Abstract
Our previous genetic and proteomic studies demonstrated that dynamin 1 is significantly associated with nicotine dependence (ND) in human smokers and its expression is highly modulated by nicotine in the brains of animals. To provide further molecular evidence for the involvement of dynamin 1 in the etiology of ND, we investigated the regulatory effect of nicotine on the expression of dynamin 1 using both in vivo and in vitro approaches. With quantitative real-time RT-PCR, we found that dynamin 1 mRNA was significantly downregulated, by 30%, 31%, and 38%, in the striatum, hippocampus, and medial basal hypothalamus (MBH), respectively, of nicotine-treated rats (P < 0.01 for all three regions). Further, dynamin 1 protein was downregulated by nicotine in the ventral tegmental area (VTA: 39.5%; P < 0.01), hippocampus (13.4%, P < 0.05), MBH (24.6%, P < 0.01), and amygdala (15.7%, P < 0.05). We also determined the effect of nicotine on human SH-SY5Y cells and found that dynamin 1 mRNA was significantly down-regulated by nicotine after treatment (51.4%; P < 0.01), and a consistent decrease in the amount of the protein was also observed (36.6%; P < 0.05). Taken together, our findings provide further molecular evidence for the involvement of dynamin 1 in the etiology of ND.
Keywords: nicotine, dynamin 1, rat brain, SH-SY5Y, expression
Tobacco smoking is a severe health problem in the United States, resulting in approximately 438,000 deaths and $157 billion in health-related costs annually [20, 31]. Nicotine, the primary addictive substance in tobacco smoke [28, 31, 32], rapidly crosses the plasma membrane and alters gene expression through the nicotinic receptor-mediated signaling pathway, leading to persistent changes in the neural plasticity of the brain and therefore in nicotine dependence (ND). Numerous twin and family studies revealed that ND is a complex trait influenced by both genetic and environmental factors, as well as by their interactions [2, 29, 30]. A meta-analysis of 17 twin studies indicates that the weighted mean heritability for ND is 0.56 [18].
By using a proteomics approach, we previously reported that nicotine significantly modulated the expression of dynamin 1 in several animal brain regions, suggesting that dynamin 1 plays an important role in drug addiction [9]. To determine if this gene is involved in the etiology of ND, we recently conducted association studies of 602 nuclear families of either African-American (AA) or European-American (EA) origin and found dynamin 1 to be significantly associated with ND in smokers [34].
Dynamin has three isoforms, all of which are expressed in nerve terminals, with dynamin 1 having the greatest expression [27]. In neurons, dynamin 1 pinches off synaptic vesicles, frees them from the membrane, and allows them to enter the synaptic vesicle pool to be refilled for future release [3, 4]. It has been well documented that dynamin 1 plays a role in synaptic function. For example, the study of a temperature-sensitive mutant of dynamin 1 in Drosophila suggested that in the absence of dynamin 1, synapses lose their ability to release neurotransmitter as a consequence of ineffective synaptic vesicle recycling [16]. In addition, analysis of dynamin 1-depleted neurons showed the accumulation of synaptic vesicles at the plasma membranes and a decrease in the releasable synaptic vesicle pool [17]. More recent studies revealed that the reduced amount of dynamin 1 impair neuronal transport and vesicle trafficking by interactions with other endocytic accessory proteins in hippocampal neurons [10, 13, 15], whereas the study of dynamin 1-knockout mice showed that synaptic vesicle endocytosis was severely impaired during strong exogenous stimulation [6]. Given that dynamin 1 is significantly associated with ND [34] and plays a central role in the neuronal system, in the current study, we investigated if nicotine has regulatory effects on the expression of dynamin 1 in the rat brain and SH-SY5Y cells.
Adult male Holtzman rats (250–350 g; HSD, Madison, WI) were randomly divided into nicotine-treated or control groups, with at least seven animals per group. For the nicotine-treated rats, nicotine bitartrate was administered through osmotic minipumps (Model 2ML1; Azlet Corp., Palo Alto, CA) in a daily dose of 3.15 mg/kg (calculated as free base) in saline (pH 7.0–7.2) for 7 days [19]. Rats in the control group were handled and treated exactly the same, except that only saline was delivered with the minipump. All rats were housed at 22°C on a 12 h light/dark cycle. Standard laboratory rat chow and water were freely available. All animal-related experimental protocols were approved by the Institutional Animal Use Committee of University of Virginia.
After seven days of nicotine treatment, rats were anesthetized with isoflurane, and the brains were removed immediately for sectioning [24]. Coronal 2-mm sections were prepared using a Stoelting tissue slicer (Chicago, IL). Brain punches were excised from the amygdala, the nucleus accumbens (NA), the prefrontal cortex (PFC), the striatum, the medial basal hypothalamus (MBH), the hippocampus, and the ventral tegmental area (VTA) using a tissue slicer from NeuroLab.com (St Louis, MO). Brain punches used for real-time RT-PCR and Western blotting experiments were from two independent animal experiments under an identical treatment regimen.
The human neuroblastoma cell line SH-SY5Y was purchased from the American Tissue Cell Culture Inc. (Manassas, VA) and grown in DMEM supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. The medium was changed every other day. For nicotine treatment, cells were cultured to about 90% confluence and then treated with 1 mM nicotine tartrate (pH 7.0–7.2; free base, Sigma, St. Louis, MO). Cells were grown for an additional period of various hours prior to harvesting for RNA and protein extraction for real-time RT-PCR and Western blotting analysis.
Real-time RT-PCR was used to assess expression of dynamin 1 mRNA in rat brain regions and human SH-SY5Y cells. Briefly, the total RNA of each sample was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). One microgram of total RNA was reverse-transcribed in a final volume of 20 µl containing 4 µl of 5× first-strand buffer (250 mM Tris HCl, pH 8.3; 375 mM KCl; 15 mM MgCl2), 10 mM DTT, 0.5 mM each dNTP, 40 U RNaseOUT™, 1 µl of 50 nM random hexamers, and 200 U of Superscript II RNase H− reverse transcriptase (Invitrogen). The TaqMan® probes and primers Rn00589865_m1 for rat brain or Hs00189369–m1 for human SH-SY5Y cells was used for the standard TaqMan PCR procedures in the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The sequences for the primers and probes cannot be reported, as the manufacturer declines to provide this information to users. Amplification of 2 µl of cDNA mixture was carried out in a total volume of 20 µl according to the manual for TaqMan® Gene Expression Assays (Applied Biosystems). The standard PCR procedures were: 2 min at 50°C and 10 min at 95°C followed by 40 cycles of 25 s at 95°C and 1 min at 60°C. Each treatment was performed in triplicate or quadruplicate, and data were analyzed with the comparative Ct method [33] and normalized to 18S rRNA of the corresponding sample.
Total protein was extracted from individual frozen brain tissue punches by homogenization with a sonicator in RIPA buffer (50 mM Tris HCl, pH 8.0; 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS), and the protein concentration was determined by the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA). Ten micrograms of total protein was separated by 10% SDS-PAGE followed by transfer to 0.45-µm nitrocellulose membranes at 25 V overnight at 4°C. The membrane was first incubated in a blocking buffer (5% non-fat milk and 0.2% Tween 20) for 1.5 h at room temperature and then 1.5 h at room temperature in the blocking buffer containing rat anti-dynamin 1 antibody (dilution 1:1000; Abcam Inc, Cambridge, MA). After three washes in TBST (10 mM Tris HCl, pH 8.0; 0.15 M NaCl; 0.2% Tween 20) for 10 min each, the membrane was exposed to horseradish peroxidase-conjugated secondary antibody at room temperature for 1.5 h and then exposed to X-ray film. After hybridization with the antibody of interest, membranes were stripped and re-probed with antibody to tubulin (dilution 1:2000; Abcam) for normalization of the protein concentration and testing of the loading efficiency of each sample. The films were scanned for quantitative analysis with ImageQuant 5.2 (Molecular Dynamics, Sunnyvale, CA). The procedures used for Western blotting analysis for dynamin 1 in SH-SY5Y cells were the same as described above, except the first antibody was human anti-dynamin 1 (dilution 1:5000; Clontech, Mountain View, CA).
The significance of differences between the nicotine-treated and control groups was analyzed by Student’s t-test, and a P value of ≤ 0.05 was considered statistically significant.
To identify the regulatory effects of nicotine treatment on the expression of dynamin 1 mRNA, we examined RNA concentrations in seven brain regions of rats treated with nicotine and control animals using real-time RT-PCR. Figure 1A shows a statistical analysis of the results. After normalization by the corresponding 18S rRNA of each sample, we found that dynamin 1 mRNA was significantly downregulated, by 30%, 31%, and 38%, in the striatum, hippocampus, and MBH, respectively, of the nicotine-treated rats (P < 0.01 for all three regions). No significant differences in dynamin 1 mRNA were detected in the VTA, PFC, NA, or amygdala.
Figure 1.

Comparison of mRNA (A) and protein (B) of dynamin 1 in seven rat brain regions after 7 days of nicotine or saline administration. With real-time RT-PCR, we found that nicotine significantly decreased the amount of dynamin 1 mRNA in the striatum, hippocampus, and MBH. Values are given as means ± S.E.M. (**P < 0.01; n = 5–8/group). For protein expression analysis, by normalizing dynamin 1 protein to the corresponding tubulin value, we found that dynamin 1 was significantly decreased by nicotine in the VTA, hippocampus, MBH, and amygdala. Values are given as means ± S.E.M. (*P < 0.05, **P < 0.01; n = 4 or 5/group).
To determine if the protein concentration of dynamin 1 also is altered by nicotine, we performed Western blotting analysis of the seven brain regions in the two experimental groups. After normalization to tubulin, we found that the dynamin 1 protein concentration was reduced by 39.5% (P < 0.01) in the VTA, 13.4% (P < 0.05) in the hippocampus, 24.6% (P < 0.01) in the MBH, and 15.7% (P < 0.05) in the amygdala of the nicotine-treated rats. No expression differences were detected in the other three brain regions (Figure 1B).
By comparing the expression trends of dynamin 1 in response to nicotine treatment, we found similar expression patterns for mRNA and protein in the hippocampus and MBH. On the other hand, we noticed a different expression trend for RNA and protein in the VTA, amygdala, and striatum. In the VTA and amygdala, we detected a significant difference at the protein level (P < 0.01 and P < 0.05, respectively) but found no significant difference at the mRNA level. For the striatum, no significant difference was found at the protein level, but a significant difference was obtained at the mRNA level (30%; P < 0.01).
To determine whether nicotine treatment also has regulatory effects on human cells, we examined the expression of dynamin 1 at both the RNA and protein levels in SH-SY5Y cells. As shown in Figure 2, dynamin 1 mRNA was down-regulated by nicotine at 1, 24, and 48 h of treatment (~51.0 % reduction for all three time points; P < 0.01). To determine whether the protein also reflected downregulation by nicotine and considering a consistent decrease in RNA expression level at three time points, Western blot analysis was employed to determine protein after 1 h of nicotine treatment under the identical experimental conditions, which revealed that dynamin 1 was significantly downregulated, by 36.6% (Figure 3; P < 0.05).
Figure 2.
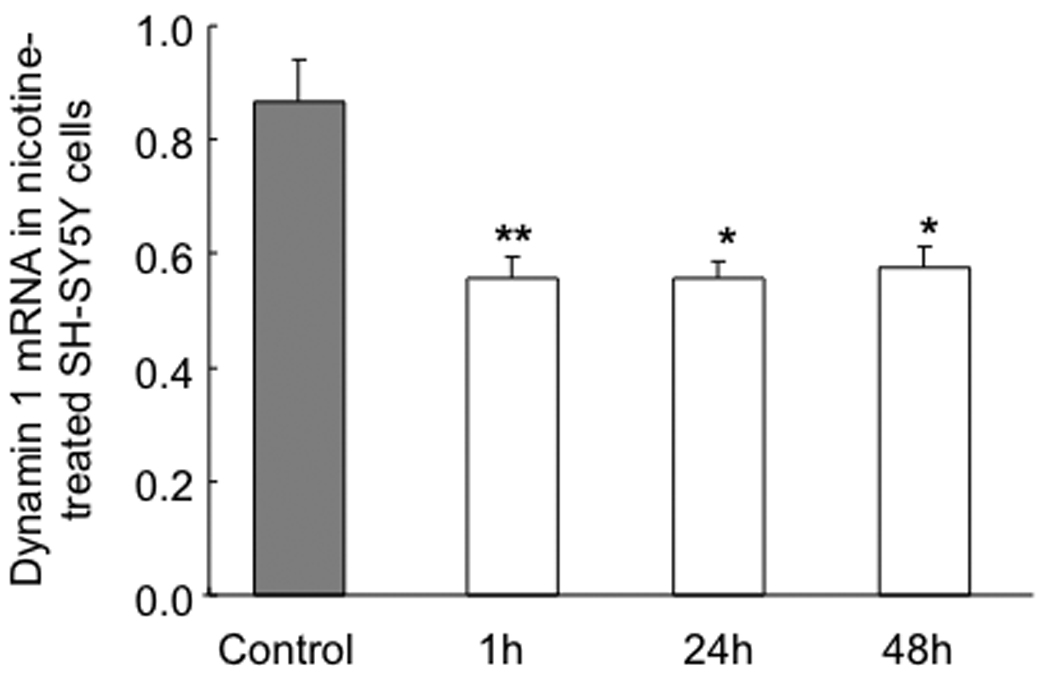
Comparison of mRNA concentration of dynamin 1 in nicotine-treated or control SH-SY5Y cells after 1, 24, and 48 h. With real-time RT-PCR, we found that nicotine significantly decreased dynamin mRNA 1 at 1, 24 and 48 h. Values are given as means ± S.E.M. (* P < 0.05, ** P < 0.01; n = 6/group).
Figure 3.
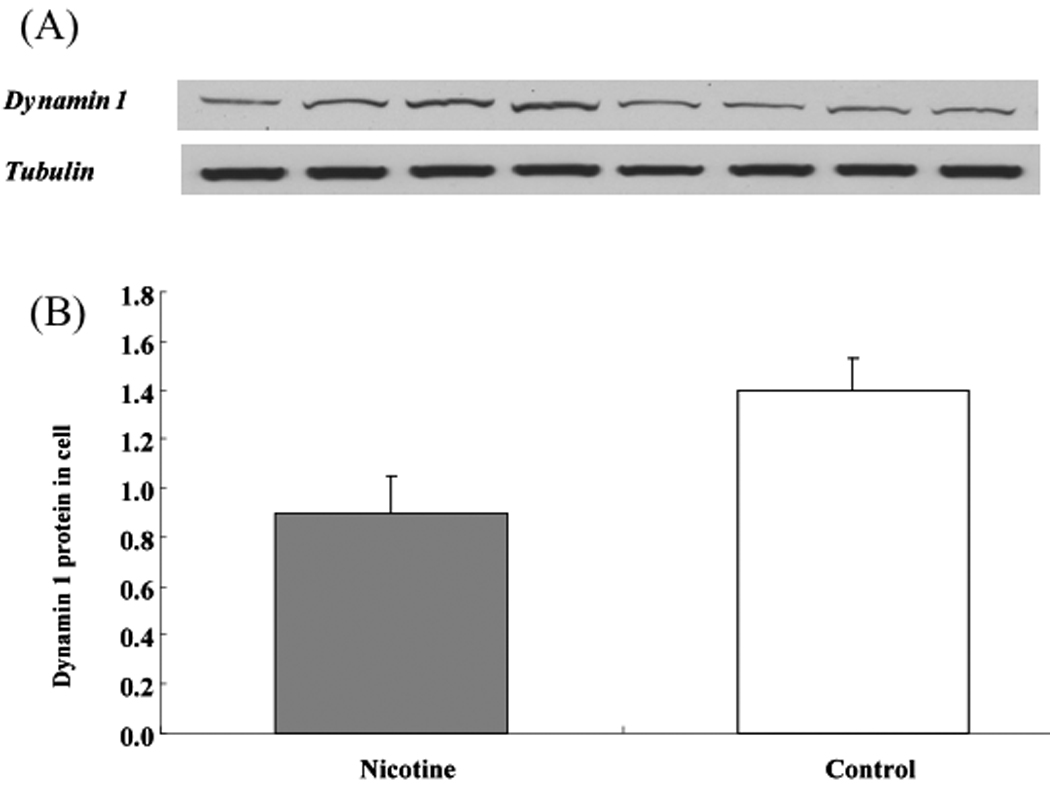
Western blotting analyses of dynamin 1 in SH-SY5Y cell treated with nicotine for 1 h. (a) Representative images for dynamin 1 and tubulin. (b) When the amount of dynamin 1 protein was normalized to the corresponding tubulin value, it was apparent that dynamin 1 was significantly downregulated, by 36.6%, in cells treated with nicotine. Values are given as means ± S.E.M. (**P < 0.01; n = 6/group).
Previously, our proteomics study demonstrated that nicotine decreases the expression of dynamin 1 in animals [9]. Moreover, our genetic association study revealed that dynamin 1 is significantly associated with ND in an EA sample and, to a lesser extent, in an AA sample [34]. In the current study, we confirmed our early finding that dynamin 1 is highly modulated by nicotine in rodent brain regions as well as in human SY-SH5Y cells. Together, our investigations at both the RNA and protein levels in tissues and cells from the two species provide convincing evidence that dynamin 1 is highly regulated by nicotine and plays an important role in the etiology of ND in humans.
By using different molecular approaches and with independent animal experiments, we confirmed the finding from our proteomics study that dynamin 1 is significantly modulated by nicotine in multiple brain regions at both the RNA and protein levels. Specifically, nicotine treatment modulated dynamin 1 mRNA expression substantially in the striatum, hippocampus, and MBH and expression of the protein in the VTA, hippocampus, MBH, and amygdala. Of note, we observed some inconsistency in the change of gene expression between RNA and protein in three brain regions: for the VTA and amygdala, a significant decrease in the protein versus no change in mRNA, whereas for the striatum, there was no change in protein versus a 30% decrease in mRNA. Such discrepancy probably is attributable to differential regulation at the transcription or post-translation level. As for the NA, we detected nicotine-induced upregulation of dynamin 1 by 10% at the protein level in the current study and by 30% in a previous study [9]. Such a difference in the degree of upregulation likely is secondary to the use of different techniques for protein quantification in the two studies. In addition, some differences in responses among the brain regions may be attributable to the different response mechanisms utilized by the seven regions and to the presence of tissue heterogeneity for some brain regions because of their proximity and the techniques used to collect these brain tissues.
Further, by detecting the expression of dynamin 1 in human neuronal cells treated with nicotine over various periods of exposure, we found that nicotine has a significant effect on the expression of dynamin 1 at both the RNA and protein levels. In view of the prior evidence that dynamin 1 plays an important role in synaptic function, these expressional changes in both in vivo and in vitro systems may be part of the cellular mechanism responsible for the initial physiological response to nicotine administration. Interestingly, a previous study from our group indicated that nicotine can upregulate the expression of miR-140* in PC12 cells treated for 1 h. The miR-140* targets the 3′-untranslated region of the dynamin 1 gene by basepairing and represses gene translation by inducing mRNA degradation in dynamin 1 expression analysis [8]. Also, dynamin 1 was revealed to bind the β2 subunit of nAChR in a protein-interaction analysis [11]. Based on the aforementioned findings, it seems that at least two pathways mediate nicotine-induced regulation of dynamin 1 in the cell model. Regardless, further experiments are required to determine how nicotine modulates the expression of dynamin 1, whether nAChR is involved, and, if so, which nAChR takes part in that action.
As a nerve terminal-trafficking protein, dynamin 1 has been suggested to have important functions in different cellular processes by interaction with other proteins. In hippocampal neurons, dynamin 1 is associated with mGLuR5 [5] and Aβ [13, 14]. The mGLuR5 play an important role in the adaptive changes needed for long-term depression or potentiation of neuronal synaptic connectivity [7], and Aβ is toxic to neurons and may be responsible for Alzheimer’s disease [25]. Furthermore, both mGLuR5 and Aβ were reported to be regulated by nicotine [12, 21, 26], so it may be easy to understand the association between dynamin 1 and ND. In addition, LTD-associated neural activity has been reported to augment expression of dynamin 1 in rat striatum [23]. Blocking the recruitment of dynamin 1 prevents LTD in the NA [1]. Recently, a synaptic proteomics approach in morphine-treated rats revealed enriched post-synaptic localization of dynamin 1 in the hippocampus [22]. Collectively, these results imply that dynamin 1 plays an important role in the neural plasticity induced by nicotine and other drugs of abuse.
Research Highlights.
Dynamin 1 plays an important role in various neuronal processes;
Dynamin 1 is highly associated with smoking dependence;
Dynamin 1 is highly regulated by nicotine at both RNA and protein levels;
Dynamin 1 represents a valuable candidate for further genetic study on addiction.
Acknowledgments
This study was supported by National Institutes of Health grants DA-12844 and DA-13783 to MDL and the Fundamental Research Funds for the Central Universities 2009JBM107 to QX. All experiments were conducted in the laboratory located at the University of Virginia. The authors thank Dr. David L Bronson for his helpful editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, Phelps L, Phillips AG, Wang YT. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310:1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- 2.Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking--a study of male twins. N Engl J Med. 1992;327:829–833. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- 3.Clark SG, Shurland DL, Meyerowitz EM, Bargmann CI, van der Bliek AM. A dynamin GTPase mutation causes a rapid and reversible temperature-inducible locomotion defect in C. elegans. Proc Natl Acad Sci U S A. 1997;94:10438–10443. doi: 10.1073/pnas.94.19.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farr CD, Gafken PR, Norbeck AD, Doneanu CE, Stapels MD, Barofsky DF, Minami M, Saugstad JA. Proteomic analysis of native metabotropic glutamate receptor 5 protein complexes reveals novel molecular constituents. J Neurochem. 2004;91:438–450. doi: 10.1111/j.1471-4159.2004.02735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, O'Toole E, Flavell R, Cremona O, Miesenbock G, Ryan TA, De Camilli P. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- 7.Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W, Li MD. Nicotine modulates expression of miR-140*, which targets the 3'-untranslated region of dynamin 1 gene (Dnm1) Int J Neuropsychopharmacol. 2009;12:537–546. doi: 10.1017/S1461145708009528. [DOI] [PubMed] [Google Scholar]
- 9.Hwang YY, Li MD. Proteins differentially expressed in response to nicotine in five rat brain regions: identification using a 2-DE/MS-based proteomics approach. Proteomics. 2006;6:3138–3153. doi: 10.1002/pmic.200500745. [DOI] [PubMed] [Google Scholar]
- 10.Jiang S, Avraham HK, Kim TA, Rogers RA, Avraham S. Receptor-type PTP-NP inhibition of Dynamin-1 GTPase activity is associated with neuronal depolarization. Cell Signal. 2006;18:1439–1446. doi: 10.1016/j.cellsig.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Kabbani N, Woll MP, Levenson R, Lindstrom JM, Changeux JP. Intracellular complexes of the beta2 subunit of the nicotinic acetylcholine receptor in brain identified by proteomics. Proc Natl Acad Sci U S A. 2007;104:20570–20575. doi: 10.1073/pnas.0710314104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane JK, Hwang Y, Konu O, Loughlin SE, Leslie FM, Li MD. Regulation of Homer and group I metabotropic glutamate receptors by nicotine. Eur J Neurosci. 2005;21:1145–1154. doi: 10.1111/j.1460-9568.2005.03945.x. [DOI] [PubMed] [Google Scholar]
- 13.Kelly BL, Ferreira A. beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. J Biol Chem. 2006;281:28079–28089. doi: 10.1074/jbc.M605081200. [DOI] [PubMed] [Google Scholar]
- 14.Kelly BL, Vassar R, Ferreira A. Beta-amyloid-induced dynamin 1 depletion in hippocampal neurons. A potential mechanism for early cognitive decline in Alzheimer disease. J Biol Chem. 2005;280:31746–31753. doi: 10.1074/jbc.M503259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitzmueller E, Krapfenbauer K, Hoeger H, Weitzdoerfer R, Lubec G, Lubec B. Life-long effects of perinatal asphyxia on stress-induced proteins and dynamin 1 in rat brain. Neurochem Res. 2004;29:1767–1777. doi: 10.1023/b:nere.0000035813.73790.b5. [DOI] [PubMed] [Google Scholar]
- 16.Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosaka T, Ikeda K. Possible temperature-dependent blockage of synaptic vesicle recycling induced by a single gene mutation in Drosophila. J Neurobiol. 1983;14:207–225. doi: 10.1002/neu.480140305. [DOI] [PubMed] [Google Scholar]
- 18.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 19.Malin DH. Nicotine dependence: studies with a laboratory model. Pharmacol Biochem Behav. 2001;70:551–559. doi: 10.1016/s0091-3057(01)00699-2. [DOI] [PubMed] [Google Scholar]
- 20.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Jama-J Am Med Assoc. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 21.Moore SA, Huckerby TN, Gibson GL, Fullwood NJ, Turnbull S, Tabner BJ, El-Agnaf OM, Allsop D. Both the D-(+) and L-(−) enantiomers of nicotine inhibit Abeta aggregation and cytotoxicity. Biochemistry. 2004;43:819–826. doi: 10.1021/bi035728h. [DOI] [PubMed] [Google Scholar]
- 22.Moron JA, Abul-Husn NS, Rozenfeld R, Dolios G, Wang R, Devi LA. Morphine administration alters the profile of hippocampal postsynaptic density-associated proteins: a proteomics study focusing on endocytic proteins. Mol Cell Proteomics. 2007;6:29–42. doi: 10.1074/mcp.M600184-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Napolitano M, Marfia GA, Vacca A, Centonze D, Bellavia D, Di Marcotullio L, Frati L, Bernardi G, Gulino A, Calabresi P. Modulation of gene expression following long-term synaptic depression in the striatum. Brain Res Mol Brain Res. 1999;72:89–96. doi: 10.1016/s0169-328x(99)00213-2. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. The Rat Brainin Stereotaxic Coordinates. Sydney: Academic Press; 1986. [Google Scholar]
- 25.Selkoe DJ. Alzheimer's disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994;53:438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Seo J, Kim S, Kim H, Park CH, Jeong S, Lee J, Choi SH, Chang K, Rah J, Koo J, Kim E, Suh Y. Effects of nicotine on APP secretion and Abeta- or CT(105)-induced toxicity. Biol Psychiatry. 2001;49:240–247. doi: 10.1016/s0006-3223(00)01124-0. [DOI] [PubMed] [Google Scholar]
- 27.Sontag JM, Fykse EM, Ushkaryov Y, Liu JP, Robinson PJ, Sudhof TC. Differential expression and regulation of multiple dynamins. J Biol Chem. 1994;269:4547–4554. [PubMed] [Google Scholar]
- 28.Stolerman IPaJ, J M. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14–20. [DOI] [PubMed] [Google Scholar]
- 29.Swan G, Hudmon K, Jack L, Hemberger K, Carmelli D, Khroyan T, Ring H, Hops H, Andrews J, Tildesley E. Environmental and Genetic Determinants of Tobacco Use. Cancer Epidemiology Biomarkers & Prevention. 2003;12:994. [PMC free article] [PubMed] [Google Scholar]
- 30.Swan GE, Lessov CN. Gene-environment interaction in nicotine addiction: the need for a large-scale, collaborative effort. Subst Use Misuse. 2004;39:2083–2085. [PubMed] [Google Scholar]
- 31.USDHHS. Atlanta, Georgia: US Department of Health & Human Services, Center for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promortion; Reducing tobacco use: A report of the Surgeon General. 2000
- 32.WHO. World Health Organization; The World Health Report 2002. 2002 doi: 10.1054/midw.2002.0343. [DOI] [PubMed]
- 33.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 34.Xu Q, Huang W, Payne TJ, Ma JZ, Li MD. Detection of genetic association and a functional polymorphism of dynamin 1 gene with nicotine dependence in European and African Americans. Neuropsychopharmacology. 2009;34:1351–1359. doi: 10.1038/npp.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]


