SUMMARY OF RECENT ADVANCES
The roles of the basal ganglia (BG) in motor control are much debated. Many influential hypotheses have grown from studies in which output signals of the BG were not blocked, but pathologically-disturbed. A weakness of that approach is that the resulting behavioral impairments reflect degraded function of the BG per se mixed together with secondary dysfunctions of BG-recipient brain areas. To overcome that limitation, several studies have focused on the main skeletomotor output region of the BG, the globus pallidus internus (GPi). Using single-cell recording and inactivation protocols these studies provide consistent support for two hypotheses: the BG modulates movement performance (“vigor”) according to motivational factors (i.e., context-specific cost/reward functions) and the BG contributes to motor learning. Results from these studies also add to the problems that confront theories positing that the BG selects movement, inhibits unwanted motor responses, corrects errors online, or stores and produces well-learned motor skills.
Introduction
What are the functions of the Basal Ganglia (BG)? Despite decades of intense study and mushrooming volumes of experimental results, the question is still widely debated. Indeed, there sometimes seem to be as many hypotheses as there are groups working on the subject. Among the most influential hypotheses, one may cite: selection of action and suppression of potentially competing actions and reflexes [1–3], control of the scale of movement and related cost functions [4•,5••,6••], online correction of motor error [7–8], motor learning [9–10,11••], and the retention and recall of well-learned or natural motor skills [10,12–13,14••]. Note that this list is not exhaustive nor are all of the hypotheses mutually exclusive. These hypotheses are elaborated in the references cited above. The present review summarizes recent experimental results that, in our opinion, buttress a subset of the hypotheses and add to the list of difficulties that challenge many of the others.
Function versus Dysfunction
The desire to understand normal functions of the BG is driven, in part, by the many neurologic and psychiatric disorders associated with pathology or abnormality within the BG. The examples of Parkinson’s disease (PD [15]), Huntington’s Disease (HD [16]), types of dystonia [17] and Tourette’s syndrome [18] illustrate the fact that most BG-associated clinical conditions involve some form of striatal dysfunction. In other words, clinical signs occur when the principal input nucleus of the BG network is affected (Box 1). Interestingly, a very different outcome is observed following discrete lesions of the main output regions of the BG [the globus pallidus internus, GPi, or substantia nigra pars reticulata, SNr (Box 1)]. In that case, behavioral effects are typically subtle or imperceptible [4•,19], consistent with the fact that surgical ablation of large portions of the GPi (“pallidotomy”) is an effective treatment for striatal-associated disorders such as PD and dystonia [20–21,22•].
Box 1 . Basal Ganglia Anatomy.
Two organizing principles guide our understanding of the roles of the BG in the control of movement and other aspects of behaviors. Recent advances corroborate the overall validity of these classical concepts. (For detailed reviews of BG anatomy see [1, and 104].) First, all regions of the BG shares a common basic circuit plan (Box 1, Fig. a). The striatum, principal input nucleus of the BG, receives massive excitatory inputs from most cortical areas and from particular thalamic nuclei (the intralaminar nuclei, primarily). Direct and indirect pathways through the BG originate in the striatum and converge ultimately in the primary output nuclei of the BG, the globus pallidus internus (GPi) or the substantia nigra reticulata (SNr). In a major recent advance, years of debate have been resolved by confirmation that the direct and indirect pathways originate from biochemically- and morphologically-distinct types of striatal projection neurons [97••,105]. Consistent with the classical model, direct and indirect pathway neurons of the striatum express D1- and D2-type dopamine receptors, respectively. It has also become clear, however, that neurons of the direct and indirect pathways collateralize far more than proposed in classical models [106] or summarized here. The second major source of input to the BG arises from excitatory projections from the frontal cortices to the subthalamic nucleus (STN). The principal output pathway from the BG consists of GABAergic projections from the GPi and SNr, which tonically inhibit targets in the thalamus and brainstem.
Second, parallel “loop” circuits from cortex, through the BG, thalamus and back to cortex mediate distinct motor, associative, and limbic functions (Box 1, Fig. b). Different regions of the striatum, GPe, and STN are devoted to these different functions. The circuit that projects to the motor cortices (i.e., the “skeletomotor circuit”) passes through a posterior-ventral region of GPi. Circuits sending information to prefrontal “associative” cortical areas occupy more anterior and dorso-medial regions of the GPi and portions of the SNr. Limbic circuits pass primarily through the SNr. Debate continues on the degree to which information is shared between functional circuits. For example, a recent study showed that sub-regions of the BG circuit that projects to the primary motor cortex receives inputs from limbic cortical areas [107], thereby opening the possibility for relatively direct communication of motivation-related information to motor cortex. The general concept that anatomically-segregated circuits through the BG contribute to different aspects of behavior has been confirmed in recent years by a series of studies showing that pharmacologic activation of different functional circuits elicits behavioral disorders consistent with the circuit being activated [108•]. The existence of multiple closed loop circuits makes it clear that the BG contributes not only to the control of movement, but also to functions such as executive control, working memory, and motivation. The parallel circuit architecture and the common basic design of each circuit has led many to propose that different circuits perform analogous operations on different types of information. For this reason, understanding the operations of one circuit (e.g., how the skeletomotor circuit transforms the information it receives) is likely to shed light on the operations performed by other BG circuits as well.
Together, these observations can seem paradoxical. BG-associated disorders arise primarily from pathology in the principal input nucleus, the striatum, and can be alleviated by lesions of a BG output nucleus. The seeming contradiction can be explained by the concept that it is better to block BG output completely than allow faulty signals from the BG to pervert the normal operations of motor areas that receive BG output [15]. Abnormalities in striatal function, whether from frank lesions [23–24] or neurotransmitter imbalance [25–27], induce grossly-abnormal “pathologic” patterns of neuronal activity in the inhibitory output neurons of the BG. These abnormal firing patterns are thought to disrupt the normal operations of BG-recipient brain regions. Although the actual mechanisms mediating that disruption remain to be determined, one possibility supported by biologically-realistic computational models [28••,29••] is that pathologic firing patterns in BG-thalamic afferents degrade the ability of thalamic neurons to transmit information reliably. In this way, pathologic BG output may block effective cortico-thalamo-cortical communication [30]. In agreement with this idea, therapeutic deep brain stimulation (DBS) within GPi or the subthalamic nucleus (source of excitatory input to the BG output nuclei, GPi and SNr; Box 1) has been shown to reduce pathologic firing patterns in BG efferent neurons [31–32]. Moreover, the therapeutic efficacies of different forms of DBS (stimulation at different frequencies and degrees of regularity) correlate well with their ability to restore the fidelity of cortico-thalamic communication in computational models [29••]. Results from functional imaging studies are also consistent with this idea. Pallidotomy and DBS normalize patterns of brain activity in non-BG brain regions [33–34]. Abnormalities in GPi activity also change toward normal firing patterns during effective pharmacotherapy [35].
In summary, growing evidence suggests that the therapeutic efficacy of pallidotomy, and DBS as well most likely, comes from its ability to block the spread of pathologic activity from the BG to other brain regions. A corollary of this insight is that many of the symptoms of BG disorders, and the behavioral sequelae of experimental manipulations of the striatum, represent dysfunctions of BG-recipient brain regions rather than ‘negative images’ of normal BG functions. This view runs contrary to a frequent assumption that the primary problem in these disorders is loss of normal BG functions (i.e., loss or corruption of the normal task-related information transmitted through the BG). As a consequence, it is difficult to infer normal functions of the BG accurately from the behavioral impairments that accompany clinical disorders or experimental manipulations of the striatum. The possibility that a subset of clinical signs may reflect normal BG functions is considered below.
Timing and Characteristics of BG Output Signals
The loop organization of BG-thalamocortical circuits makes it difficult to disentangle the relative roles of different stages of the circuit. One productive approach to this problem has been to investigate how the BG circuit ‘transforms’ the information it receives from cortical and thalamic inputs. Ultimately, this amounts to determining the nature and timing of information encoded in the activity of BG output neurons. Current understanding regarding this point can be summarized as three key facts about the BG circuit devoted to skeletomotor function.
First, movement-related changes in firing in GPi are almost always influenced by specific characteristics of a movement such as its direction, amplitude, and speed (i.e., movement kinematics) [36, and references therein]. However, motor activity in GPi neurons is also often influenced by the context of the behavioral task being performed. Single-cell responses in GPi can differ depending on the memory requirements of a task [37], whether the movement is discrete or part of a movement sequence [38], the reward contingencies of the task (i.e., whether or not a primary reward is expected to follow the movement [39]), and the learning context [40]. Similar influences of behavioral context have been observed in the oculomotor circuit in animals performing eye movement tasks [3]. These observations suggest that the BG motor circuit is not involved directly in movement execution, but rather that it brings cognitive and motivation-related signals together with signals related to movement kinematics [37].
Second, during the performance of a choice reaction time task, peri-movement changes in neuronal activity begin later in the striatum and globus pallidus than in connected regions of cortex. In GPi, for example, onset latencies of peri-movement changes in neural firing are typically clustered around the time of earliest agonist muscle activity (“EMG”; 50–80 milliseconds before movement onset; see [36] for references) and after the activation of primary motor cortex (~120 milliseconds before movement). Interestingly, peri-movement increases in GPi firing have later onset times than peri-movement decreases [36], a point that will be revisited later. The timing of movement-related activity in GPi makes it impossible for GPi output to contribute to processes that are completed prior to the initial activation of a movement’s prime moving muscles (e.g., selecting which agonist muscles to activate or triggering their activation). Based on timing, GPi activity may modulate the ongoing commands issued by BG-recipient motor control centers.
Third, movement-related changes in discharge consist of an increase in firing in 60–80% of GPi neurons (the exact percentage varying between behavioral tasks) [36,41]. Given that increases in GPi firing inhibit activity in recipient motor control circuits, this observation has been cited as evidence that an important function of output from the BG motor circuit is to suppress or inhibit patterns of motor activity and reflexes that would be inappropriate or in conflict with the movement being performed [1–3]. The late timing of peri-movement GPi activity appears to conflict with that concept, particularly that of increases in firing [36],. To be more specific, the rest activity of antagonist muscles [42–43] and the gain of reflexes that might interfere with a desired movement [44–45] are suppressed tens of millisecond before activation of a movement’s prime moving muscle. At the cortical level, suppression of potentially-competing activity patterns also begins before the initial activation of agonist muscles [46–47]. Thus, the known inhibitory processes that contribute to movement selection begin too early to be mediated by output from the BG. Cortical mechanisms may mediate most aspect of movement selection [6••,48]. A potential role for GPi movement-related activity in the control of movement vigor is discussed below.
Interrupting BG Output
A complementary approach to disentangling BG functions is to determine what aspects of motor behavior are impaired and, just as importantly, spared following transient inactivation or permanent lesion of the GPi. Because the GPi is the principal output nucleus for the BG skeletomotor circuit, inactivation of the skeletomotor region of the GPi essentially disconnects the BG from the rest of the motor control apparatus (Box 1). Several studies over the last three decades have investigated the effects of GPi inactivation on motor performance in neurologically normal animals [4•,49–54,55••]. Although very different motor tasks were used and minor disparities were sometimes noted [4•], results from these studies are surprisingly consistent. Overall, they reveal a relatively discrete group of deficits and a wide range of preserved functions. Five points are particularly noteworthy.
First, GPi inactivation does not lengthen reaction times (RTs; Fig. 1e [4•,50–52]), consistent with the frequent clinical observation that pallidotomy, if anything, speeds movement initiation [21,22•]. These observations are not consistent with the idea that the BG contributes to the selection or initiation of movement.
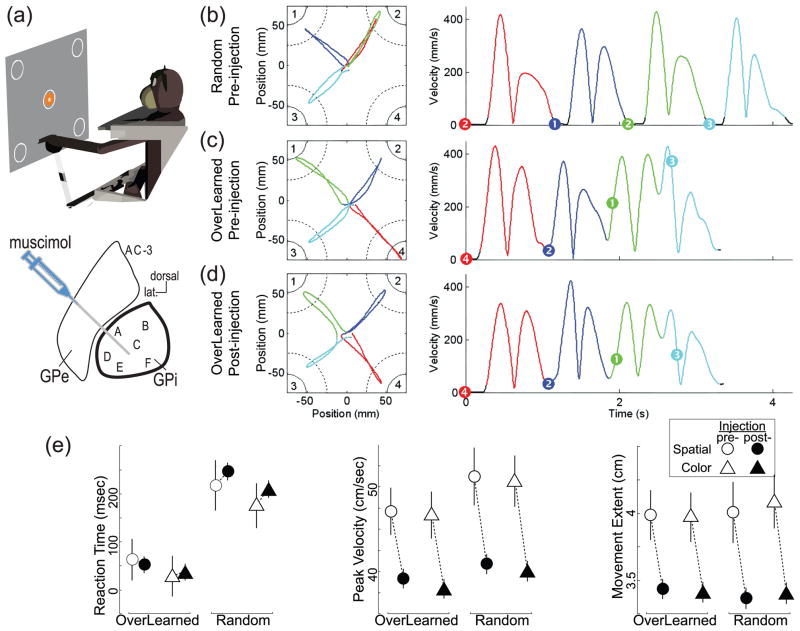
Figure 1.
Disconnection of the BG skeletomotor circuit does not impair movement initiation or performance of an overlearned motor sequence, but selectively affects movement speed and extent. Animals moved a joystick (a, top) through a series of four out-and-back component movements ((b-d) red, blue, green, and cyan traces, respectively) before and after an injection of muscimol (a long-acting GABAergic inhibitory agent) into the GPi. (a, bottom) illustrates sites of injections (letters) in a typical coronal plane through GPe and GPi. Performance is illustrated for single trials under the Random pre-injection (b), OverLearned pre-injection (c) and OverLearned post-injection (d) conditions. The left and right panel show position and velocity data, respectively. Black sections of the velocity curves indicate periods of immobility (velocity<25-mm/s). Left: Continuous arcs in corners indicate positions of the instruction cues. Dotted arcs indicate the peripheral target zones for cursor movements. Right: Dots on the velocity curves indicate the instant of presentation of the instruction cue. Under the OverLearned condition (c), outward movements to capture a peripheral target were often anticipatory, beginning before the instruction cue was presented, and this anticipatory performance persisted post-injection (d). Numbers define targets (left) and which target was indicated by each instruction cue (right). The figures are scaled to show the central region of the workspace. (e) Inactivations had a negligible effect on reaction times [RTs; left, compare pre-injection (open symbols) versus post-injection means (filled symbols)]. This was true irrespective of whether animals performed OverLearned sequences or Random sequences, or whether the target to capture was indicated by a cue’s spatial location (circles) or its color (triangles). In contrast, muscimol injections consistently reduced movement velocity (middle) and extent (right) under all conditions. Symbols indicate means±SEM from 19 separate injections of muscimol into the contralateral GPi of two animals. (Fig. 1b-d is from [55••] used with permission from the Society for Neuroscience. Fig. 1e is adapted from [109].)
Second, GPi inactivation does not perturb on-line error correction processes [4•] or the generation of discrete corrective sub-movements in a single-joint movement task [52]. These findings are consistent with the observation that rapid hand-path corrections are preserved in PD patients [56], but present challenges for the idea that the BG mediates the on-line correction of motor error [7–8].
Third, GPi inactivation does not affect the execution of overlearned or externally-cued sequences of movements. This was shown in two recent studies in monkeys [4•,55••](Fig. 1b-e). The animals were trained to perform four out-and-back reaching movements in quick succession toward four possible target locations. The targets were either chosen at random with replacement (Random) or presented in an immutable, completely predictable order (OverLearned). Before GPi inactivation, the animals practiced both tasks for 6 months and more than 50,000 trials. At the end of this intensive training, task performance was very different for the two experimental conditions. Under the Random condition, the animals stopped after each movement and a standard RT (~190-ms) was observed following presentation of each target. Under the OverLearned condition, there was little or no pause between component movements of the sequence and, in a majority of trials, RTs were clearly predictive (<100-ms) or even negative (i.e., initiated prior to target presentation). Transient inactivations in the skeletomotor territory of GPi using muscimol, a GABAA agonist, impaired specific facets of motor performance (see below), but had absolutely no effect on sequencing-related aspects of task performance. In particular, GPi inactivation did not affect an animal’s ability to chain independent movements together in quick succession (under the Random condition) or to reproduce an OverLearned sequence as a fluid predictive arpeggio. Importantly, GPi inactivation did not alter the animals habit-like tendency to reproduce the OverLearned sequence as a predictive whole when, by coincidence, the initial targets of a Random trial matched the OverLearned sequence. These results are consistent with a previous study showing that GPi blockade does not impair the reach-to-retrieval transition in a simple reach-grasp-and-retrieve task [54]. They also agree with reports that pallidotomy does not impair the execution of well-learned motor skills in patient populations [21,22•] and the consistent observation that lesions of the BG homolog in the song-bird have little impact on the execution of already-learned song sequences [9]. By contrast, these finding contradict claims, based on neuroimaging and clinical evidence, that the BG is involved in the long-term storage of overlearned motor sequences [13] or the ability to string together successive motor acts [56].
Fourth, GPi inactivation reduces movement velocity and acceleration. This is, without a doubt, the most consistent impairment found across studies [4•,49,51,53–54,55••](Fig. 1e). This slowing mirrors the bradykinesia commonly observed in PD patients [57••]. It is interesting to note that bradykinetic-like slowing has also been observed as a sequela of pallidotomy in previously non-bradykinetic PD patients [58] and as a common side-effect of DBS of the GPi for dystonia, HD or Tourette’s syndrome [59,60•]. An earlier study in neurologically-normal monkeys showed that DBS-like stimulation of the GPi slows movement and reduces the magnitude of movement-related EMG without affecting movement accuracy or the sequential organization of agonist-antagonist activity [49]. Opinions still differ on whether inactivation-induced slowing arises primarily from increased muscle co-contraction [51,53], consistent with the suppression hypothesis, or an under-scaling of the motor commands sent to the muscles [4•,49].
Fifth, for fast targeted movements, GPi inactivation causes a marked hypometria (undershooting of the desired movement extent, Fig. 1e) that is a consistent across directions of movement but is not accompanied by changes in movement linearity or directional accuracy [4•,53]. The degree of hypometria induced by an inactivation correlates closely with early markers of movement slowing (peak velocity, acceleration, and agonist EMG) [4•]. A similar form of global hypometria with no directional bias is observed in PD patients [61–62]. These results present challenges for the suppression hypothesis in which movement-related increases in GPi activity are proposed to inhibit competing motor commands and reflexes [51,54]. It is difficult to conceive of a general disturbance in motor command selection or muscle agonist/antagonist balance that would affect movement extent and speed equally for all directions of movement, but have no effect on the initial direction of movement, hand-path curvature, or final directional accuracy [4•,53].
BG and movement gain
Many of the observations summarized above can be explained by a classic and seemingly simplistic concept that the BG regulates the speed and size of movement (i.e., “movement gain” [49,63]). This concept arose first from observations that clinical disorders of the BG are marked by a deficient scaling of the initial burst of agonist EMG to meet the demands of a motor task [63–64]. Parkinson’s disease, for example, is associated with impaired gain control in reaching (bradykinesia and hypometria [61]), hand-writing (micrographia [62•]), and speech (hypophonia [65]). Divining the functional significance of this impairment is complicated, however, by the difficulties of inferring normal functions of the BG from clinical disorders of the striatum (see above).
The movement gain hypothesis has been rejuvenated by a convergence of results from theories of motor control [6••], studies of motivation and decision-making in rodents [66], and new insights into the motor impairments associated with BG disconnection [4•,5••,55••]. A series of single unit recording studies provided evidence consistent with the gain hypothesis by showing that movement-related activity in the pallidum is frequently correlated with the amplitude or velocity of limb movements (see [37], and references therein), although not all studies supported that conclusion [e.g., 67]. Corroborating evidence has come from a remarkable number of neuroimaging studies in healthy humans demonstrating close relationships between brain activation in skeletomotor regions of the BG and gain adjustments or adaptations for a variety of different motor tasks and end effectors (for recent examples, see Fig. 2 and [68•,69,70•,71]). Together, these studies provide evidence that activity in the BG skeletomotor circuit encodes information related to motor gain. It is important to recognize, however, that this encoding is not exclusive in that activity in the circuit encodes other behavioral and sensory dimensions as well [e.g., 8,37,38,39,67]. Furthermore, recording and imaging approaches are correlative and provide little insight into how information encoded in the BG is used by downstream BG-recipient centers. Thus, complementary experimental approaches are required.
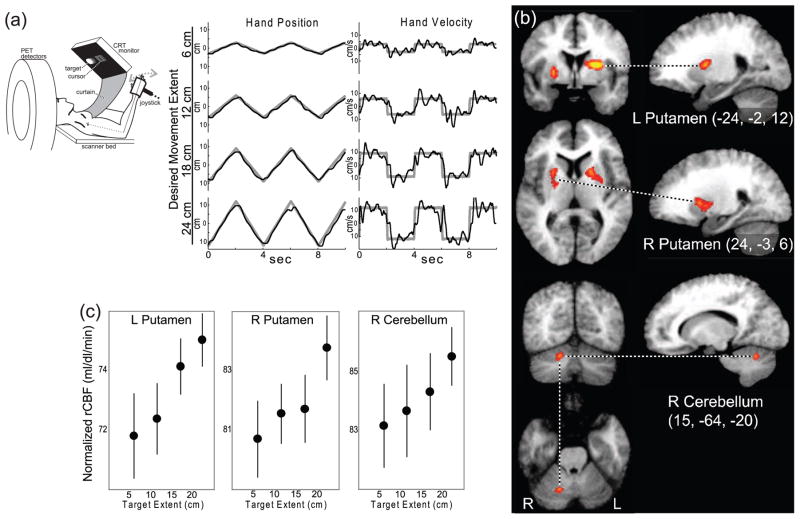
Figure 2.
Activity in skeletomotor regions of the BG correlates closely with movement gain (extent and velocity). (a) Healthy human subjects performed a continuous visuo-manual tracking task by moving a hand-held joystick (black traces illustrate representative performance of one subject) to follow constant-velocity displacements of an on-screen target (gray traces). The extent and velocity of hand movements differed between scans by training subjects during periods between scans on one of four different joystick-to-cursor scaling factors. (b) Areas of increasing cerebral blood flow (CBF) with increasing movement gain are shown in orange-yellow (P<0.001 uncorrected). Significant changes were identified at only three sites: left dorsal putamen (upper panel), right dorsal putamen (middle panel), and right cerebellum (lower panel). (c) Brain activity (normalized CBF mean±SEM) increased monotonically with movement extent at the identified sites in the BG and cerebellum. (Adapted from [71] with copyright permission from the American Physiological Society.)
An independent line of evidence regarding the gain hypothesis originates from behavioral observations that, at certain stages of motor planning, “movement gain” (the extent and speed of movement in a given workspace) is controlled independently of movement direction [72,73, and references therein]. Consistent with those observations, current models of motor control recognize the need for a mechanism that identifies optimal balances between the “costs” of movement (e.g., physical work, elapsed time, and control complexity) and the rewards available in a given behavioral setting [6••,74]. Motor cost terms, which scale with velocity, amplitude, and other aspect of motor performance, may link an animal’s previous experience of the cost/benefit contingencies of a task [75] to it’s current allocation of energy to meet the demands of a specific task [57••,66]. We and others have conjectured that a breakdown in that link would yield motor impairments similar to those observed following GPi inactivation [4•,6••]. In essence, the BG motor circuit may compute and store cost functions that modulate motor performance based on an animal’s previous experience of the requirements of a task and the rewards available.
This role for the BG motor circuit is consistent with an emerging view that the BG as a whole, including its dopaminergic innervation, regulates action motivation or response “vigor” [66,75]. Limbic circuits of the BG, for example, have been implicated in the appropriate scaling of a subject’s rate of responding or choice of effortful responses to match the cost/benefit tradeoff of a task [76,77•]. This idea is supported further by observations that focal damage in the BG is often accompanied by abulia or “auto-activation deficit” in which patients suffer from a marked deficit in motivation to perform spontaneous acts despite an absence of overt motor impairment [78]. Schmidt et al. [5••] provided a striking demonstration of this disorder by showing that patients with bilateral lesions of the putamen or pallidum are able to control grip forces normally in response to explicit sensory instructions, but do not increase grip force spontaneously despite full understanding that higher forces will earn them more money. The authors concluded that BG lesions specifically block the influence of task incentives on movement vigor.
From this perspective, parkinsonian bradykinesia and hypometria become candidates for the subset of motor signs that actually do reflect normal functions of the BG (unlike, e.g., akinesia). Building on this idea, Mazzoni and colleagues [57••] presented evidence that PD patients are capable of moving as fast as healthy subjects, but that they implicitly prefer to move more slowly, thereby expending less energy. Mazzoni et al. concluded that parkinsonian bradykinesia reflects an impairment in the link between motivation and the control of movement gain (e.g., “movement vigor,” [57••]). Alternate accounts, such as the proposal that parkinsonian bradykinesia is a byproduct of selective impairment of internally-generated movements [79], are not supported by recent studies showing that sensory cues and urgent conditions increase movement speed equally in healthy subjects and PD patients [70•,80]. Parkinsonian subjects in these studies were systematically slower than healthy subjects across all conditions, suggesting that the link between motivation and movement gain is weakened universally in PD, irrespective of other aspects of the behavioral context. The slowing and hypometria induced by experimental inactivation of GPi are similarly immune to many aspects of behavioral context (e.g., differences in memory contingency and sensory cueing [4•,53,55••]).
The hypothesis that BG modulates movement gain has been challenged on the grounds that movement-related activity in the striatum and globus pallidus begins later than activity in motor regions of cortex [15]. Both psychophysical [81–82] and electrophysiological [83–84] evidence suggests, however, that movement gain can be modulated after the earliest stages of movement initiation. Furthermore, electrical stimulation of the GPi can modify the speed of reaching movements even when stimulation is delivered solely at the time of agonist EMG onset (i.e., at latencies similar to those of movement-related GP activity [49]). Thus, the latencies of movement-related activity in the GPi may be appropriate for a role in modulating movement gain. BG output may exert its scaling influence both at the cortical level (via thalamo-cortical pathways) and at brainstem and spinal motor centers via descending outputs from GPi (Box 1).
BG and learning, but not retention
Growing evidence suggests that the connectivity and physiology of the BG is ideally-suited for fast “directed” formation of reward-relevant associations, which over the course of practice train slower Hebbian learning in thalamocortical circuits [7,85••]. This view predicts that BG circuits are intimately involved in and necessary for new skill learning, but are of far less importance in the retention and recall of well-learned motor skills. This view constitutes a major revision of the long-standing and highly influential theory that memory traces underlying motor skills are stored long-term in the BG [14••,86]. The general concept that the BG and its dopaminergic innervation play central roles in many different forms of learning is noncontroversial and supported by a vast literature (for recent reviews, see [10,14••,87]). This section focuses on evidence related to the concept that BG circuits may be involved selectively in reward-driven acquisition, but not in long-term retention or recall of well-learned motor skills.
Single unit recording studies have demonstrated major changes in neuronal activity in the BG as animals learn procedural tasks [88–90], and a few of these studies provided evidence that learning-related activity appears earlier in the course of learning in the striatum than in connected regions of cortex [89–90]. Importantly, several reports have indicated that, after a motor skill becomes well-learned, the prevalence of task-related activity in the motor striatum declines and neuronal response latencies shift to follow movement onset (see [91•] for references). These studies suggest that the BG motor circuit is activated preferentially during the learning process. Note that some task-related activity is still present in the BG in over-trained animals [38,88,91] thereby suggesting either that BG activity is not involved solely in learning or that a certain degree of learning persists even in overtrained animals.
As mentioned earlier, pallidotomy is an effective therapy for PD and dystonia with few deleterious side-effects [21,22•], even following bilateral surgery [20]. One of the sequelae most consistently associated with pallidotomy is an impaired ability to learn new motor sequences [22•,92] and arbitrary stimulus-response associations [e.g., 93]. An important but often overlooked point is that pallidotomy does not degrade, but typically improves, a patient’s ability to perform overlearned motor skills such as grooming, dressing, and handwriting (many of which are assayed by the “activities of daily living” scale, see pallidotomy references above). Given that pallidotomy produces large well-localized lesions centered on the motor territory of GPi, these studies provide some of the best evidence that BG output is necessary for motor skill learning, but not for the retention and recall or well-practiced skills.
Combined with the observation that transient inactivation of the GPi does not degrade the performance of well-learned skills in neurologically-normal animals [4•,55••], these observations suggest that the BG functions as a kind of tutor, being important for learning, but not for storage or recall of already-learned information. It is likely that the motor cortices play a central role in the long-term retention and recall of skills based on the evidence for slow synaptic modification [94], the emergence of task-specific activity [95], and even macro-scale reorganization at the cortical level [96] in response to long-term training on a skill. This concept fits well with the idea that the responses of nigrostriatal dopamine neurons mediate fast reinforcement-driven synaptic plasticity in the BG [97••]. Cortical plasticity appears to be inherently slower than striatal plasticity because it is insensitive to phasic dopaminergic training signals and thus governed by Hebbian learning rules [98]. Long-term retention at the cortical level may bring advantages, however, due to greater processing efficiency (i.e., lower conduction times and numbers of synaptic delays [85••]).
A tutor-like role for the BG is also supported by research on the neural basis of song-learning in birds. The song behaviors of birds bear many similarities to the sequential motor skills of mammals [99] and homologues have been identified in the bird anterior forebrain pathway (AFP, Fig. 3a) for most components of the mammalian BG-thalamocortical system [100]. Of greatest significance here, disconnection of the AFP completely blocks a young bird’s ability to learn a new song, but the same lesion has virtually no effect on an older bird’s ability to execute well-learned ‘crystallized’ songs [9]. AFP lesions or stimulation in adults, while not disrupting song production, do interfere with experience-dependent plasticity of song [11••,101•]. In a recent example of this, Andalman et al. [11••], showed that tetrodotoxin (TTX)-induced disconnection of the AFP blocks the expression of adaptive changes to a song that are newly-acquired (i.e., within hours of acquisition, Fig. 3b-g), but has little effect on adaptive changes after ~24 hours. The authors hypothesize that learned changes in song are represented initially in the AFP, but become incorporated into motor execution pathways by ~24 hours post-learning. Other recent studies suggest that the AFP promotes song learning by introducing variability in song performance [101•] which drives plasticity at the cortical level [102].
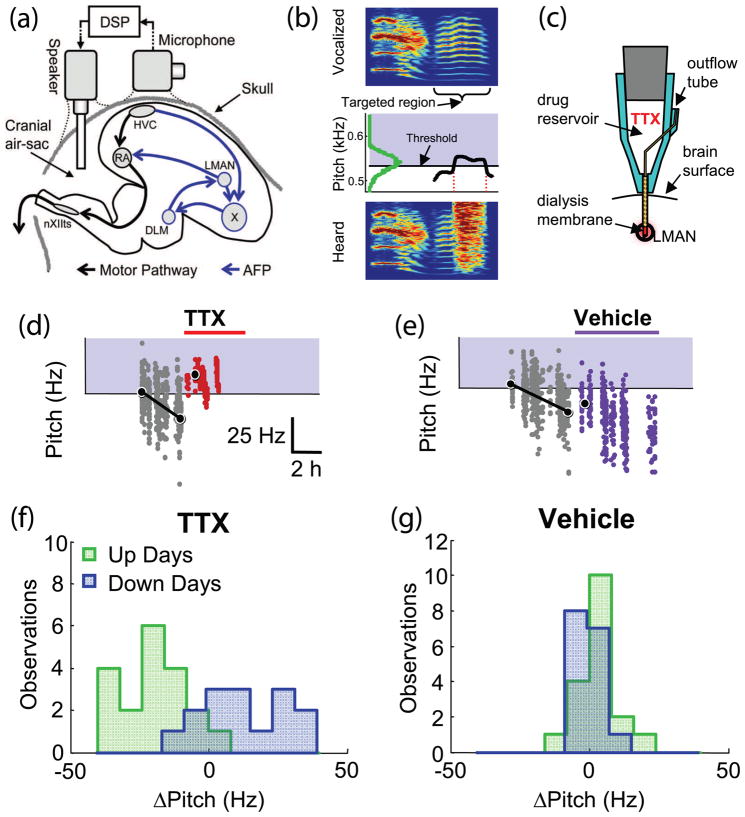
Figure 3.
Disconnection of the BG homologue in the songbird blocks the expression of newly-acquired song adaptive changes. (a) The bird anterior forebrain pathway (AFP) contains homologues to most structures of the mammalian BG. Output from the AFP affects motor execution pathways via the premotor-like LMAN nucleus. Andalman et al. [11••] perturbed singing selectively using a head-mounted microphone and speaker system. (b) White noise perturbations were delivered when the fundamental frequency of one song syllable (“Targeted region”) crossed a specific pitch threshold (red vertical lines, middle panel). The white noise burst grossly altered the song heard by the animal (bottom). On different days, the noise perturbation either targeted pitches above the mean syllable frequency (“Down days”, illustrated in (b)) or pitches below the mean (“Up days”, not shown). (c) Tetrodotoxin (TTX) was infused into LMAN bilaterally using the reverse microdialysis technique. (d) Before TTX infusion, animals responded to noise perturbations by progressively changing the fundamental frequency of the targeted syllable so as to avoid the perturbation. TTX infusion resulted in an immediate loss of that adaptive change. (e) Infusion of vehicle alone had no effect on noise-avoiding adaptive changes. (f-g) TTX infusions caused rapid maladaptive changes in the targeted syllable’s fundamental frequency (i.e., an increase in pitch on “Down days” and a decrease on “Up days”). (Adapted from Figures 1 and 2 of [11••] with permission from the authors and the National Academy of Sciences.)
To summarize, multiple lines of evidence indicate that the BG promotes new skill learning, but that other parts of the brain (cortex in particular) take over the storage and production of well-practiced skills. The unique neuromodulatory milieu of the striatum provides an ideal substrate for rapid reinforcement-driven plasticity, but cortex is better suited for long-term retention and execution. The tutor-like role proposed for the BG in skill learning is analogous the role proposed for medial temporal areas in the acquisition of declarative memories, but not their long-term storage [103].
Conclusions
Over the last three decades, a remarkable range of motor functions have been proposed for the BG. In this review we suggest that two of those hypotheses stand up particularly well to detailed scrutiny: (1) the specification or communication of cost functions related to movement gain and (2) motor learning. Recent experimental results present serious challenges to alternative hypotheses that the BG is involved in movement selection, inhibition of unwanted motor responses, on-line error correction, or the production of overlearned motor skills. Clearly, additional studies are needed to test and extend the ideas outlined here. In particular, the relationship between cost functions and motor learning requires elucidation. If the motor circuit does regulate specific cost functions related to movement gain, then is the circuit’s involvement in learning also restricted to vigor-related cost functions? A more likely alternative is that the BG motor circuit facilitates the learning of a wide gamut of different aspects of motor function, but for overlearned skills such as reaching, most aspects of motor function are controlled at the cortical level and the BG’s involvement is restricted largely to the regulation of movement gain. Why the BG maintains preferential involvement in the control of movement gain might be related to the close relationships between movement gain and the often varying costs and benefits presented by different tasks and environments. Another major unanswered question is how fast reinforcement-driven plasticity in the BG might facilitate learning at the cortical level. Ashby and colleagues proposed that BG-thalamic inputs aid Hebbian learning in cortex by coordinating the co-activation of appropriate pairs of pre- and post-synaptic cortical neurons [85••]. Although the general feasibility of these ideas is supported by computational modeling [85••], they have yet to be tested empirically.
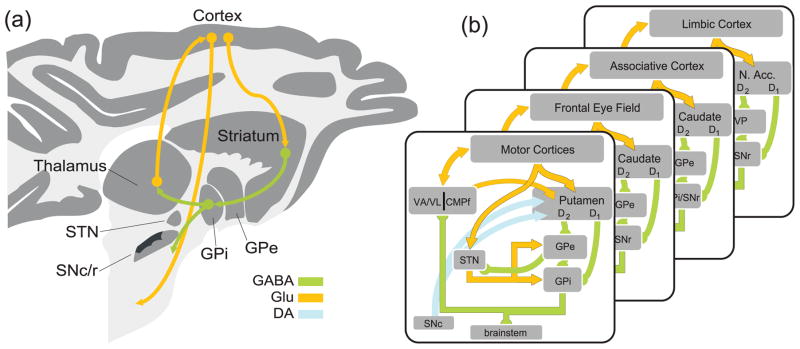
Box 1 Figure.
Circuit diagrams of the BG and associated input–output connections. (a) The positions of key BG structures involved in skeletomotor control and their basic input-output connectivity superimposed on a parasagittal section through the macaque brain. The basic loop circuit includes an excitatory glutamatergic (Glu) projection from the neocortex to the striatum (caudate nucleus and putamen) and then inhibitory (γ-amino butyric acid-containing; GABAergic) striatal projection (the ‘direct pathway’) to the internal globus pallidum (GPi). GABAergic neurons in GPi project to targets in the thalamus and brainstem. The main thalamic target of this circuit (VA/VL, ventral anterior/ventrolateral nucleus of the thalamus) projects to the frontal cortex including parts of the premotor and primary motor cortex. (b) Internal connectivity of the BG motor circuit (front sub-panel) showing principal pathways only. Direct and indirect pathways start in projection neurons of the putamen (part of the striatum) that express D1- and D2-type dopamine receptors, respectively. D2-type neurons project to the external globus pallidus (GPe). GPe projects to the subthalamic nucleus (STN) and GPi. STN also receives monosynaptic Glu input from the motor cortices and projects to GPi and GPe. GPi sends GABAergic projections to VA/VL and the centre median–parafascicular intralaminar complex (CMPf) of the thalamus. CMPf closes another loop by projecting back to the striatum. GPi also projects to brainstem regions such as the pedunculopontine nucleus. Dopaminergic (DA) neurons of the substantia nigra pars compacta (SNc) innervate the striatum and, less densely, the GP and STN. Successive subpanels represent the parallel BG circuits that sub-serve oculomotor, associative, and limbic functions. Note that these circuits pass through anatomically-distinct regions at each stage, including different regions of the STN and thalamus (not shown in figure).
Acknowledgments
This work was supported by P01 NS044393 to RST.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robert S. Turner, Email: rturner@pitt.edu.
Michel Desmurget, Email: michel.desmurget@isc.cnrs.fr.
References and annotations
- 1.Mink J. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 2.Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- 3.Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- •4.Desmurget M, Turner RS. Testing Basal Ganglia motor functions through reversible inactivations in the posterior internal globus pallidus. J Neurophysiol. 2008;99:1057–1076. doi: 10.1152/jn.01010.2007. This study examines the contributions of the BG to motor control by performing in-depth analyses of the effects of GPi inactivation on visually-targeted reaching movements in non-human primates. The strongest and most consistent effects were slowing and hypometria for all directions of movement, which correlated with reductions in the magnitude of movement-related EMG. Contrary to several current hypotheses, the following aspect to motor control were preserved during GPi inactivations: (1) movement initiation; (2) on-line correction of misdirected reaches; and (3) sequencing of muscle activation including appropriate suppression of antagonist activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••5.Schmidt L, d’Arc BF, Lafargue G, Galanaud D, Czernecki V, Grabli D, Schupbach M, Hartmann A, Levy R, Dubois B, et al. Disconnecting force from money: effects of basal ganglia damage on incentive motivation. Brain. 2008;131:1303–1310. doi: 10.1093/brain/awn045. This ground-breaking study provides detailed insight into the behavioral impairments associated with bilateral lesions of the BG in humans. Auto-activation deficit (AAD) has long been recognized in the clinical literature as a common correlate of damage to the BG. Here, Schmidt et al. found that AAD patients did not modulate grip forces in response to the monetary incentives offered in a task, even though the patients were able to modulate force levels appropriately in response to explicit instructions. Interestingly, galvanic skin responses suggested that the AAD patients retained affective awareness of the incentive value of different monetary rewards. The authors conclude that the BG links incentive motivation to the appropriate scaling of motor output. [DOI] [PubMed] [Google Scholar]
- ••6.Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. This review forges new ground by proposing potential mappings between concepts from modern theories of motor control (state estimation, optimization, prediction, and cost functions) and motor control regions of the brain. Of greatest significance here, the authors argue that the BG provides a “motor vigor” signal that modulates motor performance according to the expected costs and rewards of a given task or environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houk JC, Bastianen C, Fansler D, Fishbach A, Fraser D, Reber PJ, Roy SA, Simo LS. Action selection and refinement in subcortical loops through basal ganglia and cerebellum. Philos Trans R Soc Lond B Biol Sci. 2007;362:1573–1583. doi: 10.1098/rstb.2007.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tunik E, Houk JC, Grafton ST. Basal ganglia contribution to the initiation of corrective submovements. Neuroimage. 2009;47:1757–1766. doi: 10.1016/j.neuroimage.2009.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brainard MS. Contributions of the anterior forebrain pathway to vocal plasticity. Ann N Y Acad Sci. 2004;1016:377–394. doi: 10.1196/annals.1298.042. [DOI] [PubMed] [Google Scholar]
- 10.Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehericy S, Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- ••11.Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc Natl Acad Sci U S A. 2009;106:12518–12523. doi: 10.1073/pnas.0903214106. This paper shows that inactivation of the AFP in songbirds blocks the expression of newly acquired adaptive responses, but has little effect on adaptive responses ~24 hr after responses have been learned. These results are fully consistent with the concept that the BG is essential during early stages of skill learning, but not for long-term retention or recall of motor skills. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldridge JW, Berridge KC, Rosen AR. Basal ganglia neural mechanisms of natural movement sequences. Can J Physiol Pharmacol. 2004;82:732–739. doi: 10.1139/y04-061. [DOI] [PubMed] [Google Scholar]
- 13.Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••14.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. This review provides a comprehensive introduction to the psychology and neurobiology of habit-like behaviors, including topics ranging from automatized motor procedures to cultural rituals. Evidence is presented for involvement of the BG in the learning of habits and in their long-term storage. [DOI] [PubMed] [Google Scholar]
- 15.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 16.Vonsattel JP. Huntington disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:55–69. doi: 10.1007/s00401-007-0306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanabe LM, Kim CE, Alagem N, Dauer WT. Primary dystonia: molecules and mechanisms. Nat Rev Neurol. 2009;5:598–609. doi: 10.1038/nrneurol.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLong MR, Georgopoulos AP. Motor functions of the basal ganglia. In: Brookhart JM, Mountcastle VB, Brooks VB, Geiger SR, editors. Handbook of Physiology. The Nervous System. Motor Control. Sect. 1. Pt 2. II. American Physiological Society; 1981. pp. 1017–1061. [Google Scholar]
- 20.Cersosimo MG, Raina GB, Piedimonte F, Antico J, Graff P, Micheli FE. Pallidal surgery for the treatment of primary generalized dystonia: long-term follow-up. Clin Neurol Neurosurg. 2008;110:145–150. doi: 10.1016/j.clineuro.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 21.York MK, Lai EC, Jankovic J, Macias A, Atassi F, Levin HS, Grossman RG. Short and long-term motor and cognitive outcome of staged bilateral pallidotomy: a retrospective analysis. Acta Neurochir (Wien) 2007;149:857–866. doi: 10.1007/s00701-007-1242-x. discussion 866. [DOI] [PubMed] [Google Scholar]
- •22.Obeso JA, Jahanshahi M, Alvarez L, Macias R, Pedroso I, Wilkinson L, Pavon N, Day B, Pinto S, Rodriguez-Oroz MC, et al. What can man do without basal ganglia motor output? The effect of combined unilateral subthalamotomy and pallidotomy in a patient with Parkinson’s disease. Exp Neurol. 2009;220:283–292. doi: 10.1016/j.expneurol.2009.08.030. Results are presented from a remarkably extensive evaluation of one patient who received unilateral lesions of both the GPi and STN as treatments for PD. The patient demonstrated a preservation or improvement in most aspects of motor function in the limbs contralateral to the lesioned hemisphere. Only two aspects of motor function were impaired: (1) learning of new motor sequences, and (2) use of previously-experienced task probabilities to speed movement initiation. The discussion provides a detailed up-to-date review of the clinical literature, which supports the conclusion that pallidotomy impairs only a discrete subset of motor functions. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang P, Li Y, Hallett M. Neuronal activity in the basal ganglia and thalamus in patients with dystonia. Clin Neurophysiol. 2004;115:2542–2557. doi: 10.1016/j.clinph.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Sachdev RN, Gilman S, Aldridge JW. Bursting properties of units in cat globus pallidus and entopeduncular nucleus: the effect of excitotoxic striatal lesions. Brain Res. 1991;549:194–204. doi: 10.1016/0006-8993(91)90458-8. [DOI] [PubMed] [Google Scholar]
- 25.Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–151. [PubMed] [Google Scholar]
- 26.McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain. 2009;132:2125–2138. doi: 10.1093/brain/awp142. [DOI] [PubMed] [Google Scholar]
- 27.Gernert M, Hamann M, Bennay M, Loscher W, Richter A. Deficit of striatal parvalbumin-reactive GABAergic interneurons and decreased basal ganglia output in a genetic rodent model of idiopathic paroxysmal dystonia. J Neurosci. 2000;20:7052–7058. doi: 10.1523/JNEUROSCI.20-18-07052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••28.Guo Y, Rubin JE, McIntyre CC, Vitek JL, Terman D. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J Neurophysiol. 2008;99:1477–1492. doi: 10.1152/jn.01080.2007. This publication, and related papers from the same investigators, provides a plausible explanation for how abnormal neuronal activity exiting the GPi in PD might cause the motor signs of parkinsonism. The authors present a biologically-realistic computational model in which abnormally-patterned output from the GPi is shown to degrade the fidelity of information encoding in GPi-recipient thalamic neurons. This specific paper validates earlier results by incorporating single unit GPi recordings obtained from parkinsonian primates. The paper also presents evidence that therapeutic DBS may work by restoring the fidelity of information transmission through the thalamus. [DOI] [PubMed] [Google Scholar]
- ••29.Dorval AD, Kuncel AM, Birdno MJ, Turner DA, Grill WM. Deep brain stimulation alleviates parkinsonian bradykinesia by regularizing pallidal activity. J Neurophysiol. 2010 doi: 10.1152/jn.00103.2010. The study described here tests predictions of the model proposed in [28••] by studying the therapeutic efficacies of different forms of DBS (i.e., differing degrees of stimulus regularity). The authors found a close correlation between the ability of a form of stimulation to restore thalamic relay fidelity in the computation model and the actual therapeutic efficacy of that form of stimulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCairn KW, Turner RS. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J Neurophysiol. 2009;101:1941–1960. doi: 10.1152/jn.91092.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grafton ST, Turner RS, Desmurget M, Bakay R, Delong M, Vitek J, Crutcher M. Normalizing motor-related brain activity: subthalamic nucleus stimulation in Parkinson disease. Neurology. 2006;66:1192–1199. doi: 10.1212/01.wnl.0000214237.58321.c3. [DOI] [PubMed] [Google Scholar]
- 34.Grafton ST, Sutton J, Couldwell W, Lew M, Waters C. Network analysis of motor system connectivity in Parkinson’s Disease: Modulation of thalamocortical interactions after pallidotomy. Human Brain Mapping. 1994;2:45–55. [Google Scholar]
- 35.Heimer G, Rivlin-Etzion M, Bar-Gad I, Goldberg JA, Haber SN, Bergman H. Dopamine replacement therapy does not restore the full spectrum of normal pallidal activity in the 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine primate model of Parkinsonism. J Neurosci. 2006;26:8101–8114. doi: 10.1523/JNEUROSCI.5140-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner RS, Anderson ME. Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neurophysiol. 1997;77:1051–1074. doi: 10.1152/jn.1997.77.3.1051. [DOI] [PubMed] [Google Scholar]
- 37.Turner RS, Anderson ME. Context-dependent modulation of movement-related discharge in the primate globus pallidus. J Neurosci. 2005;25:2965–2976. doi: 10.1523/JNEUROSCI.4036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mushiake H, Strick PL. Pallidal neuron activity during sequential arm movements. J Neurophysiol. 1995;74:2754–2758. doi: 10.1152/jn.1995.74.6.2754. [DOI] [PubMed] [Google Scholar]
- 39.Pasquereau B, Nadjar A, Arkadir D, Bezard E, Goillandeau M, Bioulac B, Gross CE, Boraud T. Shaping of motor responses by incentive values through the basal ganglia. J Neurosci. 2007;27:1176–1183. doi: 10.1523/JNEUROSCI.3745-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inase M, Li BM, Takashima I, Iijima T. Pallidal activity is involved in visuomotor association learning in monkeys. Eur J Neurosci. 2001;14:897–901. doi: 10.1046/j.0953-816x.2001.01701.x. [DOI] [PubMed] [Google Scholar]
- 41.Mink J, Thach W. Basal ganglia motor control. I. nonexclusive relation of pallidal discharge to five movement modes. J Neurophysiol. 1991;65:273–300. doi: 10.1152/jn.1991.65.2.273. [DOI] [PubMed] [Google Scholar]
- 42.Hallett M, Shahani BT, Young RR. EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry. 1975;38:1154–1162. doi: 10.1136/jnnp.38.12.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zattara M, Bouisset S. Posturo-kinetic organisation during the early phase of voluntary upper limb movement. 1. Normal subjects. J Neurol Neurosurg Psychiatry. 1988;51:956–965. doi: 10.1136/jnnp.51.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seki K, Perlmutter SI, Fetz EE. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci. 2003;6:1309–1316. doi: 10.1038/nn1154. [DOI] [PubMed] [Google Scholar]
- 45.Hasbroucq T, Akamatsu M, Burle B, Bonnet M, Possamai CA. Changes in spinal excitability during choice reaction time: the H reflex as a probe of information transmission. Psychophysiology. 2000;37:385–393. [PubMed] [Google Scholar]
- 46.Meynier C, Burle B, Possamai CA, Vidal F, Hasbroucq T. Neural inhibition and interhemispheric connections in two-choice reaction time: a Laplacian ERP study. Psychophysiology. 2009;46:726–730. doi: 10.1111/j.1469-8986.2009.00818.x. [DOI] [PubMed] [Google Scholar]
- 47.Michelet T, Duncan GH, Cisek P. Response competition in the primary motor cortex: corticospinal excitability reflects response replacement during simple decisions. J Neurophysiol. 2010;104:119–127. doi: 10.1152/jn.00819.2009. [DOI] [PubMed] [Google Scholar]
- 48.Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci. 2007;362:1585–1599. doi: 10.1098/rstb.2007.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson ME, Horak FB. Influence of the globus pallidus on arm movements in monkeys. III. Timing of movement-related information. J Neurophysiol. 1985;54:433–448. doi: 10.1152/jn.1985.54.2.433. [DOI] [PubMed] [Google Scholar]
- 50.Horak FB, Anderson ME. Influence of globus pallidus on arm movements in monkeys. I. Effects of kainic acid-induced lesions. J Neurophysiol. 1984;52:290–304. doi: 10.1152/jn.1984.52.2.290. [DOI] [PubMed] [Google Scholar]
- 51.Mink J, Thach W. Basal ganglia motor control. III. pallidal ablation: normal reaction time, muscle cocontraction, and slow movement. J Neurophysiol. 1991;65:330–351. doi: 10.1152/jn.1991.65.2.330. [DOI] [PubMed] [Google Scholar]
- 52.Kato M, Kimura M. Effects of reversible blockade of basal ganglia on a voluntary arm movement. J Neurophysiol. 1992;68:1516–1534. doi: 10.1152/jn.1992.68.5.1516. [DOI] [PubMed] [Google Scholar]
- 53.Inase M, Buford JA, Anderson ME. Changes in the control of arm position, movement, and thalamic discharge during local inactivation in the globus pallidus of the monkey. J Neurophysiol. 1996;75:1087–1104. doi: 10.1152/jn.1996.75.3.1087. [DOI] [PubMed] [Google Scholar]
- 54.Wenger KK, Musch KL, Mink JW. Impaired reaching and grasping after focal inactivation of globus pallidus pars interna in the monkey. J Neurophysiol. 1999;82:2049–2060. doi: 10.1152/jn.1999.82.5.2049. [DOI] [PubMed] [Google Scholar]
- ••55.Desmurget M, Turner RS. Motor sequences and the basal ganglia: kinematics, not habits. J Neurosci. 2010;30:7685–7690. doi: 10.1523/JNEUROSCI.0163-10.2010. This study shows that an animal’s fluid predictive performance of a well-learned sequence of movement is preserved during transient inactivation of the GPi using muscimol. Effects of GPi inactivation on movement kinematics [4•] were not exacerbated for overlearned sequences as a whole, or as function of the rank-order of movements in the sequence. In addition, GPi inactivation did not degrade an animal’s ability to switch task performance with ease between blocks of OverLearned and Random sequences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desmurget M, Gaveau V, Vindras P, Turner RS, Broussolle E, Thobois S. On-line motor control in patients with Parkinson’s disease. Brain. 2004;127:1755–1773. doi: 10.1093/brain/awh206. [DOI] [PubMed] [Google Scholar]
- ••57.Mazzoni P, Hristova A, Krakauer JW. Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J Neurosci. 2007;27:7105–7116. doi: 10.1523/JNEUROSCI.0264-07.2007. In studying the ability of PD patients to generate reaching movements of different speeds the authors make the interesting observation that PD patients are capable of moving as rapidly as normal subjects, but that they are “reluctant” to do so. Additional analyses show that this reluctance cannot be attributed to abnormal speed-accuracy relationships in PD patients (i.e., reaching in PD patients is not inherently more variable). The authors propose that parkinsonian bradykinesia may be attributed to an impaired link between a task’s incentives and the regulation of movement vigor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bastian AJ, Kelly VE, Perlmutter JS, Mink JW. Effects of pallidotomy and levodopa on walking and reaching movements in Parkinson’s disease. Mov Disord. 2003;18:1008–1017. doi: 10.1002/mds.10494. [DOI] [PubMed] [Google Scholar]
- 59.Ostrem JL, Marks WJ, Jr, Volz MM, Heath SL, Starr PA. Pallidal deep brain stimulation in patients with cranial-cervical dystonia (Meige syndrome) Mov Disord. 2007;22:1885–1891. doi: 10.1002/mds.21580. [DOI] [PubMed] [Google Scholar]
- •60.Berman BD, Starr PA, Marks WJ, Jr, Ostrem JL. Induction of bradykinesia with pallidal deep brain stimulation in patients with cranial-cervical dystonia. Stereotact Funct Neurosurg. 2009;87:37–44. doi: 10.1159/000195718. This is one of several recent publications to report that DBS of the GPi often induces bradykinesia-like side-effects. In this study, GPi DBS reduced cranial-cervical dystonic signs significantly, but resulted in a marked slowing of previously-normal limb movements in 10 of 11 patients. Although GPi DBS reduces the abnormal GPi activity that contributes to the genesis of dystonia, it may also interfere with the transmission of normal movement gain-related information through the GPi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. Basal ganglia network mediates the control of movement amplitude. Exp Brain Res. 2003;153:197–209. doi: 10.1007/s00221-003-1593-3. [DOI] [PubMed] [Google Scholar]
- •62.Viviani P, Burkhard PR, Chiuve SC, Corradi-Dell’Acqua C, Vindras P. Velocity control in Parkinson’s disease: a quantitative analysis of isochrony in scribbling movements. Exp Brain Res. 2009;194:259–283. doi: 10.1007/s00221-008-1695-z. This is the most recent of a series of publications from this group supporting the view that movement gain and movement direction are specified independently at certain stages of motor planning (i.e., the “vectorial planning” hypothesis). In this publication, the authors perform in-depth analyses of the movements of PD patients during “free scribbling” movements. They conclude that PD patients display a selective impairment in scaling the size and velocity of arm movements. [DOI] [PubMed] [Google Scholar]
- 63.Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- 64.Pfann KD, Buchman AS, Comella CL, Corcos DM. Control of movement distance in Parkinson’s disease. Mov Disord. 2001;16:1048–1065. doi: 10.1002/mds.1220. [DOI] [PubMed] [Google Scholar]
- 65.Ramig LO, Fox C, Sapir S. Speech treatment for Parkinson’s disease. Expert Rev Neurother. 2008;8:297–309. doi: 10.1586/14737175.8.2.297. [DOI] [PubMed] [Google Scholar]
- 66.Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gdowski MJ, Miller LE, Parrish T, Nenonene EK, Houk JC. Context dependency in the globus pallidus internal segment during targeted arm movements. J Neurophysiol. 2001;85:998–1004. doi: 10.1152/jn.2001.85.2.998. [DOI] [PubMed] [Google Scholar]
- •68.Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol. 2007:00239.02007. doi: 10.1152/jn.00239.2007. The authors used fMRI to identify brain regions where activity correlates with the force exerted during isometric pinches. They found that activity in GPi and STN correlates closely with pinch force (a correlate of motor gain) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pope P, Wing AM, Praamstra P, Miall RC. Force related activations in rhythmic sequence production. Neuroimage. 2005;27:909–918. doi: 10.1016/j.neuroimage.2005.05.010. [DOI] [PubMed] [Google Scholar]
- •70.Thobois S, Ballanger B, Baraduc P, Le Bars D, Lavenne F, Broussolle E, Desmurget M. Functional anatomy of motor urgency. Neuroimage. 2007;37:243–252. doi: 10.1016/j.neuroimage.2007.04.049. PET imaging was used to identify brain regions involved in the implicit scaling of movement speed according to conditions of urgency (i.e., reaching to catch a falling ball). Among other results, the authors found that activity in the globus pallidus correlated closely with the speed of movement. [DOI] [PubMed] [Google Scholar]
- 71.Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST. Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol. 2003;90:3958–3966. doi: 10.1152/jn.00323.2003. [DOI] [PubMed] [Google Scholar]
- 72.Vindras P, Desmurget M, Viviani P. Error parsing in visuomotor pointing reveals independent processing of amplitude and direction. J Neurophysiol. 2005;94:1212–1224. doi: 10.1152/jn.01295.2004. [DOI] [PubMed] [Google Scholar]
- 73.Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci. 2000;20:8916–8924. doi: 10.1523/JNEUROSCI.20-23-08916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guigon E, Baraduc P, Desmurget M. Computational motor control: redundancy and invariance. J Neurophysiol. 2007;97:331–347. doi: 10.1152/jn.00290.2006. [DOI] [PubMed] [Google Scholar]
- 75.Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- 76.Croxson PL, Walton ME, O’Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29:4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •77.Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, Frith CD. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316:904–906. doi: 10.1126/science.1140459. In neurologically-normal human subjects, subliminal visual cues were used to indicate the monetary rewards available during a force exertion task. fMRI identified the ventral pallidum (part of the BG limbic circuit) as part of the network of brain regions that link motivation to response vigor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Habib M. Athymhormia and disorders of motivation in Basal Ganglia disease. J Neuropsychiatry Clin Neurosci. 2004;16:509–524. doi: 10.1176/jnp.16.4.509. [DOI] [PubMed] [Google Scholar]
- 79.Majsak MJ, Kaminski T, Gentile AM, Flanagan JR. The reaching movements of patients with Parkinson’s disease under self- determined maximal speed and visually cued conditions. Brain. 1998;121:755–766. doi: 10.1093/brain/121.4.755. [DOI] [PubMed] [Google Scholar]
- 80.Ballanger B, Thobois S, Baraduc P, Turner RS, Broussolle E, Desmurget M. “Paradoxical Kinesis” is not a Hallmark of Parkinson’s disease but a general property of the motor system. Mov Disord. 2006 doi: 10.1002/mds.20987. [DOI] [PubMed] [Google Scholar]
- 81.Marinovic W, Plooy A, Tresilian JR. The time course of amplitude specification in brief interceptive actions. Exp Brain Res. 2008;188:275–288. doi: 10.1007/s00221-008-1360-6. [DOI] [PubMed] [Google Scholar]
- 82.Ghez C, Favilla M, Ghilardi MF, Gordon J, Bermejo R, Pullman S. Discrete and continuous planning of hand movements and isometric force trajectories. Exp Brain Res. 1997;115:217–233. doi: 10.1007/pl00005692. [DOI] [PubMed] [Google Scholar]
- 83.Hepp-Reymond M, Kirkpatrick-Tanner M, Gabernet L, Qi HX, Weber B. Context-dependent force coding in motor and premotor cortical areas. Exp Brain Res. 1999;128:123–133. doi: 10.1007/s002210050827. [DOI] [PubMed] [Google Scholar]
- 84.Ashe J. Force and the motor cortex. Behav Brain Res. 1997;86:1–15. doi: 10.1016/s0166-4328(96)00145-3. [DOI] [PubMed] [Google Scholar]
- ••85.Ashby FG, Ennis JM, Spiering BJ. A neurobiological theory of automaticity in perceptual categorization. Psychological Review. 2007;114:632–656. doi: 10.1037/0033-295X.114.3.632. This review presents arguments similar to those found in the present paper, but for roles of the BG in perceptual categorization rather than in motor control. The authors propose that the BG plays an essential role in initial procedural learning of perceptual categories, but that purely cortical pathways become dominant as the categorization skill is automatized by extended practice. A detailed computational model of this process is shown to account for a variety of single unit recording and behavioral observations. [DOI] [PubMed] [Google Scholar]
- 86.Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 87.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- 89.Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- 90.Williams ZM, Eskandar EN. Selective enhancement of associative learning by microstimulation of the anterior caudate. Nat Neurosci. 2006;9:562–568. doi: 10.1038/nn1662. [DOI] [PubMed] [Google Scholar]
- •91.Tang CC, Root DH, Duke DC, Zhu Y, Teixeria K, Ma S, Barker DJ, West MO. Decreased firing of striatal neurons related to licking during acquisition and overtraining of a licking task. J Neurosci. 2009;29:13952–13961. doi: 10.1523/JNEUROSCI.2824-09.2009. This is one of several studies from the West group showing that neurons in the skeletomotor region of the rat striatum are far more likely to show task-related activity during initial stages of training on a task than after extensive training. These studies, as a whole, stand out for their care in recording from identified single body part-related neurons. Results from this specific study: (1) suggest that the decline in prevalence of task-related activity is not simply a correlate of habit formation and (2) corroborate the observation that, in a minority sub-population of striatal neurons, task-related activity persists and is even accentuated with overtraining. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown RG, Jahanshahi M, Limousin-Dowsey P, Thomas D, Quinn NP, Rothwell JC. Pallidotomy and incidental sequence learning in Parkinson’s disease. Neuroreport. 2003;14:21–24. doi: 10.1097/00001756-200301200-00004. [DOI] [PubMed] [Google Scholar]
- 93.Sage JR, Anagnostaras SG, Mitchell S, Bronstein JM, De Salles A, Masterman D, Knowlton BJ. Analysis of probabilistic classification learning in patients with Parkinson’s disease before and after pallidotomy surgery. Learn Mem. 2003;10:226–236. doi: 10.1101/lm.45903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matsuzaka Y, Picard N, Strick PL. Skill representation in the primary motor cortex after long-term practice. J Neurophysiol. 2007;97:1819–1832. doi: 10.1152/jn.00784.2006. [DOI] [PubMed] [Google Scholar]
- 96.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••97.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. This provides definitive evidence that direct- and indirect-pathway neurons of the striatum can be distinguished by their differential expression of D1 and D2 dopamine receptors along with other differences in cell physiology. Moreover, the study demonstrates a selective involvement of dopamine in long-term potentiation in D1-expressing direct pathway neurons, and in long-term depression in D2-expressing indirect pathway neurons. These results provide a substrate for the proposed learning-related functions of the BG and may explain the imbalance in activation of indirect- vs. direct-pathways that is thought to contribute to parkinsonism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 100.Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •101.Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. The authors show that song-triggered electrical stimulation of an output nucleus of the AFP (homologue of the mammalian BG) alters parameters of song execution in real-time without altering overall song structure or sequencing. In many ways, these results parallel and corroborate those of an earlier study of the effects of electrical stimulation in the BG performed in non-human primates [49]. The authors conclude that the AFP may contribute to motor learning by biasing processing in brain circuits devoted to song execution. [DOI] [PubMed] [Google Scholar]
- 102.Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- 103.Squire LR. Mechanisms of Memory. Science. 1986;232:1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- 104.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parent M, Parent A. Axon collateralization in primate basal ganglia and related thalamic nuclei. Thalamus & Related Systems. 2002;2:71–86. [Google Scholar]
- 107.Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–459. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- •108.Grabli D, McCairn K, Hirsch EC, Agid Y, Feger J, Francois C, Tremblay L. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain. 2004;127:2039–2054. doi: 10.1093/brain/awh220. This is the first of an ongoing series of publications from the Tremblay group showing that focal activations of neuronal activity in different regions of the BG (in this case, using bicuculline injections into the GPe) induce distinct behavioral disorders, the nature of which depend on the area activated. These studies provide convincing support for the existence of parallel circuits through the BG mediating skeletomotor, associative, and limbic functions. [DOI] [PubMed] [Google Scholar]
- 109.Turner RS, McCairn KW, Simmons D, Bar-Gad I. Sequential motor behavior and the basal ganglia. Evidence from a serial reaction time task in monkeys. In: Bolam JP, Ingham CA, Magill PJ, editors. Basal Ganglia VIII (Advances in Behavioral Biology) Vol. 56 Plenum: 2005. pp. 563–574. [Google Scholar]