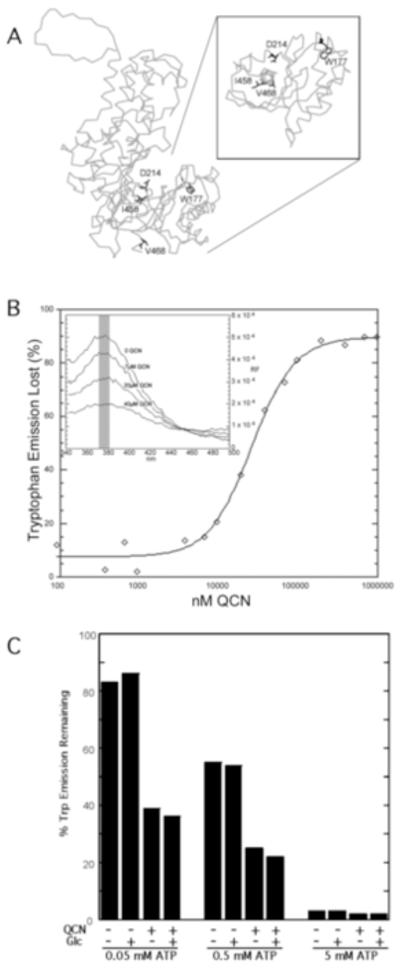
QCN bound to rTbHK1 alters Trp177 fluorescence. (A) TbHK1 was modeled to the crystal structure of S. cerevisiae hexokinase PII (Kuser, et al., 2000). The active site, Asp214, and Trp177 are indicated, along with residues that are involved in ATP binding in the C-terminal tail. For clarity, the lower lobe of the protein has been isolated and rotated to position the C-terminal tail perpendicular to the page. (B) Trp177 emission is inhibited by QCN. TbHK1 (1 μg) was incubated with increasing concentrations of QCN in a 100 mM TEA (pH 7.4) solution and, following excitation at 280 nm, emission monitored from 340-500 nm. To assess the percentage of Trp emission lost, the region between 370-380 nm (inset) was calculated and the % reduction in response to QCN determined. (C) ATP and QCN quench Trp177. Spectra were acquired from samples containing different amounts of ATP as described in 2B, and percentage of Trp emission lost calculated. Glucose (20 mM) was added as indicated.
To further resolve the impact QCN was having on Trp177 emission, substrates of TbHK1 were included to determine their impact on fluorescence. Addition of glucose (20 mM) alone did not alter Trp177 emission at 370 nm (not shown). However, addition of ATP (0.05 mM) quenched the Trp177 emission ~20%, while additional ATP (to 5 mM) nearly eliminated emission (Fig. 2C). Addition of glucose with the ATP did not alter quenching, yielding emission loss similar to ATP alone.
3.3 QCN localizes in part to glycosomes in live trypanosomes
Little is known about the mechanisms of action of QCN in the African trypanosome but the observation that the compound inhibits the essential TbHK1 led us to consider if the two shared subcellular localization. To explore this, live PF parasites expressing glycosomally targeted mCherry were incubated with QCN and the fluorescence both scored by microscopy (Fig. 3A). QCN accumulated in distinct foci, yielding punctate fluorescence that co-localized with the mCherry-bearing glycosomes. Additional QCN fluorescence was observed as a light haze throughout the cell, suggesting that localization was not limited to glycosomes. (Please note, parasite autofluorescence was not observed when similar exposure times were used on cells not incubated with QCN, suggesting the diffuse signal is due to the compound.) Similarly, live BSF parasites yielded QCN fluorescence in punctate foci, a distribution suggesting subcellular localization, potentially glycosomal in nature (Fig 3A). Additional staining was observed associated with the flagellum.
Fig. 3.
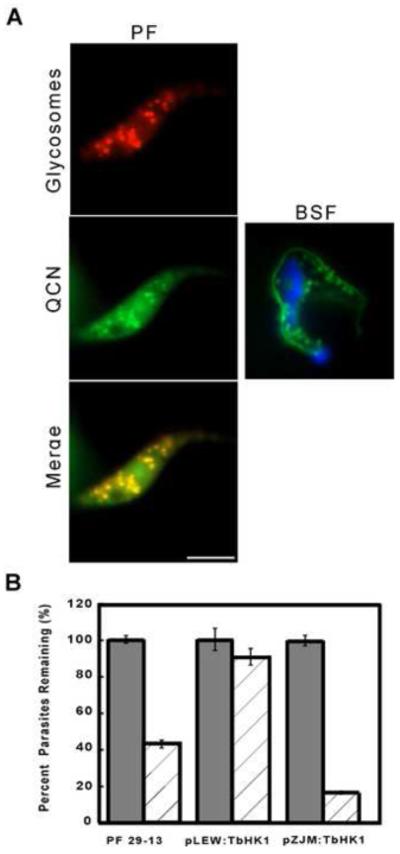
Localization and genetic manipulation studies to explore the biological consequences of QCN on T. brucei. BSF and PF parasites incubated with QCN (100 μM, 15 and 10 min for PF and BSF, respectively) were stained with DAPI and imaged. Glycosome localization in live PF cells was visualized by mCherry emission. Fluorescence of the QCN does not bleed into the mCherry spectra and was captured using a 488 nm filter set. Scale bar = 10 μm. (B) RNAi of TbHK1 enhances QCN toxicity, while over-expression of TbHK1 tempers it. Parental PF trypanosomes (29-13) or parasites transformed with pLEW111(2T7):TbHK1 or pZJM:TbHK1 were induced with tetracycline (1 μg/ml) for 24 hours to either express or silence TbHK1, QCN (50 μM, dashed columns) added, and cell viability scored after an additional 24 hour incubation. All assays were performed in triplicate, with cell numbers normalized to untreated controls. The p-values for untreated and treated parental 29-13 and pZJM:TbHK1 bearing cells were statistically significant (p < 0.05 in both cases), indicating that the differences between untreated and treated cells were significant, while the value for pLEW111(2T7):TbHK1 harboring cells incubated with or without QCN was not (p > 0.05).
The glycosomes, a peroxisome-like organelle, compartmentalizes the majority of the glycolytic enzymes in the trypanosome, many of which have been demonstrated genetically to be essential, including TbHK1 (Chambers, et al., 2008). The localization of a compound that inhibits TbHK1 in vitro to the compartment that houses the essential enzyme perhaps explains the cytotoxicity of the compound. Previous research demonstrated that QCN is toxic to T. b. gambiense (LD50 = 10 μM) (Mamani-Matsuda, et al., 2004). To confirm that our 427 T. b. brucei lab strain was also susceptible to QCN, parasites were incubated for 24 hr in the presence of compound and growth monitored by cell counting. QCN was found to be toxic to both BSF 90-13 and PF 29-13 trypanosomes (LD50 = 7.5 μM and 35 μM, respectively).
3.4 Exploring potential in vivo targets of QCN
Unlike BSF parasites (which rely exclusively on glycolysis for ATP production), genetic manipulation of glycolytic enzymes, including over-expression and RNAi-based silencing, can be tolerated in PF parasites if the trypanosomes are first provided an opportunity to down-regulate hexose metabolism (Morris, et al., 2002, Morris, et al., 2006). If QCN is toxic as a result of its inhibition of glycolysis, increased cellular TbHK1 polypeptide could temper the toxicity of the compound. To explore this, PF cells over-expressing TbHK1 from the inducible ectopic expression vector pLew111(2T7) were incubated with 50 μM QCN and cell growth compared to parental cell lines grown under similar conditions (Fig. 3B). After 24 hrs, cells over-expressing TbHK1 displayed cellular HK activity 2.1 greater than control cells. Additionally, these cells were more resistant to QCN with growth reduced only 10.1 ± 0.5% while growth of QCN-treated control parasites was repressed 55.5± 0.5%.
The incomplete penetrance of RNAi (and the slowly developing phenotypes associated with the silencing) allows manipulation of TbHK1 abundance without detectable cell toxicity in PF parasites (Morris, et al., 2002). Therefore, we have explored the consequence of silencing TbHK1 on QCN sensitivity, with the prediction being that these cells may be more sensitive to the compound (that is, the remaining TbHK1 that results from incomplete penetrance will be inhibited more readily by QCN). RNAi of TbHK1 using pZJM(TbHK1), which targets the distinct 3′UTR of TbHK1 (Morris, et al., 2002), was induced for 24 hours before cells were passed into medium containing QCN and cell growth compared to parental PF 29-13 parasites after an additional 24 hours. Silencing TbHK1 led to enhanced sensitivity to QCN, with cells induced to silence TbHK1 reduced in number to 16.4 ± 0.6% of the corresponding untreated cell lines (Fig. 3B).
4. Discussion
BSF T. brucei generate ATP exclusively by glycolysis, indicating that inhibitors of enzymes in the pathway may be therapeutic lead compounds. The trypanosome glycolytic enzymes, TbHK1 and TbHK2, are potential drug targets. These proteins, which are 30-33% identical to those from yeast, plants, and mammals, have a number of unique biochemical features (in addition to amino acid composition) that suggest that identification of trypanosome-specific HK inhibitors may be possible. These differences include mulitmerization of the enzyme into hexamers, localization of the protein to the glycosome, and sensitivity of the TbHKs to fatty acids (Chambers, et al., 2008, Misset and Opperdoes, 1984, Morris, et al., 2006).
Genetic studies using RNAi have shown that both TbHKs are essential to the parasite (Albert, et al., 2005, Chambers, et al., 2008), and chemical inhibitors of the enzyme have demonstrated anti-parasitic activity in vitro (Chambers, et al., 2008, Sharlow, et al., Willson, et al., 2002). Here, we report that QCN, a previously recognized anti-trypanosomal flavanoid (Mamani-Matsuda, et al., 2004), inhibits recombinant TbHK1. Amongst the mammalian enzymes that have been reported to be sensitive to QCN, including a tyrosine protein kinase, a phosphorylase kinase (Srivastava, 1985), a phosphatidyl 3-kinase (Matter, et al., 1992), and a DNA topoisomerase (Boege, et al., 1996), the IC50s for QCN are similar to that of TbHK1, ranging from 3-300 μM. This suggests that there may be limited increase in therapeutic range for the compound. However, the observation that QCN is not toxic to mammals at trypanocidal concentrations (Hu, et al., 2001) supports the possibility that the partial localization of QCN to glycosomes (and the potential for concentration of the compound therein) may increase the therapeutic ratio favorably.
Many of the other enzymes that have been reported to be sensitive to QCN share the feature of binding nucleotides or nucleotide triphosphates. It has been suggested that QCN inhibits Tyr kinases (like pp60v-src) by forming a hydrogen-bonded complex with ATP, which would mimic the transition state of ATP and the tyrosyl residue of the enzyme (Granot, 2002). A similar mechanism is unlikely in TbHK1, as QCN alone can quench Trp177 fluorescence.
QCN has proven to be a potent anti-kinetoplastid agent, with toxicity toward Leishmania donavani (Mittra, et al., 2000) and T. brucei gambiense (Mamani-Matsuda, et al., 2004). In the former case, QCN inhibits DNA synthesis, leading to cell cycle arrest that triggers apoptosis. Similarly, QCN causes T. brucei death through apoptosis (Mamani-Matsuda, et al., 2004), though we propose the mechanism may be distinct. In mammalian cells, mitochondrial associated HK activity is required to prevent apoptosis (Gottlob, et al., 2001) and increased HK activity prevents oxidant-induced apoptosis (Bryson, et al., 2002). Our observation that QCN inhibits TbHK1 suggests that the apoptosis may be the result of a mechanism that measures HK activity, similar to that found in mammalian cells. The TbHKs are unusual in a number of ways, including the finding that they oligomerize into hexamers (Chambers, et al., 2008) and localize to the peroxisome-like glycosome (Misset and Opperdoes, 1984). These characters suggest novel mechanisms connecting TbHK to cell signaling, which remain to be resolved.
Acknowledgements
The authors would like to thank Stephen Carek and Tom Caldwell for their technical assistance. This work was supported in part by the US National Institutes of Health 1R15AI075326 to JCM.
Biography
Author Vitae
Heidi C. Dodson is a graduate student at Clemson University. Dr. Todd L. Lyda (Ph.D., Genetics, Clemson University,2009) is a Post-doctoral Fellow at the NIH. Dr. Jeremy W. Chambers (Ph.D., Biochemistry and Molecular Biology, Clemson University, 2008) is a Research Associate at The Scripps Research Institute. Dr. Meredith T. Morris (Ph.D., Biochemistry, The University of Georgia, 2000) is a Research Assistant Professor in the Department of Genetics and Biochemistry at Clemson University. Dr Kenneth A. Christensen (Ph.D., Chemistry, University of Michigan) is an Assistant Professor in the Chemistry Department. Dr. James C. Morris (Ph.D., Cellular Biology, The University of Georgia) is an Associate Professor in Genetics and Biochemistry at Clemson University.
Vitae Photos:
 Heidi Dodson
Heidi Dodson
 Todd Lyda
Todd Lyda
 Jeremy Chambers
Jeremy Chambers
 Meredith Morris
Meredith Morris
 Kenneth Christensen
Kenneth Christensen
 James Morris
James Morris
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- BSF
- bloodstream form
- G6-P
- glucose-6-phosphate
- G6PDH
- glucose-6-phosphate dehydrogenase
- HK
- hexokinase
- LND
- lonidamine
- PF
- procyclic form
- TbHK1
- recombinant T. brucei hexokinase 1
- TbHK2
- recombinant T. brucei heoxkinase 2
- TbHK
- T. brucei hexokinase
- TEA
- triethanolamine
- QCN
- quercetin
References
- 1.Albert MA, Haanstra JR, Hannaert V, Van Roy J, Opperdoes FR, Bakker BM, Michels PA. Experimental and in silico analyses of glycolytic flux control in bloodstream form Trypanosoma brucei. Journal of Biological Chemistry. 2005;280:28306–28315. doi: 10.1074/jbc.M502403200. [DOI] [PubMed] [Google Scholar]
- 2.Barrett MP, Burchmore RJ, Stich A, Lazzari JO, Frasch AC, Cazzulo JJ, Krishna S. The trypanosomiases. Lancet. 2003;362:1469–1480. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- 3.Blattner J, Dorsam H, Clatyon CE. Function of N-terminal import signals in trypanosome microbodies. FEBS Letters. 1995;360:310–314. doi: 10.1016/0014-5793(95)00128-v. [DOI] [PubMed] [Google Scholar]
- 4.Boege F, Straub T, Kehr A, Boesenberg C, Christiansen K, Andersen A, Jakob F, Kohrle J. Selected novel flavones inhibit the DNA binding or the DNA religation step of eukaryotic topoisomerase I. Journal of Biological Chemistry. 1996;271:2262–2270. doi: 10.1074/jbc.271.4.2262. [DOI] [PubMed] [Google Scholar]
- 5.Bryson JM, Coy PE, Gottlob K, Hay N, Robey RB. Increased hexokinase activity, of either ectopic or endogenous origin, protects renal epithelial cells against acute oxidant-induced cell death. Journal of Biological Chemistry. 2002;277:11392–11400. doi: 10.1074/jbc.M110927200. [DOI] [PubMed] [Google Scholar]
- 6.Chambers JW, Fowler ML, Morris MT, Morris JC. The anti-trypanosomal agent lonidamine inhibits Trypanosoma brucei hexokinase 1. Molecular and Biochemical Parasitology. 2008;158:202–207. doi: 10.1016/j.molbiopara.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Chambers JW, Kearns MT, Morris MT, Morris JC. Assembly of heterohexameric trypanosome hexokinases reveals that hexokinase 2 is a regulable enzyme. Journal of Biological Chemistry. 2008;283:14963–14970. doi: 10.1074/jbc.M802124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colasante C, Ellis M, Ruppert T, Voncken F. Comparative proteomics of glycosomes from bloodstream form and procyclic culture form Trypanosoma brucei brucei. Proteomics. 2006;6:3275–3293. doi: 10.1002/pmic.200500668. [DOI] [PubMed] [Google Scholar]
- 9.Floridi A, Lehninger AL. Action of the antitumor and antispermatogenic agent lonidamine on electron transport in Ehrlich ascites tumor mitochondria. Archives of Biochemistry and Biophysics. 1983;226:73–83. doi: 10.1016/0003-9861(83)90272-2. [DOI] [PubMed] [Google Scholar]
- 10.Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes and Development. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granot Y. The ATP site of protein kinases as target for drug development: from natural compounds to gleevec. Israel Medical Association Journal. 2002;4:633–635. [PubMed] [Google Scholar]
- 12.Graziani Y. Bioflavonoid regulation of ATPase and hexokinase activity in Ehrlich ascites cell mitochondria. Biochimica et Biophysica Acta. 1977;460:364–373. doi: 10.1016/0005-2728(77)90221-3. [DOI] [PubMed] [Google Scholar]
- 13.Graziani Y, Erikson E, Erikson RL. The effect of quercetin on the phosphorylation activity of the Rous sarcoma virus transforming gene product in vitro and in vivo. European Journal of Biochemistry. 1983;135:583–589. doi: 10.1111/j.1432-1033.1983.tb07692.x. [DOI] [PubMed] [Google Scholar]
- 14.Guharay J, Sengupta B, Sengupta PK. Protein-flavonol interaction: fluorescence spectroscopic study. Proteins. 2001;43:75–81. doi: 10.1002/1097-0134(20010501)43:2<75::aid-prot1019>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Hu CC, Chen WK, Liao PH, Yu WC, Lee YJ. Synergistic effect of cadmium chloride and acetaldehyde on cytotoxicity and its prevention by quercetin and glycyrrhizin. Mutation Research. 2001;496:117–127. doi: 10.1016/s1383-5718(01)00214-5. [DOI] [PubMed] [Google Scholar]
- 16.Kuser PR, Krauchenco S, Antunes OA, Polikarpov I. The high resolution crystal structure of yeast hexokinase PII with the correct primary sequence provides new insights into its mechanism of action. Journal of Biological Chemistry. 2000;275:20814–20821. doi: 10.1074/jbc.M910412199. [DOI] [PubMed] [Google Scholar]
- 17.Mamani-Matsuda M, Rambert J, Malvy D, Lejoly-Boisseau H, Daulouede S, Thiolat D, Coves S, Courtois P, Vincendeau P, Mossalayi MD. Quercetin induces apoptosis of Trypanosoma brucei gambiense and decreases the proinflammatory response of human macrophages. Antimicrobial Agents and Chemotherapy. 2004;48:924–929. doi: 10.1128/AAC.48.3.924-929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchetti MA, Tschudi C, Kwon H, Wolin SL, Ullu E. Import of proteins into the trypanosome nucleus and their distribution at karyokinesis. Journal of Cell Science. 2000;113(Pt 5):899–906. doi: 10.1242/jcs.113.5.899. [DOI] [PubMed] [Google Scholar]
- 19.Matter WF, Brown RF, Vlahos CJ. The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochemical and Biophysical Research Communication. 1992;186:624–631. doi: 10.1016/0006-291x(92)90792-j. [DOI] [PubMed] [Google Scholar]
- 20.Misset O, Opperdoes FR. Simultaneous purification of hexokinase, class-I fructose-bisphosphate aldolase, triosephosphate isomerase and phosphoglycerate kinase from Trypanosoma brucei. European Journal of Biochemistry. 1984;144:475–483. doi: 10.1111/j.1432-1033.1984.tb08490.x. [DOI] [PubMed] [Google Scholar]
- 21.Mittra B, Saha A, Chowdhury AR, Pal C, Mandal S, Mukhopadhyay S, Bandyopadhyay S, Majumder HK. Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Molecular Medicine. 2000;6:527–541. [PMC free article] [PubMed] [Google Scholar]
- 22.Molnar J, Beladi I, Domonkos K, Foldeak S, Boda K, Veckenstedt A. Antitumor activity of flavonoids on NK/Ly ascites tumor cells. Neoplasma. 1981;28:11–18. [PubMed] [Google Scholar]
- 23.Morris JC, Wang Z, Drew ME, Englund PT. Glycolysis modulates trypanosome glycoprotein expression as revealed by an RNAi library. EMBO Journal. 2002;21:4429–4438. doi: 10.1093/emboj/cdf474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris MT, DeBruin C, Yang Z, Chambers JW, Smith KS, Morris JC. Activity of a second Trypanosoma brucei hexokinase is controlled by an 18-amino-acid C-terminal tail. Eukaryotic Cell. 2006;5:2014–2023. doi: 10.1128/EC.00146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motyka SA, Drew ME, Yildirir G, Englund PT. Overexpression of a cytochrome b5 reductase-like protein causes kinetoplast DNA loss in Trypanosoma brucei. Journal of Biological Chemistry. 2006;281:18499–18506. doi: 10.1074/jbc.M602880200. [DOI] [PubMed] [Google Scholar]
- 26.Paggi MG, Fanciulli M, Perrotti N, Floridi A, Zeuli M, Silvestrini B, Caputo A. The role of mitochondrial hexokinase in neoplastic phenotype and its sensitivity to lonidamine. Annals of the New York Academy of Sciences. 1988;551:358–360. doi: 10.1111/j.1749-6632.1988.tb22362.x. [DOI] [PubMed] [Google Scholar]
- 27.Sengupta B, Sengupta PK. The interaction of quercetin with human serum albumin: a fluorescence spectroscopic study. Biochemical and Biophysical Research Communication. 2002;299:400–403. doi: 10.1016/s0006-291x(02)02667-0. [DOI] [PubMed] [Google Scholar]
- 28.Sharlow ER, Lyda TA, Dodson HC, Mustata G, Morris MT, Leimgruber SS, Lee KH, Kashiwada Y, Close D, Lazo JS, Morris JC. A target-based high throughput screen yields Trypanosoma brucei hexokinase small molecule inhibitors with antiparasitic activity. PLoS Neglected Tropical Diseases. 4:e659. doi: 10.1371/journal.pntd.0000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava AK. Inhibition of phosphorylase kinase, and tyrosine protein kinase activities by quercetin. Biochemical and Biophysical Research Communicaiton. 1985;131:1–5. doi: 10.1016/0006-291x(85)91761-9. [DOI] [PubMed] [Google Scholar]
- 30.Suolinna EM, Buchsbaum RN, Racker E. The effect of flavonoids on aerobic glycolysis and growth of tumor cells. Cancer Research. 1975;35:1865–1872. [PubMed] [Google Scholar]
- 31.Wang Z, Morris JC, Drew ME, Englund PT. Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. Journal of Biological Chemistry. 2000;275:40174–40179. doi: 10.1074/jbc.M008405200. [DOI] [PubMed] [Google Scholar]
- 32.Willson M, Sanejouand YH, Perie J, Hannaert V, Opperdoes F. Sequencing, modeling, and selective inhibition of Trypanosoma brucei hexokinase. Chemistry and Biology. 2002;9:839–847. doi: 10.1016/s1074-5521(02)00169-2. [DOI] [PubMed] [Google Scholar]


