Abstract
Opioids have immunomodulatory functions and may alter susceptibility to immune disorders. Behavioral studies also indicate that chemokines, molecules expressed by immune cells, block opioid induced analgesia in the periaqueductal grey (PAG). Bi-directional heterologous desensitization of opioid and chemokine receptors has been described in cell systems. We report the anatomical and functional interactions of chemokine receptors with the mu-opioid receptor (MOR) in the rat brain. The chemokine receptors, CXCR4 and CX3CR1, as well as their chemokine substrates, CXCL12 and CX3CL1, are widely expressed in the central nervous system (CNS). Immunohistochemical techniques were utilized to investigate MOR-CXCR4 and MOR-CX3CR1 receptor colocalization in multiple brain areas. Our results demonstrate co-expression of these receptors on individual neurons in several regions including cingulate cortex, hippocampus and PAG, suggesting functional receptor interactions. Whole-cell patch-clamp recordings of PAG neurons in a rat brain slice preparation were used to examine morphine or chemokine (CXCL12, CX3CL1) effects alone or in combination on neuronal membrane properties. Morphine (10 µM) hyperpolarized and reduced input resistance of PAG neurons. CXCL12 and CX3CL1 (10 nM) had no impact on either parameter. In the presence of CXCL12, morphine’s electrophysiological effects were blocked in all neurons, whereas with CX3CL1, morphine’s effects were blocked in 57% of neurons. The data provide electrophysiological evidence for MOR-CXCR4 and MOR-CX3CR1 heterologous desensitization in the PAG at the single cell level. These interactions may contribute to the limited utility of opioid analgesics for inflammatory pain treatment and supports chemokines as neuromodulators.
Keywords: SDF-1α/CXCL12, CXCR4, fractalkine/CX3CL1, CX3CR1, morphine, mu-opioid receptor, heterologous desensitization, periaqueductal grey, electrophysiology, immunohistochemistry
Opioids function as immunomodulators and appear to impact susceptibility to various immune system conditions and diseases (Stefano et al., 1996). Specifically, morphine, a highly efficacious opiate utilized in the clinical setting for pain management, has been reported to compromise the immune system in in vivo animal studies (Lorenzo et al., 1987; Starec et al., 1991). Morphine exerts its functions primarily via the mu-opioid receptor (MOR), which is widely distributed throughout the central nervous system (CNS) (Arvidsson et al., 1995; Mansour et al., 1995). Opioids modulate immune system functions via MORs localized in the CNS (Fecho et al., 1996; Hernandez et al., 1993) or in the periphery (Stefano et al., 1996).
Chemokines (chemoattractant cytokines) comprise a family of small (7–11 kDa), secreted proteins that bind to chemokine receptors located mainly on immune cells. These chemoattractant molecules mediate leukocyte trafficking, inflammation, angiogenesis, and neuronal migration/patterning (D’Ambrosio et al., 2003). Chemokines are present and functionally active within the CNS. These immune proteins and their receptors localize to neurons and glia in specific brain regions (Banisadr et al., 2002; Coughlan et al., 2000; Horuk et al., 1997). For example, Banisadr et al. (2002) reported expression of CXCR4, the receptor for the chemokine stromal cell-derived factor (SDF)-1α/CXCL12, on neurons in the cerebral cortex, striatum, ventral tegmental area, supraoptic and paraventricular hypothalamic nuclei, and substantia nigra. The chemokine receptor CX3CR1 is also expressed on microglia and neurons in the hippocampus, cortex, thalamic nuclei, spinal cord, and dorsal root ganglia (Hughes et al., 2002; Meucci et al., 2000; Verge et al., 2004). Furthermore, chemokines present in the normal brain are over-expressed in response to inflammation where they function to induce transmigration of monocytes from the periphery into the CNS (D’Ambrosio et al., 2003). Thus, the release of endogenous CNS chemokines may contribute to the development of neuroimmune diseases including meningitis, HIV-associated dementia, encephalitis, and multiple sclerosis (Schmidtmayerova et al., 1996; Sørensen et al., 1999; Sprenger et al., 1996).
Endogenous opioids and chemokines also localize to sites of inflammation in the brain and periphery (Glabinski and Ransohoff, 1999; Mennicken et al., 1999). Behavioral and molecular studies have demonstrated opioid and chemokine G-protein coupled receptor (GPCR) interactions via heterologous desensitization (Chen et al., 2004; Steele et al., 2002; Szabo et al., 2001; Szabo et al., 2002). This process occurs when a ligand binds to a specific GPCR, causing the inactivation/desensitization of a different, unrelated, and ligand unstimulated GPCR. For example, pretreatment with mu- and delta-opioids inhibits the chemotaxis of neutrophils and monocytes in response to complement-derived chemotactic factors and to CCL3, CCL5, CCL2, or CXCL8 (Grimm et al., 1998; Liu et al., 1992). In these studies, the administration of mu- or delta-opioid agonists reduced chemokine-directed chemotaxis of human peripheral blood neutrophils and monocytes. Heterologous desensitization of these receptors appears to be bi-directional as evidenced by inhibition of opioid-induced analgesia via chemokines acting at CXCR4, CX3CR1, CCR5 or CXCR1 in the periaqueductal grey (PAG) (Chen et al., 2007; Szabo et al., 2002).
The PAG region highly expresses MOR, is involved in pain signal processing, and is a primary site of action for analgesic compounds. In the PAG, MOR agonists function to hyperpolarize PAG neurons via an increase in potassium conductance (Chieng and Christie, 1994). Chemokine receptors, expressed on neurons and/or glia in brain regions with known MOR expression or activation could function as neurophysiologic substrates for pain associated with neuroinflammatory diseases. The potential cross-talk between chemokine and opioid GPCRs on PAG neurons may contribute to the limited utility of opioid analgesics in inflammatory pain treatments (Szabo et al., 2003).
Chemokine actions in the CNS may be due to their ability to activate chemokine receptors localized on neurons and/or glia to modulate neurotransmitter and/or neuropeptide storage, release, and reuptake. Both CXCL12 and CX3CL1 impact neuronal physiology in several different brain regions (Guyon and Nahon, 2007; Heinisch and Kirby, 2009a; 2009b; Limatola et al., 2000; Meucci et al., 1998; Ragozzino et al., 2002; Skrzydelski et al., 2007). The present study was designed to investigate the neuroanatomical relationship of chemokine receptors, CXCR4 and CX3CR1, to MOR in the rat brain, and to examine the functional interactions between these receptors using whole-cell patch-clamp recordings of PAG neurons in a rat brain slice preparation.
Methods
Animals
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY), 10 weeks of age (for immunohistochemistry experiments) and 4–5 weeks of age (for electrophysiological experiments), were housed 2–3 per cage on a 12 h light schedule (lights on at 07:00 AM) in a temperature-controlled (20°C) colony room. Rats were given access to standard rat chow and water ad libitum. Animal protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance to the NIH Guide for the Care and Use of Laboratory Animals.
As stated above, electrophysiology and anatomy studies employed subjects of differing ages (juvenile and adult, respectively). At the 4–5 week age range used for electrophysiology studies, many brain systems are still developing and have not attained their fully-functional adult status. However, it is necessary to utilize this age range since cells in brain slices prepared from animals greater than 5 weeks are considerably less viable, appear shrunken under the microscope, and exhibit altered ion conductance (Haj-Dahmane, 1991; Alger et al., 1984; Gibb & Edwards, 1994). The adult age range used for anatomical studies was employed for comparability of the data to earlier published anatomical studies of chemokine and opioid receptors in adult rats (Mansour et al., 1995; Banisadr et al., 2002; Hughes et al., 2002; Verge et al., 2004). This age discrepancy, however, should not compromise interpretation of the data as both the opioid and chemokine systems examined in the present study function at an early age. The chemokine system makes critical contributions to embryonic brain development (Mennicken et al., 1999; Tran and Miller, 2003). For example, CXCR4 knockout mice exhibit extensive CNS malformations and fail to survive (Lu et al., 2002). While mu-opioid receptor expression and function is regulated during development (Hauser and Mangoura, 1998), the presence of a mu-opioid response in juvenile PAG neurons that is similar in quality and magnitude to that observed in older rats (Chieng and Christie, 1994) is evidence that this particular response is stable across postnatal development. Furthermore, studies directly comparing juvenile and adult neurons in other early developing systems such as the serotonin system (Azmitia & Whitaker-Azmitia, 1991) have shown no differences in a number of different electrophysiological characteristics (Vandermaelen & Aghajanian, 1983). These collective observations support the validity of comparing electrophysiogical findings from juvenile brains to anatomical findings from adult brains in the present study.
Antibodies
Details regarding the primary and secondary antibodies used in our immunohistochemistry experiments are provided in Figure 1A.
Figure 1. Antibody details and blocking experiment.
A table with information about the primary antibodies used in our immunolabeling studies, including the primary antibody species, clonality, dilution, and catalog number is illustrated (A). The secondary antibody used to detect the primary antibody is also listed and specifics regarding the secondary antibody species and dilution are included. Immunohistochemical labeling detected CXCR4 and CX3CR1 in the CA1 hippocampal pyramidal cell layer (B). Coronal rat hippocampal slices (30 µm) pre-absorbed with a 10-fold excess of the peptide used to generate each antibody eliminated the staining previously detected. SC, Santa Cruz; TP, Torrey Pines; T, Sigma; AB, MAB and CBL, Millipore; Scale Bars = 25 µm.
To detect CXCR4-immunoreactivity in the rat brain, we used a polyclonal goat antibody prepared from a 20 amino acid peptide to the C-terminus of CXCR4 receptor of human origin obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). In western blot analysis, the antibody revealed a 45 kD band in both cultured neurons and rat brain samples, corresponding to the expected molecular mass for CXCR4 (Pujol et al., 2005). Preincubation of the polyclonal goat CXCR4 antibody overnight with a tenfold excess of the immunogenic peptide (sc-6190P, Santa Cruz Biotechnology, Inc.), eliminated specific staining (Figure 1B), confirming preabsorption controls for this antibody (Banisadr et al., 2002). Furthermore, immunolabeling with this antibody produced a localization profile matching in situ hybridization for CXCR4 mRNA in neurons within the ventricular ependyma, olfactory bulb, cerebral cortex, hippocampus, amygdala, caudate putamen, and cerebellum (Lu et al., 2002; Stumm et al., 2002; Stumm et al., 2007). The immunolocalization of CXCR4 within brain slices was consistent with previous studies in the cerebral cortex, caudate putamen, globus pallidus, substantia innominata, supraoptic and paraventricular hypothalamic nuclei, ventromedial thalamic nucleus, substantia nigra, and hippocampus using the Santa Cruz CXCR4 antibody (Banisadr et al., 2002; Trecki et al., 2010) as well as other CXCR4 antibodies (Stumm et al., 2002).
A polyclonal rabbit CX3CR1 antibody purified from E. coli expressed rat CX3CR1 mapping to amino acids 2–22 in the N-terminal domain was obtained from Torrey Pines Biolabs (East Orange, NJ). Western blot analysis revealed a specific band at approximately 40 kD corresponding to the molecular weight of CX3CR1 (Meucci et al., 2000). The specificity of the immunohistochemical staining of the CX3CR1 antibody was tested in previous preabsorption studies (Meucci et al., 2000; Moon et al., 2006) and by our preabsorption experiments in brain slices using a tenfold excess of the immunogenic peptide (Figure 1B). Staining patterns with the Torrey Pine CX3CR1 antibody corresponded with in situ hybridization studies for the mRNA of CX3CR1 in the rat spinal cord and dorsal root ganglia (Verge et al., 2004). The staining of brain sections with this antibody produced a pattern of CX3CR1 immunoreactivity that was consistent with previous labeling of neurons and microglia in the hippocampus, cortex, thalamic nuclei, spinal cord, and dorsal root ganglia using the same antibody (Hughes et al., 2002; Verge et al., 2004).
Furthermore, a polyclonal guinea pig antibody directed against a synthetic peptide (NHQLENLEAETAPLP) corresponding to amino acids 384–398 of the C-terminal domain of the cloned rat MOR1 was obtained from Chemicon (Temecula, CA). The labeling pattern observed with this antibody corresponds to previous autoradiography and immunolabeling studies (Arvidsson et al., 1995; Mansour et al., 1995; Wang et al., 1996).
The staining pattern and specificity of the NeuN and CD11b antibodies used in our studies are well established in the rat brain. When brain tissue is stained with these antibodies, it produced a pattern that is identical to previously published reports for NeuN and CD11b (Karuppagounder et al., 2007). Additionally, tests for secondary antibody specificity were conducted on brain slices with omission of primary antibodies and no specific staining was detected.
Immunohistochemistry
(Antibody details in Antibodies section)
Rats were deeply anesthetized with pentobarbital (60 mg/kg, i.p.) and transcardially perfused with saline and 4% paraformaldehyde prior to brain extraction. Brains were cryoprotected in a 20% sucrose solution, frozen at −80°C, and sectioned coronally (30 µm) by cryostat. Brain slices were preincubated with blocking solution containing 3% normal donkey serum and 0.05% Triton X-100 in phosphate-buffered saline for 30 min.
To analyze colocalization of MOR with the chemokine receptor (CXCR4 or CX3CR1) of interest, brain slices were initially incubated with either goat anti-CXCR4 antibody (1:100; Santa Cruz Biotechnology, Inc.) or rabbit anti-CX3CR1 antibody (1:100; Torrey Pines Biolabs) overnight at 4°C. Sections were then incubated in guinea pig anti-MOR1 antibody (1:5000; Chemicon) overnight at 4°C. Subsequently, immunohistochemical labeling was detected with an Alexa 647-conjugated donkey anti-goat or anti-rabbit secondary antibody (1:200; Molecular Probes, Eugene, OR) and a FITC-conjugated donkey anti-guinea pig secondary antibody (1:100; Jackson ImmunoResearch, West Grove PA) for 1 h at room temperature in the dark.
Triple-labeling immunohistochemistry of MOR with CXCR4 or CX3CR1 with a mouse anti-NeuN antibody (1:100; Chemicon) was used to determine the localization of MOR, CXCR4, and CX3CR1 to neurons. An AMCA-conjugated donkey anti-mouse secondary antibody (1:100; Jackson ImmunoResearch) was used to detect anti-NeuN labeling.
CX3CR1 localization to microglia was determined by the sequential incubation of brain slices with a rabbit anti-CX3CR1 antibody (1:100; Torrey Pines Biolabs) and mouse anti-CD11b FITC-conjugated antibody (1:100; Chemicon). Antibody incubations were conducted overnight at 4°C, and the slices were kept in the dark following the addition of the anti-CD11b FITC-conjugated antibody. Immunohistochemical labeling was visualized using an Alexa 647-conjugated donkey anti-rabbit secondary antibody (1:200; Molecular Probes) for 1 h at room temperature in the dark.
For all immunohistochemical experiments, sections were rinsed with PB solution (3 × 10 min) between incubations. Sections were mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and cover slipped with Prolong Gold Antifade Reagent (Molecular Probes).
Fluorescence microscopy
Fluorescent images of MOR and the chemokine receptor (CXCR4 or CX3CR1) of interest were captured with a Leica DMIRE TCS SL-Conformation confocal microscope using a 40× oil immersion objective and Leica operating software (Leica Microsystems, Exton, PA). The laser power and emission filters were adjusted for both the red and green fluorophors so that there was minimal possibility of a false positive result. Fluorographs were produced from six stacks with four point line averaging. The image format was 1285 by 1285 pixels, and the scan speed was 400 image-lines/s. Fluorescent images were also captured with a Nikon E800 fluorescent microscope (Nikon Instruments, Melville, NY), using a Retiga EXi Fast 1394 digital camera and QCapture Suite imaging software (Quantitative Imaging Corp., Surrey, BC, Canada).
Slice Preparation
Rats were rapidly decapitated and the head placed in ice cold artificial cerebrospinal fluid (ACSF) in which sucrose (248 mM) was substituted for NaCl. The brain was rapidly removed and trimmed to isolate the brainstem region. Slices 300 µm thick were cut throughout the rostro-caudal extent of the PAG using a Vibratome 3000 Plus (Vibratome, St. Louis, MO) and placed in a holding vial containing ACSF at 35°C bubbled with 95% O2/5% CO2 for 1 h. Slices were then maintained in room temperature ACSF bubbled with 95% O2/5% CO2. The composition of the ACSF was (mM), NaCl 124, KCl 2.5, NaH2PO4 2, CaCl2 2.5, MgSO4 2, Dextrose 10 and NaHCO3 26.
Electrophysiological Recordings
Slices were transferred to a recording chamber (Warner Instruments, Hamden, CT) and continuously perfused with ACSF at 1.5–2.0 ml/min at 32–34°C maintained by an in-line solution heater (TC-324, Warner Instruments). Neurons were visualized using a Nikon E600 upright microscope fitted with a 40X water-immersion objective, differential interference contrast and infrared filter (Optical Apparatus, Ardmore, PA). The image from the microscope was enhanced using a CCD camera and displayed on a computer monitor. Whole-cell recording pipettes were fashioned on a P-97 micropipette puller (Sutter Instruments, Novato, CA) using borosilicate glass capillary tubing (1.2 mm OD, 0.69 mm ID; Warner Instruments). The resistance of the electrodes was 4–8 MΩ when filled with an intracellular solution of (in mM) Kgluconate 130, NaCl 5, MgCl2 1, EGTA 0.02, HEPES 10, Naphosphocreatinine 10, MgATP 2, Na2GTP 0.5, 0.1% Biocytin, pH 7.3.
Recordings were conducted in cells located in the lateral and ventrolateral subdivisions of the PAG at the mid-caudal levels (corresponding to −5.88 mm −6.84 caudal to bregma in Paxinos and Watson (2005). A visualized cell was approached with the electrode, a gigaohm seal established and the cell membrane ruptured to obtain a whole-cell recording using a HEKA patch clamp EPC-10 amplifier (HEKA Elecktronik, Pfalz, Germany). Series resistance was monitored throughout the experiment. If the series resistance was unstable or exceeded four times the electrode resistance, the cell was discarded. Once the whole-cell recording was obtained, cell membrane potential and input resistance were obtained in current-clamp mode (I = 0 pA).
Experimental Protocols
Baseline membrane potential was initially recorded for 5 min to ensure that the cell was stable. Morphine (10 µM), CXCL12 (10 nM), or CX3CR1 (10 nM) was then added to the perfusion bath and recorded for 10 min or until a drug effect on the resting membrane potential was observed. If a drug effect was present, then the cell was recorded for an additional 5 min following the stabilization of the membrane potential to its new baseline. The drug was subsequently removed from the perfusion bath to determine if the cell would return to its initial baseline membrane potential.
For drug combination studies the effect of CXCL12 or CX3CL1 in combination with morphine on the membrane potential and input resistance of PAG neurons was determined. CXCL12 (10 nM) or CX3CR1 (10 nM) was added to the perfusion bath, and the membrane characteristics recorded for 5 min. In the continued presence of the chemokine, morphine (10 µM) was added to the perfusion bath. The membrane characteristics were then recorded for a maximum of 10 minutes. If no drug effect was observed within 10 min, the cell was considered a “non-responder”. If a drug effect was present, the drug solution was removed from the perfusion bath for post-drug washout as described above. In a subset of cells, the sodium channel blocker tetrodotoxin (TTX; 1 µM) was added to the perfusion bath prior to the drug combination as described above in order to determine if drug combination effects were direct (i.e. mediated by receptors located on the recorded PAG neuron) or indirect (i.e. mediated by receptors located on synaptic afferents). In another subset of cells, the receptor specificity of the morphine and chemokine effects were determined by pretreatment with receptor-selective antagonists or blocking antibodies. Receptor antagonists (mu-opioid antagonist D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP; 1 µM) or CXCR4 antagonist AMD 3100 (2 µM)) were added to the perfusion bath 6 min prior to addition of the respective agonist (morphine or CXCL12) and remained in bath for the duration of the experiment. To block CX3CR1 receptors, slices were incubated for 1 hr in anti-CX3CR1 blocking antibody (2 µg/ml) prior to being transferred to the recording chamber to examine the effect of the agonist, CX3CL1.
The concentration of CXCL12 used in these studies (10 nM) is 10 times the reported Kd of CXCL12 for its receptor CXCR4 (Fedyk et al., 1999). Previous studies of CXCL12 using functional assays and electrophysiological techniques applied comparable doses. For example, CXCL12 dose-dependently activates the biologic activity of Jurkat cells with an EC50 of 18 nM (Hesselgesser et al., 1998). CXCL12 dose-dependently reduces excitatory postsynaptic current amplitude in Purkinje neurons with an IC50 of 0.34 nM (Ragozzino et al., 2002). In dopaminergic neurons, CXCL12 increases the frequency of GABA postsynaptic currents, enhances an outward G-protein activated inward rectifier current (Guyon et al., 2006) and increases the amplitude of total high-voltage-activated Ca2+ currents (Guyon et al., 2008) at 0.01–10 nM concentrations.
The concentration of CX3CL1 used in these studies (10 nM) is 10–100 times the reported Kd of CX3CL1 for its receptor CX3CR1 (Imai et al., 1997). Previous studies of CX3CL1 using in vitro bioassays and electrophysiological techniques have also employed similar doses. For example, CX3CR1 dose-dependently stimulates calcium mobilization in CX3CR1-expressing cells with an EC50 of 2 nM (Imai et al., 1997). CX3CL1 also dose-dependently reduces glutamate-induced excitotoxicity in hippocampal neurons with an EC50 of 0.7 nM (Limatola et al., 2005). Finally, CX3CL1 dose-dependently inhibits field excitatory postsynaptic potentials (Bertollini et al., 2006) and evoked excitatory postsynaptic current amplitude (Ragozzino et al., 2006) in hippocampal neurons with an IC50 of 0.7 and 1.0 nM, respectively.
Data Analysis
Resting membrane potential was collected from the baseline, maximal drug, and washout phases for each cell trace. The maximum drug steady-state value was reported as the drug effect. Data were analyzed using pClamp 9.0 software (Molecular Devices, Sunnyvale, CA), and the effects of morphine, CXCL12, and CX3CR1 on the membrane potential and input resistance were analyzed by paired Student’s t-test or Wilcoxon Signed Rank test for non-normally distributed data. Combined drug studies using CXCL12 and morphine or CX3CR1 and morphine were analyzed by one-way ANOVA with post-hoc Student-Newman-Keuls tests for pairwise comparisons or Kruskal-Wallis one-way ANOVA on ranks with pairwise comparisons using post-hoc Dunn’s tests for non-normally distributed data. A probability of p < 0.05 was considered significant. Group data are reported as mean ± SEM.
The downward deflections on each trace (see Figures 5; 6) are voltage responses to intracellular injections of −300 pA current pulses. The voltage responses were measured using pClamp 9.0 software (Molecular Devices). Input resistance was calculated by dividing the measured voltage responses by the amount of injected current (−300 pA). Input resistance was calculated based on the average of three measured voltage responses taken at baseline or when the drug effect had reached steady-state.
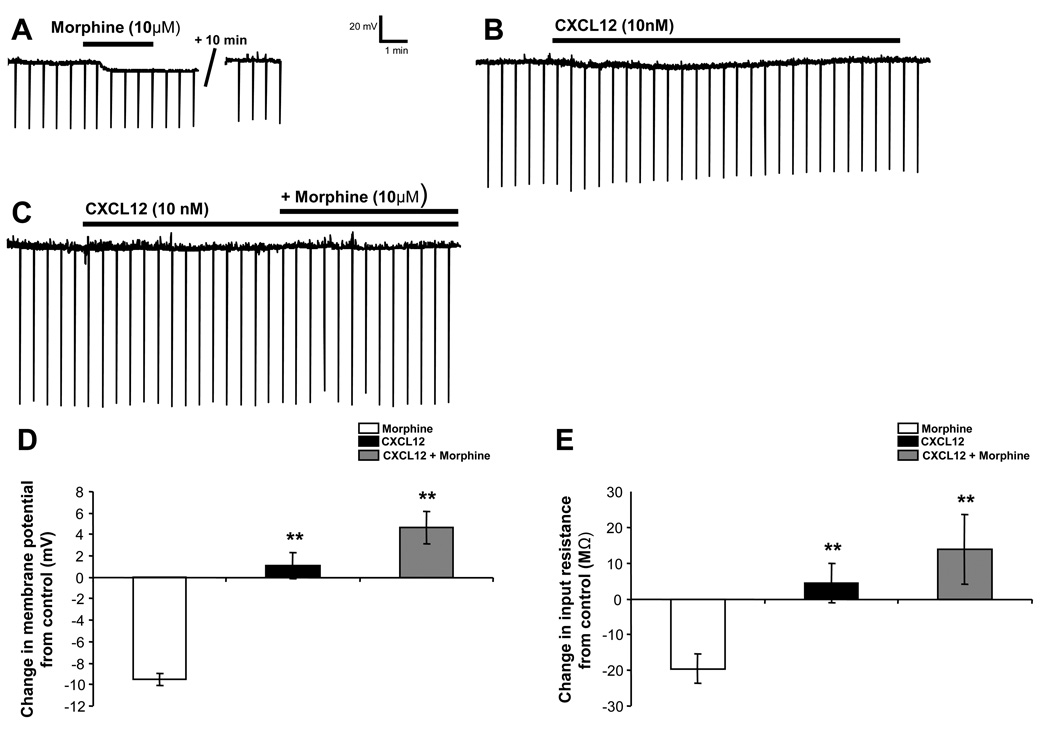
Figure 5. CXCL12 blocks morphine-induced hyperpolarization and reduction of input resistance in PAG neurons.
Morphine (10 µM) hyperpolarizes PAG neurons (A, D) accompanied by a reduction of input resistance (A, E). CXCL12 (10 nM) has no effect on resting membrane potential or input resistance (B–E). Pretreatment with CXCL12 blocks morphine’s effect on resting membrane potential and input resistance (C–E). * indicates significant difference from the morphine group (p < 0.01) by post-hoc Dunn’s tests (E) or Student-Newman-Keuls tests (F). Group data are presented as mean ± SEM.
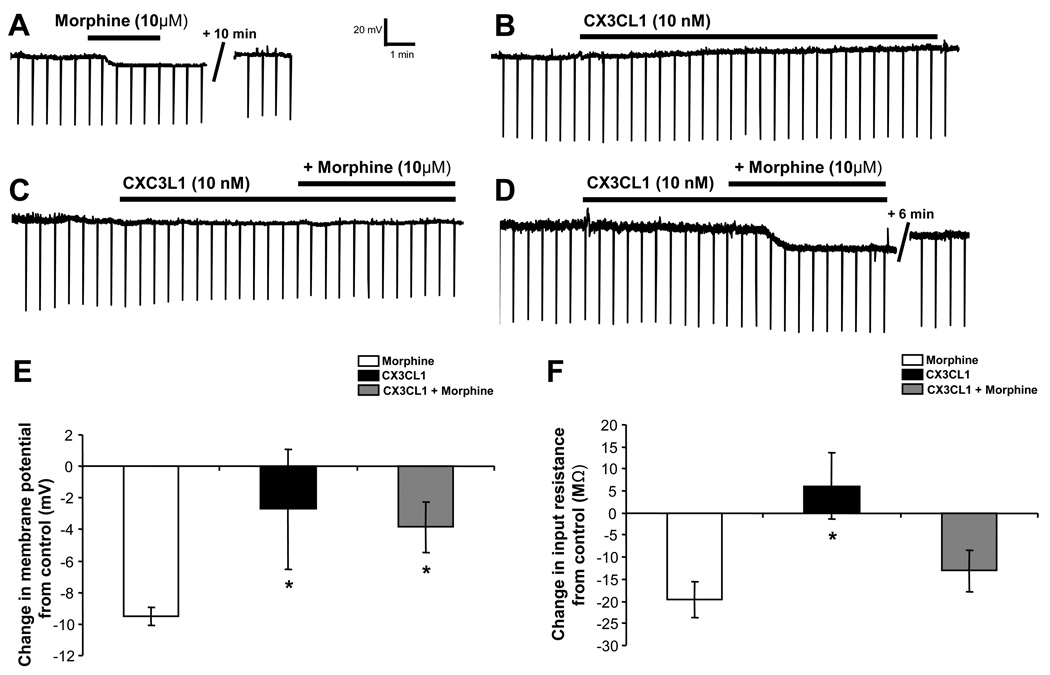
Figure 6. CX3CL1 reduces morphine-induced hyperpolarization and decrease of input resistance in a group of PAG neurons.
Morphine (10 µM) hyperpolarizes PAG neurons (A, D, F) accompanied by a reduction of input resistance (A, D, E). CX3CL1 (10 nM) has no effect on resting membrane potential (RMP) or input resistance (B–E). The combined application of fractalkine and morphine, blunts morphine-mediated hyperpolarization (E) and reduction of input resistance (F) in the combined data from a group of PAG neurons. * indicates significant difference from the morphine group by post-hoc Dunn’s tests (p < 0.05). Group data are presented as mean ± SEM.
Drugs
Most chemicals for making the ACSF and the electrolyte solution were obtained from Sigma-Aldrich. Morphine and CTAP, made by Research Triangle Institute and generously supplied by the National Institute on Drug Abuse, were dissolved in dH20 at 10 mM and 1 mM, respectively, and stored at 4°C protected from light. CXCL12 (recombinant human/rhesus macaque/feline CXCL12/SDF-1α carrier free) and CX3CL1 (recombinant rat CX3CL1/Fractalkine carrier free) were obtained from R&D Systems (Minneapolis, MN), dissolved in dH20 at 10 µM, and stored at 4°C for 1-week following rehydration. TTX was obtained from EMD Chemicals, San Diego, CA. The anti-CX3CR1 blocking antibody was obtained from Torrey Pines Biolabs (TP 502), dissolved in dH20 to 1.0 mg/ml concentration and stored in 5 µl aliquots at −20°C. The final drug concentrations were: morphine = 10 µM, CXCL12 = 10 nM, and CX3CR1 = 10 nM, TTX = 1 µM, CTAP = 1 µM, AMD 3100 = 2 µM and anti-CX3CR1 blocking antibody = 2 µg/ml.
Results
The chemokines CXCL12 and CX3CL1 are widely expressed in the CNS, as are their receptors, CXCR4 and CX3CR1. We used immunohistochemistry to determine the localization of CXCR4 and CX3CR1 in relation to MOR on neurons, and the brain regions in which the relationship is observed. We examined the functional implications of opioid-chemokine receptor colocalization with whole-cell electrophysiology in the PAG.
Colocalization of MOR-CXCR4 and MOR-CX3CR1 in the Hippocampus, Cingulate Cortex, and Periaqueductal Grey
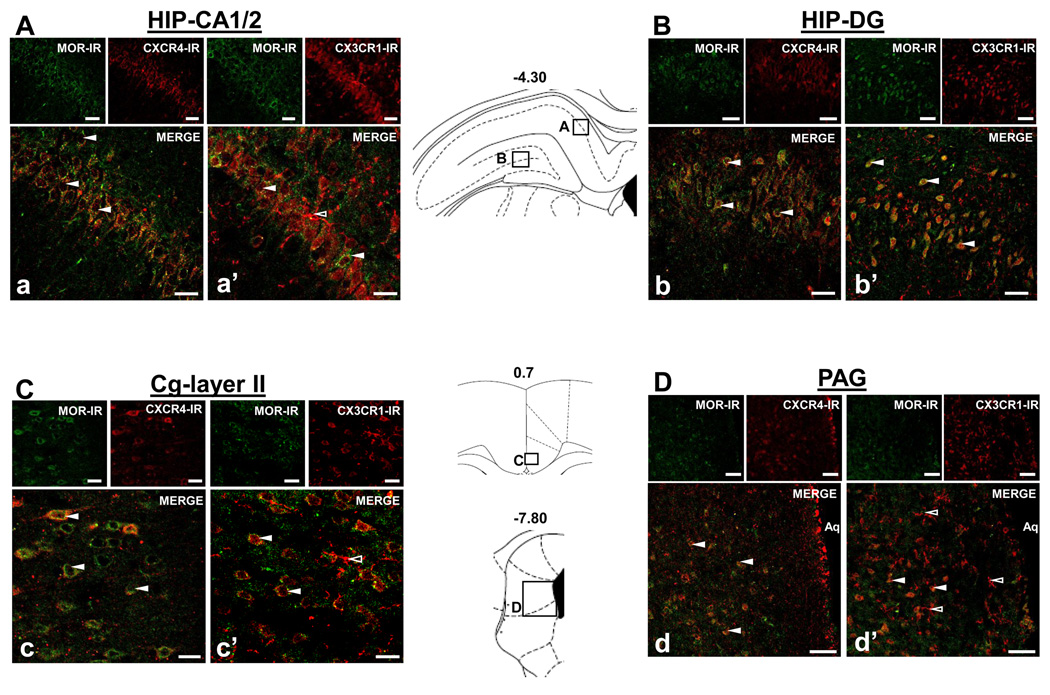
Fluorescent photomicrographs of MOR, CXCR4, and CX3CR1 containing cells in the hippocampus (HIP) pyramidal cell layer and dentate gyrus, in layer 2 of the cingulate cortex (Cg), and in the PAG are shown in Figures 2 and 3. In Figure 2, MOR-immunoreactivity is shown in green and CXCR4- or CX3CR1-immunoreactivity in red taken in the xy-plane, indicating a generalized colocalization of MOR-CXCR4 and MOR-CX3CR1 in the HIP pyramidal cells of the CA1/2 region (A), dentate gyrus (DG) (B) and CA2–3 (data not shown); the Cg, layers II (C) and III–IV (data not shown); and the PAG (D). Numerous yellow/orange cell bodies and process seen in the merge images demonstrate that cells in these brain regions co-express MOR-CXCR4 as well as MOR-CX3CR1 (white arrows, Figure 2Aa, a’; Bb, b’; C, c’; D, d’). MOR-CXCR4 and MOR-CX3CR1 colocalization are observed on cells whose morphology and localization identify them as neurons. In Figure 3, a neuronal marker, NeuN, and triple-labeling immunohistochemistry verify receptor colocalization on neurons (solid white arrows, Aa, a’). MOR-CXCR4 colocalize to the outer cellular membrane and processes of neurons, yet CX3CR1 exhibits a unique perinuclear distribution in these neurons.
Figure 2. Colocalization of CXCR4-MOR and CX3CR1-MOR in hippocampus (HIP), cingulate cortex (Cg), and periaqueductal grey (PAG).
Fluorescent photomicrographs of MOR- with CXCR4- or CX3CR1- containing cells in coronal sections (30 µm) through the HIP CA1/2 and dentate gyrus (DG) (Aa, a’; Bb, b’), Cg (Cc, c’), and PAG (Dd, d’). In each brain region examined, the upper left panels show MOR-immunoreactivity (IR) in green, the upper right panels show CXCR4-IR or CX3CR1-IR in red, and the large panel illustrates the merge image with numerous colocalized cells (solid white arrows A–D). CX3CR1-IR was also single-labeled on cells morphologically similar to microglia (outlined white arrows, a’; c’; d’). Schematics from Paxinos and Watson (2005) are included to indicate the locations of the panels within their respective brain regions. Scale Bars: 50 µm.
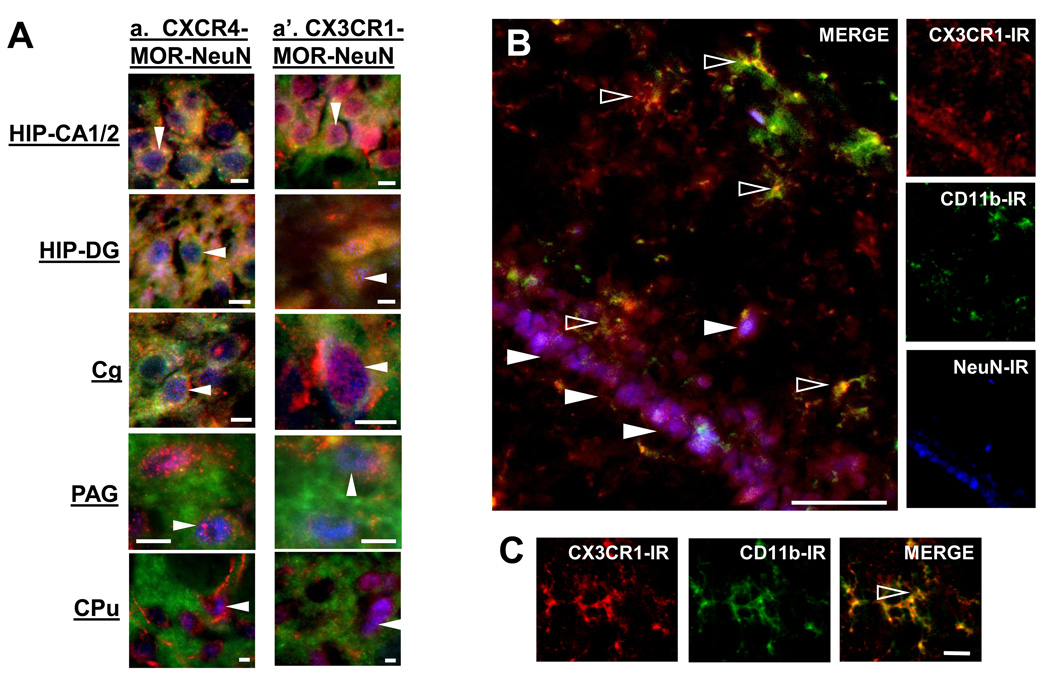
Figure 3. Neuronal localization of CXCR4 and CX3CR1, and microglial localization of CX3CR1 in the brain.
Colocalization of CXCR4 and CX3CR1 to neurons in the HIP CA1/2, DG, Cg, PAG, and CPu was confirmed with triple-labeling immunohistochemistry including the neuronal marker NeuN in blue (solid white arrows, A). Fluorescent photomicrographs of CX3CR1 containing microglia (anti-CD11b) and neurons (anti-NeuN) in coronal HIP sections (30 µm) are shown in panel B. Additional CX3CR1 containing microglia in HIP are shown in Panel C. CX3CR1-IR is shown in red, CD11b-IR in green and NeuN-IR in blue. CX3CR1 exhibits a dual microglia (outlined white arrows, B; C) and neuronal (solid white arrows, Aa’; B) localization. Scale Bars: 20 µm (Aa, a’; C) and 50 µm (B).
Interestingly, single-labeled CX3CR1 fibers and cells, which morphologically resemble microglia, were also detected in the HIP, Cg, and PAG (open white arrows, Figure 2Aa’; Cc’; Dd’). Also, in Figure 3B, the neuronal CA1 HIP pyramidal cell layer appears purple since CX3CR1 in red colocalizes with NeuN in blue (solid white arrows). Microglia were detected as yellow-green cells throughout the HIP since they co-label for both CD11b in green and CX3CR1 in red. A magnified image of a microglial cell in HIP co-expressing CX3CR1 and CD11b (open white arrow) is shown in Figure 3C. At a subcellular level, CX3CR1 exhibits an extensive localization throughout the soma and processes of the microglial cell.
Localization of MOR, CXCR4 and CX3CR1 in the Caudate Putamen and Habenular Nuclei
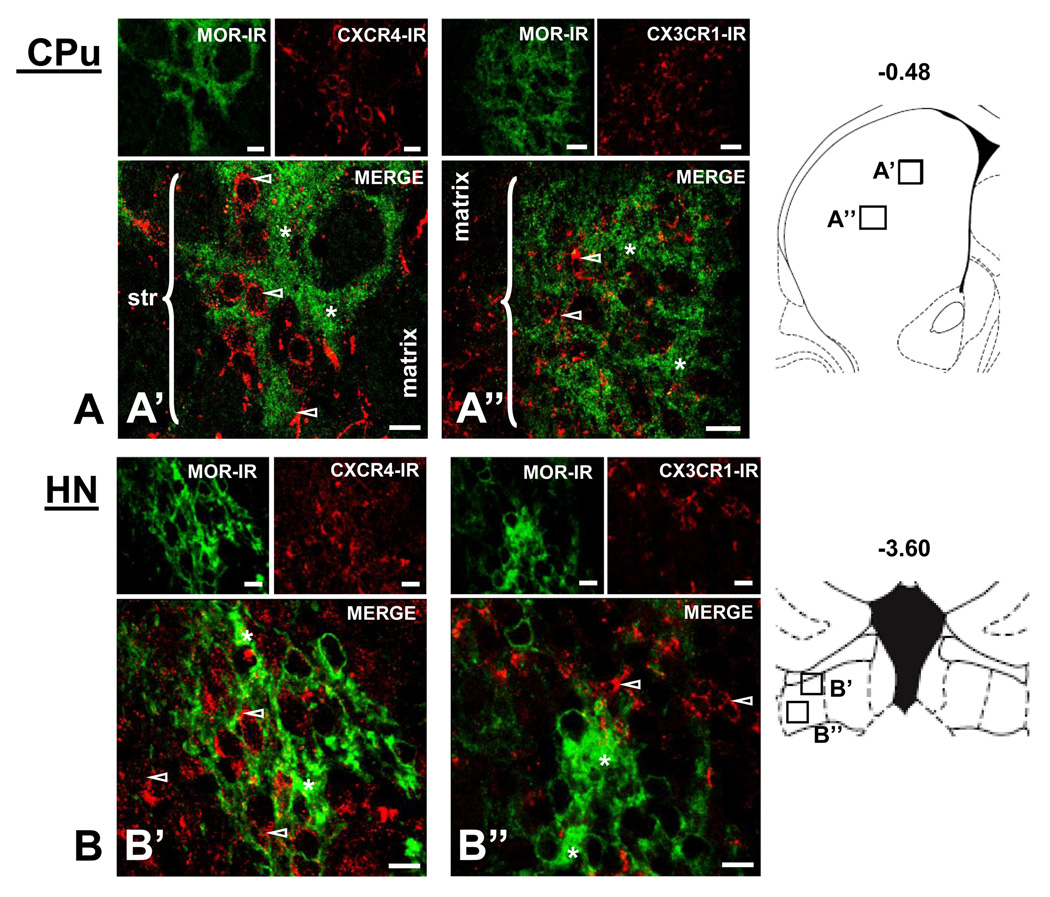
Fluorescent photomicrographs of MOR, CXCR4, and CX3CR1 containing cells in the caudate putamen (CPu) and habenular nuclei (HN) are illustrated in Figure 4. Individual panels of MOR-immunoreactivity in green and CXCR4- or CX3CR1-immunoreactivity in red taken in the xy-plane of brain sections indicate the distinct localization profiles of MOR, CXCR4, and CX3CR1 throughout the CPu, particularly its striosome-matrix (Figure 4A) and subcallosal streak (data not shown) compartments, as well as the HN (Figure 4B). In these brain regions, CXCR4 and CX3CR1 exhibit a general localization on cells and fibers (open white arrows) in all striatal compartments, including the matrix, striosomes, and subcallosal streak (data not shown) as well as the medial and lateral (data not shown) habenula. Triple-labeling immunohistochemistry also indicates CXCR4 and CX3CR1 localization on neurons within striosomes (CPu solid white arrows, Figure 3A).
Figure 4. Differential localization of CXCR4-MOR and CX3CR1-MOR in the striosome and matrix compartments of the caudate putamen (CPu) and in the habenular nuclei (HN).
Fluorographs of MOR- with CXCR4- or CX3CR1- containing cells in coronal sections (30µm). The upper left panels show MOR-IR in green, the upper right panels show CXCR4-IR or CX3CR1-IR in red, and the large panel illustrates the merge image (A’, A’’). MOR labeling concentrates in striosomes (str) indicated by brackets, where it exhibits a diffuse, punctate localization profile (*, A’, A’’). CXCR4-IR and CX3CR1-IR are detected in cells and fibers throughout the striosome-matrix compartments (outlined white arrows), but they do not colocalize with MOR in striosomes (outlined white arrows, A’, A’’). The medial habenula identified by green MOR-IR labeling is shown in panels B’ and B”. The upper left panels indicate MOR-IR in green, the upper right panels show CXCR4-IR or CX3CR1-IR in red, and the large panel illustrates the merge image. MOR labeling is concentrated in the HN, where it exhibits a dense, fibrous network localization profile (*). CXCR4-IR and CX3CR1-IR are detected in fibers/processes in the HN and surrounding regions, however, they fail to colocalize with MOR (outlined white arrows, B’, B’’). Schematics from Paxinos and Watson (2005) are included to indicate the locations of the panels within their respective brain regions. Scale Bars: 20 µm (A’, A’’) and 10 µm (B’, B’’).
Furthermore, MOR is present as dense puncta (asterisks) in these brain regions, particularly throughout the “patchy” striosome compartment of the CPu (brackets, Figure 4A) and medial habenula (Figure 4B). The distinct localization of MOR to striosomes has made it a typical marker for this compartment within the striatum (Wang et al., 1996). Interestingly, unlike the widespread localization of CXCR4 and CX3CR1 within the CPu, MOR localizes only to the striosome compartment of the CPu.
In summary, colocalization of MOR-CXCR4 and MOR-CX3CR1 is present on individual neurons and fibers in several brain regions including the HIP (areas CA1, CA2, CA3, and DG), Cg (layers II-IV), PAG, and other regions including the nucleus accumbens, ventral tegmental area, and globus pallidus (data not shown). However, MOR colocalization with CXCR4 and CX3CR1 is not uniform throughout certain brain regions including the CPu and HN. CPu striosome, matrix, and subcallosal streak compartments as well as HN medial and lateral regions are void of receptor colocalization. Each receptor appears to localize to discrete fibers and/or cells in these brain regions.
CXCR4-MOR Interactions in Periaqueductal Grey Neurons: Electrophysiology Studies
The resting membrane potential was recorded under current clamp conditions (I = 0 pA) in neurons within the lateral and ventrolateral subdivisions of the PAG (Figure 5). We examined the effects of a single application of morphine (10 µM) and CXCL12 (10 nM) as well as the sequential application of CXCL12 followed by morphine on the membrane potential and input resistance of PAG neurons.
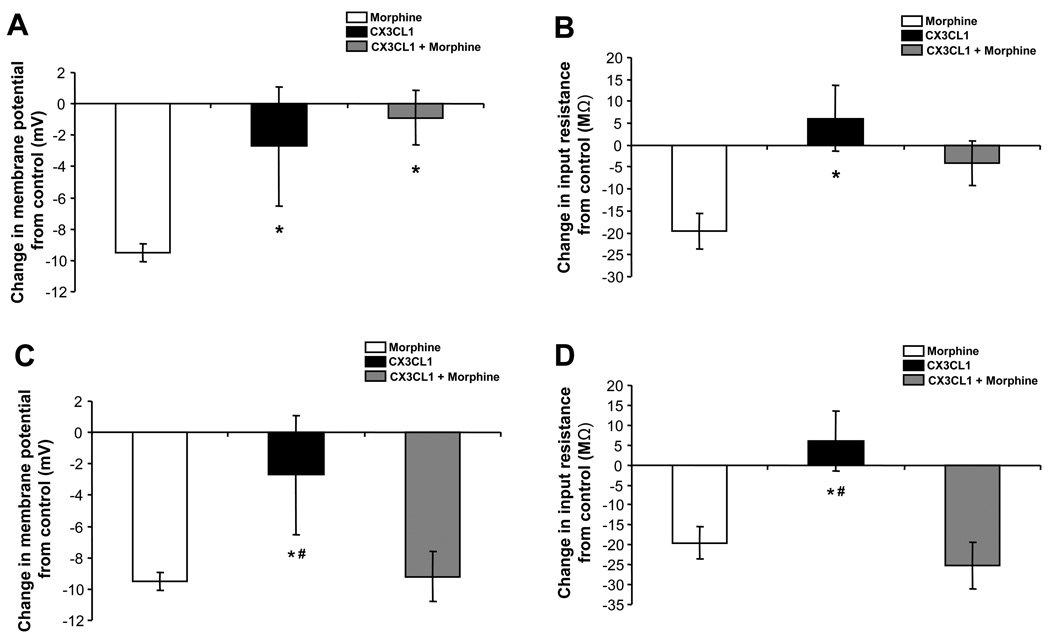
An individual PAG neuron shown in Figure 5A hyperpolarizes from a baseline level of −69.4 mV to −76.8 mV in response to 10 µM morphine. The morphine-induced hyperpolarization returns to a baseline level of −69.5 mV following drug washout. Morphine hyperpolarizes PAG neurons from an average baseline of −48.2 ± 3.0 mV to −57.8 ± 2.9 mV (N = 18), producing a mean hyperpolarization of −9.5 ± 0.6 mV (Figure 5D). This hyperpolarization effect is mediated by mu-opioid receptors as it is prevented by pretreatment with the mu-opioid selective antagonist CTAP (1 µM; −59.0 ± 2.2 to −60.3 ± 2.0 mV; N = 7, n.s.). 89% of PAG neurons showed at least a 7 mV hyperpolarization response to morphine. CXCL12 application did not change the average membrane potential from baseline levels in PAG neurons (N = 19). However, CXCL12 pretreatment blocks morphine-induced hyperpolarization (N = 12) in 100% of PAG neurons examined (Figure 5A vs. 5C). A summary of the change in membrane potential mediated by morphine, CXCL12, and CXCL12 pretreatment followed by morphine application (CXCL12 + morphine) is shown in Figure 5D. There is a statistically significant drug effect when comparing the treatment groups by one-way ANOVA on ranks (H(2) = 31.50, p < 0.01). Post-hoc Dunn’s test indicates that morphine-mediated hyperpolarization is statistically greater than CXCL12 (p < 0.01) and CXCL12 + morphine (p < 0.01). To verify the receptor specificity of this CXCL12 effect, AMD 3100 was used to block the CXCR4 receptor prior to application of CXCL12 and morphine. In the presence of AMD 3100 (2 µM), CXCL12 did not prevent a significant morphine-induced hyperpolarization (from −52.0 ± 5.1 to −59.0 ± 4.6 mV; N = 13, p < 0.01). In summary, CXCL12 pretreatment (CXCL12 + morphine) was able to block the morphine-induced hyperpolarization, an effect mediated by the CXCR4 receptor.
Changes in input resistance produced by morphine, CXCL12, or CXCL12 + morphine application are depicted in Figure 5E. The downward deflections in the traces in Figure 5A–C are representations of voltage responses to −300 pA current injections, which permit the changes in the cellular input resistance to be determined. The example in Figure 5A shows 10 µM morphine reducing the input resistance of a PAG neuron from a baseline of 180 MΩ to 153 MΩ with a return to baseline following drug washout (177 MΩ). Morphine reduction of input resistance in PAG neurons is in agreement with the effects of other mu-opioid agonists in previous studies (Chieng and Christie, 1994). In addition, morphine’s reduction of input resistance was significantly blocked by the mu-opioid selective antagonist CTAP (1 µM; 250.2 ± 22.4 to 247.7 ± 22.1 MΩ; N = 7, n.s.). There is a statistically significant drug effect when comparing the treatment groups by one-way ANOVA (F(2) = 6.46, p < 0.01). Post-hoc Student-Newman-Keuls tests indicate that the morphine-mediated reduction of input resistance was greater than the effect of CXCL12 alone (p < 0.01) or in combination with morphine (p < 0.01). Furthermore, in the presence of AMD 3100 (2 µM) to block CXCR4 receptors, CXCL12 did not prevent a significant morphine-induced reduction of input resistance (from 265.8 ± 41.7 to 233.0 ± 29.9 MΩ; N = 13, p < 0.05). In summary, CXCL12 does not change the membrane potential or input resistance of PAG neurons. However, CXCL12 pretreatment blocks both morphine-mediated hyperpolarization as well as morphine-mediated reduction in input resistance in these neurons, effects mediated by the CXCR4 receptor.
In an additional group of cells, TTX (1 µM) was added prior to the drug combination. In the presence of TTX, CXCL12 pretreatment was still able to block both morphine-mediated hyperpolarization as well as morphine-mediated reduction of input resistance. For comparison, the mean change in membrane potential produced by morphine + CXCL12 was 4.6 ± 1.5 mV in the absence of TTX (N = 12) vs. 3.0 ± 0.9 mV in the presence of TTX (N = 6) (vs. −9.5 ± 0.6 mV produced by morphine alone; N = 18). The mean change in input resistance produced by morphine + CXCL12 was 13.8 ± 9.8 MΩ in the absence of TTX vs. 4.3 ± 4.5 MΩ in the presence of TTX (vs. −19.6 ± 4.1 MΩ produced by morphine alone). These data indicate that MOR-CXCR4 interactions are mediated by receptors located on PAG neurons, not on synaptic afferents.
CX3CR1-MOR Interactions in Periaqueductal Grey Neurons: Electrophysiology Studies
The resting membrane potential was recorded as previously described for CXCL12-morphine experiments. We examined the effects of a single application of morphine (10 µM) and CX3CL1 (10 nM) as well as the consecutive application of CX3CL1 followed by morphine, to investigate the individual and combined effects of these drugs on the membrane potential and input resistance of PAG neurons. An individual PAG neuron (Figure 6A) hyperpolarizes in response to 10 µM morphine, as described in the previous section (CXCR4-MOR Interactions in Periaqueductal Grey Neurons: Electrophysiology Studies). CX3CL1 treatment does not change the membrane potential in PAG neurons (baseline membrane potential: −52.9 ± 2.5 mV, CX3CL1: −52.0 ± 2.4 mV; N = 20). CX3CL1 pretreatment prior to morphine application attenuates morphine-induced hyperpolarization of PAG neurons (N = 14). The mean morphine-induced hyperpolarization of −9.5 ± 0.6 mV was reduced in the presence of CX3CL1 to −3.8 ± 1.6 mV (Figure 6E).
The net change in membrane potential mediated by morphine, CX3CL1, and CX3CL1 + morphine application is shown in Figure 6E. There is a statistically significant drug effect on membrane potential by one-way ANOVA on ranks (H(2) = 21.35, p < 0.01). Post-hoc Dunn's tests indicate that morphine-mediated hyperpolarization is statistically greater than CX3CL1 (p < 0.05) and the CX3CL1 + morphine group (p < 0.05). Importantly, CX3CL1 pretreatment (CX3CL1 + morphine) attenuates morphine-induced hyperpolarization in PAG neurons. Interestingly, there appears to be an all-or-none blockade of morphine’s hyperpolarizing actions by the pretreatment of CX3CL1 in two populations of PAG neurons (compare Figure 6C vs. 6D). Our results show that 8/14 PAG neurons pretreated with CX3CL1 do not exhibit a morphine-mediated hyperpolarization and we described these cells as “non-responders” to morphine (Figures 6C; 7A). However, 6/14 of recorded neurons continued to show morphine-mediated hyperpolarization after CX3CL1 application and are characterized as “responders” to morphine (Figures 6D; 7C). In the “responders,” application of CX3CL1 (10 nM) followed by morphine hyperpolarizes the membrane potential by −9.2 ± 1.7 mV (N = 6). No change in membrane potential was evident in the “non-responders” (baseline: −49.7 ± 4.2 mV, CX3CL1 + morphine: −50.5 ± 4.1 mV; N = 8). Furthermore, the magnitude of morphine-mediated hyperpolarization on PAG neurons treated with CX3CL1 in the “responders” is similar to PAG neurons treated only with morphine (Figure 7C). Thus, the net “group” effect of CX3CL1’s ability to attenuate morphine-induced PAG membrane hyperpolarization appears to be entirely mediated by the “non-responders.” To verify the receptor specificity of this CX3CL1 effect, slices were pretreated with anti-CX3CR1 blocking antibody (2 µg/ml) as no CX3CR1 receptor antagonists are currently available. Following pretreatment with the blocking antibody, CX3CL1 did not prevent a significant morphine-induced hyperpolarization (from −52.4 ± 4.2 to −57.1 ± 5.3 mV; N = 9, p < 0.05). In summary, CX3CL1 pretreatment (CX3CL1 + morphine) was able to block the morphine-induced hyperpolarization, an effect mediated by the CX3CR1 receptor.
Figure 7. CX3CL1 exhibits an all-or-none effect on subsets of PAG neurons treated with morphine.
Pretreatment with CX3CL1 (10 nM) blocks morphine’s neurophysiological effects (hyperpolarization and reduction of input resistance) in 8/14 PAG neurons (A, B) but not in the other 6/14 PAG neurons (C, D), resulting in a blunting of morphine’s effect when data from all cells are combined (Figure 6E, F).
* indicates significant difference from the morphine group and # indicates significant difference from the MOR + CX3CL1 group by post-hoc Dunn’s tests (p < 0.05). Group data are presented as mean ± SEM.
There was a significant overall drug effect on input resistance (H(2) = 11.48, p < 0.01). Unlike morphine’s inhibition of input resistance, a single application of CX3CL1 has no impact on the input resistance of PAG neurons (baseline: 285.9 ± 22.1 MΩ, CX3CL1: 292.0 ± 21.3 MΩ; N = 20). In addition, CX3CL1 pretreatment only partially reduced the morphine-mediated reduction in input resistance. The mean morphine-induced reduction of input resistance of −19.6 ± 4.1 MΩ was significantly different from the effect of CX3CL1 alone (p < 0.05; Dunn’s test) and was reduced to −13.1 ± 4.8 MΩ in the presence of CX3CL1 (Figure 6F, N = 14), though this difference from morphine alone did not reach statistical significance. Following pretreatment with the CX3CR1 blocking antibody, CX3CL1 did not prevent a significant morphine-induced reduction of input resistance (from 347.0 ± 75.2 to 312.1 ± 63.1 MΩ; N = 9, p < 0.05), indicating that this effect of CX3CL1 was mediated by the CX3CR1 receptor.
Furthermore, the ability of CX3CL1 to modulate morphine-mediated actions on input resistance appears to be cell-specific, consistent with its effects on morphine-mediated hyperpolarization already described above. Data from the “responders” is presented in Figure 7D, indicating reductions of input resistance from an average of 281.6 ± 22.9 MΩ to 255.3 ± 25.5 MΩ (N = 6) whereas the “non-responders” (Figure 7B) did not exhibit any change (baseline: 270.8 ± 35.9 MΩ, CX3CL1 + morphine: 267.5 ± 38.5 MΩ; N = 8) in PAG neurons pretreated with CX3CL1. Therefore, CX3CL1 functions to inhibit both morphine-mediated hyperpolarization and input resistance reduction primarily in a specific population of PAG neurons, the “responders,” and does not impact either parameter in another group of PAG neurons, the “non-responders.”
In an additional group of cells, TTX (1 µM) was added prior to the drug combination. In the presence of TTX, CX3CL1 pretreatment was still able to attenuate both morphine-mediated hyperpolarization as well as morphine-mediated reduction of input resistance. For comparison, the mean change in membrane potential produced by morphine + CX3CL1 was −3.8 ± 1.6 mV in the absence of TTX (N = 14) vs. −3.6 ± 0.9 mV in the presence of TTX (N = 9) (vs. −9.5 ± 0.6 mV produced by morphine alone; N = 18). The mean change in input resistance produced by morphine + CX3CL1was −13.1 ± 4.6 MΩ in the absence of TTX vs. 9.6 ± 10.6 MΩ in the presence of TTX (vs. −19.6 ± 4.1 MΩ produced by morphine alone). In addition, the same proportions of PAG neurons pretreated with CX3CL1 were characterized as “responders” and “non-responders” to morphine: 6/14 and 8/14 in the absence of TTX vs. 4/9 and 5/9 in the presence of TTX. These data indicate that MOR-CXC3R1 interactions are mediated by receptors located on PAG neurons, not on synaptic afferents.
Discussion
The present results indicate CXCR4 and CX3CR1 anatomical colocalization with MOR on neurons in a region-specific manner throughout the rat brain, and that these G-protein coupled receptors neurophysiologically interact at a single-cell level in the PAG.
CXCR4 expression has been reported to be limited to areas associated with neural stem cells (Stumm et al., 2003; Tran et al., 2007; Stumm and Hollt, 2007), however, its localization appears to also be present in adult neurons in a number of different brain regions as we show in the current study. Some of this variability may relate to the method of detecting CXCR4 and the species in which expression was examined (immunohistochemistry or in situ hybridization, gene expression using CXCR4-EGPF transgenic mice). In addition, variability may exist between CXCR4 antibodies available from different manufacturers including the one used in the present study (Fischer et al., 2008). However, there is a large body of evidence to support the specificity of the antibody used in this study (see Antibodies section of Methods) as well as the widespread distribution of CXCR4 in adult neurons. When examining CXCR4 in neural progenitor cells, Tran et al. (2007) compared the expression of CXCR4 using fluorescent in situ hybridization relative to neural progenitor cells using nestin as a progenitor cell marker. In this study, nestin-EGFP transgenic mice were used with immunohistochemistry for EGFP to identify neural progenitor cells. These investigators clearly confirmed CXCR4 expression in neural progenitor cells but also showed that CXCR4 expression greatly exceeded EGFP expression, indicating that CXCR4 is on more cells than just progenitor cells in the CNS. The GENSAT BAC CXCR4 database using CXCR4-EGFP transgenic mouse data shows CXCR4 gene expression in the adult mouse in olfactory bulb, entorhinal cortex, hippocampus, septum, ventral striatum, basal forebrain, amygdala, thalamus, hypothalamus, midbrain, pons, medulla, cerebellum, spinal cord and ventricle (The Gene Expression Nervous System Atlas (GENSAT) Project, NINDS Contracts N01NS02331 & HHSN271200723701C to The Rockefeller University (New York, NY)).
Banisadr et al. (2002) using the same Santa Cruz antibody as in the current manuscript showed expression of the receptor in cerebral cortex, caudate putamen, globus pallidus, substantia innominata, supraoptic and paraventricular hypothalamic nuclei, ventromedial thalamic nucleus and substantia nigra of the rat. A recent publication by Trecki et al. (2010) also using the same antibody as in the current study showed CXCR4 expression in the caudate putamen and lateral shell of the nucleus accumbens. Given these collective findings using multiple anatomical techniques and rodent species, there is strong evidence for CXCR4 expression in the developing as well as the adult CNS. Furthermore, in support of the anatomical studies presented here, electrophysiological data are presented as functional evidence for localization of CXCR4 on neurons of the PAG where they interact with opioid receptors, supporting the concept that CXCR4 has a role in the CNS that extends beyond its developmental effects.
We report colocalization of MOR-CXCR4 and MOR-CX3CR1 in the hippocampus, cingulate cortex, periaqueductal grey, nucleus accumbens, ventral tegmental area, and globus pallidus. Only a few studies have previously demonstrated chemokine and opioid receptor co-expression in neurons. Zhang et al. (2004) showed MOR-CCR1 colocalization in DRG neurons in the rat brain. Patel et al. (2006) showed MOR-CXCR4 colocalization in rat cortical neuronal cultures. Our study is the first to demonstrate MOR colocalization with the selected chemokine receptors CXCR4 and CX3CR1 on neurons in the rodent brain and to compare the patterns of receptor colocalization in several CNS regions.
The extensive regional colocalization of these receptors suggests that chemokine-opioid interactions may impact memory, cognition, analgesia, reward, and motor behaviors. Furthermore, triple-labeling with a neuronal nuclei marker, NeuN, confirms MOR-CXCR4 and MOR-CX3CR1 neuronal colocalization. As the cellular localization of CX3CR1 has been somewhat controversial in the literature with some reports indicating exclusively microglial expression (Harrison et al., 1998; Nishiyori et al., 1998; Maciejewski-Lenoir et al., 1999; Verge et al., 2004) and other reports showing expression also on neurons (Meucci et al., 2000; Hughes et al., 2002), we conducted additional triple-labeling studies with the microglial marker CD11b and showed CX3CR1-labeled neurons and microglia in the hippocampus, confirming both types of cellular localization for this receptor. Neuronal localization of CX3CR1 provides anatomical support for the observed neuroprotective actions of CX3CL1 in the CNS. For example, Mizuno et al. (2003) reported CX3CL1-induced suppression of neuronal apoptosis and reduction of the neurotoxic compounds nitric oxide, IL-6, and TNF-α by activated microglia. Meucci et al., 1998; 2000) demonstrated that CX3CL1 prevents hippocampal neurotoxicity induced by the HIV envelope protein gp 120. Limatola et al. (2005) also showed that CX3CL1 inhibits glutamate-mediated excitotoxicity in the hippocampus, an effect that may be mediated by the ability of CX3CL1 to reduce glutamate synaptic activity in hippocampal neurons (Bertollini et al., 2006). On a subcellular level, CXCR4 and MOR primarily localize to neuronal membranes and processes. However, as seen in other immunohistochemical studies (Hughes et al., 2002), CX3CR1 is present in the perinuclear region, indicating that CX3CR1 activation may occur when it is primed on the membrane or translocated to the membrane surface.
Interestingly, neuronal colocalization is not a uniform phenomenon throughout the brain. In a representative subject, analysis of the percentage of neurons co-expressing MOR-CXCR4 and MOR-CX3CR1 in the principal cell layer in CA1/2 and DG of the HIP and layer II of the CC indicate a uniform receptor coexpression. Within the ventrolateral PAG, 88.8% (40/45) and 91.6% (44/48) of neurons examined expressed both MOR-CXCR4 and CX3CR1 respectively. The remaining neurons labeled with CXCR4 or CX3CR1, but did not express MOR. However, in other regions such as the caudate putamen and habenular nuclei, the receptors are segregated to different compartments and thus no colocalization was observed.
The presence of both MOR-CXCR4 and MOR-CX3CR1 on individual neurons is not observed in the CPu and HN, although labeling for each receptor is evident in both regions. In the CPu, MOR localizes to striosomes/patches and the subcallosal streak, where it concentrates as dense puncta in cell bodies and axonal fibers. CXCR4 and CX3CR1 also localize to striosomes/patches, subcallosal streak, and matrix compartments, however they remain uniquely segregated to neurons and fibers void of MOR in these regions.
Furthermore, morphine, a MOR-agonist, has well-characterized effects on PAG neurons (Chieng and Christie, 1994; Vaughan and Christie, 1997). Consistent with earlier studies (Chieng and Christie, 1994), morphine (10 µM) produced a neuronal membrane hyperpolarization and reduction of input resistance in PAG neurons that reflects a MOR-mediated increase in potassium conductance. Immunohistochemically, MOR-CXCR4 and MOR-CX3CR1 were co-expressed on PAG neurons suggesting a functional interaction between these GPCRs.
Baseline recordings of CXCL12 (10 nM) indicate its inability to affect either membrane potential or input resistance in PAG neurons. However, in the presence of CXCL12, morphine’s electrophysiological actions were blocked in all PAG neurons tested. In fact, when compared to predrug baseline, the combination of CXCL12 and morphine produced a small but significant depolarization of PAG neurons accompanied by a trend for an increase in input resistance. While we can speculate that this depolarization is the result of decreased potassium conductance, further experiments would be necessary to determine the ionic basis of this effect produced by the combined activation of MOR and CXCR4 receptors. CXCL12’s ability to reverse morphineinduced hyperpolarization and input resistance reduction in all PAG neurons in our study suggests a functional interaction between MOR-CXCR4 at a single-cell level. Furthermore, this effect of CXCL12 was preserved in the presence of TTX, an indication that the CXCR4-MOR interaction is direct, mediated by receptors located on PAG neurons rather than on synaptic afferents. Previous molecular and behavioral studies have described CXCR4 heterologous desensitization of MOR upon its activation by CXCL12. For example, the activation of cytokine receptors on dorsal root ganglion neurons heterologously desensitizes MOR co-expressed on these cells (Zhang et al., 2004). Likewise, injection of chemokines followed by injection of MOR agonists in the PAG decreases tail-flick latency to cold water in rats (Szabo et al., 2002; Chen et al., 2007), implying a reduced analgesic response via chemokine receptor mediated inhibition of MOR function. Thus, our electrophysiological results provide support for heterologous desensitization between MOR-CXCR4 at the single-cell level in the brain. As the role of ventrolateral PAG neurons in opioid analgesia has been well-characterized, these data provide a cellular mechanism for chemokine modulation of traditional nociceptive and analgesic pathways. However, heterologous desensitization was also observed in lateral PAG neurons, a subdivision of the PAG that has been implicated in active defensive behaviors, autonomic reactions, and non-opioid rather than opioid analgesia (Bandler and Shipley, 1994). Therefore, the functional consequences of chemokine-opioid interactions in lateral PAG neurons remain to be determined.
Unlike CXCL12, CX3CL1 pretreatment incompletely eliminates morphine-induced membrane hyperpolarization and input resistance reduction. CX3CL1 blocks morphine’s actions in only 57% of the PAG neurons tested, whereas the remaining 43% show a membrane hyperpolarization and input resistance reduction of equal magnitude to a single application of morphine. As a result of the heterogeneity of this response, the combined group data indicate a CX3CL1-mediated reduction rather than complete elimination of morphine’s effects. This effect of CX3CL1, like the effect of CXCL12, was preserved in the presence of TTX, indicating that MOR-CX3CR1 interactions are direct, mediated by receptors located on PAG neurons. However, the mixed actions of morphine and CX3CL1 application on membrane properties suggest the presence of two distinct neuronal subpopulations in the PAG which may selectively express the CX3CR1 receptor. Since 89% of PAG neurons tested with morphine alone show a physiological response, the majority of “non-responder” neurons likely express MOR. However, the lack of response to morphine potentially indicates a functional expression of heterologous desensitization by CX3CR1 activation. The “responder” neurons express MOR, but these neurons may lack CX3CR1 expression or activate a separate, non-interacting signaling pathway. Previous behavioral studies support a heterologous desensitization mechanism of MOR-CX3CR1 in the PAG (Chen et al., 2007), implying that receptor interaction may be sufficient to produce behavioral desensitization. Further investigation is needed to decipher the receptor distribution on and potential mechanism(s) that contribute to the dual effects of CX3CL1 and morphine treatment in PAG neurons.
Furthermore, earlier antagonist/blocking antibody studies provide evidence for receptor specificity of the 10 nM concentration of the chemokines used in the current study. The CXCR4 antagonist AMD3100 was used to block the electrophysiological effects of CXCL12 in a dorsal raphe nucleus (DRN) brain slice preparation (Heinisch and Kirby, 2010). In the DRN, unlike in PAG, CXCL12 at the same 10 nM concentration produced measurable electrophysiological effects that were successfully blocked by the antagonist. Similarly, CXC3L1 effects, which also produced measurable, though distinct, electrophysiological effects in DRN neurons at the 10 nM concentration, were blocked by pretreatment of the slices with an anti-fractalkine blocking antibody (Heinisch and Kirby, 2009). Morphine-induced activation of K+ channels in PAG neurons in a brain slice preparation is mimicked by the μ-selective agonist DAMGO (Chieng and Christie, 1994; Chiou, 2001) and blocked by naloxone (Chiou and How, 2001) which has at least 10-fold higher affinity for mu than delta or kappa opioid receptors. Thus, the receptors examined show specificity for their respective agonists.
In the brain slice preparation employed in these studies, the recorded neuron receives inputs from both neurons and glia (oligodendrocytes, microglia, astrocytes). Thus, the effects of chemokines on the mu-opioid receptor response may in part be contributed to by the surrounding glial network. The brain’s numerous supportive glia participate in information processing in the brain by influencing neuronal activity. For example, previous studies indicate that glutamate released from glia is capable of modulating spontaneous and evoked synaptic transmission in neurons (Araque et al., 1998). In order to decipher glia and neuronal contributions, cell culture techniques can be utilized to permit the isolation of specific cell populations. However, we utilized the brain slice as it maintains local circuit connections and therefore is a closer representation of the in vivo environment. Future studies examining MOR-CXCR4 and MOR-CX3CR1 interactions in a neuronal culture system will be needed to confirm that chemokine desensitization of the mu-opioid receptor response, as we demonstrated in brain slices, is still present in a neuronal culture system. This finding would confirm our hypothesis that opioidchemokine interactions are mediated by receptors located on the same neuron, a hypothesis further supported by our anatomical colocalization data.
In conclusion, the present study offers evidence that chemokines can differentially regulate the neurophysiological effects of morphine at the single-cell level in PAG neurons. The study gives additional perspective into the unique and complex actions of chemokines in the brain. We also provide support for chemokine interactions with the opioid neuropeptide system and this unique interaction may contribute to the limited utility of opioid analgesics in the treatment of inflammatory pain and to hyperalgesia associated with inflammatory conditions (Szabo et al., 2002). Chemokines and their receptors may serve as novel pharmacological targets for the treatment of pain in neuroimmune disease (Gosselin et al., 2008).
Acknowledgements
We would like to thank Dr. Patrick Piggot, members of his laboratory, and Emily Freeman-Daniels for their technical assistance. We also thank Dr. Mary Barbe and Dr. Martin Adler for their helpful advice and guidance in interpreting our anatomical data and examining our research report.
This work was supported by the National Institutes of Health [Grants DA 20126, DA 06650 and DA 13429]; and the Pennsylvania Health Research Formula Fund.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- CPu
caudate putamen
- CNS
central nervous system
- Cg
cingulate cortex
- CTAP
D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2
- DG
dentate gyrus
- GPCR
G-protein coupled receptor
- HN
habenular nuclei
- HIP
hippocampus
- IR
immunoreactivity
- MOR
mu-opioid receptor
- PAG
periaqueductal grey
- SEM
standard error of the mean
- str
striosome
- SDF
stromal cell-derived factor
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alger BE. Characteristics of a slow hyperpolarizing synaptic potential in rat hippocampal pyramidal cells in vitro. J. Neurophysiol. 1984;52:892–910. doi: 10.1152/jn.1984.52.5.892. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur. J. Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J. Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, Whitaker-Azmitia PM. Awakening the sleeping giant:anatomy and plasticity of the brain serotonergic system. J. Clin. Psychiatry. 1991;52:4–16. [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Melik PS. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur. J. Neurosci. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Bertollini C, Ragozzino D, Gross C, Limatola C, Eusebi F. Fractalkine/CX3CL1 depresses central synaptic transmission in mouse hippocampal slices. Neuropharmacology. 2006;51:816–821. doi: 10.1016/j.neuropharm.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur. J. Pharmacol. 2004;483:175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Chen X, Geller EB, Rogers TJ, Adler MW. The chemokine CX3CL1/fractalkine interferes with the antinociceptive effect induced by opioid agonists in the periaqueductal grey of rats. Brain Res. 2007;1153:52–57. doi: 10.1016/j.brainres.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurons in vitro. Br. J. Pharmacol. 1994;113:121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou LC. Differential antagonism by naloxone benzoylhydrazone of the activation of inward rectifying K+ channels by nociceptin and a mu-opioid in rat periaqueductal grey slices. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363:583–589. doi: 10.1007/s002100100402. [DOI] [PubMed] [Google Scholar]
- Chiou LC, How CH. ATP-sensitive K+ channels and cellular actions of morphine in periaqueductal gray slices of neonatal and adult rats. J. Pharmacol. Exp. Ther. 2001;298:493–500. [PubMed] [Google Scholar]
- Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VM, Wolf B, Doms RW, Kolson DL. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio D, Panina-Bordignon P, Sinigaglia F. Chemokine receptors in inflammation: an overview. J. Immunol. Methods. 2003;273:3–13. doi: 10.1016/s0022-1759(02)00414-3. [DOI] [PubMed] [Google Scholar]
- Fecho K, Maslonek KA, Dykstra LA, Lysle DT. Assessment of the involvement of central nervous system and peripheral opioid receptors in the immunomodulatory effects of acute morphine treatment in rats. J. Pharmacol. Exp. Ther. 1996;276:626–636. [PubMed] [Google Scholar]
- Fedyk ER, Ryyan DH, Ritterman I, Springer TA. Maturation decreases responsiveness of human bone marrow B lineage cells to stromal-derived factor 1 (SDF-1) J. Leukoc. Biol. 1999;66:667–673. doi: 10.1002/jlb.66.4.667. [DOI] [PubMed] [Google Scholar]
- Fischer T, Nagel F, Jacobs S, Stumm R, Schulz S. Reassessment of CXCR4 chemokine receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-2. PLoS One. 2008;3:e4069. doi: 10.1371/journal.pone.0004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb AJ, Edwards FA. Patch clamp recording from cells in sliced tissues. In: Ogden D, editor. Microelectrode techniques. The Plymouth Workshop handbook. Cambridge: The Company of Biologists Limited; 1994. pp. 255–274. [Google Scholar]
- Glabinski AR, Ransohoff RM. Chemokines and chemokine receptors in CNS pathology. J. Neurovirol. 1999;5:3–12. doi: 10.3109/13550289909029740. [DOI] [PubMed] [Google Scholar]
- Gosselin RD, Dansereau MA, Pohl M, Kitabgi P, Beaudet N, Sarret P, Melik Parsadaniantz S. Chemokine network in the nervous system: a new target for pain relief. Curr. Med. Chem. 2008;15:2866–2875. doi: 10.2174/092986708786242822. [DOI] [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J. Exp. Med. 1998;188:317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Skrzydelsi D, Rovère C, Rostène W, Parsadaniantz SM, Nahon JL. Stromal cell-derived factor-1alpha modulation of the excitability of rat substantia nigra dopaminergic neurones: presynaptic mechanisms. J. Neurochem. 2006;96:1540–1550. doi: 10.1111/j.1471-4159.2006.03659.x. [DOI] [PubMed] [Google Scholar]
- Guyon A, Nahon JL. Multiple actions of the chemokine stromal cell-derived factor-1α on neuronal activity. J. Mol. Endocrinol. 2007;38:365–376. doi: 10.1677/JME-06-0013. [DOI] [PubMed] [Google Scholar]
- Guyon A, Skrzydelski D, Rovère C, Apartis E, Rostène W, Kitabgi P, Mélik Parsadaniantz S, Nahon JL. Stromal-cell-derived factor 1alpha /CXCL12 modulates high-threshold calcium currents in rat substantia nigra. Eur. J. Neurosci. 2008;28:862–870. doi: 10.1111/j.1460-9568.2008.06367.x. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Hammon M, Lanfumey L. K+ channel and 5-hydroxytryptamine1A autoreceptor interactions in the rat dorsal raphe nucleus: an in vitro electrophysiological study. Neuroscience. 1991;41:495–500. doi: 10.1016/0306-4522(91)90344-n. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Mangoura D. Diversity of the endogenous opioid system in development. Novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect. Dev. Neurobiol. 1998;5:437–449. [PubMed] [Google Scholar]
- Heinisch S, Kirby LG. Fractalkine/CX3CL1 enhances GABA synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuroscience. 2009;164:1210–1223. doi: 10.1016/j.neuroscience.2009.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch S, Kirby LG. SDF-1α/CXCL12 enhances GABA and glutamate synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuropharmacol. 2010;58:501–514. doi: 10.1016/j.neuropharm.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MC, Flores LR, Bayer BM. Immunosuppression by morphine is mediated by central pathways. J. Pharmacol. Exp. Ther. 1993;267:1336–1341. [PubMed] [Google Scholar]
- Hesselgesser J, Liang M, Hoxie J, Greenberg M, Brass LF, Orsini MJ, Taub D, Horuk R. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J. Immunol. 1998;160:877–883. [PubMed] [Google Scholar]
- Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper SC. Expression of chemokine receptors by subsets of neurons in the central nervous system. J. Immunol. 1997;158:2882–2890. [PubMed] [Google Scholar]
- Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia. 2002;37:314–327. [PubMed] [Google Scholar]
- Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Shi Q, Xu H, Gibson GE. Changes in inflammatory processes associated with selective vulnerability following mild impairment of oxidative metabolism. Neurobiol. Dis. 2007;26:353–362. doi: 10.1016/j.nbd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limatola C, Giovannelli A, Maggi L, Ragozzino D, Castellani L, Ciotti MT, Vacca F, Mercanti D, Santoni A, Eusebi F. SDF-1alpha-mediated modulation of synaptic transmission in rat cerebellum. Eur. J. Neurosci. 2000;12:2497–2504. doi: 10.1046/j.1460-9568.2000.00139.x. [DOI] [PubMed] [Google Scholar]
- Limatola C, Lauro C, Catalano M, Ciotti MT, Bertollini C, Di Angelantonio S, Ragozzino D, Eusebi F. Chemokine CX3CL1 protects rat hippocampal neurons against glutamate-mediated excitotoxicity. J. Neuroimmunol. 2005;166:19–28. doi: 10.1016/j.jneuroim.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Liu Y, Blackbourn DJ, Chuang LF, Killam KF, Jr, Chuang RY. Effects of in vivo and in vitro administration of morphine sulfate upon rhesus macaque 180 polymorphonuclear cell phagocytosis and chemotaxis. J. Pharmacol. Exp. Ther. 1992;263:533–539. [PubMed] [Google Scholar]
- Lorenzo P, Portolés A, Jr, Beneit JV, Ronda E, Portolés A. Physical dependence to morphine diminishes the interferon response in mice. Immunopharmacol. 1987;14:93–99. doi: 10.1016/0162-3109(87)90033-6. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc. Natl. Acad. Sci. USA. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski-Lenoir D, Chen S, Feng L, Maki R, Bacon KB. Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J. Immunol. 1999;163:1628–1635. [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J. Chem. Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- Mennicken F, Maki R, de Souza EB, Quirion R. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharm. Sci. 1999;20:73–78. doi: 10.1016/s0165-6147(99)01308-5. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc. Natl. Acad. Sci. USA. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc. Natl. Acad. Sci. USA. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Kawanokuch iJ, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979:65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- Moon SO, Kim W, Sung MJ, Lee S, Kang KP, Kim DH, Lee SY, So JN, Park SK. Resveratrol suppresses tumor necrosis factor-alpha-induced fractalkine expression in endothelial cells. Mol. Pharmacol. 2006;70:112–119. doi: 10.1124/mol.106.022392. [DOI] [PubMed] [Google Scholar]
- Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429:167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- Patel JP, Sengupta R, Bardi G, Khan MZ, Mullen-Przeworski A, Meucci O. Modulation of neuronal CXCR4 by the micro-opioid agonist DAMGO. J. Neurovirol. 2006;12:492–500. doi: 10.1080/13550280601064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 2005. [Google Scholar]
- Pujol F, Kitabgi P, Boudin H. The chemokine SDF-1 differentially regulates axonal elongation and branching in hippocampal neurons. J. Cell Sci. 2005;118:1071–1080. doi: 10.1242/jcs.01694. [DOI] [PubMed] [Google Scholar]
- Ragozzino D, Di AS, Trettel F, Bertollini C, Maggi L, Gross C, Charo IF, Limatola C, Eusebi F. Chemokine fractalkine/CX3CL1 negatively modulates active glutamatergic synapses in rat hippocampal neurons. J. Neurosci. 2006;26:10488–10498. doi: 10.1523/JNEUROSCI.3192-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino D, Renzi M, Giovannelli A, Eusebi F. Stimulation of chemokine CXC receptor 4 induces synaptic depression of evoked parallel fibers inputs onto Purkinje neurons in mouse cerebellum. J. Neuroimmunol. 2002;127:30–36. doi: 10.1016/s0165-5728(02)00093-0. [DOI] [PubMed] [Google Scholar]
- Schmidtmayerova H, Sherry B, Bukrinsky M. Chemokines and HIV replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- Skrzydelski D, Guyon A, Dauge V, Rovere C, Apartis E, Kitabgi P, Nahon JL, Rostene W, Parsadaniantz SM. The chemokine stromal cell-derived factor-1/CXCL12 activates the nigrostriatal dopamine system. J. Neurochem. 2007;102:1175–1183. doi: 10.1111/j.1471-4159.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- Sørensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger H, Rösler A, Tonn P, Braune HJ, Huffmann G, Gemsa D. Chemokines in the cerebrospinal fluid of patients with meningitis. Clin. Immunol. Immunopathol. 1996;80:155–161. doi: 10.1006/clin.1996.0109. [DOI] [PubMed] [Google Scholar]
- Starec M, Rouveix B, Sinet M, Chau F, Desforges B, Pocidalo JJ, Lechat P. Immune status and survival of opiate- and cocaine-treated mice infected with Friend virus. J. Pharmacol. Exp. Ther. 1991;259:745–750. [PubMed] [Google Scholar]
- Steele AD, Szabo I, Bednar F, Rogers TJ. Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine Growth Factor Rev. 2002;13:209–222. doi: 10.1016/s1359-6101(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Scharrer B, Smith EM, Hughes TK, Jr, Magazine HI, Bilfinger TV, Hartman AR, Fricchione GL, Liu Y, Makman MH. Opioid and opiate immunoregulatory processes. Crit. Rev. Immunol. 1996;16:109–144. doi: 10.1615/critrevimmunol.v16.i2.10. [DOI] [PubMed] [Google Scholar]
- Stumm R, Hollt V. CXC chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J. Mol. Endocrinol. 2007;38:377–382. doi: 10.1677/JME-06-0032. [DOI] [PubMed] [Google Scholar]
- Stumm R, Kolodziej A, Schulz S, Kohtz JD, Hollt V. Patterns of SDF-1alpha and SDF-1gamma mRNAs, migration pathways, and phenotypes of CXCR4-expressing neurons in the developing rat telencephalon. J. Comp. Neurol. 2007;502:382–399. doi: 10.1002/cne.21336. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J. Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J. Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Wetzel M, McCarthy L, Steele A, Henderson EE, Howard MZ, Oppenheim JJ, Rogers TJ. Interactions of opioid receptors, chemokines, and chemokine receptors. Adv. Exp. Med. Biol. 2001;493:69–74. doi: 10.1007/0-306-47611-8_8. [DOI] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc. Natl. Acad. Sci. USA. 2002;99:10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Wetzel MA, Zhang N, Steele AD, Kaminsky DE, Chen C, Liu-Chen LY, Bednar F, Henderson EE, Howard OM, Oppenheim JJ, Rogers TJ. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. J. Leuk. Biol. 2003;74:1074–1082. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat. Rev. Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J. Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecki J, Brailoiu GC, Unterwald EM. Localization of CXCR4 in the forebrain of the adult rat. Brain Res. 2010;1315:53–62. doi: 10.1016/j.brainres.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J. Physiol. 1997;498:463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur. J. Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural immunocytochemical localization of mu opioid receptors and Leu5-enkephalin in the patch compartment of the rat caudate-putamen nucleus. J. Comp. Neurol. 1996;375:659–674. doi: 10.1002/(SICI)1096-9861(19961125)375:4<659::AID-CNE7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Zhang N, Rogers TJ, Caterina M, Oppenheim JJ. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. J. Immunol. 2004;173:594–599. doi: 10.4049/jimmunol.173.1.594. [DOI] [PubMed] [Google Scholar]