Abstract
The relationship between psychosocial factors and an increased risk for disease has been related to a heightened pro-inflammatory status reflected in increased circulating levels of pro-inflammatory cytokines and/or C-reactive protein (CRP). Routinely, epidemiological studies rely on measurements of inflammatory markers in serum or plasma, but the use of biological fluids such as saliva or oral mucosal transudate (OMT) may offer potential advantages. This study investigated correlations among plasma CRP and levels of IL-6 and soluble IL-6 receptor (sIL-6R) in plasma, saliva and OMT in a population of middle aged women with histories of past intimate partner violence (IPV). A total of 67 women without existing chronic diseases participated in the study, which included two visits each in which psychological tests were administered, and blood, saliva and OMT samples were collected. Although significantly higher plasma CRP levels were found in past IPV sufferers compared to controls, there were no significant differences in IL-6 or sIL-6R levels in plasma, saliva or OMT between the two groups. There were only relatively modest correlations between IL-6 levels in plasma and those in saliva or OMT and between plasma IL-6 and CRP levels. A significant correlation between IL-6 and sIL6R levels in both saliva and OMT, but not in plasma, was also detected. No significant correlations were found between levels of IL-6 in saliva or OMT and periodontal health measures. Results indicate that IL-6 and sIL-6R levels in saliva or OMT do not closely reflect those in plasma, and therefore are not a good surrogate for systemic levels.
Keywords: C-reactive protein (CRP), interleukin-6 (IL-6), intimate partner violence (IPV), oral mucosal transudate (OMT), plasma, saliva, soluble interleukin-6 receptor (sIL-6R)
INTRODUCTION
The existence of a relationship between psychosocial factors and an increased risk for disease and death has been well established (Hemingway and Marmot, 1999; Krause et al., 2004). This risk has been shown to be associated with increased circulating levels of a variety of inflammatory markers, including pro-inflammatory cytokines such as tumor necrosis factor-α (TNFα), interleukin-1 (IL-1) and interleukin −6 (IL-6); soluble forms of several cytokine receptors such as soluble IL-6 receptors (sIL-6R) and soluble TNF receptors I and II; and acute phase proteins such as C-reactive protein (CRP) (Papanicolaou et al.,1998; Harris et al., 1999; Maes, 1999; Appels et al., 2000; Ridker et al., 2002; Grassi-Oliveira et al., 2009). The activation of the hypothalamic-pituitary-adrenal (HPA) axis by these pro-inflammatory mediators is thought to be a contributing factor in the development of a variety of chronic inflammatory diseases (Lyson and McCann, 1991; Mastorakos et al., 1993; Kiecolt-Glaser et al., 2003; Danese et al., 2007; Davis et al., 2008).
IL-6 is a pleiotropic cytokine involved not only in inflammatory responses but also in immune regulation, promoting antibody production and the differentiation of Th17 CD4+ T lymphocytes, a subset that has been associated with autoimmune disorders (Betelli et al., 2006). Its importance in studies relating psychosocial factors and inflammatory mechanisms is based on the fact that IL-6 is considered a key cytokine in inflammatory responses and the major regulator of acute phase protein responses by the liver (Hirano et al., 1990; Ohzato et al., 1992; Kishimoto, 2005). In addition, its levels in serum and plasma are more consistently detected and measured compared to other pro-inflammatory cytokines such as TNFα and IL-1. The levels of IL-6 in serum or plasma are increased in an array of pathologic conditions including both acute and chronic inflammatory diseases (Hirano et al., 1990). Elevated systemic IL-6 levels have also been reported in association with a variety of health-related psychosocial factors such as chronic stress conditions, depression and low socioeconomic level, suggesting a potential association with a heightened inflammatory status (Maes et al., 2001; Kiecolt-Glaser et al., 2003; Brydon et al., 2004; Danese et al., 2007; Davis et al., 2008).
IL-6 is produced by a wide variety of cells, including T lymphocytes, macrophages, glial cells, fibroblasts, keratinocytes, endothelial cells and adipocytes (Hirano et al., 1990; Kishimoto, 2005). Consistent with its role in inflammatory responses, the expression of IL-6 is normally induced by a variety of pro-inflammatory stimuli such as the cytokines TNFα and IL-1 and bacterial lipopolysaccharide (LPS) (Hirano et al., 1990; Kishimoto, 2005). The activity of IL-6 is mediated by a heterodimeric membrane receptor formed by an IL-6-binding chain (gp80 or IL-6R) and a signal-transducing subunit (gp130 or CD130) (Ward et al., 1994; Kishimoto, 2005). Both of these receptor subunits are also released as soluble proteins (sIL-6R and sgp130) in biological fluids (Fernandez-Botran et al., 2002). Unlike most soluble cytokine receptors, the sIL-6R acts as an agonist, binding IL-6 and allowing it to signal on cells that do not express membrane IL-6R but only the gp130 protein, such as hemopoietic cells, osteoclasts and neuronal cells, by a process known as “trans-signaling” (Mackiewicz et al., 1992; Jones et al., 2002). Thus, the sIL-6R is considered as an important regulator of IL-6 activity (Jones et al., 2002). The levels of sIL-6R in serum have been reported to be elevated in conditions such as autoimmune diseases, multiple myeloma and sepsis (Gaillard et al., 1999; Jones and Rose-John, 2002; Kallen, 2002; Peake et al., 2006). Although potential alterations related to psychosocial factors have not been widely investigated, there is evidence that increased sIL-6R levels occur in psychotic disorders and patients with post-traumatic stress disorder (PTSD) with concurrent major depression (Kallen, 2002) and that negative correlations exist between sIL6R levels and scores on a purpose of life scale (Friedman et al., 2007).
In most cases, the measurements of cytokine levels related to psychosocial factors are performed in serum or plasma. However, the ease and lower costs of collection without the need for trained personnel have generated interest in the use of other biological fluids such as saliva or oral mucosal transudate (OMT) for epidemiological studies involving a variety of markers (Nishanian et al., 1998). For example, cortisol analyses in saliva have been used by many investigators, while OMT testing is routinely done for antibodies against HIV-1 (Gallo et al., 1997; Preussner et al., 1997). These samples, however, have not been extensively evaluated for their suitability for measuring cytokines and soluble cytokine receptors in psychobiological studies. The purpose of the present study was to investigate the correlations among measurements of IL-6 and sIL6R in plasma, saliva and OMT as part of a study of inflammatory markers in a population of healthy middle aged women with a history of past intimate partner violence (IPV), a stressor that is often chronic and that has been positively associated with the presence of chronic medical conditions (Tjaden and Thoennes, 2000; Breiding et al., 2008). Results of the parent study, including associations of plasma IL-6 and CRP levels with different IPV dimensions (i.e., cumulative IPV history, and the specific IPV types physical assault, sexual coercion, and stalking), have been reported elsewhere (Newton et al., 2010).
MATERIALS AND METHODS
Overall Study Design
The current study was part of a larger project investigating pro-inflammatory markers in post-menopausal women with past histories of IPV. A detailed description of the overall study design has been recently reported (Newton et al., 2010). Briefly, the study included a group of healthy postmenopausal women with divorce histories who were recruited after a phone interview and psychological and biomedical evaluation. The subjects completed two research visits during which they had blood drawn, provided saliva and OMT samples, and completed questionnaires about past intimate relationships. Based on their responses to the Revised Conflict Tactics Scale (described below), forty-six women were included in the “IPV+” group and twenty-one in the “IPV−” (control) group. Results of the measurements for IL-6 and sIL-6R in plasma, saliva and OMT samples, as well as plasma CRP, were then used for this study.
Participant Recruitment and Selection
Mailings and community advertisements recruited women ever divorced or separated from extremely stressful relationships. Eligibility assessment included: a) a phone interview; b) mental status interviews at research visits; and c) laboratory tests conducted on blood and urine sampled at the first research visit. Initial eligibility required English language skills, no ongoing divorce-related legal issues, no recent psychiatric hospitalization, and no current IPV (i.e., IPV involving an ex-partner in the preceding year, or any IPV history with a current partner) defined as a score of 1 or greater on the 3-item STaT, a screening tool for IPV with a sensitivity and specificity of 96% and 76% for lifetime IPV (Paranjape and Liebschutz, 2003), and 95% and 37% for ongoing IPV (Paranjape et al., 2006). Biomedical eligibility criteria were age 45 to 60; 12-month cessation of menses (except women ages 45 to 54 who reported hysterectomy without bilateral ovariectomy (Johnson et al., 2004); free of chronic disease other than unmedicated hypertension; no use of street drugs, prescription or over-the-counter medications (including botanicals) with potential anti-inflammatory effects; no blood or needle phobia and no current alcohol use disorder defined as an AUDIT-C score of 5 or greater, which yields sensitivities ≥ 72% and specificities ≥ 88% (Dawson et al., 2005).
Procedure
Research visits began between 8 a.m. and 1 p.m. First, in order to ensure that women were not too psychologically vulnerable to complete the study protocol, mental status interviews were conducted to screen for suicidal ideation (visits 1 and 2), and for psychosis and cognitive impairment (visit 1 only). Afterwards, a female nurse assessed acute medical conditions, evaluated signs of illness or infection, took blood pressure, pulse, and body measurements, and obtained a urine sample for a toxicology screen and urinalysis (visit 1 only). Blood, drawn once at each visit via antecubital venipuncture, was collected next with sodium citrate (for IL-6 and sIL-6R) or lithium heparin (for high-sensitivity CRP, comprehensive metabolic profile, thyroid stimulating hormone [TSH], ethanol, and follicle-stimulating hormone [FSH]), or EDTA (for complete blood count [CBC] and HbA1c). Women then completed computer-administered questionnaires at both visits. Previous to visit 2, women were reminded that the focus of that visit would be past abuse and violence and given opportunity to decline participation if they wished to do so. Those choosing to participate provided blood, saliva, and OMT samples and then completed IPV measures and interviews about anxiety symptoms related to IPV or other stressors. Saliva and OMT samples were collected during visits 1 and 2, both immediately before and immediately after the completion of the questionnaires (samples A and B, respectively). Times elapsed between collection of samples A and B were normally 1.5–2 h for visit 1 and 2.5–3 h for visit 2. Interleukin-6, sIL-6R and CRP were assayed at both visits. All other assays were conducted at visit 1 to evaluate eligibility.
IPV Classification
The Revised Conflict Tactics Scale (Straus et al., 2003) was used to assess frequency (0 = never, 6 = more than 20 times) of minor and severe forms of physical assault, sexual coercion, injury and psychological aggression across women’s adult intimate relationships collectively. Women reporting no or minor instances of physical assault or sexual coercion (e.g., being pushed or shoved) served as the control (IPV−) group (n=21), whereas women reporting one or more cases of severe physical assault or sexual coercion (e.g., being beat up) were classified in the IPV+ group (n=46).
Periodontal Health Assessment
Periodontal health was assessed during the first visit using a self-reported questionnaire including three “yes/no” items. One item, “Do your gums usually bleed?,” (1 = yes, 2 = yes, when brushing my teeth, 3 = no) assessed self-reported symptoms of gingivitis. This item has sensitivity of .42 and specificity of .76, as indexed against a clinical evaluation of bleeding on probe (Buhlin et al., 2002; Blicher et al., 2005). Two additional items, “Has any dentist/hygienist told you that you have deep pockets?” (1 = yes, 2 = no) (Buhlin, 2002) and “Have you ever been told that you need periodontal or gum treatment?” (1 = yes, 2 = no) (Pitiphat, 2002; Blicher et al., 2005) have sensitvities of .55 and .65, and specificities of .90 and .64, respectively, as indexed against either number and depth of pockets or radiographic determination of alveolar bone loss.
Biological Measures
Eligibility Labs
Comprehensive metabolic profile was measured on an Ortho Clinical Diagnostics Vitros® 5,1 FS analyzer by dry slide technology (Rochester, NY). FSH and TSH were measured by immunometric assay with electrochemiluminescent detection on a Roche E170 Analyzer (Hoffman-LaRoche AG, Basel, Switzerland). Ethanol (plasma) and HbA1c (whole blood) were assayed on a Roche Integra 800. The ethanol assay is enzymatic and the HbA1c assay is immunoturbidometric. The CBC was analyzed on a Sysmex XE-2100 analyzer (Sysmex Corporation, Kobe, Japan). A urine toxicology screen was analyzed on the Roche Integra 800. Measurement of CRP. High-sensitivity CRP was measured by turbidimetry on a Roche Integra 800 Analyzer. For the Roche Integra CRP assay, within-run coefficients of variation are 0.9 and 0.7% at 3.3 and 8.0 mg/L, respectively, and between-run coefficients of variation are 3.5 and 2.2%. The limit of detection is 0.1 mg/L.
Isolation of Plasma
Peripheral blood (10 ml) was collected in sodium citrate-containing Vacutainer tubes and used for the isolation of plasma. After venipuncture, the tubes were centrifuged for 10 min at 350 × g. The plasma was then separated by aspiration, aliquoted (0.25 ml) into cryovials and stored frozen at −80°C until assay.
Collection of Saliva and OMT
Saliva and OMT samples were collected using Saliva Sampler (Saliva Diagnostic Systems, Medford, NY) and OraSure collection devices (OraSure Technologies, Bethlehem, PA), respectively. The Saliva Sampler consists of a cellulose pad attached to a polypropylene stem that is placed under the tongue until a built-in indicator changes color, alerting that 1 ml of saliva has been collected. The pad is then inserted in a tube containing 1 ml of preserving buffer solution. The OraSure device consists of a cotton fiber pad treated with a hypertonic salt solution that enhances the transport of OMT across the gingival crevice and oral mucosa (Gallo et al., 1997; Ferri, 1998). The device is placed between the subject’s lower cheek and gum for 3 min and then inserted into a transport tube containing a preserving buffer solution. After collection, saliva and OMT samples were kept refrigerated until delivered to the laboratory for processing, usually within 2–4 hours of collection. In order to collect the samples for assay, the applicator pads of both devices were separated from the rest of the applicator with a pair of forceps, inserted into a 5 ml syringe and the plunger used to release the fluid, which was collected in a 2ml microcentrifuge tube. After centrifugation (60 seconds at 10,000g), the supernatants were transferred to a clean tube and kept frozen until assay. OMT differs from saliva in that it is a transudate, lacking most salivary proteins and having a higher content of IgG antibodies over IgA (Ferri, 1998).
Measurement of IL-6 and sIL-6R by ELISA
The concentrations of IL-6 and sIL-6R in plasma, saliva or OMT were measured by commercial two-site ELISAs according to the manufacturer’s instructions. An Opti-EIA kit (BD Pharmingen, San Diego, CA) was used for the measurement of IL-6, while a Duo Set R&D kit (R&D Diagnostics, Minneapolis, MN) was used for sIL-6R. Each sample was tested in triplicate. Assay sensitivities were 0.3 pg/ml and 1 pg/ml for IL-6 and sIL6R, respectively. In order to minimize and control for inter-assay variability, analysis of the samples was deferred until a minimum of plasma and supernatants from 15 participants could be run together. Recovery of IL-6 from the Saliva and OMT collection devices in samples spiked with recombinant IL-6 (10 pg/ml) were 90±9% and 93±5%, respectively. Recovery of sIL-6R was 95±4% and 98±4% for samples spiked with recombinant sIL-6R (1 ng/ml) from the Saliva and OMT collection devices, respectively. Intra- and inter-assay coefficients of variation (CV) for the IL-6 ELISA were 6.9 and 9.6%, respectively.
Data Analysis
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Data distribution was analyzed using the D’Agostino and Pearson omnibus normality test. Comparisons between groups were performed using the Kruskal Wallis test followed by Dunns post-hoc analysis. Correlation analyses were performed on ranked data by the Spearman’s method. All p values are two-tailed. Values of p<0.05 were considered statistically significant.
RESULTS
IL-6 levels in Biological Fluids
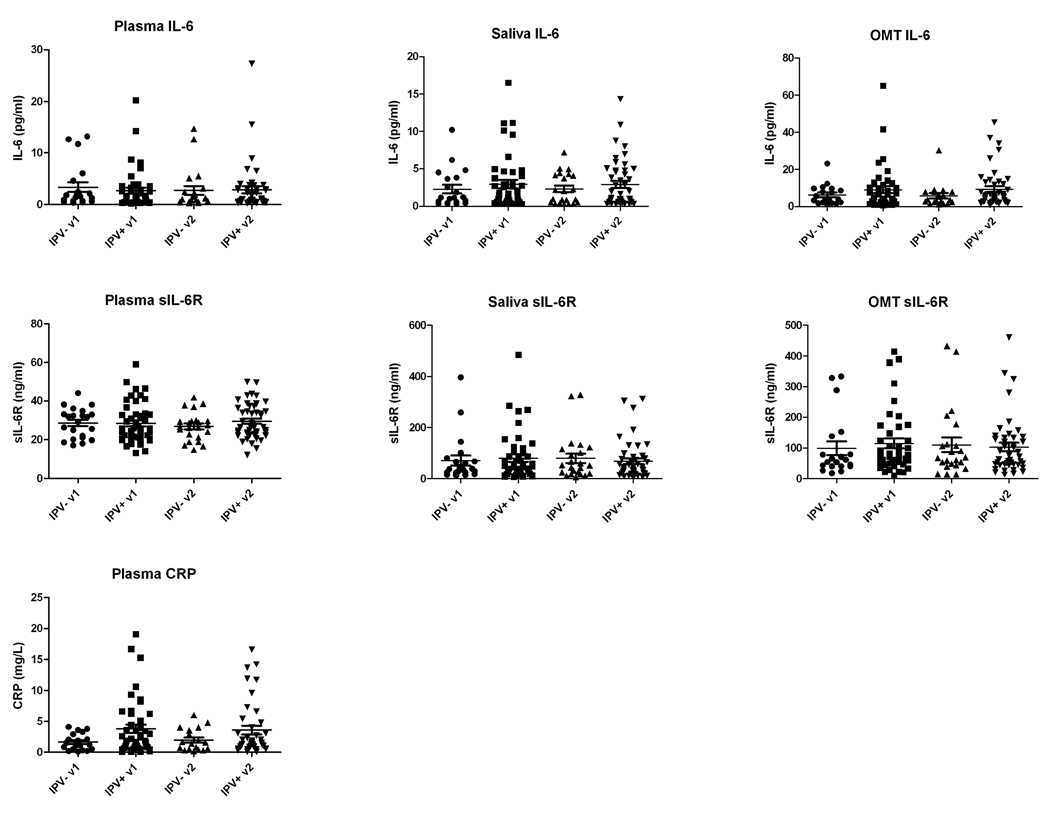
The distributions of IL-6 and sIL6R levels in plasma, saliva and OMT, as well as hsCRP in plasma for the IPV+ and IPV− (control) groups for visits 1 and 2 are shown in Figure 1, while Table 1 summarizes the median with 25th and 75th percentiles plus the mean and standard error of the data. In general, the data did not follow a normal distribution and therefore ranked statistical methods were used for analysis. Overall, similar levels of IL-6 were found in plasma and saliva (median of 0.9 –1.7 pg/ml), whereas OMT samples had IL-6 levels that were approximately three times higher than those in plasma (median of 3.6–5.2 pg/ml). There were no statistically significant differences when comparing IL-6 levels in plasma, saliva and OMT between the two research visits (e.g., visit 1 vs. visit 2) or when comparing IL-6 levels in saliva or OMT between samples taken before and after the interviews (samples A and B). Although examination of the scatter plots in Figure 1 pointed to some potential differences in distribution of IL-6 concentrations in plasma, saliva and OMT when comparing the IPV− (control) to the IPV+ group on both visits, statistical analysis failed to show significant differences. Despite a trend for higher mean IL-6 levels in both saliva and OMT in the IPV+ group compared to controls, these differences were also not statistically significant.
Figure 1. Distribution of the levels of IL-6, sIL-6R and CRP in plasma, saliva and OMT samples.
Plasma, saliva and OMT samples collected during visits 1 and 2 were assayed for IL-6 and sIL-6R by ELISA and for CRP by turbidimetry. Figure shows the individual values for the IPV− (control) and IPV+ groups on visits 1 (v1) and 2 (v2). In the case of saliva and OMT, the average values of determinations for samples A and B (before and after completion of the psychological questionnaires) were used. The horizontal bar shows the mean and S.E.M. (n=21 for the IPVand n=46 for the IPV+ group).
Table 1.
IL-6, sIL-6R and hsCRP levels in plasma, saliva and OMT in the IPV− (control) and IPV+ groupsa.
| IPV− visit 1 | IPV+ visit 1 | IPV−visit 2 | IPV+ visit 2 | |
|---|---|---|---|---|
| IL-6 (pg/ml) | ||||
| Plasma (Median) | 1.65 (0.70–4.05) | 1.20 (0.40–3.30) | 0.95 (0.50–2.93) | 0.90 (0.60–3.10) |
| (Mean) | 3.34 (0.94) | 2.64 (0.60) | 2.73 (0.91) | 2.86 (0.73) |
| Saliva (Median) | 1.13 (0.48–3.76) | 1.48 (0.64–3.94) | 1.50 (0.49–4.19) | 1.10 (0.55–4.75) |
| (Mean) | 2.28 (0.58) | 2.96 (0.55) | 2.30 (0.44) | 2.92 (0.48) |
| OMT (Median) | 3.85 (2.15–9.70) | 4.30 (2.95–11.05) | 3.63 (2.41–7.84) | 5.20 (2.56–12.89) |
| (Mean) | 6.30 (1.24) | 9.05 (1.89) | 5.76 (1.42) | 9.34 (1.56) |
| CRP (mg/L) | ||||
| (Median) | 1.10 (0.40–2.65) | 1.90 (0.70–6.20)* | 1.10 (0.43–3.73) | 1.50 (0.85–5.10) |
| (Mean) | 1.54 (0.33) | 3.90 (0.73)* | 1.89 (0.47) | 3.69 (0.75) |
| sIL6R (ng/ml) | ||||
| Plasma (Median) | 29.0 (21.0–32.9) | 25.0 (20.5–33.2) | 27.0 (19.4–29.6) | 26.4 (22.8–35.9) |
| (Mean) | 28.0 (1.66) | 28.1 (1.60) | 26.5 (1.63) | 29.5 (1.32) |
| Saliva (Median) | 38.3 (24.3–70.1) | 47.5 (18.5–102.5) | 52.0 (20.5–115.5) | 40.5 (20.0–81.3) |
| (Mean) | 71.3 (19.4) | 80.9 (14.1) | 80.2 (18.3) | 68.3 (11.2) |
| OMT (Median) | 58.0 (40.1–123.6) | 74.0 (48.3–158.8) | 68.0 (44.5–112.5) | 67.3 (45.8–133.4) |
| (Mean) | 99.1 (22.3) | 115.0 (16.2) | 110.2 (23.7) | 103.1 (14.1) |
Results are expressed as the median (25th and 75th percentiles) and mean (standard error). Data for Saliva and OMT represent pooled results for specimens collected before (A) and after (B) the interviews.
sIL-6R levels in Biological Fluids
Most subjects showed levels of sIL6R in plasma in the 20–40 ng/ml range, with no statistically significant differences between visits or between IPV− (control) and IPV+ groups. Levels of sIL-6R in saliva and OMT were, in general, two to three times higher (median of 38–74 ng/ml) compared to those in plasma (median of 25–29 ng/ml). Again, there were no statistically significant differences between visits or between IPV− (control) and IPV+ groups for saliva and OMT sIL-6R levels. In contrast to the levels of IL-6, which were in the single pg/ml range (approximately 0.1 pM), the levels of sIL-6R in plasma, saliva and OMT were in the 10–100 ng/ml range (approximately 0.2–2 nM), which means that sIL6R concentrations in these fluids are at least over a thousand-fold higher on a molar basis than those of the ligand, IL-6. This would imply that most of the IL-6 in biological fluids exists in the form of IL6/sIL-6R complexes, and is thus biologically active and able to mediate trans-signaling on cells expressing only gp130 (Jones and Rose-John, 2002).
Plasma CRP levels
As shown in Table 1, analysis of plasma CRP levels showed higher levels in the IPV+ compared to the IPV− (control) group for both visits (median values of 1.90 vs. 1.10 mg/L for visit 1 and 1.50 vs 1.10 mg/L for visit 2 when comparing IPV+ to the IPV− group). The difference for visit 1 was statistically significant (p<0.05).
Correlation between IL-6, sIL-6R and CRP levels in plasma, saliva and OMT
As a next step, a non-parametric method (Spearman’s rank correlation) was used to analyze the correlations between the levels of IL-6, sIL6R and CRP in the different biological fluids. This analysis included all samples, without segregation into IPV groups. Justification for pooling the two groups was based on the lack of statistically significant differences for 38 out of 40 correlation coefficients calculated separately for the IPV+ and IPV− groups, and compared using z-values obtained through Fisher’s −r to −z transformation. For the saliva and OMT measurements, IL-6 and sIL-6R values from samples taken at the same time as the blood samples (sample A) were used. Results are summarized in Table 2. There was a moderate to high degree of correlation when comparing measurements of the same analyte collected during different visits in the case of plasma, saliva and OMT (visit 1 vs. visit 2). These comparisons demonstrated statistically significant correlations for IL-6 measurements in plasma (r=0.780; p<0.001), saliva (r=0.513; p<0.001) and OMT (r=0.380; p=0.003), as well as for plasma CRP measurements (r=0.859; p<0.001). When the relations between the levels of IL-6 in different biological fluids were analyzed (e.g., plasma vs. saliva, or plasma vs. OMT), the correlations were relatively lower, although still statistically significant. For example, IL-6 levels in plasma showed correlation coefficients of only 0.289 and 0.411 with corresponding IL-6 levels in saliva and OMT, respectively (visit 1). The correlation between IL-6 levels in saliva and OMT was somewhat higher but still in the modest range (r=0.474 and 0.540 for visits 1 and 2, respectively). For visit 2, no significant correlations were found between plasma and saliva or OMT IL-6 levels (r=0.103 and 0.131, respectively).
Table 2.
Matrix of correlation coefficients between IL-6, sIL6R and CRP levels in different biological fluids
| Plasma | Plasma | Saliva | Saliva | OMT | OMT | Plasma | Plasma | |
|---|---|---|---|---|---|---|---|---|
| IL-6-v1 | IL-6-v2 | IL-6-v1 | IL-6-v2 | IL-6-v1 | IL-6-v2 | CRP-v1 | CRP-v2 | |
| Plasma | 0.780 | 0.289 | 0.411 | 0.272 | ||||
| IL-6-v1 | (<0.001)** | (0.019)** | (0.001)** | (0.033)* | ||||
| Plasma | 0.103 | 0.131 | 0.160 | |||||
| IL-6-v2 | (0.410) | (0.295) | (0.237) | |||||
| Saliva | 0.513 | 0.474 | 0.069 | |||||
| IL-6-v1 | (<0.001)** | (<0.001)** | (0.599) | |||||
| Saliva | 0.540 | 0.151 | ||||||
| IL-6-v2 | (<0.001)** | (0.267) | ||||||
| OMT | 0.380 | 0.229 | ||||||
| IL-6-v1 | (0.003)** | (0.089) | ||||||
| OMT | 0.002 | |||||||
| IL-6-v2 | (0.991) | |||||||
| Plasma | 0.859 | |||||||
| hsCRP-v1 | (<0.001)** | |||||||
| Plasma | 0.068 | 0.063 | −0.122 | −0.112 | ||||
| sIL-6R–v1 | (0.587) | (0.617) | (0.351) | (0.389) | ||||
| Plasma | 0.140 | 0.256 | 0.199 | −0.182 | ||||
| sIL-6R–v2 | (0.263) | (0.038)* | (0.110) | (0.180) | ||||
| Saliva | 0.286 | 0.564 | 0.457 | 0.064 | ||||
| sIL-6R–v1 | (0.020)* | (<0.001)** | (0.001)** | (0.624) | ||||
| Saliva | −0.028 | 0.638 | 0.373 | 0.183 | ||||
| sIL-6R–v2 | (0.821) | (<0.001)** | (0.002)** | (0.176) | ||||
| OMT | 0.138 | 0.329 | 0.604 | 0.148 | ||||
| sIL-6R–v1 | (0.292) | (0.010)* | (<0.001)** | (0.281) | ||||
| OMT | 0.004 | 0.426 | 0.588 | 0.024 | ||||
| sIL-6R–v2 | (0.974) | (<0.001)** | (<0.001)** | (0.859) | ||||
Table depicts Spearman’s correlation coefficients and p values (in parentheses). In the case of saliva and OMT, Sample A values (taken at the same time as blood samples) were used for calculations.
(p<0.05)
(p<0.01)
Inasmuch as IL-6 is a known regulator of acute phase proteins, the correlation between IL-6 levels in the different fluids and plasma CRP concentrations was also investigated. Results depicted in Table 2 showed a statistically significant but low correlation between plasma IL-6 and CRP levels (r=0.272, p=0.033 for visit 1). However, no significant correlations were found between IL-6 levels in saliva or OMT and plasma CRP levels. Additional correlation analyses using samples separated into IPV+ and IPV− groups produced a set of results similar to those shown by Table 2 (pooled samples), with the exception that stronger correlations between plasma IL-6 and CRP levels were seen for the IPV+ (r=0.345, p=0.025) compared to IPV− samples (r=0.015, p=0.953) for visit 1.
Finally, a correlation was also investigated between the levels of IL-6 and sIL-6R in the different biological fluids. As shown in Table 2, there was no significant correlation between plasma IL-6 and sIL6R levels (r=0.068, p=0.587 for visit 1; r=0.140, p=0.263 for visit 2), but the correlations between IL-6 and sIL-6R levels in saliva (r=0.564; p<0.001; r=0.638; p<0.001 for visits 1 and 2, respectively) and OMT (r=0.604, p<0.001; r=0.588, p<0.001 for visits 1 and 2, respectively) were statistically significant.
Relationship between Periodontal health measures and IL-6 or sIL-6R levels in Saliva and OMT
Table 3 summarizes the responses to the periodontal health assessment. Overall, only 20% of women reported potential periodontal health problems with no significant differences between the IPV+ and IPV− (control) groups. Statistical analysis showed no significant correlation between periodontal health measures and IL-6 levels in saliva or OMT. However, there was a modest and statistically significant correlation between the levels of sIL-6R in saliva (visit 1) and scores for deep gingival pockets (r=0.263, p=0.036) and a trend for gum bleeding (r=0.276, p=0.026). No such correlation was observed with sIL-6R levels in OMT.
Table 3.
Summary of Periodontal health assessment
| IPV+ group | IPV− group | Overall | |
|---|---|---|---|
| Deep pockets | |||
| 1 (yes) | 6 (13%) | 7 (21%) | 13 (19%) |
| 2 (no) | 40 (87%) | 14 (66%) | 54 (81%) |
| Gum bleeding | |||
| 1 (yes) | 1 (3%) | 2 (10%) | 3 (5%) |
| 2 (yes, on brushing) | 8 (17%) | 2 (10%) | 10 (15%) |
| 3 (no) | 37 (80%) | 17 (80%) | 54 (80%) |
| Need dental treatment | |||
| 1 (yes) | 11 (24%) | 4 (19%) | 15 (22%) |
| 2 (no) | 35 (76%) | 17 (81%) | 52 (78%) |
Responses to the Periodontal health assessment by group and overall. IPV+ (n=46); IPV− (n=21).
DISCUSSION
The aim of this work was to investigate the relationships among measurements of IL-6, sIL6R and CRP in plasma, saliva and OMT as part of a larger study of inflammatory markers in a population of middle aged women with a history of past intimate partner violence (Newton et al., 2010). Thus, we focus mainly on the levels and correlations between different fluids. Results from this study indicate that although statistically significant, there was only a relatively modest correlation between IL-6 levels in plasma with those in saliva and OMT, suggesting that IL-6 measurements in oral fluids may not closely reflect systemic levels.
Previous studies by Minetto et al. (2005) and Sjögren et al. (2005) investigated the correlation between serum and saliva IL-6 levels in post-exercise conditions and in relation to psychosocial factors, respectively. In both of these cases, no significant correlation was found, prompting the inference that saliva IL-6 measurements could not be used as an alternative to serum IL-6. Because only a very modest correlation was found in our studies, we agree with these authors that saliva or OMT IL-6 levels cannot be used as surrogates for plasma IL-6 levels. The situation with IL-6 is in contrast with that of cortisol, in which good agreement between serum and saliva levels has been reported (Kristenson et al., 1998). However, whereas salivary cortisol may originate exclusively through microfiltration of the circulating cortisol, a substantial portion of the IL-6 in oral fluids may originate locally in the salivary glands and/or the oral mucosa. The fact that the concentrations of IL-6 in OMT were 2–3 times higher than those in plasma or saliva would suggest, in fact, that the oral mucosa is able to produce IL-6. The physiological and health-related significance of IL-6 in oral fluids, if any, remains to be determined.
Because IL-6 is one of the main regulators of acute phase responses and CRP measurements are also routinely used to assess inflammatory conditions, we investigated the relationship between IL-6 concentrations in the different biological fluids with plasma CRP levels. Our data showed a low, although statistically significant, correlation between plasma IL-6 and CRP. However, no significant correlations were found between plasma CRP levels and saliva or OMT IL-6 levels. Although a stronger correlation between plasma IL-6 and CRP levels was expected, given that IL-6 is one of the main inducers of the synthesis of CRP (Hirano et al., 1990; Kishimoto, 2005), it is possible that plasma IL-6 levels may be more susceptible to change in response to physiological and psychosocial stimuli compared to CRP levels, which are likely to be the result of a more chronic, cumulative effect. Moreover, it is well known that cytokines other than IL-6, such as TNFα and IL-1, and adipokines such as resistin, also influence CRP synthesis (Calabro et al., 2005).
It is not clear why, in contrast to visit 1, no statistically significant correlation was observed between IL-6 levels in plasma and those in saliva or OMT for visit 2. The fact that there was only moderate correlation between the levels of IL-6 in saliva and OMT between visits 1 and 2 (r values of 0.513 and 0.380, respectively) suggests that there was a substantial degree of variability in saliva/OMT levels between visits, which may have been influenced, at least in part, by the anticipatory stress of the second interview, which the subjects knew would center on past abuse and violence. Indeed, anticipatory stress, worry and rumination have been associated with enhanced cardiovascular, endocrinological and immunological activity (Brosschot et al., 2006). In this regard, the average changes within samples from the same subject taken before and after interviews for visit 1 were 12.3 and 9.4% for saliva and OMT, whereas average changes for visit 2 were 22.0 and 23.3%, respectively.
Measurements of sIL-6R levels in plasma indicated a relatively tight distribution, with most subjects having concentrations of sIL-6R between 20 and 40 ng/ml. Moreover, no correlation was found between plasma IL-6 and sIL6R levels, indicating that systemic sIL6R production or release may not be regulated by IL-6. In contrast, the sIL6R levels in saliva and OMT showed a positive and statistically significant correlation with the levels of IL-6 in the same fluids, suggesting in this case that sIL-6R production may be under partial IL-6 regulation in the oral mucosa.
The significance of sIL6R in biological fluids lies in their ability to bind free IL-6 and mediate trans-signaling on cells expressing membrane gp130, even in the absence of membrane IL-6R. This type of signaling has been reported to be particularly important in a variety of inflammatory conditions (Kallen, 2002). However, another function of sIL6R might be to protect IL-6 molecules from clearance and degradation, as described for other soluble cytokine receptors (Fernandez-Botran et al., 2002). The role of sIL6R in oral fluids, however, remains to be elucidated. In this regard, it will have to be taken into consideration that the activity of IL6/sIL-6R complexes is regulated by soluble forms of gp130 (Jones and Rose-John, 2002; Kallen, 2002), so their contribution will also have to be weighed.
Periodontal disease has been reported to result in increased local levels of pro-inflammatory cytokines (Takahashi et al., 1994; Dongari-Bagtzoglou and Ebersole, 1998; Orozco et al., 2007), and thus, it may represent a potential confounding factor when measuring levels of pro-inflammatory cytokines in oral fluids. In our study, we did not observe any significant correlation between levels of IL-6 levels in saliva or OMT and periodontal health measures as assessed by a self-reported questionnaire. There was, however, a significant correlation between saliva (but not OMT) sIL-6R levels and measures related to gum bleeding and gingival pockets. These results suggest that measurements of sIL-6R levels in saliva may be more influenced by periodontal health status compared to levels in OMT.
As indicated previously, the decision to pool the IPV+ and IPV− samples for the correlation analysis was based on the overall lack of statistically significant differences between correlations coefficients calculated separately for the two groups. Exceptions were a significantly higher correlation between CRP values for visits 1 and 2 and between OMT IL-6 and sIL-6R levels (visit 1) for the IPV+ group. This suggests that some differences between the two groups may potentially exist, which may represent a limitation to our approach. However, no significant differences were found when comparing the correlations of the pooled samples with those of the IPV+ group.
In conclusion, these results demonstrate that although oral fluids may offer certain advantages for epidemiological studies, the levels of IL-6 and sIL-6R in these fluids do not seem to correlate closely with systemic levels and/or with other inflammatory markers such as CRP. However, potential changes in the levels of IL-6 in oral fluids in response to psychosocial factors still need to be further investigated.
ACKNOWLEDGMENTS
The authors thank Ms. Cristina Fernandez and Ms. Jie Zhang for their technical help. This research was funded by a grant from the National Institute on Aging (1 R21 AG0249902-01A2), and a University of Louisville Research on Women grant, and conducted with support from the University of Louisville Hospital Clinical Research Center and its staff.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
REFERENCES
- Appels A, Bär FW, Bär J, Bruggeman C, de Baets M. Inflammation, depressive symptomatology, and coronary artery disease. Psychosom. Med. 2000;62:601–605. doi: 10.1097/00006842-200009000-00001. [DOI] [PubMed] [Google Scholar]
- Betelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Blicher B, Joshipura K, Eke P. Validation of self-reported periodontal disease: A systematic review. J. Dental Res. 2005;84:881–890. doi: 10.1177/154405910508401003. [DOI] [PubMed] [Google Scholar]
- Breiding MJ, Black MC, Ryan GW. Chronic disease and health risk behaviors associated with intimate partner violence--18 U.S. states/territories, 2005. Ann. Epidemiol. 2008;18:538–544. doi: 10.1016/j.annepidem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. J. Pyschosom. Res. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brydon L, Edwards S, Mohamed-Ali V, Steptoe A. Socioeconomic status and stressinduced increases in interleukin-6. Brain Behav. Immunol. 2004;18:281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Buhlin K, Gustafsson A, Andersson K, Hakansson J, Klinge B. Validity and limitations of self-reported periodontal health. Community Dent. Oral Epidemiol. 2002;30:431–437. doi: 10.1034/j.1600-0528.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Calabro P, Chang DW, Willerson JT, Yeh ETH. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J. Am. Col. Cardiol. 2005;46:1112–1113. doi: 10.1016/j.jacc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Zautra AJ, Younger J, Motivala SJ, Attrep J, Irwin MR. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: Implications for fatigue. Brain Behav. Immunol. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the Derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcoholism: Clin. Exp. Res. 2005;29:844–854. doi: 10.1097/01.alc.0000164374.32229.a2. [DOI] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A, Ebersole JL. Increased presence of IL-6 and IL-8-secreting fibroblast subpopulations in adult periodontitis. J. Periodontol. 1998;69:899–910. doi: 10.1902/jop.1998.69.8.899. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R, Crespo FA, Sun X. Soluble cytokine receptors in biological therapy. Exp. Opin. Biol. Ther. 2002;2:585–605. doi: 10.1517/14712598.2.6.585. [DOI] [PubMed] [Google Scholar]
- Ferri RS. Oral mucosal transudate testing for HIV-1 antibodies: A clinical update. J. Assoc. Nurses AIDS Care. 1998;9:68–72. doi: 10.1016/S1055-3290(98)80062-9. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Hayney M, Love GD. Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychol. 2007;26:305–313. doi: 10.1037/0278-6133.26.3.305. [DOI] [PubMed] [Google Scholar]
- Gaillard J-P, Pugniere M, Tresca J-P, Mani J-C, Klein B, Brochier J. Interleukin 6 receptor signaling. II. Bio-availability of interleukin-6 in serum. Eur. Cytokine Netw. 1999;10:337–344. [PubMed] [Google Scholar]
- Gallo D, George JR, Fichen HH, Goldstein AS, Hindahl MS. Evaluation of a system using oral mucosal transudate for HIV-1 antibody screening and confirmation testing. JAMA. 1997;277:254–258. [PubMed] [Google Scholar]
- Grassi-Oliveira R, Brietzke E, Pezzi JC, Lopes RP, Teixeira AL, Bauer ME. Increased soluble tumor necrosis factor-α receptors in patients with major depressive disorder. Psych. Clin. Neurosci. 2009;63:202–208. doi: 10.1111/j.1440-1819.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis if coronary heart disease. Systematic review of prospective cohort studies. Br. Med. J. 1999;318:1460–1467. doi: 10.1136/bmj.318.7196.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin-6. Immunol. Today. 1990;11:443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Merz CNB, Braunstein GD, Berga SL, Bittner V, Hodgson TK, Gierach GL, Reis SE, Vido DA, Sharaf BL, Smith KM, Sopko G, Kelsey SF. Determination of menopausal status in women: The NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) Study. J. Women's Health. 2004;13:872–887. doi: 10.1089/jwh.2004.13.872. [DOI] [PubMed] [Google Scholar]
- Jones S, Rose-John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim. Biophys. Acta. 2002;1592:251–263. doi: 10.1016/s0167-4889(02)00319-1. [DOI] [PubMed] [Google Scholar]
- Kallen KJ. The role of trans-signalling via the agonistic soluble IL-6 receptor in human diseases. Biochim. Biophys. Acta. 2002;1592:323–343. doi: 10.1016/s0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: from basic science to medicine - 40 years in Immunology. Annu. Rev. Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- Krause N, Shaw BA, Cairney J. A descriptive epidemiology of lifetime trauma and the physical health status of older adults. Psychol. Aging. 2004;19:637–648. doi: 10.1037/0882-7974.19.4.637. [DOI] [PubMed] [Google Scholar]
- Kristenson M, Orth-Gomer K, Kucinskiene Z, Bergdahl B, Calkauskas H, Balinkyiene I. Attenuated cortisol response to a standard stress test in Lithuanian vs. Swedish men: The LiVicordia study. J. Behav. Med. 1998;5:17–30. doi: 10.1207/s15327558ijbm0501_2. [DOI] [PubMed] [Google Scholar]
- Lyson K, McCann S. The effect of interleukin-6 on pituitary hormone release in vivo and in vitro. Neuroendocrinology. 1991;54:262–266. doi: 10.1159/000125884. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A, Schooltink H, Heinrich PC, Rose-John S. Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute phase proteins. J. Immunol. 1992;149:2021–2202. [PubMed] [Google Scholar]
- Maes M. Major depression and activation of the inflammatory response system. Adv. Exp. Med. Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J. Affect. Disord. 2001;63:85–92. doi: 10.1016/s0165-0327(00)00156-7. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Chrousos G, Weber J. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J. Clin. Endocrinol. Metab. 1993;77:1690–1694. doi: 10.1210/jcem.77.6.8263159. [DOI] [PubMed] [Google Scholar]
- Minetto M, Rainoldi A, Gazzoni M, Terzolo M, Borrione P, Termine A, Saba L, Dovio A, Angeli A, Paccotti P. Differential responses of serum and salivary interleukin-6 to acute strenuous exercise. Eur. J. Appl. Physiol. 2005;93:679–686. doi: 10.1007/s00421-004-1241-z. [DOI] [PubMed] [Google Scholar]
- Newton TL, Fernandez-Botran R, Miller JJ, Lorenz DJ, Burns VE, Fleming KE. C-reactive Protein levels, Interleukin-6, and Past Intimate Partner Violence in Midlife Women: A Preliminary Study. J. Traum. Stress. Under Revision. 2010 [Google Scholar]
- Nishanian P, Aziz N, Chung J, Detels R, Fahey JL. Oral fluids as an alternative to serum for measurement of markers of immune activation. Clin. Diag. Lab. Immunol. 1998;5:507–512. doi: 10.1128/cdli.5.4.507-512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohzato H, Yoshizaki K, Nishimoto N, Ogata A, Tagoh H, Monden H, et al. Interleukin-6 as a new indicator of inflammatory status: detection of serum levels of interleukin 6 and C-reactive protein after surgery. Surgery. 1992;111:201–209. [PubMed] [Google Scholar]
- Orozco A, Gemmell E, Bickel M, Seymour GJ. Interleukin-18 and periodontal disease. J. Dental Res. 2007;86:586–593. doi: 10.1177/154405910708600702. [DOI] [PubMed] [Google Scholar]
- Papanicolaou D, Wilder R, Manolagas S, Chrousos G. The pathophysiology of interleukin-6 in human disease. Ann. Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Paranjape A, Liebschutz J. STaT: A three-question screen for intimate partner violence. J. Women's Health. 2003;12:233–239. doi: 10.1089/154099903321667573. [DOI] [PubMed] [Google Scholar]
- Paranjape A, Rask K, Liebschutz J. Utility of STaT for the identification of recent intimate partner violence. J. Natl. Med. Assoc. 2006;98:1663–1669. [PMC free article] [PubMed] [Google Scholar]
- Peake NJ, Khawaja K, Myers A, Nowell MA, Jones SA, Rowan AD, Cawston TE, Foster HE. Interleukin-6 signalling in juvenile idiopathic arthritis is limited by proteolytically cleaved soluble interleukin-6 receptor. Rheumatology. 2006;45:1485–1489. doi: 10.1093/rheumatology/kel154. [DOI] [PubMed] [Google Scholar]
- Pitiphat W, Gracia RI, Doluglass CW, Joshipura KJ. Validation of self-reported oral health measures. J. Public Health Dent. 2002;62:122–128. doi: 10.1111/j.1752-7325.2002.tb03432.x. [DOI] [PubMed] [Google Scholar]
- Preussner J, Wolf O, Hellhammer D, Buske-Kirschbaum A, Von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- Sjögren E, Leanderson P, Kristenson M, Ernerudh J. Interleukin-6 levels in relation to psychosocial factors: Studies on serum, saliva, and in vitro production by blood mononuclear cells. Brain, Behav. Immun. 2005;20:270–278. doi: 10.1016/j.bbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Warren WL. The Conflict Tactics Scales Handbook. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Takahashi K, Takashiba S, Nagai A. Assessment of interleukin-6 in the pathogenesis of periodontal disease. J. Periodontol. 1994;65:147–153. doi: 10.1902/jop.1994.65.2.147. [DOI] [PubMed] [Google Scholar]
- Tjaden P, Thoennes N. Extent, nature, and consequences of intimate partner violence. Washington, D.C: U.S. Department of Justice, National Institute of Justice; 2000. [Google Scholar]
- Ward LD, Howlett GJ, Discolo G, Yasukawa K, Hammacher A, Morotz EL, Simpson RG. High affinity interleukin-6 receptor is a hexameric complex consisting of two molecules each of interleukin-6, interleukin-6 receptor and gp130. J. Biol. Chem. 1994;269:23286–23289. [PubMed] [Google Scholar]