Abstract
Despite advances into our understanding of how nutrient oversupply and triacylglycerol (TAG) anabolism contribute to hepatic steatosis, little is known about the lipases responsible for regulating hepatic TAG turnover. Recent studies have identified adipose triglyceride lipase (ATGL) as a major lipase in adipose tissue although its role in the liver is largely unknown. Thus, we tested the contribution of ATGL to hepatic lipid metabolism and signaling. Adenoviral-mediated knockdown of hepatic ATGL resulted in steatosis in mice and decreased hydrolysis of TAG in primary hepatocyte cultures and in vitro assays. In addition to altering TAG hydrolysis, ATGL is shown to play a significant role in partitioning hydrolyzed fatty acids between metabolic pathways. Whereas ATGL gain- and loss-of-function did not alter hepatic TAG secretion, fatty acid oxidation was increased by ATGL overexpression and decreased by ATGL knockdown. The effects on fatty acid oxidation coincided with decreased expression of PPAR-α and its target genes in mice with suppressed hepatic ATGL expression. However, PPAR-α agonism was unable to normalize the effects of ATGL knockdown on PPAR-α target gene expression suggesting that ATGL influences PPAR-α activity independent of ligand-induced activation. Taken together, these data show that ATGL is a major hepatic TAG lipase that plays an integral role in fatty acid partitioning and signaling to control energy metabolism.
Keywords: ATGL, β-oxidation, PPAR-α, TAG
Introduction
Hepatic steatosis represents the most common form of liver disease in both adults and children in the US (1-3). In addition to being a precursor to fibrosis, cirrhosis and cancer, hepatic steatosis is also tightly linked to Type 2 Diabetes, obesity and cardiovascular disease (4). Because TAG content defines steatosis, the regulation of hepatic lipid metabolism is an integral part of disease etiology. To date, most studies on hepatic steatosis have focused upon enzymes involved in TAG synthesis or how energy oversupply leads to TAG accumulation. However, little is known about lipases responsible for controlling hepatic TAG hydrolysis and how this process contributes to the development of steatosis.
Hormone-sensitive lipase has received the most research attention for its role in regulating lipolysis especially in adipose tissue. However, in 2004, several groups indentified a novel TAG lipase that is highly expressed in adipose tissue (5-7). This lipase, which is commonly known as ATGL (aliases include desnutrin, phospholipase A2-ζ and pigment epithelium-derived factor receptor), is a member of the patatin domain-containing family. Characterization of this lipase revealed that it has high substrate specificity for TAG [10 fold over diacylglycerol (DAG)] especially compared to hormone-sensitive lipase, which preferentially hydrolyzes DAG (6). ATGL null mice have impaired rates of lipolysis and, consequently, have increased adipose tissue mass (6, 8). Because of its high expression and prominent role in white adipose tissue, most research has focused upon ATGL in the context of this tissue. However, ATGL is expressed at lower levels in non-adipose tissues such as heart, muscle and liver (6, 9). Its importance outside of adipose tissue is evidenced by the ectopic lipid accumulation in most tissues of ATGL null mice including increased TAG in cardiac muscle (21-fold), skeletal muscle (3-fold) and liver (2.3-fold) (6, 8). Despite these changes it is difficult to determine if the effects of global ATGL ablation on hepatic metabolism are direct or due to the broad effects of ATGL on other tissues. In this report, we show that ATGL is an important hepatic lipase that governs TAG turnover and lipid partitioning and signaling to influence the development of steatosis.
Materials and Methods
Animals, Diets and Adenoviral Administration
All animal protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee. Eight week old C57/Bl6 male mice were purchased from Jackson Laboratory and housed under controlled temperature and lighting (20-22°C; 12:12-h light-dark cycle). Mice were allowed to acclimate for 1 wk prior to adenoviral injections. Adenoviruses that encode mouse ATGL shRNA and control shRNA that targets a non-specific mRNA sequence were generated as described previously (10). Mice were injected with 1 × 109 plaque forming units of adenovirus containing ATGL shRNA or non-targeting shRNA control via the tail vein. Mice had free access to water and were fed with either chow (TD.94045) or a 45% fat diet (TD.09404) from Harlan Teklad Premier Laboratory Diets following adenovirus administration. The chow diet contained 19% protein, 64% carbohydrate and 17% fat as a % of total calories and the fat source was soybean oil (70g/kg). The high fat diet contained 19% protein, 35% carbohydrates and 45% fat with lard (195 g/kg) and soybean oil (30 g/kg) comprising the fat sources. Exactly one week following adenovirus injection, mice were sacrificed for tissue and serum collection after an overnight fast. For studies involving administration of fenofibrate, starting one day after adenovirus injection, mice were gavaged daily with fenofibrate (125 mg/kg body weight) suspended in 0.5% carboxymethylcellulose. This dosage of fenofibrate is within the range of doses used to affect PPAR-α and hepatic energy metabolism in mice (11-13).
Primary Hepatocyte Isolation and Culture
Mouse primary hepatocytes were isolated by the collagenase perfusion method from 10-12 wk old C57/Bl6 male mice with free access to water and chow diet. Hepatocytes were isolated and cultured exactly as we have described previously (14).
Cell Adenoviral Transduction, Radiolabeling, and Lipid Analysis
Adenovirusus expressing either ATGL or green fluorescent protein (GFP), which serves as a control virus, were generated as described previously (10). After 4 h of plating, cells were exposed to either adenovirus expressing GFP or ATGL at 10 MOI for 24 h. For knockdown studies, cells were treated with adenovirus containing ATGL shRNA and control shRNA and were cultured in maintenance media for 66 h unless otherwise noted. After 24 h for overexpression studies and 66 h for knockdown studies, cells were pulsed with 500 μM [1-14C]oleate bound to fatty acid-free BSA in a 3:1 molar ratio for 1.5 h. Some cells were harvested to measure radiolabel incorporation into cellular lipid fractions. Parallel incubations were washed with PBS and the wells were replaced with fresh media lacking labeled fatty acids for an additional 6 h of chase period followed by collection of cells for lipid extraction. Lipid extracts were further fractionated and radioisotopes quantified (14). Fatty acid oxidation to acid-soluble metabolites (ASM) and CO2 was measured as described previously (15).
Statistical Analysis
Results are expressed as mean ± standard error of the mean. Statistical analysis was performed using unpaired Student t-test. Values of P<0.05 were considered statistically significant.
See Supporting Information for additional descriptions of Materials and Methods.
Results
Adenovirus delivery of ATGL shRNA efficiently suppresses ATGL expression and TAG hydrolase activity
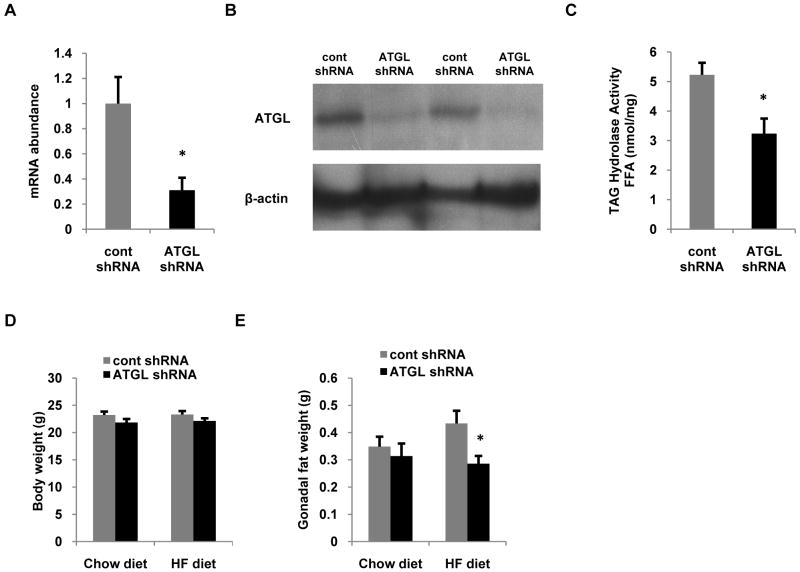
To determine the effects of hepatic ATGL, we injected mice via the tail vein with adenoviruses expressing scrambled shRNA (cont shRNA) or shRNA targeted against ATGL (ATGL shRNA). After 7 d, hepatic ATGL mRNA in ATGL shRNA treated mice was reduced ∼70% (Fig. 1A) and ATGL protein was suppressed ∼80% (Fig. 1B) compared to mice treated with cont shRNA. We next measured hepatic TAG hydrolase activity from the different treatment groups using [3H]trioleate as the substrate. Consistent with a robust knockdown of ATGL, the ATGL shRNA decreased TAG hydrolase activity ∼40% relative to controls (Fig. 1C). ATGL knockdown did not influence body weight in mice fed chow or high fat diets for 7 d following transduction (Fig. 1D); a negative control group injected with saline showed similar ATGL expression and body weight as those receiving cont shRNA (data not shown). High fat feeding for 7 d following administration of adenoviruses resulted in a significant increase in gonadal fat pad weight in control mice, however, this increase was completely abrogated in mice treated with ATGL shRNA (Fig. 1E) suggesting that manipulating hepatic ATGL impacted adipose metabolism.
Figure 1. Adenovirus-mediated shRNA suppresses hepatic ATGL expression and TAG hydrolase activity.
C57/Bl6 mice at 8-10 weeks of age were infected with control or ATGL shRNA adenovirus (n = 8-10 per group) and were fed with either chow or high fat (HF) diets. After an overnight fast, animals were sacrificed 7 d post-infection followed by determination of mRNA (A) and protein (B) expression of hepatic ATGL liver in chow fed mice. (C) Cytosolic extracts were also used to measure hepatic TAG hydrolase activity from chow-fed mice (n = 3-4). Body (D) and gonadal fat (E) weights of mice fed chow or HF diets. Data are presented as means ± SEM. *P<0.05 vs control shRNA group.
Hepatic ATGL knockdown causes steatosis
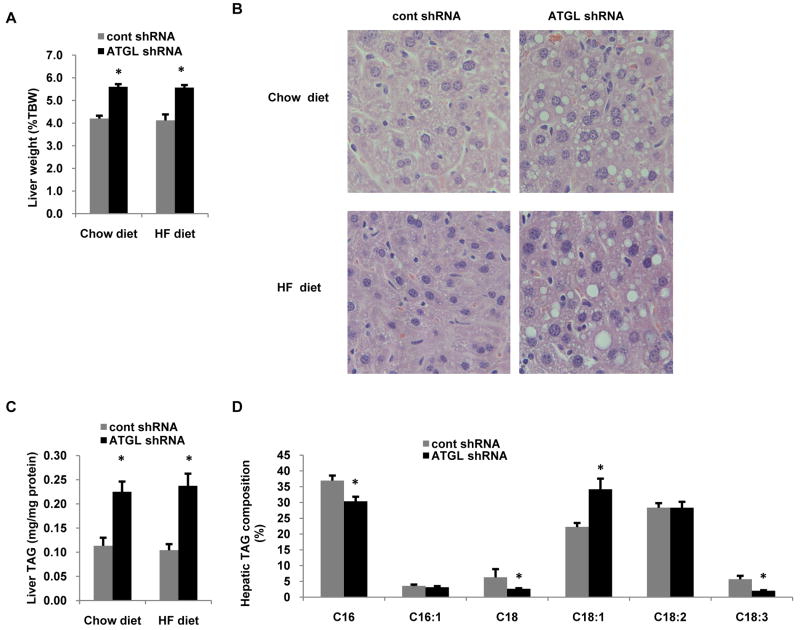
Despite its relatively low expression in the liver, ATGL null mice have hepatic steatosis although it is unclear if this is a direct effect of loss of hepatic ATGL or due to changes in extrahepatic metabolism (6, 9). Thus, we evaluated the liver-specific effects of ATGL knockdown on the development of steatosis. After 7 d following adenoviral treatments, ATGL knockdown resulted in a ∼30% increase in liver weight (Fig. 2A) regardless of diet. Histological analysis of liver specimens revealed abundant lipid droplet accumulation following ATGL knockdown in chow and high fat diets (Fig. 2B). Further analysis revealed that hepatic TAG content was more than doubled in mice treated with ATGL shRNA confirming that suppression of ATGL leads to steatosis (Fig. 2C). Additionally, the role of ATGL on influencing TAG composition is not known. Therefore, we quantified the composition of fatty acids in TAG from mice treated with cont or ATGL shRNA. Knockdown of ATGL caused a significant reduction in C16:0, C18:0 and C18:3, but increased C18:1 content in TAG by ∼40% (Fig. 2D). Thus, ATGL is an important hepatic lipase that regulates both TAG content and composition.
Figure 2. Hepatic ATGL knockdown induces steatosis.
(A) Liver weights in mice treated with control or ATGL shRNA in both chow and HF diet groups (n = 8-10). (B) Liver sections from these mice were stained with hematoxylin and eosin and imaged at 20× magnification. (C) Liver TAG was quantified from mice fed chow or high fat diets (n = 8-10). (D) In order to determine composition of TAG, lipids extracted from livers of chow-fed mice were separated by TLC and TAG was methylated with 5% HCl in methanol to produce fatty acid methyl esters (FAMEs), which were analyzed by GC (n = 6). Data are presented as means ± SEM. *P<0.05 vs control shRNA group.
Hepatic ATGL alters TAG turnover
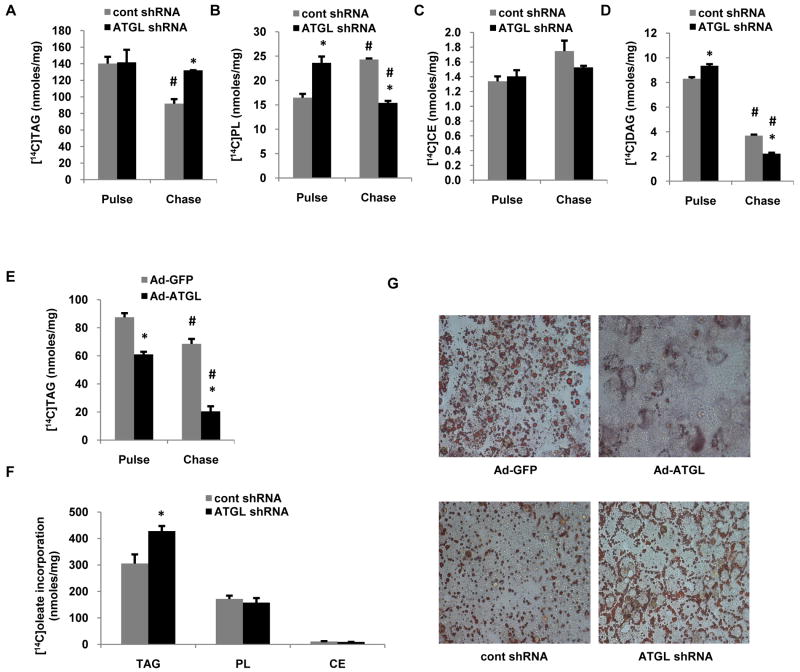
Given the increase in hepatic TAG content and reduced TAG hydrolase activity in mice with suppressed hepatic ATGL expression, we next sought to characterize the effects of ATGL on TAG turnover. To do so, we performed pulse-chase experiments with [1-14C]oleate in primary mouse hepatocytes treated with ATGL knockdown or overexpression adenoviruses. ATGL knockdown did not influence the amount of oleate incorporated into TAG during the 1.5 h pulse period, but blunted the loss of [14C]TAG during the chase period by ∼80% compared to cells transduced with control shRNA (Fig. 3A). Similar effects of ATGL on hepatic TAG turnover were also observed with a second siRNA (data not shown). ATGL knockdown did not influence cholesterol ester metabolism, but increased oleate incorporation into PL and DAG during the pulse, and enhanced radiolabled PL and DAG loss during the chase period (Fig. 3B-D). Consistent with the above data, pulse-chase experiments in hepatocytes overexpressing ATGL showed that ATGL decreased incorporation of [14C]oleate during the pulse period and increased the rate of [14C]TAG loss during the chase period (Fig. 3E). Longer term (8 h) labeling, which more closely reflects the effects of ATGL on lipid turnover revealed that ATGL knockdown increased [14C]TAG by approximately 30% without affecting PL and CE (Fig. 3F). To further characterize the role of ATGL in mediating fatty acid-induced lipid accumulation, hepatocytes were treated with overexpression or knockdown adenoviruses and then exposed to 500 μM oleate for 30 h. As shown in Fig. 3G, overexpression of ATGL almost completely prevented lipid droplet accumulation as evidenced by Oil Red O staining, whereas, ATGL knockdown resulted in increased lipid droplet formation as expected. ATGL also promoted smaller lipid droplets, which has been reported previously (16).
Figure 3. Hepatic ATGL regulates TAG turnover.
Primary mouse hepatocytes were isolated from 8-10 wk old C57/Bl6 chow-fed mice and cells were transduced with control or ATGL shRNA adenovirus for 66 h, at which time pulse (1.5 h) and chase (6 h) experiments were performed with 500 μM [1-14C]oleate. (A) TAG, (B) PL, (C) CE and (D) DAG were isolated from cells by lipid extraction and separation by TLC to measure incorporation of radiolabeled oleate into different lipid species (n = 3-5). (E) Cells were transduced with Ad-GFP and Ad-ATGL virus for 24 h, at which time pulse (1.5 h) and chase (6 h) experiments were performed with 500 μM [1-14C]oleate (n = 3). (F) Cells were transduced with control and ATGL shRNA for 66 h, at which time cells were pulsed with 500 μM [1-14C]oleate for 8 h. TAG, PL & CE were isolated from harvested cells by TLC to measure incorporation of radiolabeled oleate into different lipid species (n = 3). (G) Primary hepatocytes were transduced with ATGL knockdown or overexpression adenovirus as described above and then exposed to 500 μM of oleate for 30 h, at which time cells were washed, fixed and stained with Oil Red O followed by imaging with light microscopy at 20× magnification (representative of 3 experiments). Data are presented as means ± SEM. CE, cholesteryl ester; DAG, diacylglycerol; PL, phospholipid. *P< 0.05 vs control shRNA group. #P<0.05 vs pulse period.
Hepatic ATGL does not influence TAG secretion
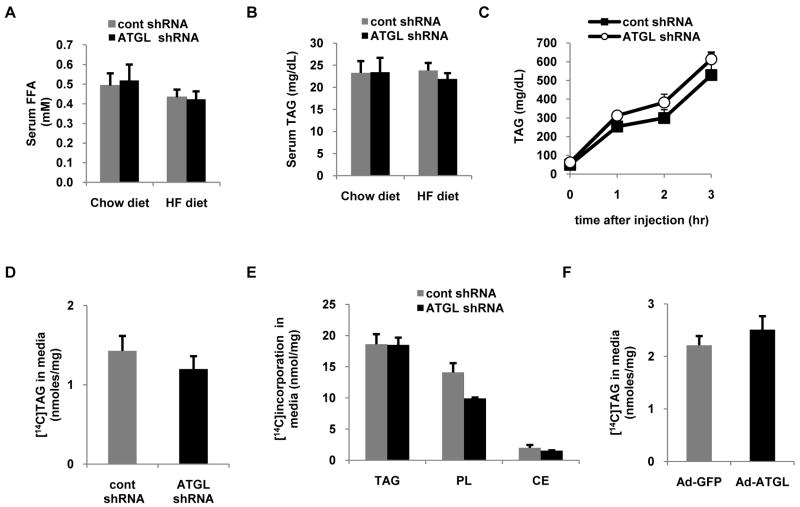
To gain insight into the effects of hepatic ATGL knockdown on whole-body fatty acid metabolism, we quantified serum FFA and TAG. Regardless of diet, the concentrations of these serum metabolites were unaltered following hepatic ATGL knockdown (Fig. 4A,B). Previous studies have shown that cytosolic TAG undergoes hydrolysis to DAG or monoacylglycerol prior to resterification and incorporation into VLDL (17). Thus, it was surprising that mice treated with ATGL shRNA had unaltered fasting serum TAG levels despite having attenuated hepatic TAG hydrolysis. Based upon these findings, we next questioned if ATGL regulates the rate of hepatic TAG secretion. To quantify rates of hepatic TAG secretion, fasted mice were injected with Tyloxapol, an inhibitor of lipoprotein lipase. In agreement with the similar fasting TAG values between treatment groups, rates of hepatic TAG secretion were unchanged in response to ATGL knockdown (Fig. 4C).
Figure 4. Hepatic ATGL does not regulate TAG secretion.
Mice fed the chow diet were treated with control or ATGL shRNA adenovirus and were fasted overnight followed by harvesting of serum for analysis of (A) free fatty acid (FFA) and (B) TAG (n = 8-10). (C) For measurement of hepatic TAG production, overnight fasted mice fed the chow diet were treated with Tyloxapol and blood collections were performed at 0, 1, 2 and 3 h intervals (n = 4-6) and serum TAG was subsequently analyzed. (D) Primary hepatocytes were transduced with control or ATGL shRNA for 66 h, at which time pulse (1.5 h) and chase (6 h) experiments were performed with 500 μM [1-14C]oleate. [14C]TAG was quantified during the chase period. (E) Primary hepatocytes were transduced with control or ATGL shRNA for 66 h followed by 8 h of pulse with 500 μM [1-14C]oleate. Cells were harvested and radiolabeled oleate incorporation into different lipid fractions was determined (n = 3). (F) Primary hepatocytes were also transduced with Ad-GFP or Ad-ATGL adenoviruses and after 24 h pulse (1.5 h) and chase (6 h) experiments were performed with 500 μM [1-14C]oleate and [14C]TAG secretion during the chase period was measured. Data are presented as means ± SEM.
We next extended these studies to primary hepatocyte cultures. Cells were pulsed with 500 μM [1-14C]oleate for 1.5 h, at which time media was changed and appearance of [14C]TAG in the media was measured after 6 h to quantify the contribution of ATGL and TAG hydrolysis to hepatic TAG secretion. In support of the in vivo data, ATGL knockdown did not alter TAG secretion in primary hepatocytes (Fig. 4D). Similar results were obtained when cells were labeled with [1-14C]oleate for 8 h (Fig. 4E). We also overexpressed ATGL and found that despite enhanced TAG hydrolysis (Fig. 3E) ATGL overexpression did not alter TAG secretion (Fig. 4F). Thus, despite potent effects on intracellular TAG hydrolysis, ATGL does not appear to be involved in channeling hydrolyzed fatty acids to VLDL synthesis.
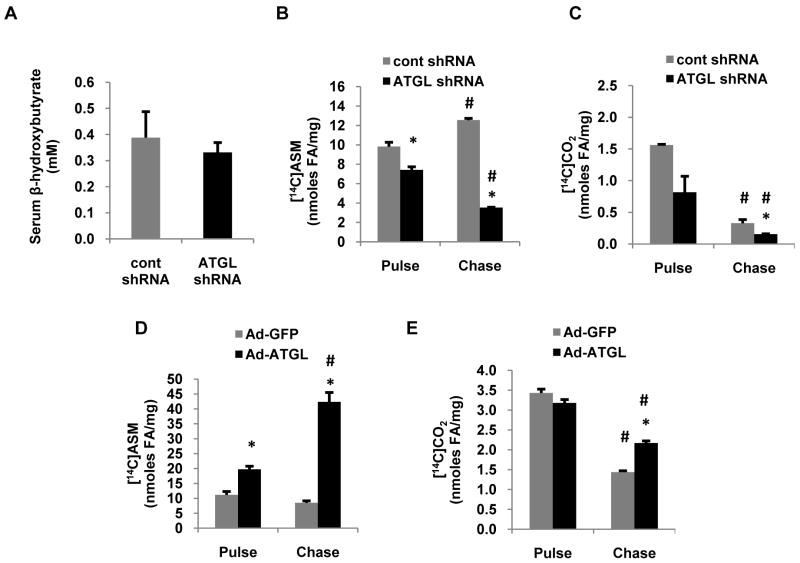
Hepatic ATGL promotes fatty acid oxidation
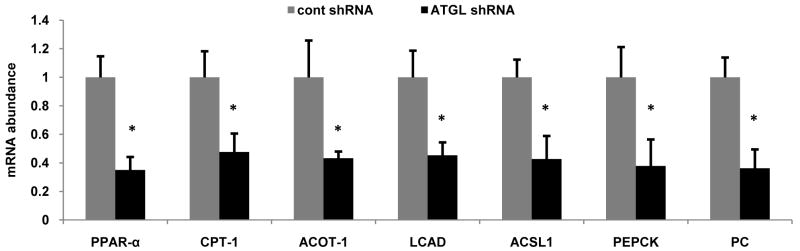
Intracellular fatty acids have two predominant routes of disposal in the liver, export via VLDL and β-oxidation. Given the pronounced effects of ATGL on hepatic TAG turnover, we next explored if ATGL influenced fatty acid oxidation. Serum β-hydroxybuytrate concentrations were similar in mice treated with control or ATGL shRNA suggesting that perhaps hepatic β-oxidation was not influenced by hepatic ATGL (Fig 5A). However, ATGL knockdown in primary hepatocytes resulted in a 30% decrease in ASM production when cells were pulsed with 500 μM [1-14C]oleate (Fig. 5B). However, these data reflect primarily oxidation of fatty acids derived from exogenous uptake. Thus, we also quantified fatty acid oxidation during the chase period, which reflects oxidation of fatty acids derived primarily from intracellular TAG hydrolysis. Under these conditions, ATGL shRNA resulted in a ∼70% decrease in fatty acid oxidation to both ASM and CO2 compared with cells treated with control shRNA (Fig. 5B,C). Similarly, we also measured fatty acid oxidation during pulse and chase periods following ATGL overexpression. In support of the above experiments, ATGL overexpression increased fatty acid oxidation to ASM 40% during the pulse period and caused an even more robust increase in fatty acid oxidation to both ASM and CO2 during the chase period (Fig. 5D,E). Previously, we have shown that overexpression of ATGL in rat hepatocytes increases PPAR-α activity (18). Moreover, gene array studies show that ATGL null mice have decreased expression of genes involved in fatty acid β-oxidation in numerous tissues (19). Since ATGL knockdown decreased fatty acid oxidation, a principal target pathway of PPAR-α, we examined the effects of ATGL knockdown in vivo on the expression of PPAR-α and its target genes. After 7 d following transduction, the mRNA abundance of PPAR-α and its target genes involved in fatty acid oxidation and utilization (CPT-1, ACOT1, LCAD, ACSL1) and gluconeogenesis (PEPCK, PC) were decreased ∼40-70% (Fig. 6A). Taken together, these data show that hepatic ATGL promotes fatty acid oxidation through altered channeling of hydrolyzed fatty acids and through changes in oxidative gene expression.
Figure 5. Hepatic ATGL promotes fatty acid oxidation.
(A) Serum β-hydroxybutyrate was measured with a colorimetric enzymatic kit in serum samples collected from chow-fed mice 7 d after infection with control or ATGL shRNA adenovirus and following an overnight fast. (B-E) Primary hepatocytes isolated from chow-fed mice were transduced with control or ATGL shRNA for 66 h and Ad-GFP or Ad-ATGL for 24 h, at which time pulse (1.5 h) and chase (6 h) experiments were performed with 500 μM [1-14C]oleate. Media from hepatocytes were harvested after pulse and chase, and CO2 and ASM were quantified as outlined in the experimental procedures to measure fatty acid oxidation. Data are presented as means ± SEM. *P<0.05 vs control shRNA group. #P<0.05 vs pulse period.
Figure 6. Hepatic ATGL regulates oxidative gene expression.
Abundance of mRNA of PPAR-α and its target genes was quantified with qRT-PCR in livers of mice fed the chow diet for 7 d after adenoviral transduction. Data are presented as means ± SEM. *P<0.05 vs control shRNA group. PPAR-α, peroxisome proliferator-activated receptor alpha; CPT-1, carnitine palmitoyltransferase I; ACOT-1, acyl-CoA thioesterase I; LCAD, long-chain acyl-CoA dehydrogenase; ACSL1, acyl-CoA synthetase 1; PEPCK, phosphoenolpyruvate carboxykinase; PC, pyruvate carboxylase. *P<0.05 vs control shRNA group.
ATGL regulates fatty acid oxidation independent of PPAR-α agonism
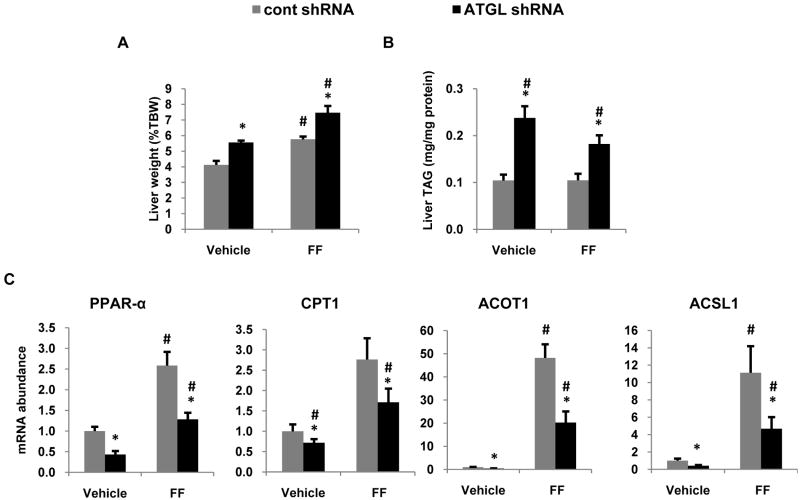
PPAR-α is activated by numerous endogenous ligands including free fatty acids (20). Given that ATGL promotes production of fatty acids from TAG hydrolysis, it is logical to speculate that ATGL mediates PPAR-α by supplying fatty acid ligands. Thus, to test this hypothesis, mice treated with the control or ATGL shRNA adenoviruses were given daily oral gavages of carboxymethycellulose (vehicle) or 125 mg/kg fenofibrate, a PPAR-α agonist. Fenofibrate administration had no effect on food intake or body weight (data not shown), but increased liver weight 30% (Figure 7A) as previously reported by others (21). Fenofibrate was unable to normalize liver weight or liver TAG content in ATGL shRNA treated mice compared to those treated with control shRNA (Fig. 7A,B). Fenofibrate caused a similar fold increase in PPAR-α target gene expression in both treatment groups, but was unable to overcome the decreased expression of PPAR-α target genes in mice treated with ATGL shRNA (Fig. 7C) suggesting that ATGL regulates PPAR-α through a ligand-independent mechanism.
Figure 7. PPAR-α agonism does not rescue the effects of ATGL knockdown.
One day after adenoviral injections, mice were treated with 125 mg/kg of fenofibrate suspended in 0.5% carboxylmethylcellulose for 6 d via oral gavage. Mice were fed with the HF diet and sacrificed 7 d after infection following an overnight fast. Liver weight (A) and triglyceride (B) were measured in mice treated with fenofibrate (FF) and compared to vehicle-treated mice (n = 7-8). (C) mRNA abundance of oxidative genes was determined in liver tissues of mice treated with FF (n=6). Abbreviations are described in the legend to Figure 6. Data are presented as means ± SEM. *P<0.05 versus control shRNA group. #P<0.05 vs pulse period.
Discussion
Despite the importance of hepatic TAG in local and systemic energy metabolism and disease etiology, the major lipases responsible for TAG mobilization in the liver are largely unknown. Herein, we show ATGL to be a principal TAG lipase in the liver consistent with its crucial role in TAG hydrolysis in other tissues such as adipose, heart and muscle. Interestingly, pulse-chase experiments in hepatocytes revealed that ATGL knockdown almost completely blocked TAG hydrolysis. However, livers from mice treated with ATGL shRNA still possessed ∼60% of TAG hydrolase activity suggesting that other lipases also contribute to hepatic TAG hydrolysis. Although these studies clearly show that ATGL impacts TAG hydrolysis, the discrepancies between TAG hydrolase activity assays and TAG turnover in cells also suggest that there are many additional factors that contribute to TAG hydrolysis that are not reflected by in vitro assays. For example, several proteins such as comparative gene identification-58, pigment epithelium-derived factor, G0/G1 switch gene 2 and numerous lipid droplet proteins have potent effects on ATGL-mediated TAG hydrolysis and are not present on synthetic lipid droplets used for in vitro assays (22-24). Thus, it is likely that the contribution of ATGL to hepatic TAG turnover likely varies depending upon the presence of the various inhibitors and activators and may in fact represent more than in vitro assays indicate.
The current studies provide evidence that ATGL acts as a branch point in partitioning hydrolyzed fatty acids between oxidative and VLDL synthetic pathways. As the complexity of the lipid droplet and control of TAG turnover is unraveled, it is becoming apparent that specific lipid droplet proteins and lipases differentially partition fatty acids to distinct metabolic fates. Recent studies have shown that ablation of triacylglycerol hydrolase, which is located exclusively on the ER, suppresses hepatic TAG turnover and TAG export, but increases oxidation (25). Coinciding with the increased fatty acid oxidation in mice lacking triacylglycerol hydrolase is an increase in oxidative gene expression. Overexpression of arylacetamide deacetylase, which posses TAG hydrolase activity, increases hepatic TAG turnover and fatty acid oxidation, but decreases TAG secretion (26). Taken together with the current findings, these data suggest that distinct lipases differentially channel fatty acids between β-oxidation and VLDL synthesis. Additionally, it is unclear how reducing TAG hydrolysis by both ATGL and triacylglycerol hydrolase result in opposing effects on oxidative gene expression. In addition to lipases, several lipid droplet proteins may also contribute to differential partitioning of hydrolyzed fatty acids. Overexpression of fat specific protein-27, a recently identified lipid droplet protein, decreases TAG turnover and fatty acid oxidation without altering TAG export from hepatocytes (27). Despite promoting TAG accumulation, perilipin 5 (also known as OXPAT) also promotes fatty acid oxidation consistent with its high expression levels in oxidative tissues (28). Thus, there appears to be a coordinated regulation of TAG metabolism by lipid droplet proteins and lipases that determines the metabolic fate of hydrolyzed fatty acids (Table 1). Defining the mechanism through which specific pools of TAG are hydrolyzed and channeled between oxidative and anabolic pathways will provide valuable insight into the regulation of hepatic energy metabolism.
The effects of ATGL on fatty acid channeling highlight the importance of TAG hydrolysis in supplying substrates for β-oxidation. Although intracellular fatty acids can be derived from numerous sources including TAG hydrolysis and exogenous uptake, the partitioning of these fatty acids between metabolic pathways appears to be dissimilar. Recently, using stable isotope infusions in humans, Kanaley et al. showed that resting muscle preferentially oxidizes fatty acids derived from TAG hydrolysis compared to those derived from exogenous uptake (29). In support, previous work in rat hepatocytes has suggested that fatty acids derived from TAG hydrolysis are more readily oxidized compared to those supplied as fatty acids in the media (30). Given the importance of intracellular TAG in supplying fatty acids for oxidation, our data would suggest that ATGL plays a critical role in controlling substrate oxidation. Additionally, although this study only focused upon male mice, effects of estrogen on ATGL have been reported (31). Thus, it remains to be determined if the effects of ATGL observed in the current study would be similar in female mice.
In addition to its direct effects on TAG hydrolysis, ATGL also appears to influence hepatic energy metabolism through changes in gene expression. The current study shows that ATGL knockdown reduces the expression of genes involved in fatty acid oxidation and gluconeogenesis. Microarray analysis of tissues from global ATGL knockout mice reveals decreased expression of oxidative genes in numerous tissues (19). Additionally, we have previously shown that ATGL overexpression increases PPAR-α activity in rat hepatocytes (18). Thus, these data suggest that ATGL may modulate fatty acid channeling at least in part through changes in oxidative gene expression. In an attempt to recover oxidative gene expression, mice were gavaged with fenofibrate following administration of adenoviruses. Fenofibrate treatment resulted in a robust induction of oxidative gene expression between both treatment groups, but was unable to normalize gene expression in ATGL shRNA treated groups to those of control animals. Thus, these data suggest that the effects of ATGL on gene expression are independent of PPAR-α ligand binding. Although the mechanism explaining the effects of ATGL on gene expression remain to be elucidated, it is likely that the alterations in gene expression contribute to the metabolic effects of ATGL knockdown. Additionally, it could be postulated that changes in PPAR-α activity could influence fatty acid partitioning through alterations in hepatic TAG export. However, the role of PPAR-α in hepatic TAG secretion is unclear. Studies in mice and hepatocytes show that PPAR-α decreases hepatic TAG secretion (32-34) although not all data are consistent (35). Fibrate administration in humans lowers serum TAG through increased VLDL clearance, but rates of hepatic TAG secretion are unaltered (36, 37). Although the direct effects of PPAR-α on hepatic TAG secretion are not resolved, perhaps changes in metabolic enzymes, such as ATGL, may mediate the effects of PPAR-α. Previous studies have shown that hepatic ATGL is activated in response to fasting and that ATGL contains a PPAR-γ responsive peroxisome proliferator response element (38, 39). Ongoing studies will further characterize the transcriptional and post-transcriptional regulation of hepatic ATGL.
The present studies shows that ATGL knockdown results in increased C18:1 and lower C16:0, C18:0 and C18:3 in hepatic TAG. Although we cannot rule out a role for ATGL in selective hepatic fatty acid uptake and esterification or in altering de novo fatty acid synthesis, the effects of ATGL knockdown on hepatic TAG fatty acid composition suggest that ATGL may show substrate specificity towards different fatty acids. Given the potent effects of ATGL on fatty acid partitioning and signaling, it is plausible that some of these effects could be due to changes in hydrolysis and metabolism of specific fatty acids rather than ATGL itself.
In summary, this study identifies ATGL as a major hepatic TAG lipase that has important roles in fatty acid trafficking and signaling. Given these important characteristics, alterations in hepatic ATGL expression or activity is likely to contribute to a host of metabolic diseases including non-alcoholic fatty liver disease.
Supplementary Material
Acknowledgments
This study was supported by grants from the NIH (RO1-DK0822574 to ASG and R56-DK085008 to DGM) and the American Diabetes Association (7-08-RA-57 to ASG and 7-07-JF-43 to DGM). The authors would also like to thank the technical support of Katie Ress and the Minnesota Obesity Center (P30-DK50456).
Abbreviations used
- ASM
acid-soluble metabolites
- ATGL
adipose triglyceride lipase
- CE
cholesterol ester
- DAG
diacylglycerol
- PL
phospholipid
- PPAR-α
peroxisome proliferator-activated receptor-α
- TAG
triacylglycerol
- VLDL
very low density lipoprotein
Footnotes
Potential conflict of interest: none
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the united states: Impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe S, Yaginuma R, Ikejima K, Miyazaki A. Liver diseases and metabolic syndrome. J Gastroenterol. 2008;43(7):509–518. doi: 10.1007/s00535-008-2193-6. [DOI] [PubMed] [Google Scholar]
- 3.Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis. 2004;8(3):549–58. doi: 10.1016/j.cld.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279(47):48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 7.Villena JA, Roy S, Sarkadi-Nagy E, Kim K, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: Ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279(45):47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 8.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312(5774):734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 9.Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: Function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55(1):148–157. [PMC free article] [PubMed] [Google Scholar]
- 10.Miyoshi H, Perfield JW, 2nd, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, Kraemer FB, et al. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem. 2007;282(2):996–1002. doi: 10.1074/jbc.M605770200. [DOI] [PubMed] [Google Scholar]
- 11.Dana SL, Hoener PA, Bilakovics JM, Crombie DL, Ogilvie KM, Kauffman RF, Mukherjee R, et al. Peroxisome proliferator-activated receptor subtype-specific regulation of hepatic and peripheral gene expression in the zucker diabetic fatty rat. Metabolism. 2001;50(8):963–971. doi: 10.1053/meta.2001.24870. [DOI] [PubMed] [Google Scholar]
- 12.Seo YS, Kim JH, Jo NY, Choi KM, Baik SH, Park JJ, Kim JS, et al. PPAR agonists treatment is effective in a nonalcoholic fatty liver disease animal model by modulating fatty-acid metabolic enzymes. J Gastroenterol Hepatol. 2008;23(1):102–109. doi: 10.1111/j.1440-1746.2006.04819.x. [DOI] [PubMed] [Google Scholar]
- 13.Kwanyuen P, Witherspoon SM, Creech DR, Colton HM, Falls JG, Cariello NF. Flow cytometric assessment of peroxisome proliferation from frozen liver of fibrate-treated monkeys. Int J Toxicol. 2006;25(1):41–47. doi: 10.1080/10915810500488395. [DOI] [PubMed] [Google Scholar]
- 14.Bu SY, Mashek MT, Mashek DG. Suppression of long chain acyl-CoA synthetase 3 decreases hepatic de novo fatty acid synthesis through decreased transcriptional activity. J Biol Chem. 2009;284(44):30474–30483. doi: 10.1074/jbc.M109.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewin TM, Wang S, Nagle CA, Van Horn CG, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-1 directs the metabolic fate of exogenous fatty acids in hepatocytes. Am J Physiol Endocrinol Metab. 2005;288(5):E835–44. doi: 10.1152/ajpendo.00300.2004. [DOI] [PubMed] [Google Scholar]
- 16.Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006;7(1):106–113. doi: 10.1038/sj.embor.7400559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiggins D, Gibbons GF. The lipolysis/esterification cycle of hepatic triacylglycerol. its role in the secretion of very-low-density lipoprotein and its response to hormones and sulphonylureas. Biochem J. 1992;284(Pt 2):457–462. doi: 10.1042/bj2840457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sapiro JM, Mashek MT, Greenberg AS, Mashek DG. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J Lipid Res. 2009;50(8):1621–1629. doi: 10.1194/jlr.M800614-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinent M, Hackl H, Burkard TR, Prokesch A, Papak C, Scheideler M, Hämmerle G, et al. Differential transcriptional modulation of biological processes in adipocyte triglyceride lipase and hormone-sensitive lipase-deficient mice. Genomics. 2008;92(1):26–32. doi: 10.1016/j.ygeno.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalwani ND, Reddy MK, Qureshi SA, Sirtori CR, Abiko Y, Reddy JK. Evaluation of selected hypolipidemic agents for the induction of peroxisomal enzymes and peroxisome proliferation in the rat liver. Hum Toxicol. 1983;2(1):27–48. doi: 10.1177/096032718300200103. [DOI] [PubMed] [Google Scholar]
- 22.Chung C, Doll JA, Gattu AK, Shugrue C, Cornwell M, Fitchev P, Crawford SE. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL) J Hepatol. 2008;48(3):471–478. doi: 10.1016/j.jhep.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in chanarin-dorfman syndrome. Cell Metab. 2006;3(5):309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, et al. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11(3):194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei E, Alam M, Sun F, Agellon LB, Vance DE, Lehner R. Apolipoprotein B and triacylglycerol secretion in human triacylglycerol hydrolase transgenic mice. J Lipid Res. 2007;48(12):2597–2606. doi: 10.1194/jlr.M700320-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Lo V, Erickson B, Thomason-Hughes M, Ko KW, Dolinsky VW, Nelson R, Lehner R. Arylacetamide deacetylase attenuates fatty-acid-induced triacylglycerol accumulation in rat hepatoma cells. J Lipid Res. 2010;51(2):368–377. doi: 10.1194/jlr.M000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, Gonzalez FJ. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008;7(4):302–311. doi: 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, Varma V, et al. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55(12):3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 29.Kanaley JA, Shadid S, Sheehan MT, Guo Z, Jensen MD. Relationship between plasma free fatty acid, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J Physiol. 2009;587(Pt 24):5939–5950. doi: 10.1113/jphysiol.2009.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lankester DL, Brown AM, Zammit VA. Use of cytosolic triacylglycerol hydrolysis products and of exogenous fatty acid for the synthesis of triacylglycerol secreted by cultured rat hepatocytes. J Lipid Res. 1998;39(9):1889–1895. [PubMed] [Google Scholar]
- 31.Wohlers LM, Spangenburg EE. 17beta-estradiol supplementation attenuates ovariectomy-induced increases in ATGL signaling and reduced perilipin expression in visceral adipose tissue. J Cell Biochem. 2010;110(2):420–427. doi: 10.1002/jcb.22553. [DOI] [PubMed] [Google Scholar]
- 32.Tordjman K, Bernal-Mizrachi C, Zemany L, Weng S, Feng C, Zhang F, Leone TC, et al. PPARalpha deficiency reduces insulin resistance and atherosclerosis in apoE-null mice. J Clin Invest. 2001;107(8):1025–1034. doi: 10.1172/JCI11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahn SE, Goldberg DM. Modulation of lipoprotein production in hep G2 cells by fenofibrate and clofibrate. Biochem Pharmacol. 1992;43(3):625–633. doi: 10.1016/0006-2952(92)90586-8. [DOI] [PubMed] [Google Scholar]
- 34.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 35.Bijland S, Pieterman EJ, Maas AC, van der Hoorn JW, van Erk MJ, van Klinken JB, Havekes LM, et al. Fenofibrate increases very low density lipoprotein triglyceride production despite reducing plasma triglyceride levels in APOE*3-leiden.CETP mice. J Biol Chem. 2010;285(33):25168–25175. doi: 10.1074/jbc.M110.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watts GF, Ji J, Chan DC, Ooi EM, Johnson AG, Rye KA, Barrett PH. Relationships between changes in plasma lipid transfer proteins and apolipoprotein B-100 kinetics during fenofibrate treatment in the metabolic syndrome. Clin Sci (Lond) 2006;111(3):193–199. doi: 10.1042/CS20060072. [DOI] [PubMed] [Google Scholar]
- 37.Fabbrini E, Mohammed BS, Korenblat KM, Magkos F, McCrea J, Patterson BW, Klein S. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95(6):2727–2735. doi: 10.1210/jc.2009-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kershaw EE, Schupp M, Guan HP, Gardner NP, Lazar MA, Flier JS. PPARgamma regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am J Physiol Endocrinol Metab. 2007;293(6):E1736–45. doi: 10.1152/ajpendo.00122.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JY, Tillison K, Lee JH, Rearick DA, Smas CM. The adipose tissue triglyceride lipase ATGL/PNPLA2 is downregulated by insulin and TNF-alpha in 3T3-L1 adipocytes and is a target for transactivation by PPARgamma. Am J Physiol Endocrinol Metab. 2006;291(1):E115–27. doi: 10.1152/ajpendo.00317.2005. [DOI] [PubMed] [Google Scholar]
- 40.Wei E, Ben Ali Y, Lyon J, Wang H, Nelson R, Dolinsky VW, Dyck JR, et al. Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab. 2010;11(3):183–193. doi: 10.1016/j.cmet.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Wei E, Quiroga AD, Sun X, Touret N, Lehner R. Altered lipid droplet dynamics in hepatocytes lacking triacylglycerol hydrolase expression. Mol Biol Cell. 2010;21(12):1991–2000. doi: 10.1091/mbc.E09-05-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magnusson B, Asp L, Bostrom P, Ruiz M, Stillemark-Billton P, Linden D, Boren J, et al. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler Thromb Vasc Biol. 2006;26(7):1566–1571. doi: 10.1161/01.ATV.0000223345.11820.da. [DOI] [PubMed] [Google Scholar]
- 43.Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, Chan L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol. 2006;26(3):1063–1076. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, et al. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem. 2008;283(19):13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.