Abstract
Variations in the serotonin transporter gene (5HTTLPR) and biased processing of face-emotion displays both have been implicated in the transmission of depression risk, but little is known about developmental influences on these relationships. Within a community sample of adolescents, we examine whether 5HTTLPR genotype moderates the link between maternal depressive history and errors in face-emotion labeling. When controlling for current levels of depression and anxiety among youth, a two-way interaction between maternal depressive history and 5HTTLPR genotype was detected. Specifically, adolescents whose mothers reported a depressive history and who had a low expressing genotype made more errors in classifying emotional faces when compared with adolescents with an intermediate or high expressing genotype, with or without maternal depression history. These findings highlight the complex manner in which maternal depression and genetic risk may interact to predict individual differences in social information processing, as it is moderated by the 5HTTLPR genotype.
Keywords: intergenerational transmission, depression, 5HT, adolescence, face processing, G X E
INTRODUCTION
Children of parents with depression face increased risk for depression themselves (Sullivan, Neale, & Kendler, 2000), particularly during adolescence (Williamson, Birmaher, Axelson, Ryan, & Dahl, 2004). Various potential mechanisms for this increased risk have been explored, including the contribution of genetics (Gross & Hen, 2004) and, more recently, biases in the processing of emotion (Gibb, Benas, Grassia, & McGeary, 2009).
Factors that affect the brain circuits implicated in major depressive disorder (MDD) also affect face-emotion identification (Drevets, 2003). Specifically, the serotonergic system influences neural processing of emotion, presumably through effects on amygdala-based circuitry (Hariri et al., 2002). Variation in 5HTT function has been linked to accuracy in identifying faces (Marsh et al., 2006), as well as both perturbed amygdala-ventrolateral prefrontal cortex (vPFC) function (Hariri et al., 2002) and risk for MDD (Gross & Hen, 2004). Specifically, lesions in the amygdala or the ventral prefrontal cortex (vPFC), two key brain structures implicated in MDD, disrupt face-emotion identification proficiency. Collectively, evidence suggests that brain circuitry implicated in MDD is also involved in face-emotion identification proficiency (e.g., Rolls, 2008; Adolphs, 2002). This suggests that research on face-emotion identification informs understandings of MDD pathophysiology.
However, key questions in this area remain. First, inconsistencies in research linking risk for MDD to the processing of emotional faces may reflect interaction with genetic risk (e.g., Lau et al., 2009). While not without controversy (Risch et al., 2009), some evidence suggests that the 5HTT low expressing allele moderates the association between life stress and depressive symptomatology (Caspi et al., 2003). Similar moderating influences might occur for the relationship between MDD and face-emotion processing; however, the role of these complex pathways in MDD risk remains imprecisely mapped. Prior work suggests that perturbations in the 5HT system early in development may relate both to later life risk for MDD and perturbed processing of facial emotions, possibly through effects on a core underlying neural architecture. For example, Monk and colleagues (2008) found that children of MDD parents, much like depressed adults and carriers of the 5HTTLPR low expressing allele, exhibit abnormal amygdala response to fearful faces. However, because this investigation did not examine the impact of 5HTTLPR on face processing and its association with familial risk, the role of serotonin remains unclear. Elucidation of the pathophysiology of MDD may derive from studies examining face processing in relation to genotype and development.
The goal of this report is to advance understanding of the relationship between risk for MDD and identification of emotional facial expressions in youth. We hypothesize that deficits in the processing of emotional faces may reflect broader deficits in emotion regulation. However, few studies examine facial processing deficits among youth at familial risk for depression, and these studies report inconsistent findings (Gibb et al., 2009; Joorman, Talbot, & Gotlib, 2007). The current investigation focuses specifically on face-emotion identification, a particularly important process that has been linked to neural system dysfunction, 5HT perturbations, and pediatric risk for mood disorders (Gibb et al., 2009; Lau et al., 2009). We examine interaction of maternal depression history and 5HTTLPR genotype in predicting face processing errors in a community sample of adolescents. We hypothesized that youth with a maternal depression history and the low expressing 5HTTLPR genotype would display more errors in correctly identifying facial emotion, relative to youth without these two risk factors. We also hypothesized that symptoms of depression and/or anxiety among the youth would not predict errors. We cast a broad net in our categorization of maternal depressive history in order to evaluate an endophenotype for depression within a general community sample.
METHODS
Participants and Design
This study examines face-emotion identification among a community sample of adolescents. Data are from the East Boston Family Study (EBFS; Wakschlag et al., 2010), an adolescent follow-up of a pregnancy cohort oversampled for prenatal smoking. This sample is at risk for depression given the robust association between prenatal smoking and depression (Pickett, Wilkinson, & Wakschlag, 2009). The current analyses included white non-Hispanic adolescents (mean age = 15.85, SD = 1.7) and their biological mothers on whom complete face processing, depressive history, and genotype data were obtained (n = 123, 56% female). In the 17 EBFS families who had multiple siblings participating, one sibling was randomly selected for participation from these families to eliminate dependency issues.
5-HTTLPR Genotyping
Saliva samples were obtained using Oragene (DNA Genotek, Ottawa, Ontario, Canada) self-collection vials. After extraction, DNA was quantitated with Quant-iT PicoGreen dsDNA Assay (Invitrogen, Carlsbad, CA) and normalized to a concentration of 10ng/uL. DNA was amplified with FAM –labeled forward primer 5’-FAM-CTG AAT GCC AGC ACC TAA CCC CTA ATG T-3’ (AppliedBiosystems, Foster City, CA) and reverse primer (with pigtail sequence in parentheses) 5’(GTTTCT)TGG GGA ATA CTG GTA GGG TGC AAG GAG AA-3’ (Applied Biosystems, Foster City, CA) using Dynazyme EXT Polymerase (Finnzymes, Espoo, Finland) with an initial denaturation step of 96°C for 12min followed by 45 cycles of 96°C for 30sec, 68°C for 45sec, 72°C for 3min, one hold at 72°C for 10min, and a final hold at 10°C. Products were digested using Msp I restriction enzyme (Promega, Madison, WI) to ascertain SNP data within this fragment. Both cut and uncut products were separated on a 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA) in the UIC Research Resources Center DNA Services Facility. Genotypes were called blind to phenotype data using Genemapper v 3.7. 5HTTLPR, including the SNP contained within the length variant, was coded into the following three categories: low (Sa/Sa, Sa/Sg n =23), intermediate (Sa/Lg, Sa/La, Sg/Lg, Sg/La, Lg/Lg, Lg/La., n = 72), and high (La/La, XLa/La, and XXLa/La, n =28) expressing.
Youth Face Processing
The Diagnostic Analysis of Nonverbal Accuracy-2 (DANVA-2; Nowicki & Carton, 1993) was computer administered to assess receptive knowledge of facial emotions. In the DANVA-2, standardized pictures of 24 adult male and female pictures displaying high- and low- intensity expressions of happiness, sadness, anger, or fear. Each emotion category includes six pictures. After viewing each picture for two seconds the participant must identify, in a forced choice format, which of the four expressions was displayed (happy, sad, angry, fearful). Total error scores across all emotions were generated by calculating the number of errors, both of commission and omission, for each emotion and then summing these errors. We chose to focus on the total error score to minimize Type I error and to align with previous research, such as that of McClure and colleagues’ (2005), which found that the total error score on the DANVA reliably predicted diagnostic group status. The paradigm possesses excellent psychometric properties and accordingly has been used widely in research on the genetic and psychiatric correlates of face-emotion labeling proficiency (e.g., Lau et al., 2009).
Maternal Depressive History
Because the current study was based in a community sample, with relatively low rates of maternal depression, maternal depressive history was broadly conceptualized. The maternal depressive history variable was assigned based on a clear maternal report of having had a lifetime episode of depression. We used the standardized clinical interview questions from the lifetime history module of the computerized YA-DISC (Shaffer et al., 2000). In particular, an endorsement of one of the hallmark depression symptoms of feeling persistently depressed for two or more weeks was captured in one consolidated question: “Since the age of five years old, was there ever a time when you felt sad or depressed, felt a loss of interest or pleasure, or seemed irritable nearly everyday for two or more weeks in a row?”
As an index of validity to this approach, we examined the extent to which this consolidated question overlapped with diagnostic level data about current MDD from the three waves of the study on the YA-DISC. Of the mothers who met criteria for MDD on the YA-DISC at one time point (n = 23), eighty-three percent were captured by the consolidated item. Only four mothers who met criteria for current MDD did not endorse the consolidated depressive history item. To maximize accuracy of our depression history classification, these four mothers were also included in the maternal depressive history group1. Overall, mothers who endorsed the depressive history item, met more criteria for MDD on the YA-DISC (M = 2.92, SD = 3.28 versus M = 0.40, SD = 1.38; t = 5.50, p < .05). As another validity check of the consolidated depression item, we examined maternal data on the Personal Well-Being Index (Dunst & Trivette, 1986). Indeed, mothers captured by the consolidated depression screen item reported poorer functioning overall (M = 39.07, SD = 11.09) when compared to those who did not endorse the depression screen item (M = 48.38, SD = 8.22). This was also reflected in the scores for emotional well being (M = 8.80, SD = 3.62 versus M = 11.88, SD = 2.39) and physical well being (M = 7.59, SD = 3.59 versus M = 10.18, SD = 3.42). The fact that women endorsing the depression screen item were functioning at a lower level than women who did not, lends some support to the use of the consolidated item as a valid screen.
Covariates
As this sample was collected based on prenatal exposure to cigarettes (Wakschlag et al., 2010), this variable was controlled in all analyses. We also included youth age as a covariate. We also controlled for current symptoms of internalizing disorders among the youth by controlling for the continuous number of MDD and GAD symptoms endorsed by both the parent and child for the previous year. Youth depression and anxiety were assessed with the Diagnostic Interview for Children (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone; 2000). Twelve adolescents met criteria for Major Depressive Disorder within the last year, whereas seven adolescents met criteria for Generalized Anxiety Disorder (GAD). These data were collected at the same assessment point as the DANVA-2. Controlling for these symptoms provides some assurance that errors in face processing did not stem from current symptomatology.
Data analyses
Total face processing errors were counts of incorrectly categorized faces across all emotions. Total errors were predicted using SAS PROC GENMOD with the following terms in the model: youth anxiety, youth depression, youth age, gender, genotype, maternal depression, nicotine exposure, genotype by maternal depression, sex by genotype, and maternal depression by sex. χ2 for Generalized Estimating Equations (GEE) are reported. Our analyses report two-tailed significance tests (alpha = .05). Contrasts were performed using χ2 tests to further examine significant main and interaction effects.
RESULTS
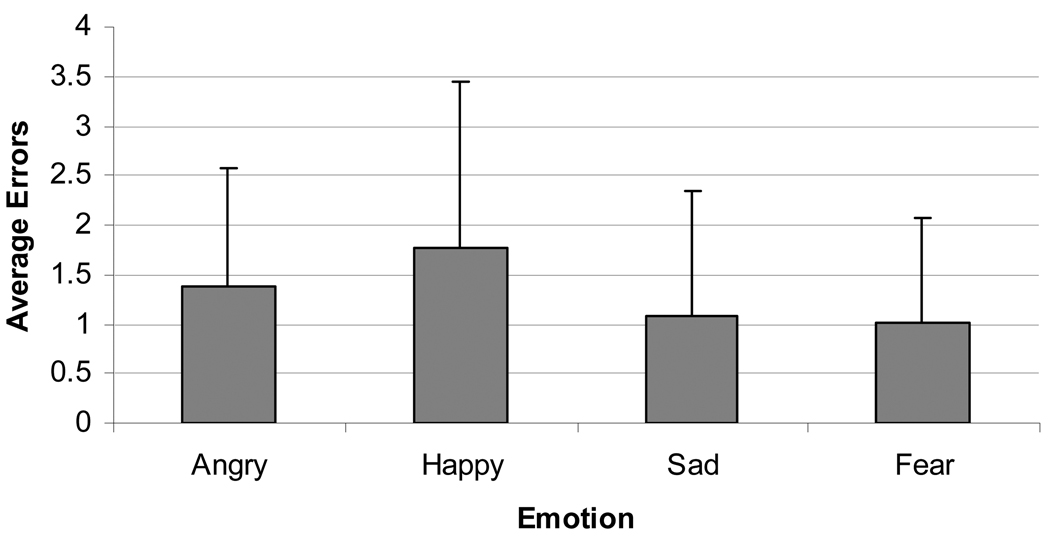
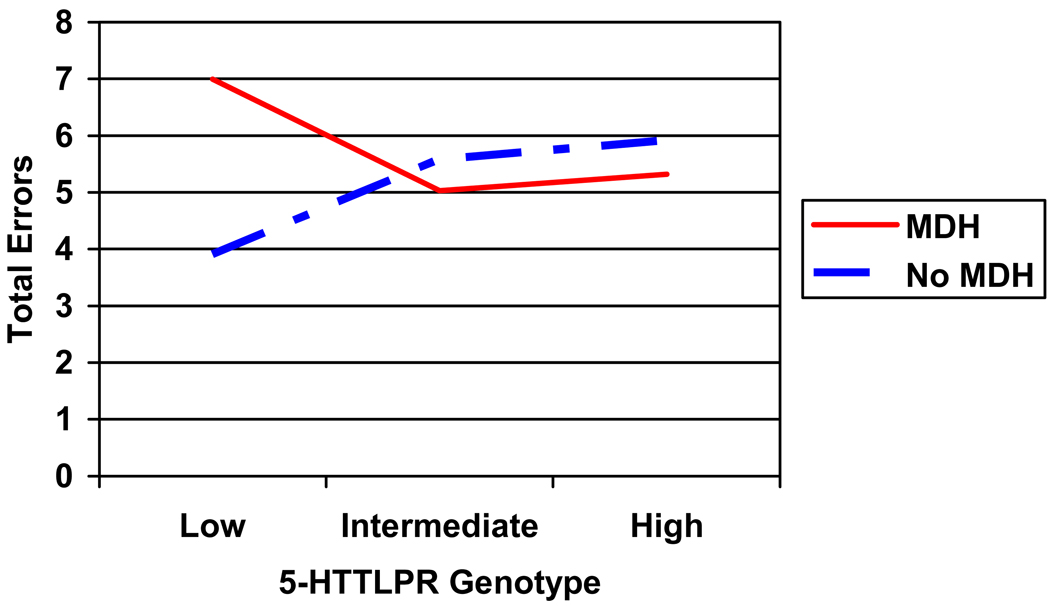
Youth anxiety and depression did not significantly contribute to the model predicting errors in face processing. However, gender significantly contributed to overall errors in classifying emotional faces, with boys making more errors (M = 6.05, SD = 0.44) than girls (M = 4.87, SD = .37)2. Figure 1 displays average errors for each emotion. A two-way interaction was detected between maternal depressive history and youth 5HTTLPR genotype as a predictor of emotional-face classification errors (see Table 1). Specifically, in the presence of the low expressing 5HTTLPR genotype, adolescents whose mothers reported a depressive history made more face-emotion identification errors than adolescents with no such maternal history (Figure 2; χ2 = −3.08 (1.12), p = .01, CI = − 5.28 – −0.89). There was also a significant difference between the low and intermediate expressing alleles in predicting total errors among youth whose mothers had a history of depression (χ2 = 1.96 (0.91), p = .03, CI = 0.18 – 3.74), implicating the low expressing allele in face processing errors. Post-hoc analyses regarding the influence of specific type of emotion revealed for a significant maternal depressive history by genotype interaction in predicting anger (χ2 = 7.61, p = .02) indicating that youth whose mother had a history of depression and who also had the low expressing genotype made more errors on angry faces.
Figure 1.
Mean errors per emotion
Table 1.
Results of GEE model predicting errors on emotional faces
| χ2 | p | |
|---|---|---|
| Adolescent Depression Symptoms | .04 | .83 |
| Adolescent Anxiety Symptoms | 1.08 | .30 |
| Maternal Depressive History | 1.25 | .26 |
| Sex | 6.36 | .01 |
| Youth Age | .54 | .46 |
| Genotype | 1.42 | .49 |
| Prenatal Nicotine Exposure | 0.64 | .42 |
| Maternal Depressive History by Genotype | 13.53 | <.01 |
| Maternal Depressive History by Sex | .00 | .96 |
| Sex by Genotype | .04 | .98 |
Figure 2.
Interaction of 5-HTTLPR genotype by maternal depressive history (MDH) in predicting total errors in classifying emotional faces
DISCUSSION
Our study documents a youth genotype-by-maternal depressive history interaction in predicting youth face-emotion labeling errors. Specifically, youth with the low expressing 5HTTLPR genotype whose mothers also reported a depressive history made more face processing errors in comparison with youth whose mothers’ reported a depressive history but had an intermediate or high expressing 5HTTLPR genotype. This pattern is particularly striking within this community sample. That is, detection of this interaction based on a broad assessment of maternal depression is noteworthy. Although we acknowledge the limitations of this approach, we also note that a broad characterization of depression would likely attenuate this pattern. We also demonstrate prediction from maternal history and genotype to a putative endophenotype (i.e. youth face processing) rather than depressive symptoms per se. These findings add support to an emerging body of work (Gibb et al., 2009) suggesting that maternal depression history may interact with genetic risk in pathways to youth depression, and that altered processing of emotional faces may be a mechanism of risk.
Other critical work has begun to examine the neural correlates of face processing in at-risk populations (e.g., Monk et al., 2008). For example, adolescents with MDD have demonstrated amygdala hypoactivation in response to passively viewed happy faces (Beesdo et al., 2009). Thus, research examining individuals’ patterns of neural activation in response to affective faces reveals group differences, sometimes even when behavioral data do not. Moving forward, methods that directly tap neural processes such as fMRI will be vital for advancing understanding these complex relations. For example, if at-risk youth can be identified based on their observable errors in face processing, this may refine our understanding of the etiology of depression as well as inform clinical diagnostic criteria. More importantly, the early identification of risk may lead to more effective prevention.
The current study contributes to the growing body of literature suggesting links among genotype, emotional biases, and depression. While maternal depression is a well recognized risk factor for depressive disorders in offspring, the mechanisms of intergenerational transmission remain poorly understood. Genetic factors are likely involved; the current data also implicate biased processing of emotional faces in the intergenerational transmission of depression. Our results are also in line with research demonstrating that serotonin manipulations affect facial emotion recognition (Merens, Van der Does, & Spinhoven, 2007). The current sample was not primed with a negative mood procedure and represented a community, rather than clinical, population, extending this previous work. Recent evidence suggests that the high expressing 5HTTLPR genotype is associated with a protective pattern of avoiding negative information and a vigilance toward the positive in processing images (Fox, Ridgewell, & Ashwin, 2009). This is consistent with our finding that the intermediate and high expressing genotypes appeared to mitigate the effect of a history of maternal depression on accuracy in face processing. In addition, recent work has examined the interplay of 5HTTLPR and the early mother-child relationship in the development of poor self-regulation abilities in early childhood (Kochanska, Philbert, & Barry, 2009). Thus, it is likely that 5HTTLPR status interacts with many environmental factors to make a child vulnerable to depression. Specific examination of these potential mechanisms in longitudinal, developmentally-based studies is needed.
Results must be interpreted within the confines of the current sample. On the one hand, our broad conceptualization of depression history in a community sample makes generalization to clinical populations uncertain. On the other hand, detecting an interaction within this sample highlights the robust nature of these candidate mechanisms. Replication of our findings within a sample that includes mothers with clinically diagnosed MDD and carefully captures a detailed history of the timing and nature of depressive episodes will be critical. Forty-four mothers endorsed the lifetime depression screen item, but did not meet DISC criteria for MDD within the last year in our analytic sample. We cannot know whether this is because these women had experienced a true depressive episode in the past and subsequently recovered, or whether these women inaccurately assessed their depressive history. We note that other studies examining depression within the community have used screeners that consist of only one or two questions. For example, the Patient Health Questionnaire is a two-item screener that has been used in primary care settings and has been found to have good specificity and sensitivity in identifying individuals with depression (Kroenke, Spitzer, & Williams, 2003). Nevertheless, this methodological limitation certainly requires more refined analysis in future work as we cannot rule out that these women represent “false positives.”
Although we have hypothesized this as an intermediary step in pathways from maternal to youth depression, we cannot assess the specificity of this pathway. For example, our findings may not be specific to depression, but may represent broader risk for internalizing disorders. In fact, recent work suggests that amygdala activity in response to emotional faces may reflect a common vulnerability to internalizing disorders (Beesdo et al., 2009). Our results are also limited by the fact that we could not test whether or not facial processing errors mediated relations between maternal and youth depression. This is because rates of depression among the youth were low. Only twelve adolescents met criteria for depression in the previous year and across the entire sample number of symptoms endorsed was low (Mean symptoms = 1, SD = 2.06). However, although rates of MDD diagnosis were low, a range of symptoms were captured in this sample. Youth depression and anxiety did not contribute to face processing errors in the current analyses, suggesting that face processing errors are not likely to be merely a manifestation of depression in the youth. However, it remains possible that such errors correlate with levels of depression. Prospective measurement of accuracy in face processing would be required to rule out this possibility. We look forward to such research as well as further work examining whether the facial processing abilities of parent or child mediate the intergenerational transmission of depression. In addition, linking information processing biases to specific brain networks (through methods such as fMRI) and depressive phenotypes may importantly elucidate neurodevelopmental mechanisms of youth depression.
Perhaps more important, however, are the potential implications of this work on the development and refinement of interventions. A deficit in accurately identifying emotion in the faces of others is likely to contribute to the social difficulties experienced by youth with MDD. Future research can examine whether or not training in the accurate identification of emotions ameliorates or prevents symptoms of depression. Another promising avenue would be promoting the development of attentional control (e.g., Reinholdt-Dunne, Mogg, & Bradley, 2009) to mitigate these biases, as attentional control in children has been related to better regulation of distress (Posner & Rothbart, 2000).
Acknowledgements
This work was supported by NIDA grant DA15223 to Dr. Wakschlag, including support to Dr Cook. Dr. Wakschlag and Dr. Cook were also supported by the Walden & Jean Young Shaw and Children’s Brain Research Foundations. We gratefully acknowledge contributions of our EBFS collaborators, Drs. Kate Pickett, Vanja Dukic and Rosalind Wright. We also thank Dr. Brian Mustanski & the Gene Environment and Development Interaction (GEDI) team, Drs. Lauren McGrath, Holly Barnard, Patrick Fowler, Aaron Metzger & Miwa Yasui, for the contribution of the GEDI discussions to conceptualization of this work. Finally, we thank Dr. Gretchen Biesecker and the EBFS research staff and Kathy Hennessy of the IJR Laboratory of Developmental Neuroscience, whose outstanding efforts were key to the success of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We subsequently ran our analyses without classifying these four mothers as having a history of depression and our results did not change.
There were no significant differences in age (F = 1.56, p = 0.21) or gender (χ2= 2.82, p = 0.24) across the three categorizations of 5HTTLPR genotype.
References
- Adolphs R. Neural systems for recognizing emotion. Current Opinions in Neurobiology. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, Mclure-Tone EB, Monk CS, Nelson EE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Annals of the New York Academy of Sciences. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38:415–426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Hen R. The developmental origins of anxiety. Nature Reviews Neuroscience. 2004;5:545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Fox E, Ridgewell A, Ashwin C. Looking on the bright side: Biased attention and the human serotonin transporter gene. Proceedings of the Royal Society B. 2009;276:1747–1751. doi: 10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joorman J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The patient health questionnaire-2: Validity of a two-item screener. Medical Care. 41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- Lau JY, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, et al. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biological Psychiatry. 2009;65:349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Philibert RA, Barry RA. Interplay of genes and early mother-child relationship in the development of self-regulation from toddler to preschool age. Journal of Child Psychology and Psychiatry. 2009;50:1331–1338. doi: 10.1111/j.1469-7610.2008.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Buzas B, Soliman N, Richell RA, Vythilingham M, et al. Impaired recognition of fear facial expressions in 5-HTTLPR S-polymorphism carriers following tryptophan depletion. Psychopharmacology. 2006;189:387–394. doi: 10.1007/s00213-006-0581-2. [DOI] [PubMed] [Google Scholar]
- McClure E, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. American Journal of Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- Merens W, Van der Does AJW, Spinhoven P. The effects of serotonin manipulations on emotional information processing and mood. Journal of Affective Disorders. 2007;103:43–62. doi: 10.1016/j.jad.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Carton J. The measurement of emotional intensity from facial expressions. The Journal of Social Psychology. 1993;133:749–750. doi: 10.1080/00224545.1993.9713934. [DOI] [PubMed] [Google Scholar]
- Pickett K, Wilkinson R, Wakschlag L. The psychosocial context of pregnancy smoking and quitting in the Millennium Cohort Study. Journal of Epidemiology and Community Health. 2009;63:474–480. doi: 10.1136/jech.2008.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Reinholdt-Dunne ML, Mogg K, Bradley BP. Effects of anxiety and attention control on processing pictorial and linguistic emotional information. Behavior Research and Therapy. 2009;47:410–417. doi: 10.1016/j.brat.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. The Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Face processing in different brain areas, and critical band masking. Journal of Neuropsychology. 2008;2:325–360. doi: 10.1348/174866407x258903. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas C, Dulcan M, Schwab-Stone M. The Diagnostic Interview Schedule for Children, Version IV (DISC-IV): Description, Differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, et al. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Molecular Psychiatry. 2010;15:928–937. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D, Birmaher B, Axelson D, Ryan N, Dahl R. First episode of depression in children at low and high familial risk for depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:291–297. doi: 10.1097/00004583-200403000-00010. [DOI] [PubMed] [Google Scholar]