Abstract
Non-specific binding of Y receptor agonists to intact CHO cells, and to CHO cell or rat brain particulates, is much greater for human neuropeptide Y (hNPY) compared to porcine peptide Y (pPYY), and especially relative to human pancreatic polypeptide (hPP). This binding of hNPY is reduced by alkali cations in preference to non-ionic chaotrope urea, while the much lower non-specific binding of pPYY is more sensitive to urea. The difference could mainly be due to the 10–16 stretch in 36-residue Y agonists (residues 8–14 in N-terminally clipped 34-peptides), located in the sector that contains all acidic residues of physiological Y agonists. Anionic pairs containing aspartate in the 10–16 zone could be principally responsible for non-specific attachments, but may also aid the receptor site binding. Two such pairs are found in hNPY, one in pPYY, and none in hPP. The hydroxyl amino acid residue at position 13 in mammalian PYY and PP molecules could lower conformational plasticity and the non-selective binding via intrachain hydrogen bonding. The acidity of this tract could also be important in agonist selectivity of the Y receptor subtypes. The differences point to an evolutionary reduction of promiscuous protein binding from NPY to PP, and should also be important for Y agonist selectivity within NPY receptor group, and correlate with partial agonism and out-of group cross-reactivity with other receptors.
Keywords: Neuropeptide Y, Peptide YY, Pancreatic polypeptide, Low-affinity binding, Partial agonism
1. Introduction
The neuropeptide Y (NPY) family of receptors comprises several chordate receptors (termed Y1, Y2, Y4-Y8; see the review in [22]), among which four subtypes (Y1, Y2, Y4 and Y5) are well expressed and sufficiently studied in the mammal. All of these receptors can use two 36-residue peptidic agonists, neuropeptide Y (NPY) and peptide YY (PYY). The N-terminally clipped form of PYY, PYY(3–36), is quite selective for the Y2 receptor (see [43] and Table 1 in this study), and pancreatic polypeptide (PP) is highly selective for the Y4 receptor [15, 30]. Neuropeptide Y (NPY), abundant in the forebrain and generally restricted to neural cells, binds at a high affinity to Y1 [14] and Y2 receptors (see [45]), and at lower affinities to the Y5 [8], and especially to the Y4 receptor [15, 30]. NPY also attaches at a significant affinity to orexin receptors [18] and GPCR83 [51], and interacts with the pituitary gonadotropin hormone -releasing hormone (GnRH) receptor [23, 41], with numerous other receptors, and also with ion channels. As different from PYY, NPY displays significant partial agonism at the Y1 receptor [44, 50]. Peptide YY, a gut peptide apparently not produced in the brain, is accepted as ligand by all Y receptors [22]), however with a lower affinity and a limited access to the Y4 site [39]. Peptide YY also is a ligand for orexin receptors [18]. Pancreatic polypeptide (which also is not produced in the brain) binds significantly only to the Y4 receptor (see Table 1), and thus far was not found to bind to receptors outside the NPY receptor group. Peptide YY thus shows the largest in-group cross-reactivity (viewed as saturable attachment to specific binding sites of other Y receptors) and NPY appears to have the largest general cross-reactivity (in terms of affecting activity of receptors and other molecules).
Table 1.
Specific binding of non-selective and subtype-selective agonists to human Y receptors in cell lines
| Receptor | [125I] hNPY | [125I]pPYY | [125I]hPP | [125I] LP-hPYY | [125I]hPYY(3–36) |
|---|---|---|---|---|---|
| Y1 | |||||
| Kd or Ki, nM | 0.195 ± 0.012 | 0.172 ± 0.021 | - - - | 0.28 ± 0.027 | >100 |
| % of NPY binding | 100 | 102 ± 12 | 1.72 | 97.8 ± 6 | <1 |
| Y2 | |||||
| Kd or Ki, nM | 0.013 ± 0.004 | 0.0078 ± 0.0009 | - - - | >100 | 0.0098 ± 0.00013 |
| % of PYY(3–36) binding | 102 ± 16 | 90.4 ± 6.7 | 4.04 | 1.52 ± 0.13 | 100 |
| Y4 | |||||
| Kd or Ki, nM | 2.52 ± 0.04 | 0.535 ± 0.12 | 0.045 ± 0.005 | 0.488 ± 0.187 | >100 |
| % of hPP binding | 24.4 ± 1.6 | 34.4 ± 13 | 100 | 81.6 ± 22 | <1 |
| Y5 a | |||||
| Kd or Ki, nM | 2.19 ± 0.6 | 1.63 ± 0.48 | 9.1 ± 0.79 | 0.887 ± 0.16 | 7.9 ± 2.1 |
| % of NPY binding | 100 | 115 ± 11 | <20 | 96.5 ±16 | 54.7 ±12 |
With Y5-selective agonist hNPY-(19–23)-[Gly1,Ser3,Gln4,Thr6,Ala31,Aib32,Gln34] hPP; [8], the principal Ki for this receptor was 1.29 ± 0.26 nM at 140% of the binding of hNPY.
Data are averages of at least three independent binding experiments, ± S.E.M. . Human Y1, Y2 and Y4 subtypes were expressed in CHO cells, and the human Y5 receptor in HEK293 cells. It should be noted that NPY and PYY have similar binding parameters at, respectively, Y1, Y2 and Y5 receptors, as shown in a number of studies with different cell lines and tissues. The Kd values for agonists considered as primary for the given receptor type are shown in bold. Uncompeted specific binding for any [125I]-Y agonist as percent of the specific binding of the [125I]-labeled agonist selective or most active for the respective receptor is shown in the row below the Kd value. Competition assays used the same concentration of labeled agonist (50 pM), and unlabeled agonists at 8–12 concentrations ranging from 3 pM to 10 nM.
The binding of NPY is known to entail a large low-affinity fraction that is poorly competed by excess of the peptide or by specific antagonists, and is commonly perceived as non-specific (i.e., as non-saturating binding to progressively mobilized cohorts of sites other than the binding sites of Y receptors). This type of binding is much less with PYY, and especially low with PP. Brief comparisons of the non-specific binding of Y peptides in CHO-Y1 expression have been performed previously in [42, 43]. The above differences obviously are important in selectivity and physiological impact of Y agonists. The first objective of the present work was therefore to compare quantitatively the non-specific binding to expressions of four most studied human Y receptors. This examination indicated that the principal determinants of non-specific binding, as well as cross-reactivity, could be in the acidic section of Y peptides. This section has a low role in the specific binding of NPY ([5]; also see [20], but its function was only studied for effects of size reduction upon binding affinity. The second goal of this study was therefore to evaluate the constituent motifs of the acidic sector in terms of known reactivities and structural propensities of similar motifs in other peptides, and formulate approaches for experimental resolving role(s) of these anionic motifs in function, selectivity and cross-reactivity of the Y agonists.
2. Materials and Methods
2.1 Materials
Human / rat neuropeptide Y (hNPY), porcine / rat peptide YY (pPYY) and human (Leu31,Pro34) peptide YY (LP-hPYY) were obtained from the American Peptide company (Sunnyvale, CA, USA), or from Bachem (King of Prussia, PA, USA). The Y1 antagonist BIBP3226 ( (R)- N2-(diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]D-arginine amide ) and the Y2 antagonist BIIE0246 N-[(1S)-4-[(aminoiminomethyl)amino]-1-[([2-(3,5-dioxo-1,2-diphenyl-1,2,4-triazolidin-4-yl)ethyl]amino)carbonyl]butyl]-1-(2-[4-(6,11-dihydro-6-oxo-5H-dibenz[b,e]azepin-11-yl)-1-piperazinyl]-2-oxoethyl)cyclopentaneacetamide) were purchased from Tocris (Ellisville, MD, USA). Monoiodinated [125I] hNPY and human peptide YY(3–36) (hPYY(3–36) ) were supplied by Phoenix Pharmaceuticals (Shadyvale, CA, USA). [125I](Leu31,Pro34)human peptide YY (LP-hPYY) and [125I]porcine peptide YY (pPYY) were from PerkinElmer (Cambridge, MA, USA). (Pro34)human PYY (P-hPYY) was iodinated by the chloramine T procedure. All [125I]-labeled Y peptides had specific activities above 1700 Ci/mmole. High purity digitonin was from Calbiochem (La Jolla, CA, USA). All other chemicals were from Sigma (St. Louis, MO, USA).
2.2 Cell cultures and labeling
The cDNAs for human Y1, Y2, Y4 and Y5 receptors packaged in Invitrogen pcDNA 3.1+ vector were donated by the University of Missouri at Rolla (MO, USA). The cDNA for mouse Y4 receptor was a gift from Dr. Herbert Herzog (Garvan Institute for Medical Research, Sydney, Australia). The Y1, Y2 and Y4 cDNAs were stably expressed in CHO-K1 cells (American Type Culture Collection, Baltimore, MD, USA), and the Y5 cDNA in HEK293 cells [36] using lipofectamine-2000 cationic lipid (Invitrogen, Carlsbad, CA, USA). The cells were cultured at 400 μg/ml geneticin in D-MEM/F12 medium (Gibco, Long Island, NY, USA) containing 5% (v/v) fetal calf serum. Experimental incubations were preceded by four washes with D-MEM/F12 medium without antibiotics, and with 0.2% proteinase-free BSA instead of fetal calf serum. The incubations were done in 48 -well plates, in a volume of 0.25 ml of the above D-MEM/F12 medium. The incubations were terminated by removal of the medium by suction, two washes with ice-cold D-MEM/F12 medium, and extraction for 10 min at 0 – 5 ° with ice-cold 0.2 M CH3COOH – 0.5 M NaCl (pH 2.7), which quantitatively dissociated the cell-surface attached Y peptides [42].
2.3 Receptor characterization
Receptor assays were done in a buffer containing 20 mM Hepes.NaOH (pH 7.4), 3 mM CaCl2, 1 mM MgCl2, 0.2% proteinase -free bovine serum albumin, 1 mM diisopropylfluorophosphate, 0.04% bacitracin, and 10 μg/ml each of proteinase inhibitors aprotinin, bestatin, chymostatin, leupeptin and pepstatin. Cells were disrupted in a Dounce homogenizer, the particulates collected by centrifugation (see below) and stored at −80 °C. Assay concentration of particulate protein (measured by the Bradford procedure) was 200 μg/ml, the assay volume was 0.10 ml, and the incubation time was 30 min at 24–25 °C, with the appropriate competitors or inhibitors. The assay was terminated by centrifugation for 12 min at 30,000 × gmax and 4 °C , the supernatants were aspirated, and the pellets surface -washed by cold assay buffer prior to counting in a γ-scintillation counter. The binding properties of cell-surface receptors were characterized on monolayer cultures in D-MEM/F12 medium containing no antibiotics, and 0.2% BSA instead of fetal bovine serum. In competition assays, the iodinated Y peptides were used at 50 pM, and the competing peptides were input at 8–10 different concentrations ranging from 3 × 10−12 to 1 × 10−6 M (see also the footnote of Table 1). Saturation assays employed [125I]-labeled peptides as specified in the footnote of Table 2.
Table 2.
Parameters of non-specific binding of agonists to Y receptors from CHO cells and rat brain cortex at 25 °C
| Receptor and cell type | [125I] tracer | Slope factor ± SE | r2 | Half-period, min |
|---|---|---|---|---|
| hY1-CHO [3] | hNPY | 0.507 ± 0.068 | 0.985 | 1.77 ± 0.4 |
| hY1-CHO [4] | LP-hPYY | 0.226 ± 0.011 | 0.993 | 1.72 ± 0.11 |
| rY1-brain cortex [3] | hNPY | 0.541 ± 0.028 | 0.955 | 2.51 ± 0.91 |
| hY2-CHO [3] | hPYY(3–36) | 0.154 ± 0.002 | 0.995 | 1.92 ± 0.32 |
| mY4-CHO [4] | hPP | 0.058 ± 0.004 | 0.985 | 1.55 ± 0.3 |
The number of saturation assays employing 8–10 concentrations of [125I]-labeled agonists in the range of 4 - 400 pM is shown in brackets after the receptor subtype. Slope factors represent increment of the non-specifically bound per pM input [125I]-agonist. The slope factors and r2 (determination) coefficients are from the corresponding least-square linear regressions. Half-periods of equilibration are from hyperbolic fits on the binding of 50 pM tracer at 100 nM unlabeled peptide over 3, 6, 10, 20 and 30 min at 25 °C. The binding to cortex particulates also included 2 nM unlabeled hPYY(3–36), to mask the small population of Y2 receptors. Using 50 pM labeled and 1 nM cold agonist at 5–35 °C, the specific binding to CHO-Y1 particulates for both hNPY and pPYY saturated to about 210 fmol/mg protein, but with slope factors of 2.06 ± 0.18 for hNPY, and 5.94 ± 0.84 for pPYY.
2.4 Sucrose gradient assays
The particulates labeled by [125I]hNPY or pPYY were surface-washed in ice, and dispersed in cold receptor assay buffer at 0.20 mg particulate protein / ml. A mixture of sodium cholate and digitonin was added to 10 mM final each, the suspensions gently passed eight times through a 25-gauge needle, and sedimented for 5 min at 10,000 × gmax. The supernatants (0.4 ml) were then loaded on linear 10–30% sucrose gradients (total volume 9.2 ml, made in the receptor assay buffer) and sedimented for 24 h at 35,000 rev/min (218,000 × gmax) and 5 °C in SW41 Ti rotor of Beckman-Spinco M8-80 ultracentrifuge. The sedimentation positions were calibrated with [125I]-labeled bovine γ-globulin (158 kDa), human iron-saturated transferrin (75 kDa) and ovalbumin (44 kDa) and the covalently colorized myosin (211 kDa), producing a linear relation of distance traveled in the gradient and molecular weight (r2 > 0.99). The gradients were divided in 0.42 -ml fractions and the fractions counted in a γ-scintillation counter before and after precipitation at 12% polyethylene glycol (see [46]).
2.5 Evaluation of structure and binding data
Secondary structure evaluations were retrieved from the Protein Data Bank (http://www.rcsb.org/pdb/ ) site. Structure predictions [48] were obtained in http://distill.ucd.ie/, using porter program for 3-type structures (Table 4 ). Alignments of peptide sequences were done in SSEARCH3 program [47], available at fasta.bioch.virginia.edu/fasta/.
Table 4.
Sequences, motifs and secondary structure of Y peptides
| Peptide | 1 | 10 | 20 | 30 | Access |
|---|---|---|---|---|---|
| Sequences | |||||
| NPY human | YPSKPDNPGEDAPAEDMARYYSALRHYINLITRQRY | s-p P01303 | |||
| PYY human | YPIKPEAPGEDASPEELNRYYASLRHYLNLVTRQRY | s-p P10082 | |||
| PYY porcine | YPAKPEAPGEDASPEELSRYYASLRHYLNLVTRQRY | s-p P01305 | |||
| PYY(3–36) human | IKPEAPGEDASPEELNRYYASLRHYLNLVTRQRY | s-p P10082 | |||
| PP human | APLEPVYPGDNATPEQMAQYAADLRRYINMLTRPRY | s-p P01298 | |||
| PP bovine | APLEPEYPGDNATPEQMAQYAAELRRYINMLTRPRY | s-p P01300 | |||
| Structures a | |||||
| NPY human | CCCCCCCCCCCCCHHHHHHHHHHHHHHHHHHHHHHC | pdb 1TZ4 | |||
| PYY human | CCCCCCCCCCCCCHHHHHHHHHHHHHHHHHHCCCCC | pdb 2DEZ | |||
| PYY porcine | CCCCCCCCCCCCCHHHHHHHHHHHHHHHHHHCCCCC | pdb 2RLK | |||
| PYY(3–36) human | CCCCCCCCCCCCCHHHHHHHHHHHHHHHHCCCCC | pdb 2DF0 | |||
| PP human | CCCCCCCCCCCCCHHHHHHHHHHHHHHHHHHCCCCC | porter | |||
| PP bovine | CCCCCCCCCCCCCHHHHHHHHHHHHHHHHHHCCCCC | pdb 1LJV | |||
C = coiled, H = helical type of structure; no sheet-type structure is indicated in Y peptides.
Changes non-conservative relative to NPY are in italics. Residues critical for the high-affinity binding are underlined. Access files are in Swiss-Protein(s-p) or in Protein Data Bank (pdb).
The receptor binding parameters were calculated in the LIGAND program [33] and in biexponential curve fits using SigmaPlot software (SPSS, Chicago, IL; version 8.02).
3. Results
3.1 Affinity, selectivity and capacity in the binding of principal agonists to Y receptors
Determinants of affinity and selectivity of Y peptides were assessed in many studies over the past 20 years, of which [5], [11] and [15] are among the most instructive. Values for the binding affinities however tend to differ appreciably with assay conditions and the receptor expression used. We therefore present a compilation of binding parameters for all Y receptors as measured in our laboratories, using stable expressions in CHO-K1 cells for human Y1, Y2 and Y4 receptors, and in HEK-293 cells for the human Y5 receptor, and the same buffer and conditions in all experiments (see section 2.3 in Methods, and Table 1). The listed affinity estimates are for the main binding component in non-linear biexponential fits. Two components of specific binding, differing in affinity by about one order of magnitude, are found for the Y2 binding [37], and are detected for other Y receptors as well. For Y receptor heteropentamers this could be linked to unequal association of the two protomers with transducers [40], but the low-affinity component is not homogenous (and especially not for NPY). The specific binding at 50 pM of [125I]peptides relative to that of the primary [125I]agonist is listed to compare agonist selectivity.
As seen in Table 1, in CHO cell expressions NPY and PYY bind to either the Y1 or the Y2 receptor with similar affinity and capacity, while the binding of hPP to these receptors is very small. The Y2 receptors (human or rodent) have similar affinity and capacity for hNPY, pPYY and the N-terminally clipped human peptide YY(3–36); this peptide has very low affinity for the Y1 and the Y4 receptor. The Y1 receptor shows similar affinity and number of sites for NPY, PYY and the Y1-selective (Leu31,Pro34)hPYY (LP-hPYY), which has a very low affinity for the Y2 receptor. The human Y4 receptor shows high affinity for hPP binding, but poorly binds pPYY, and especially hNPY [30]. The Y5 receptor has similar parameters for hNPY and pPYY, and shows a low binding of hPP.
3.2 Non-specific binding of Y agonists is similar for wildtype cells and cells expressing Y receptors
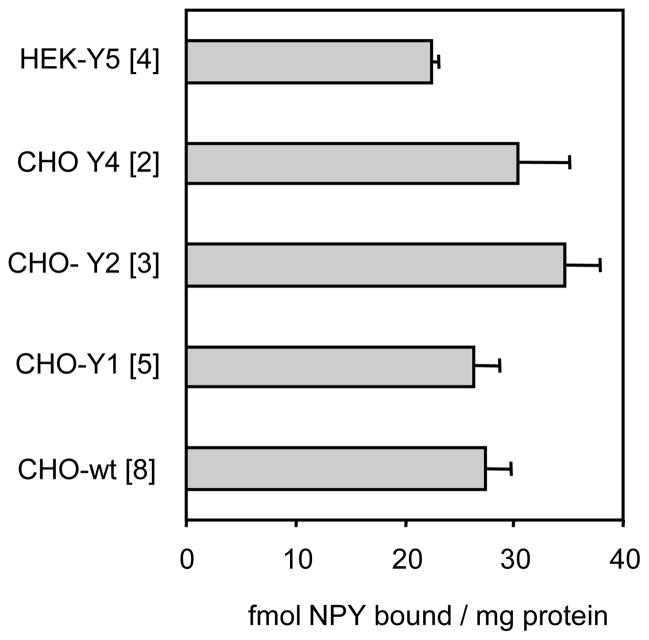
Non-specific binding of [125I]hNPY to wildtype CHO-K1 cells and to CHO and HEK293 cells expressing Y receptors is shown in Fig. 1; the corresponding specific binding values are listed in the caption. As seen, the non-specific binding of hNPY per mg of particulate protein is similar across cell lines and Y receptor expressions, with not more than 20% departure from the common mean; the binding to cell monolayers followed the same pattern. Very similar profiles were observed with agonist peptides selective for Y receptor subtypes (Fig. 2), however with large differences among three agonist types (see the next section).
Fig. 1.
Non-specific binding of NPY to cell particulates. Particulates from wildtype CHO-K1 cells or from CHO or HEK293 cells expressing the indicated human Y receptor subtype were labeled with 50 pM [125I]hNPY in the presence of 100 nM of the unlabeled hNPY. For assay details see section 2.3. Data are averages of 3–6 replicates in two or more experiments (number of experiments is shown in brackets), ± S.E.M. The specific binding in fmol/mg protein was <1 with CHO-wildtype (wt), 62.4 ± 9.6 with the CHO-Y1, 182 ± 21 with the CHO-Y2, 0.30 ± 0.13 with the CHO-Y4, and 26.1 ± 5.2 with the HEK293-Y5 expression.
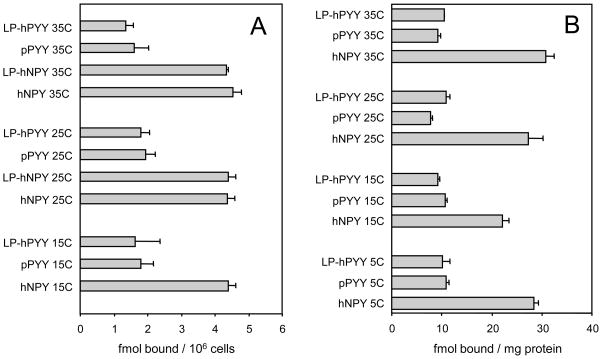
Fig. 2.
Non-specific binding of Y peptides at 5–35 °C is equilibrated in less than 30 min either with intact cells expressing human Y1 receptors (A) or with particulates from these cells (B). The 125I-labeled tracers were input at 50 pM in presence of either 100 nM of the respective non-labeled peptide or 20 μM Y1 antagonist BIBP3226. The labeling was for 30 min at the indicated temperature, in cell growth medium without fetal calf serum and antibiotics (see section 2.2) (A) or in the receptor assay buffer (B). Data are averages of two or more independent experiments. For other details see sections 2.2 and 2.3.
3.3 Non-specific binding is highly specific for Y agonist type, and equilibrates quickly
At 25 °C, the non-specific binding of the three major types of Y peptides at 100 nM of non-labeled and 4–400 pM [125I]-labeled agonist in CHO cells showed, as expected, highly linear (r2 > 0.95), non-saturating increase. The slope factors were however very different among the three major agonist types, ranging from 0.06 for hPP to >0.5 for hNPY (Table 2). The non-specific binding of hNPY to the rat Y1 receptor in particulates from rat brain cortex showed a slope similar to hY1-CHO binding of the peptide (Table 2). As expected from mass-action, with either monolayers or particulates of the CHO-Y1 expression, this binding reaches equilibrium much before 30 min of incubation at 5–35 °C (Table 2 and Fig. 2). Similar was observed with CHO-hY2 and CHO-hY4 cells (data not shown). The specific binding to CHO-Y1 particulates however showed a pronounced dependence on temperature, and a large rate difference between hNPY and pPYY. The specific CHO-Y1 binding of both hNPY and pPYY at 1 nM over 30 min saturates to similar values with increase in temperature, but the linear slope in the range of 5–35 °C is about three times lower (and the specific binding at 5 °C much higher) for hNPY (the caption of Table 2).
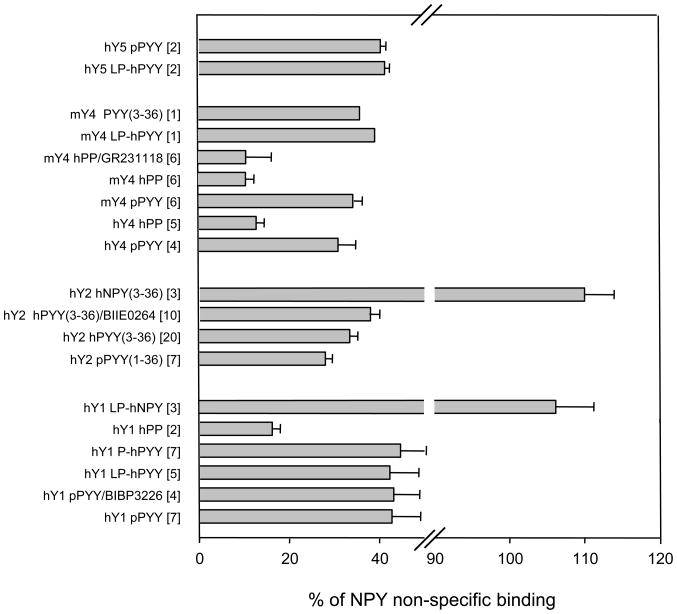
3.4 Levels of non-specific binding differ greatly among three types of human Y agonists
Non-specific binding differs considerably among three principal types of Y peptides, decreasing steeply in order of hNPY > pPYY > hPP (Figures 2 and 3; see also [42, 43] for the Y1 receptor-expressing CHO cells). These profiles are in Fig. 3 shown in relation to the binding of NPY to the respective expressions. It should be emphasized that the Y1-selective agonists LP-hNPY, LP-hPYY and P-hPYY and the Y2-selective agonists hNPY(3–36) and hPYY(3–36) have non-specific binding close to the parent peptides, i.e. there is a consistent large difference between NPY- and PYY- type peptides (Fig. 3). The binding of agonists to Y1-CHO at 20 μM of the Y1-selective antagonist BIBP3226, to Y2-CHO at 2 μM of the Y2-selective antagonist BIIE0246, and to Y4-CHO at 100 nM of the partial agonist GR231118 (1229U91) was very similar to the respective values at 100 nM of peptide agonists (Fig. 3). The above profiles are not likely to be significantly different with NPY and PYY of other mammals, and even of other vertebrates, which have sequences highly similar or even identical to human peptides (e.g. alligator NPY, s-p P09640). However, pancreatic polypeptides across vertebrates (and even among mammals) differ significantly in sequence, and some could have larger low-affinity interactions, e.g. porcine PP (s-p P01300, 10–16 sequence DDATPEQ) and turkey PP (s-p P68249, 10–16 sequence DDAPVED, lacking T13), as indicated by [17].
Fig. 3.
Non-specific binding of Y agonists relative to NPY in cells expressing Y receptors and in wildtype (wt) CHO-K1 cells. All 125I – labeled agonist were input at 50 pM in the presence of 100 nM of the respective unlabeled peptide. As shown, the Y1 binding was also assessed at 20 μM of Y1-selective antagonist BIBP3226, the Y2 binding at 2 μM of the Y2-selective antagonist BIIE0246, and the Y4 binding at 100 nM of the Y4 partial agonist GR231118 (1229U91). For other details see sections 2.2 and 2.3.
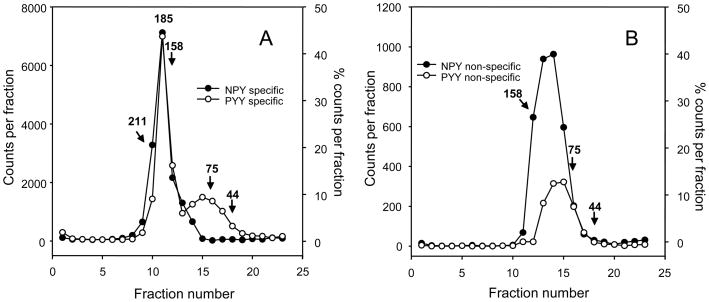
3.5 Solubilized non-specific binding of NPY and PYY sediments in the range of 50–150 kDa
The non-specific binding of NPY and PYY upon solubilization at 5 °C shows a broad distribution of molecular size (50–150 kDa, Fig. 4); less than 10% of the solubilized radioactivity sediments below 50 kDa. Most of the 50–150 kDa material could be recovered by precipitation with polyethyleneglycol. These profiles would be consistent with a fairly stable binding to a number of protein targets. Mixing [125I]-labeled hNPY or pPYY with the solubilizing detergents (10 mM each digitonin and cholate), or solubilization of [125I]pPYY -labeled Y1 or Y2 receptor by Y agonist-detaching detergents (CHAPS [40] or Triton X-100) did not result in appreciable Y agonist sedimentation past 10 kDa, indicating lack of a stable micellar association with the detergents (see [57] about removal of digitonin from rhodopsin by gradient ultracentrifugation).
Fig. 4.
Comparison of the Y agonist-competed (specific; graph A) and non-specific (graph B) binding of hNPY and pPYY to CHO cells expressing the human Y1 receptor. The 125I – labeled agonists were added to particulates at 50 pM without (graph A) or with (graph B) 100 nM of the corresponding unlabeled parent peptide. Data in (A) are the difference of total and non-specific binding (which is shown in (B) ). After labeling for 30 min at 25 °C, the particulates were sedimented, surface-washed, and lysed with 10 mM each digitonin and cholate, followed by sedimentation through 10–30% sucrose gradients for 24 h at 218,000 × gmax, and gradient fractionation. For molecular weight calibration and other details see section 2.4. In (A), the 185 kDa peak corresponds to heteropentamer containing the Y1 receptor dimer and Giα-Gβγ heterotrimer (see e.g. [46]).
3.6 Sensitivity of Y peptide binding to chaotropes
The binding of hNPY and pPYY to the Y1 receptor was also examined for sensitivity to the non-ionic chaotrope urea and the ionic chaotrope NaClO4 (Table 3). Concentrations allowing ≥95% recovery of particulate protein at assay end were 2 M for urea, and 0.3 M for NaClO4, resulting in inhibition of the specific binding of ~80% with urea, and ~90% with perchlorate. Guanidium salts could not be appropriately tested as ionic chaotropes, due to strong competitive interference of the guanido group with the binding of Y peptides (see e.g. [38]). With both agents, IC50 values for inhibition of the specific binding were well within the concentration ranges employed, indicating the expected largely ionic association with the Y1 site [5, 37]. With urea, the non-specific binding of hNPY was inhibited only 49% at 2 M, pointing to largely ionic interactions. The much smaller non-specific binding of pPYY was however inhibited 78% at 2 M urea (Table 3), indicating a large non-ionic contribution. With NaClO4 the non-specific binding of hNPY was reduced exponentially, confirming a strong ionic component. For pPYY, the displacement by 0.3 M NaClO4 was somewhat lower than with hNPY.
Table 3.
Inhibition of the binding of hNPY and pPYY to CHO-Y1 receptor by chaotropes
| Chaotrope and [125I] peptide | IC50 for specific binding, M | fmol/mg a | % inhibitionb | IC50 for non-specific binding, M | fmol/mga | % inhibitionb |
|---|---|---|---|---|---|---|
| Urea | ||||||
| hNPY | 0.96 ± 0.08 | 126 | 83 | 2.11 ± 0.38 | 34 | 49 |
| pPYY | 0.60 ± 0.11 | 118 | 79.5 | 0.53 ± 0.14 | 13.4 | 78 |
| NaClO4 | ||||||
| hNPY | 0.196 ± 0.02 | 126 | 90 | 0.202 ± 0.048 | 34 | 65.4 |
| pPYY | 0.135 ± 0.02 | 118 | 94 | 0.162 ± 0.022 | 13.4 | 61.1 |
Binding in absence of the chaotrope, in fmol/mg particulate protein.
The binding at maximal chaotrope input as % of the binding without the chaotrope.
[125I] peptides (25 pM) were added to particulates at eight concentrations of chaotropes (in the range of 0.1 – 2 M for urea and 0.01 – 0.3 M for NaClO4), without or with 100 nM of the corresponding unlabeled peptide, and after 60 min at 21–23° the incubation was terminated by centrifugation (see section 2.3). The specific binding is the difference between binding of the [125I]-peptide without and with 100 nM of the non-labeled peptide. All data are averages of three assays.
3.7 Structure of Y peptides: agonist-binding motifs and tracts that can be important in the non-specific binding
Sequences and structures of Y peptides and a map of receptor binding motifs are presented in Table 4. Sequence of NPY is the same in the three most studied species (man, rat and mouse), and even in alligator (s-p P09640). Porcine / rodent PYY is thus far the consensus PYY agonist, with nonconservative differences from hNPY in three positions (7, 13 and 14; Table 4). The human PYY (which is rarely used) has two more non-conservative differences with hNPY (at positions 3 and 18; Table 4). However, its close analogs (Pro34)hPYY and (Leu31,Pro34)hPYY with CHO-hY1 expression show both specific and non-specific binding similar to pPYY (Table 1 and Figure 3). All secondary structures are from porter program [48]. For NPY and PYY peptides, the porter predictions are identical to those in pdb files indicated in Table 4. For human PP, the porter structure is identical to that for the closely similar bovine PP (pdb 1LJV).
The C-terminal hexad is critically involved in the specific binding of Y peptides to all Y receptors [5, 10, 11, 15, 60]. Tyr1 is the only N-terminal residue that could be clipped without loss of Y1 binding [5] and activation [24]. Residues 3–6 are of importance for high-affinity binding to Y2 (see [45]) and Y4 [15, 59] receptors. Residues 4–25 are not critical for binding to the Y2 receptor (see [45]). Residues in the helical 18–32 sector of all primary Y agonists (see pdb files 1RU5, 1RUU [27], 2DEZ [35] and 2RK [34] for PYY, 1F8P [2] and 1FVN [3] for NPY, 1BBA [29] and 1LJV [26] for bPP), and especially in the 19–23 zone (pdb files 1RU5 and 1RUU [27]) are known to be important in subtype selectivity of Y agonists. The C-terminal hexapeptide, while critical for the specific attachment of agonists to all Y receptors, may not be important in the non-specific binding, since this binding does not differ between Y1 or Y2 -selective NPY and PYY analogs and the respective parent peptides (Fig. 3).
The acidic 6–16 stretch (which carries all non-conservative differences between hNPY and pPYY; Table 4) appears to have an only auxiliary role in the high-affinity binding of Y agonists, to the extent of tolerating radically non-conservative substitutions D11 > R11 or E15 > R15 [5]. The 17–30 tract is considered as helical in most NPY, PYY and PP structures available at the Protein Data Bank. This helicity could reduce sidechain mobility and availability, and then also the potential for low-affinity binding. The 19–23 tract of Y peptides contributes to specific binding [9], structuring [28] and receptor selectivity [8], but the difference between hNPY and hPYY or pPYY is only in the conservative switch of A and S at 22–23, and the following heptads are identical (Table 4). The 24–30 sector, LRHY[I,L]NL, differs only at position 28, and conservatively, for hNPY and hPYY or pPYY (Table 4). In hPP and bPP there are non-conservative changes at positions 21 and 23 and a conservative difference with hNPY at position 30. None of the above changes appear critical for the very different non-specific binding of the three Y agonist types.
Analysis of the helical contact propensity [1] shows the greatest difference for residues 14–16 (hNPY, 2.93; all PYYs, 1.97; hPP and bPP, 2.27). The 15–16 ED pair in NPY is flanked by a small and a medium-sized neutral sidechain, which is not found for the EE pair in PYY. This could also contribute to differences in low-affinity interactions.
In the 10–16 EDAPAED motif of hNPY, P13 is flanked by small A12 and A14 residues. This motif is changed to EDASPEE in pPYY and hPYY, and to DNATPEQ in hPP and bPP (Table 4), with a potential for reduced conformational mobility of proline, and lower interactivity of residues 15–16 (see the Discussion).
4. Discussion
Of the three Y peptides examined in this work, hNPY shows the lowest selectivity across the four canonical Y receptors (although its affinity for the Y4 receptor is low [30]), pPYY has a discernible preference for the Y2 site [52], and hPP essentially does not attach to the Y1, and very little to the Y2 receptor (Table 1). These differences extend to interaction with other receptors, NPY being known for several (e.g. [7, 18, 23, 32, 41]), PYY for a few [7, 18], and PP for none. With both NPY or PYY, changes at residues 31 and 34 that prevent specific Y2 binding, and deletion of residues 1–2 (which prevents specific Y1 binding) do not affect the non-specific binding (Fig. 3).
In NPY and PYY, there is segregation of acidic residues to positions 6–18, and of basic sidechains to the 19–36 zone (with an isolated lysine at position 4 (Table 4) ). The acidic residues in hPP (and bPP) are all in the 1–24, and the basic in the 25–36 zone (Table 4). The basic tracts are indispensable for binding to all Y receptors (see [5, 10, 15], and [20] for critical affinity and chirality of the C-terminal hexad). Acidic sectors of Y agonists appear to have mainly a supporting role in the specific binding. The acidic sector of NPY retains a largely open structure in surface association with phospholipid micelles [2, 54], and could be readily available for interactions at large. In pancreatic polypeptide, however, the quite stable PP-fold [6, 26, 28] could strongly reduce the contact surface of the acidic segment.
Motifs containing DE and ED pairs are important for salt bridge stability, assembly of DNA-protein complexes, activation of kinases, helicases, caspases and disintegrins, and stable anchoring of a host of proteins to membrane systems. However, in many structural contexts Asp exhibits higher propensity than Glu for bridging cationic amino acids, possibly due to a low mobility and a significantly higher acidity of the Asp β-carboxyl compared to the Glu γ-carboxyl. Ionic bridges containing aspartate also can stabilize inactive conformations of GPCRs [25, 49]. Three aspartates in the 6–16 region of hNPY (Table 4) could bridge cationic sidechains of membrane-resident proteins, to produce the large sodium-sensitive non-specific binding of NPY (Table 3). These residues of hNPY also could engage basic sidechains in the neighborhood of the two-prong binding site of the Y1 receptor, and contribute to the partial agonism found for NPY, but not for PYY [44, 50]. Porcine or human PYY and human or bovine PP have a single aspartate in this highly accessible, largely non-helical section [6, 26, 28].
In the human Y1 receptor, H105 and K114 in the extracellular loop 1, H207 and H208 in loop 2 and H290 in loop 3 appear not to be involved in the specific binding of agonists [13, 56], and could be targets for low-affinity association with the acidic tract of Y peptides, and especially of NPY. At high levels of extracellular NPY this induces reverse agonism [50]. Presence of a hydroxyl amino acid at position 13 in mammalian PYY and PP molecules could reduce the conformational plasticity in that zone via H bonding of Ser / Thr to the Pro neighbor (e.g. [12, 58]), or to (n+3) or (n+4) residue [53], and thus decrease associations using 15–16 Glu-Glu (in PYY) or Glu-Gln (in PP). In NPY, P13 is flanked by small alanine residues, and can kink to help interactions of E15D16 (and even E10D11) within the Y1 or the Y2 molecule, or with the receptor environment. In PYY and PP, rotation of P14 would also be dampened by the absence of Ala following Pro. The hPP molecule has no acidic doublets, and that coincides with a very low non-specific binding (Fig. 3). However, this could also be linked to a considerably higher stability of PP-fold in PP compared to NPY and PYY [28].
Evolution of Y agonists [21] apparently was linked with production of PYY and PP mainly in non-neural tissues (see [4, 16]), and obviously reduced (relative to NPY) the low-affinity binding. NPY however can act both as Y receptor agonist and as a low-affinity ligand or co-agonist of other receptors [18, 23, 41]. The NPY binding not shared with PYY [55] may involve interaction with other GPCRs that transduce to G-proteins used by Y receptors, possibly with the chemokine receptors. Differences in interaction with phospholipids (which is avid for NPY [31], but thus far not quantitatively compared for the primary Y agonists) could be reflected in barrier-crossing ability (which is higher for NPY [19] relative to PYY [16] or PP [4]) as well as in the non-specific binding.
In testing the role of aspartate in the large low-affinity association of hNPY, replacements of Asp11 and Asp16 by Glu or amide amino acids could be expected to reduce the non-specific binding. Replacing the 10–16 tract of hNPY (EDAPAED) by that of hPP (DNAETPQ) could produce a similar result. This however could also affect the affinity and Y receptor selectivity of the specific binding, and stability of the PP-fold [2, 28]. Testing effects of the above changes could significantly add to understanding the multiple activities of Y peptides, and in particular of NPY.
Research Highlights.
Non-specific binding of NPY, PYY and PP is typically in 6:2:1 ratio
This relates to acidic pairs (2 in NPY, 1 in PYY, 0 in PP) and proline-13 in NPY
The above should also be important in agonist selectivity of Y receptor subtypes
The differences should also be important for partial agonism and cross-reactivity
Acknowledgments
This research was partly supported by the U.S. National Institutes of Health grant R01 HD-13703.
Abbreviations
- BIBP3226
(R)- N2-(diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]D-arginineamide
- BIIE0246
N-[(1S)-4-[(aminoiminomethyl)amino]-1-[([2-(3,5-dioxo-1,2-diphenyl-1,2,4-triazolidin-4-yl)ethyl]amino)carbonyl]butyl]-1-(2-[4-(6,11-dihydro-6-oxo-5H-dibenz[b,e]azepin-11-yl)-1-piperazinyl]-2-oxoethyl)cyclopentaneacetamide
- GR231118 or 1229U91
bis(29/31',29/31'||[Glu29,Pro30, Dpa31,Tyr32,Leu34,(Tyr-NH2)36]NPY(28–36) ||
- NPY
neuropeptide Y (h = human or rat)
- LP-hNPY
(Leu31,Pro34) human neuropeptide Y PYY, peptide YY (h = human; p = porcine or rat)
- LP-hPYY
(Leu31,Pro34) human peptide YY
- P-hPYY
(Pro34) human peptide YY
- hNPY(3–36)
human neuropeptide Y(3–36)
- hPYY(3–36)
human peptide YY(3–36)
- pdb
Protein Data Bank
- PP
pancreatic polypeptide (h = human, b =bovine)
Footnotes
Conflict of interest
The authors declare no conflict of interest in research presented in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamian L, Liang J. Helix-helix packing and interfacial pairwise interactions of residues in membrane proteins. J Mol Biol. 2001;311:891–907. doi: 10.1006/jmbi.2001.4908. [DOI] [PubMed] [Google Scholar]
- 2.Bader R, Bettio A, Beck-Sickinger AG, Zerbe O. Structure and dynamics of micelle-bound neuropeptide Y: comparison with unligated NPY and implications for receptor selection. J Mol Biol. 2001;305:307–29. doi: 10.1006/jmbi.2000.4264. [DOI] [PubMed] [Google Scholar]
- 3.Bader R, Rytz G, Lerch M, Beck-Sickinger AG, Zerbe O. Key motif to gain selectivity at the neuropeptide Y5-receptor: structure and dynamics of micelle-bound [Ala31, Pro32]-NPY. Biochemistry. 2002;41:8031–42. doi: 10.1021/bi0201419. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, Kastin AJ, Jaspan JB. Regional variation in transport of pancreatic polypeptide across the blood-brain barrier of mice. Pharmacol Biochem Behav. 1995;51:139–47. doi: 10.1016/0091-3057(94)00412-c. [DOI] [PubMed] [Google Scholar]
- 5.Beck-Sickinger AG, Wieland HA, Wittneben H, Willim KD, Rudolf K, Jung G. Complete L-alanine scan of neuropeptide Y reveals ligands binding to Y1 and Y2 receptors with distinguished conformations. Eur J Biochem. 1994;225:947–58. doi: 10.1111/j.1432-1033.1994.0947b.x. [DOI] [PubMed] [Google Scholar]
- 6.Blundell TL, Pitts JE, Tickle IJ, Wood SP, Wu CW. X-ray analysis (1.4-A resolution) of avian pancreatic polypeptide: Small globular protein hormone. Proc Natl Acad Sci U S A. 1981;78:4175–9. doi: 10.1073/pnas.78.7.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouchard P, Dumont Y, Fournier A, St-Pierre S, Quirion R. Evidence for in vivo interactions between neuropeptide Y-related peptides and sigma receptors in the mouse hippocampal formation. J Neurosci. 1993;13:3926–31. doi: 10.1523/JNEUROSCI.13-09-03926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrele C, Langer M, Bader R, Wieland HA, Doods HN, Zerbe O, Beck-Sickinger AG. The first selective agonist for the neuropeptide YY5 receptor increases food intake in rats. J Biol Chem. 2000;275:36043–8. doi: 10.1074/jbc.M000626200. [DOI] [PubMed] [Google Scholar]
- 9.Cabrele C, Wieland HA, Langer M, Stidsen CE, Beck-Sickinger AG. Y-receptor affinity modulation by the design of pancreatic polypeptide/neuropeptide Y chimera led to Y(5)-receptor ligands with picomolar affinity. Peptides. 2001;22:365–78. doi: 10.1016/s0196-9781(01)00339-4. [DOI] [PubMed] [Google Scholar]
- 10.Cox HM, Tough IR, Ingenhoven N, Beck-Sickinger AG. Structure-activity relationships with neuropeptide Y analogues: a comparison of human Y1-, Y2- and rat Y2-like systems. Regul Pept. 1998;75–76:3–8. doi: 10.1016/s0167-0115(98)00047-0. [DOI] [PubMed] [Google Scholar]
- 11.Daniels AJ, Matthews JE, Viveros OH, Leban JJ, Cory M, Heyer D. Structure-activity relationship of novel pentapeptide neuropeptide Y receptor antagonists is consistent with a noncontinuous epitope for ligand-receptor binding. Mol Pharmacol. 1995;48:425–32. [PubMed] [Google Scholar]
- 12.Deupi X, Olivella M, Govaerts C, Ballesteros JA, Campillo M, Pardo L. Ser and Thr residues modulate the conformation of pro-kinked transmembrane alpha-helices. Biophys J. 2004;86:105–15. doi: 10.1016/S0006-3495(04)74088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du P, Salon JA, Tamm JA, Hou C, Cui W, Walker MW, Adham N, Dhanoa DS, Islam I, Vaysse PJ, Dowling B, Shifman Y, Boyle N, Rueger H, Schmidlin T, Yamaguchi Y, Branchek TA, Weinshank RL, Gluchowski C. Modeling the G-protein-coupled neuropeptide Y Y1 receptor agonist and antagonist binding sites. Protein Eng. 1997;10:109–17. doi: 10.1093/protein/10.2.109. [DOI] [PubMed] [Google Scholar]
- 14.Fuhlendorff J, Gether U, Aakerlund L, Langeland-Johansen N, Thogersen H, Melberg SG, Olsen UB, Thastrup O, Schwartz TW. [Leu31, Pro34]neuropeptide Y: a specific Y1 receptor agonist. Proc Natl Acad Sci U S A. 1990;87:182–6. doi: 10.1073/pnas.87.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gehlert DR, Schober DA, Beavers L, Gadski R, Hoffman JA, Smiley DL, Chance RE, Lundell I, Larhammar D. Characterization of the peptide binding requirements for the cloned human pancreatic polypeptide-preferring receptor. Mol Pharmacol. 1996;50:112–8. [PubMed] [Google Scholar]
- 16.Hernandez EJ, Whitcomb DC, Vigna SR, Taylor IL. Saturable binding of circulating peptide YY in the dorsal vagal complex of rats. Am J Physiol. 1994;266:G511–6. doi: 10.1152/ajpgi.1994.266.3.G511. [DOI] [PubMed] [Google Scholar]
- 17.Inui A, Okita M, Miura M, Hirosue Y, Nakajima M, Inoue T, Oya M, Baba S. Characterization of the receptors for peptide-YY and avian pancreatic polypeptide in chicken and pig brains. Endocrinology. 1990;127:934–41. doi: 10.1210/endo-127-2-934. [DOI] [PubMed] [Google Scholar]
- 18.Kane JK, Tanaka H, Parker SL, Yanagisawa M, Li MD. Sensitivity of orexin-A binding to phospholipase C inhibitors, neuropeptide Y, and secretin. Biochem Biophys Res Commun. 2000;272:959–65. doi: 10.1006/bbrc.2000.2880. [DOI] [PubMed] [Google Scholar]
- 19.Kastin AJ, Akerstrom V. Nonsaturable entry of neuropeptide Y into brain. Am J Physiol. 1999;276:E479–82. doi: 10.1152/ajpendo.1999.276.3.E479. [DOI] [PubMed] [Google Scholar]
- 20.Kirby DA, Boublik JH, Rivier JE. Neuropeptide Y: Y1 and Y2 affinities of the complete series of analogues with single D-residue substitutions. J Med Chem. 1993;36:3802–8. doi: 10.1021/jm00076a007. [DOI] [PubMed] [Google Scholar]
- 21.Larhammar D. Evolution of neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept. 1996;62:1–11. doi: 10.1016/0167-0115(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 22.Larsson TA, Olsson F, Sundstrom G, Lundin LG, Brenner S, Venkatesh B, Larhammar D. Early vertebrate chromosome duplications and the evolution of the neuropeptide Y receptor gene regions. BMC Evol Biol. 2008;8:184. doi: 10.1186/1471-2148-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leblanc P, L'Heritier A, Rasolonjanahary R, Kordon C. Neuropeptide Y enhances LHRH binding to rat gonadotrophs in primary culture. Neuropeptides. 1994;26:87–92. doi: 10.1016/0143-4179(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 24.Leibowitz SF, Alexander JT. Analysis of neuropeptide Y-induced feeding: dissociation of Y1 and Y2 receptor effects on natural meal patterns. Peptides. 1991;12:1251–60. doi: 10.1016/0196-9781(91)90203-2. [DOI] [PubMed] [Google Scholar]
- 25.Leppik RA, Miller RC, Eck M, Paquet JL. Role of acidic amino acids in the allosteric modulation by gallamine of antagonist binding at the m2 muscarinic acetylcholine receptor. Mol Pharmacol. 1994;45:983–90. [PubMed] [Google Scholar]
- 26.Lerch M, Gafner V, Bader R, Christen B, Folkers G, Zerbe O. Bovine pancreatic polypeptide (bPP) undergoes significant changes in conformation and dynamics upon binding to DPC micelles. J Mol Biol. 2002;322:1117–33. doi: 10.1016/s0022-2836(02)00889-6. [DOI] [PubMed] [Google Scholar]
- 27.Lerch M, Mayrhofer M, Zerbe O. Structural similarities of micelle-bound peptide YY (PYY) and neuropeptide Y (NPY) are related to their affinity profiles at the Y receptors. J Mol Biol. 2004;339:1153–68. doi: 10.1016/j.jmb.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 28.Lerch M, Kamimori H, Folkers G, Aguilar MI, Beck-Sickinger AG, Zerbe O. Strongly altered receptor binding properties in PP and NPY chimeras are accompanied by changes in structure and membrane binding. Biochemistry. 2005;44:9255–64. doi: 10.1021/bi0501232. [DOI] [PubMed] [Google Scholar]
- 29.Li XA, Sutcliffe MJ, Schwartz TW, Dobson CM. Sequence-specific 1H NMR assignments and solution structure of bovine pancreatic polypeptide. Biochemistry. 1992;31:1245–53. doi: 10.1021/bi00119a038. [DOI] [PubMed] [Google Scholar]
- 30.Lundell I, Blomqvist AG, Berglund MM, Schober DA, Johnson D, Statnick MA, Gadski RA, Gehlert DR, Larhammar D. Cloning of a human receptor of the NPY receptor family with high affinity for pancreatic polypeptide and peptide YY. J Biol Chem. 1995;270:29123–8. doi: 10.1074/jbc.270.49.29123. [DOI] [PubMed] [Google Scholar]
- 31.McLean LR, Buck SH, Krstenansky JL. Examination of the role of the amphipathic alpha-helix in the interaction of neuropeptide Y and active cyclic analogues with cell membrane receptors and dimyristoylphosphatidylcholine. Biochemistry. 1990;29:2016–22. doi: 10.1021/bi00460a009. [DOI] [PubMed] [Google Scholar]
- 32.Mollereau C, Zajac JM, Roumy M. Staurosporine differentiation of NPFF2 receptor-transfected SH-SY5Y neuroblastoma cells induces selectivity of NPFF activity towards opioid receptors. Peptides. 2007;28:1125–8. doi: 10.1016/j.peptides.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Munson PJ, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding proteins. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 34.Neumoin A, Mares J, Lerch-Bader M, Bader R, Zerbe O. Probing the formation of stable tertiary structure in a model miniprotein at atomic resolution: determinants of stability of a helical hairpin. J Am Chem Soc. 2007;129:8811–7. doi: 10.1021/ja0716960. [DOI] [PubMed] [Google Scholar]
- 35.Nygaard R, Nielbo S, Schwartz TW, Poulsen FM. The PP-fold solution structure of human polypeptide YY and human PYY3–36 as determined by NMR. Biochemistry. 2006;45:8350–7. doi: 10.1021/bi060359l. [DOI] [PubMed] [Google Scholar]
- 36.Parker EM. Neuropeptide Y Y5 receptor expression. Methods Mol Biol. 2000;153:53–60. doi: 10.1385/1-59259-042-X:53. [DOI] [PubMed] [Google Scholar]
- 37.Parker MS, Crowley WR, Parker SL. Differences in cation sensitivity of ligand binding to Y1 and Y2 subtype of neuropeptide Y receptor of rat brain. Eur J Pharmacol. 1996;318:193–200. doi: 10.1016/s0014-2999(96)00783-2. [DOI] [PubMed] [Google Scholar]
- 38.Parker MS, Lundell I, Parker SL. Pancreatic polypeptide receptors: affinity, sodium sensitivity and stability of agonist binding. Peptides. 2002;23:291–302. doi: 10.1016/s0196-9781(01)00610-6. [DOI] [PubMed] [Google Scholar]
- 39.Parker MS, Sah R, Sheriff S, Balasubramaniam A, Parker SL. Internalization of cloned pancreatic polypeptide receptors is accelerated by all types of Y4 agonists. Regul Pept. 2005;132:91–101. doi: 10.1016/j.regpep.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Parker MS, Sah R, Balasubramaniam A, Sallee FR, Sweatman T, Park EA, Parker SL. Dimers of the neuropeptide Y (NPY) Y2 receptor show asymmetry in agonist affinity and association with G proteins. J Recept Signal Transduct. 2008;28:437–51. doi: 10.1080/10799890802447423. [DOI] [PubMed] [Google Scholar]
- 41.Parker SL, Kalra SP, Crowley WR. Neuropeptide Y modulates the binding of a gonadotropin-releasing hormone (GnRH) analog to anterior pituitary GnRH receptor sites. Endocrinology. 1991;128:2309–16. doi: 10.1210/endo-128-5-2309. [DOI] [PubMed] [Google Scholar]
- 42.Parker SL, Parker MS, Lundell I, Balasubramaniam A, Buschauer A, Kane JK, Yalcin A, Berglund MM. Agonist internalization by cloned Y1 neuropeptide Y (NPY) receptor in Chinese hamster ovary cells shows strong preference for NPY, endosome-linked entry and fast receptor recycling. Regul Pept. 2002;107:49–62. doi: 10.1016/s0167-0115(02)00094-0. [DOI] [PubMed] [Google Scholar]
- 43.Parker SL, Parker MS, Buschauer A, Balasubramaniam A. Ligand internalization by cloned neuropeptide Y Y(5) receptors excludes Y(2) and Y(4) receptor-selective peptides. Eur J Pharmacol. 2003;474:31–42. doi: 10.1016/s0014-2999(03)02039-9. [DOI] [PubMed] [Google Scholar]
- 44.Parker SL, Parker MS, Sah R, Balasubramaniam A, Sallee FR. Self-regulation of agonist activity at the Y receptors. Peptides. 2007;28:203–13. doi: 10.1016/j.peptides.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 45.Parker SL, Balasubramaniam A. Neuropeptide Y Y2 receptor in health and disease. Br J Pharmacol. 2008;153:420–31. doi: 10.1038/sj.bjp.0707445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker SL, Parker MS, Sah R, Balasubramaniam A, Sallee FR. Pertussis toxin induces parallel loss of neuropeptide Y Y(1) receptor dimers and G(i) alpha subunit function in CHO cells. Eur J Pharmacol. 2008;579:13–25. doi: 10.1016/j.ejphar.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Pearson WR. Flexible sequence similarity searching with the FASTA3 program package. Methods Mol Biol. 2000;132:185–219. doi: 10.1385/1-59259-192-2:185. [DOI] [PubMed] [Google Scholar]
- 48.Pollastri G, McLysaght A. Porter: a new, accurate server for protein secondary structure prediction. Bioinformatics. 2005;21:1719–20. doi: 10.1093/bioinformatics/bti203. [DOI] [PubMed] [Google Scholar]
- 49.Porter JE, Perez DM. Characteristics for a salt-bridge switch mutation of the alpha(1b) adrenergic receptor. Altered pharmacology and rescue of constitutive activity. J Biol Chem. 1999;274:34535–8. doi: 10.1074/jbc.274.49.34535. [DOI] [PubMed] [Google Scholar]
- 50.Sah R, Balasubramaniam A, Parker MS, Sallee F, Parker SL. Neuropeptide Y as a partial agonist of the Y1 receptor. Eur J Pharmacol. 2005;525:60–68. doi: 10.1016/j.ejphar.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Sah R, Parker SL, Sheriff S, Eaton K, Balasubramaniam A, Sallee FR. Interaction of NPY compounds with the rat glucocorticoid-induced receptor (GIR) reveals similarity to the NPY-Y2 receptor. Peptides. 2007;28:302–9. doi: 10.1016/j.peptides.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Statnick MA, Schober DA, Mayne NG, Burnett JP, Gehlert DR. Analysis of NPY receptor subtypes in the human frontal cortex reveals abundant Y1 mRNA and binding sites. Peptides. 1997;18:137–43. doi: 10.1016/s0196-9781(96)00246-x. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki M, Yagi N. Structure of the SPXX motif. Proc Biol Sci. 1991;246:231–5. doi: 10.1098/rspb.1991.0149. [DOI] [PubMed] [Google Scholar]
- 54.Thomas L, Scheidt HA, Bettio A, Huster D, Beck-Sickinger AG, Arnold K, Zschornig O. Membrane interaction of neuropeptide Y detected by EPR and NMR spectroscopy. Biochim Biophys Acta. 2005;1714:103–13. doi: 10.1016/j.bbamem.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Wahlestedt C, Regunathan S, Reis DJ. Identification of cultured cells selectively expressing Y1-, Y2-, or Y3-type receptors for neuropeptide Y/peptide YY. Life Sci. 1992;50:PL7–12. doi: 10.1016/0024-3205(92)90342-m. [DOI] [PubMed] [Google Scholar]
- 56.Walker P, Munoz M, Martinez R, Peitsch MC. Acidic residues in extracellular loops of the human Y1 neuropeptide Y receptor are essential for ligand binding. J Biol Chem. 1994;269:2863–9. [PubMed] [Google Scholar]
- 57.Wildenauer D, Khorana HG. The preparation of lipid-depleted bacteriorhodopsin. Biochim Biophys Acta. 1977;466:315–24. doi: 10.1016/0005-2736(77)90227-9. [DOI] [PubMed] [Google Scholar]
- 58.Yu MH, King J. Single amino acid substitutions influencing the folding pathway of the phage P22 tail spike endorhamnosidase. Proc Natl Acad Sci U S A. 1984;81:6584–8. doi: 10.1073/pnas.81.21.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou C, Kumaran S, Markovic S, Walser R, Zerbe O. Studies of the structure of the N-terminal domain from the Y4 receptor - a G protein-coupled receptor - and its interaction with hormones from the NPY family. Chembiochem. 2008;9:2276–84. doi: 10.1002/cbic.200800221. [DOI] [PubMed] [Google Scholar]
- 60.Zwanziger D, Bohme I, Lindner D, Beck-Sickinger AG. First selective agonist of the neuropeptide Y1-receptor with reduced size. J Pept Sci. 2009;15:856–66. doi: 10.1002/psc.1188. [DOI] [PubMed] [Google Scholar]