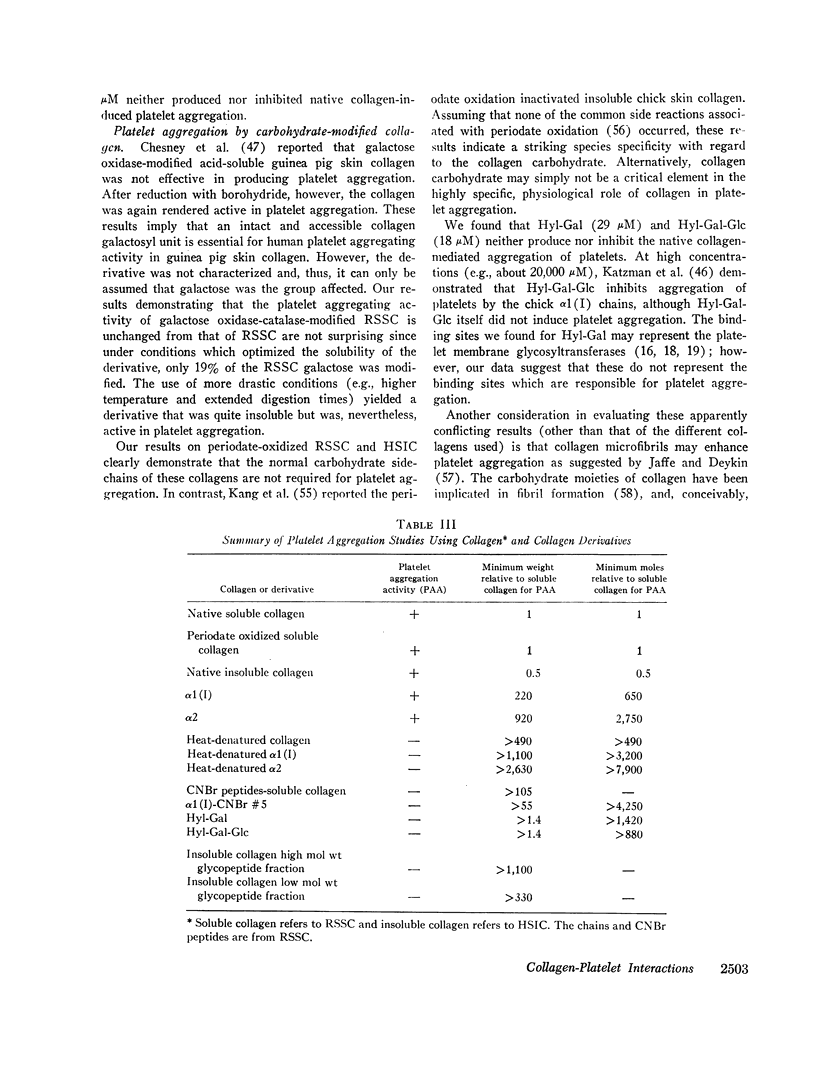
Abstract
In an effort to elucidate the nature of the collagen-platelet interaction, the effects of collagen modification on platelet aggregation have been studied. We have shown that purified rat skin (salt) soluble collagen is effective at about 20 nM in mediating platelet aggregation in human platelet-rich plasma. This concentration is somewhat greater than that required of several skin insoluble collagens (ca. 10 nM). Both the α1(I) and α2 chains from rat skin soluble collagen produced platelet aggregation, but only at concentrations of about 13 μM and 55 μM, respectively. In contrast, heat-denatured collagen and chains (e.g., 65 μM α1(I) and 160 μM α2) failed to induce platelet aggregation and to inhibit platelet aggregation by native collagen.
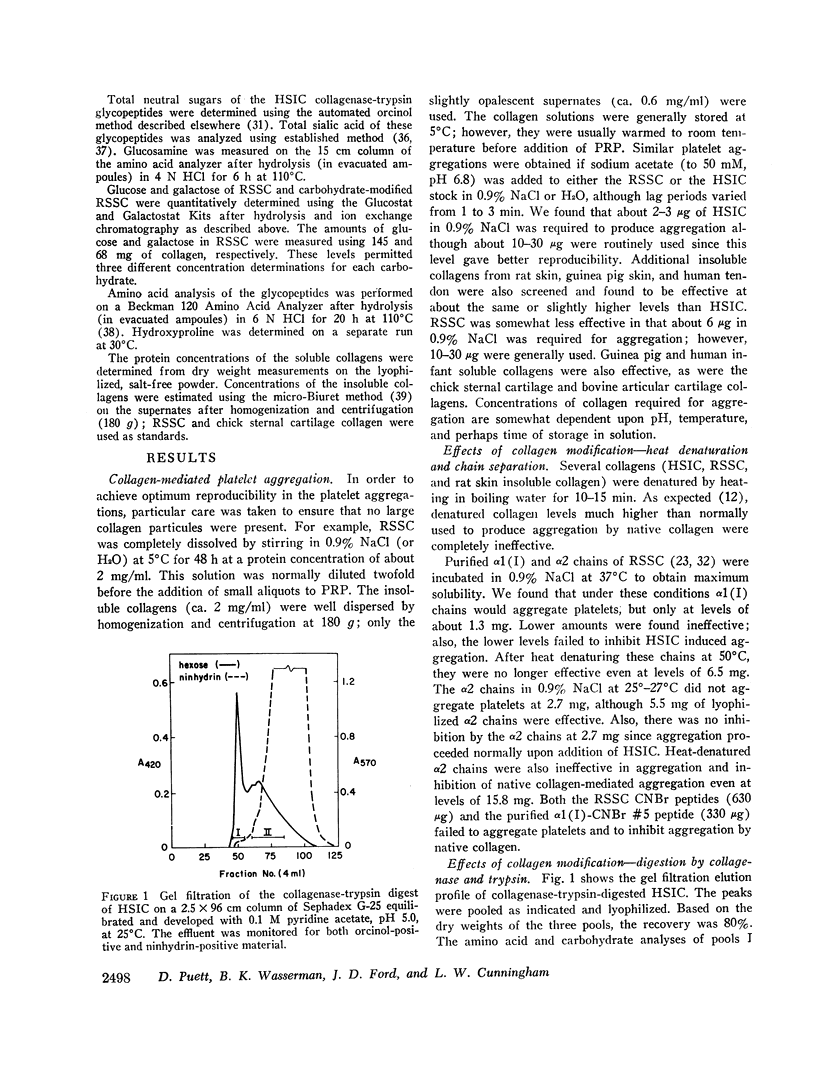
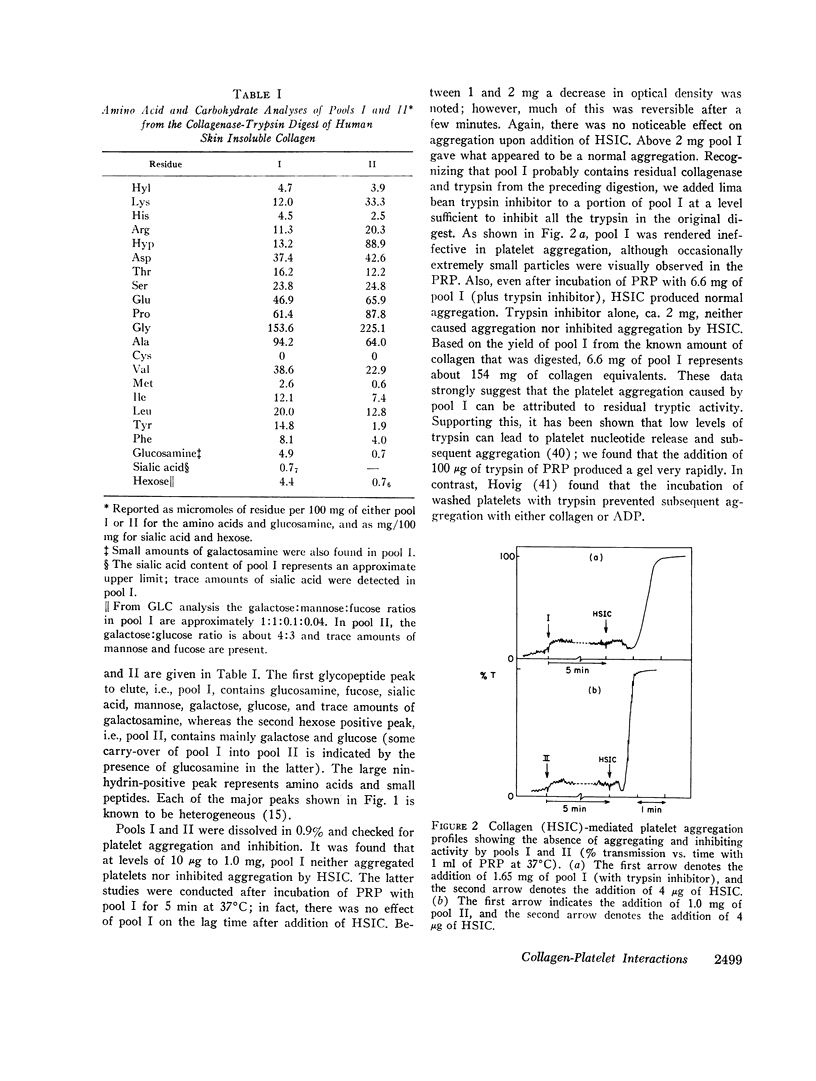
Glycopeptides were prepared from human skin insoluble collagen by extended digestion with bacterial collagenase and trypsin, and were purified by gel filtration into two classes. One class of higher molecular weight contained sialic acid, glucosamine, galactosamine, fucose, mannose, galactose, and glucose, and the other of lower molecular weight consisted primarily of a mixture of galactose and galactosyl-glucose units O-glycosidically linked to hydroxylysine-containing peptides. We found that, after the residual tryptic activity contaminating the higher molecular weight fraction was inhibited, neither of the glycopeptide classes produced nor inhibited native human skin insoluble collagen-mediated platelet aggregation at the highest concentration examined (ca. 1-2 mg glycopeptide per ml of platelet-rich plasma).
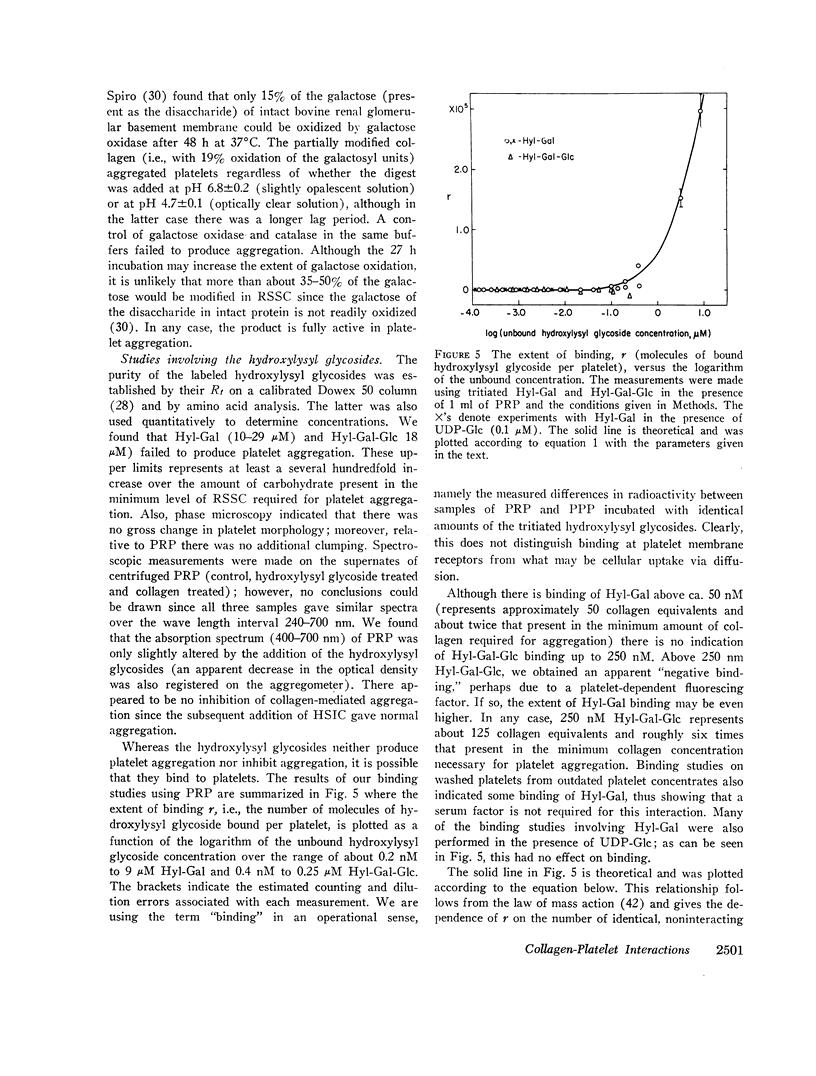
Highly purified samples of the hydroxylysyl glycosides, hydroxylysylgalactose and hydroxylysylgalactosylglucose (Hyl-Gal and Hyl-Gal-Glc, respectively), were prepared from human urine and labeled at galactose using galactose oxidase followed by reduction with tritiated borohydride. Binding studies with platelet-rich plasma showed that, at concentrations greater than 50 nM, Hyl-Gal gives apparent binding to platelets, but there was no evidence of Hyl-Gal-Glc binding to platelets at concentrations up to 250 nM. At concentrations several hundredfold higher than the equivalents present in the minimum concentration of rat skin soluble collagen required for platelet aggregation, neither Hyl-Gal (at 29 μM) nor Hyl-Gal-Glc (at 18 μM) caused platelet aggregation or inhibited platelet aggregation by native collagen. Also, at a concentration of 85 μM (which represents a concentration about two thousandfold higher than the equivalents in the minimum concentration of soluble collagen required for platelet aggregation) the Gal-Glc-containing 36 residue rat skin soluble collagen α1(I)cyanogen bromide #5 peptide had no platelet aggregating or inhibiting activity.
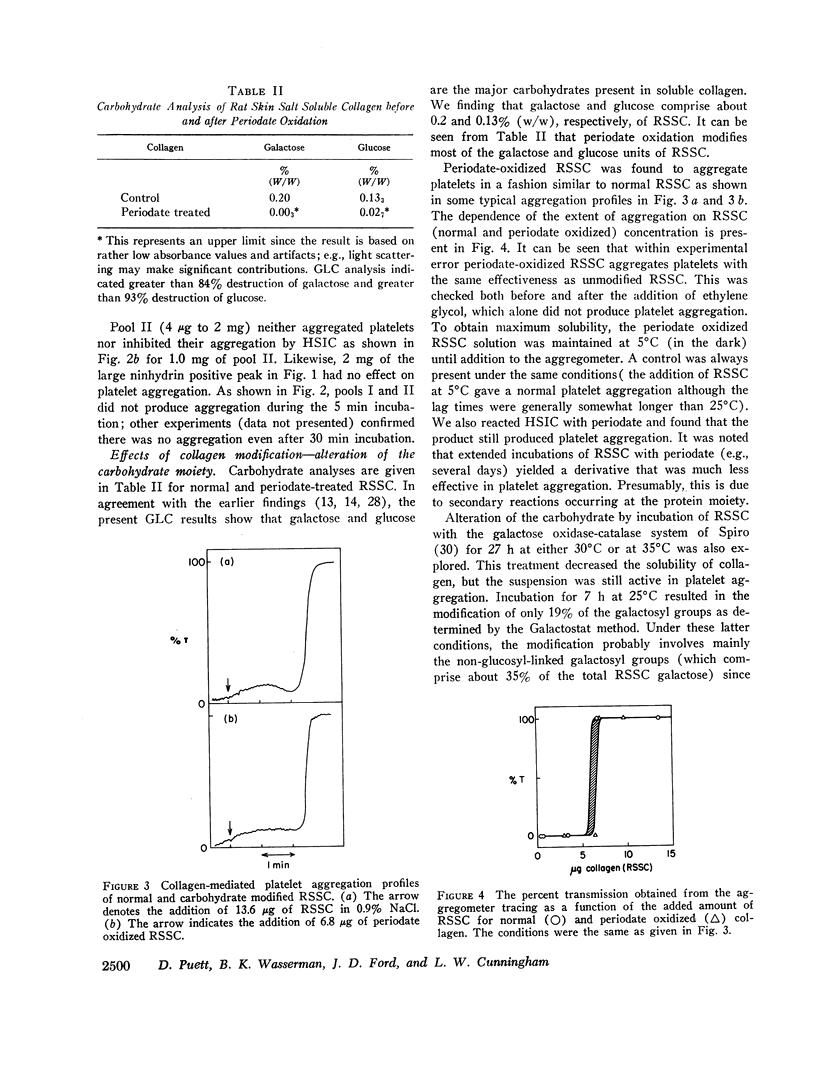
Modification of at least 90% of the rat skin soluble collagen carbohydrate by mild periodate oxidation had no effect on the platelet aggregating activity. Human skin insoluble collagen was reacted with periodate under the same conditions, and this had no demonstrable effect on its ability to induce platelet aggregation. This indicates that the normal carbohydrate side chains of these collagens are not required for the platelet interaction that produces the release of ADP and other metabolic constituents and leads to aggregation.
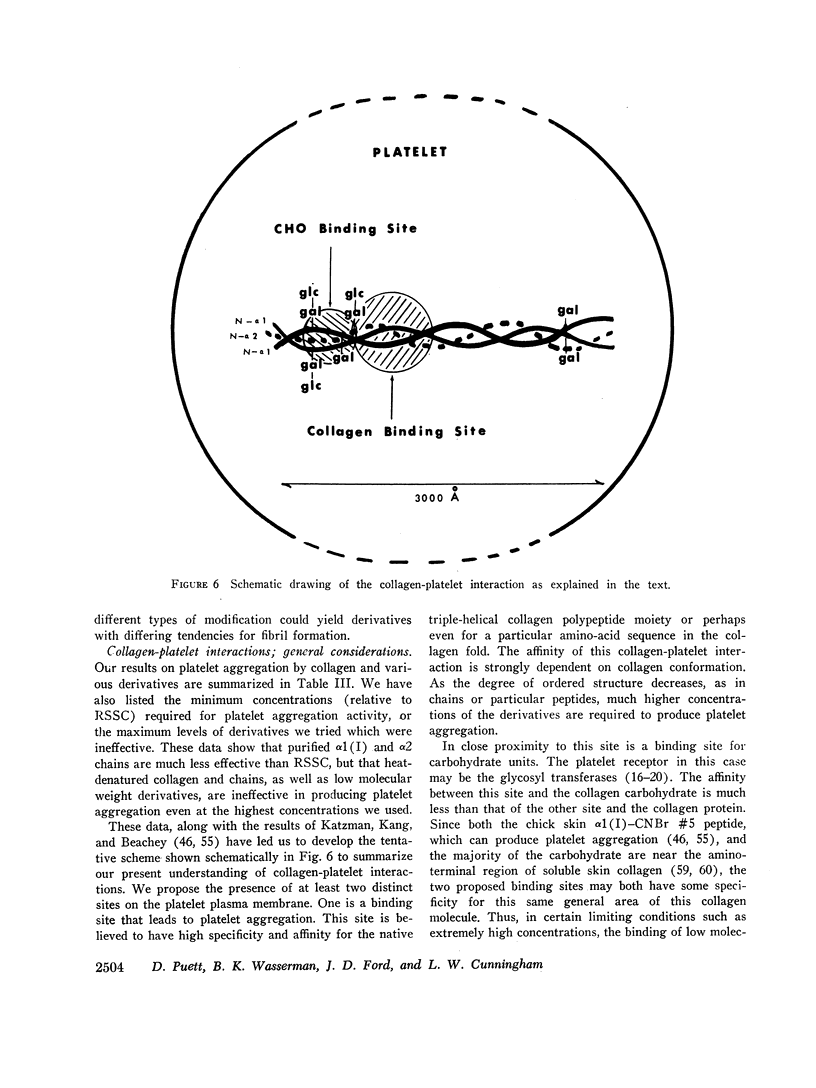
Thus, collagen-platelet interactions appear to involve at least two distinct binding sites on the platelet plasma membrane. One is a protein binding site that activates platelet aggregation and has high specificity and affinity for the collagen triple-helical fold or perhaps even for a particular amino acid sequence in the triple helix.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOUNAMEAUX Y. [The coupling of platelets with subendothelial fibers]. C R Seances Soc Biol Fil. 1959;153:865–867. [PubMed] [Google Scholar]
- Barber A. J., Jamieson G. A. Characterization of membrane-bound collagen galactosyltransferase of human blood platelets. Biochim Biophys Acta. 1971 Dec 21;252(3):546–552. doi: 10.1016/0304-4165(71)90157-7. [DOI] [PubMed] [Google Scholar]
- Barber A. J., Jamieson G. A. Platelet collagen adhesion characterization of collagen glucosyltransferase of plasma membranes of human blood platelets. Biochim Biophys Acta. 1971 Dec 21;252(3):533–545. doi: 10.1016/0304-4165(71)90156-5. [DOI] [PubMed] [Google Scholar]
- Born G. V. Observations on the rapid morphological reaction of platelets to aggregating agents. Ser Haematol. 1970;3(4):114–120. [PubMed] [Google Scholar]
- Bornstein P., Piez K. A. The nature of the intramolecular cross-links in collagen. The separation and characterization of peptides from the cross-link region of rat skin collagen. Biochemistry. 1966 Nov;5(11):3460–3473. doi: 10.1021/bi00875a012. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Platelet adhesiveness and aggregation: the collagen:glycosyl, polypeptide:N-acetylgalactosaminyl and glycoprotein:galactosyl transferases of human platelets. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1118–1124. doi: 10.1016/0006-291x(71)90578-x. [DOI] [PubMed] [Google Scholar]
- Butler W. T. Chemical studies on the cyanogen bromide peptides of rat skin collagen. The covalent structure of alpha 1-CB5, the major hexose-containing cyanogen bromide peptide of alpha 1. Biochemistry. 1970 Jan 6;9(1):44–50. doi: 10.1021/bi00803a006. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Cunningham L. W. Evidence for the linkage of a disaccharide to hydroxylysine in tropocollagen. J Biol Chem. 1966 Sep 10;241(17):3882–3888. [PubMed] [Google Scholar]
- Butler W. T., Ponds S. L. Chemical studies on the cyanogen bromide peptides of rat skin collagen. Amino acid sequence of 1-CB4. Biochemistry. 1971 May 25;10(11):2076–2081. doi: 10.1021/bi00787a018. [DOI] [PubMed] [Google Scholar]
- Chesney C. M., Harper E., Colman R. W. Critical role of the carbohydrate side chains of collagen in platelet aggregation. J Clin Invest. 1972 Oct;51(10):2693–2701. doi: 10.1172/JCI107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D. A comparison of glycopeptides derived from soluble and insoluble collagens. J Biol Chem. 1968 May 10;243(9):2390–2398. [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D., Segrest J. P. The isolation of identical hydroxylysyl glycosides from hydrolysates of soluble collagen and from human urine. J Biol Chem. 1967 May 25;242(10):2570–2571. [PubMed] [Google Scholar]
- Davey M. G., Lüscher E. F. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967 Dec 2;216(5118):857–858. doi: 10.1038/216857a0. [DOI] [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOVIG T. THE EFFECT OF VARIOUS ENZYMES ON THE ULTRASTRUCTURE, AGGREGATION, AND CLOT RETRACTION ABILITY OF RABBIT BLOOD PLATELETS. Thromb Diath Haemorrh. 1965 Mar 15;13:84–113. [PubMed] [Google Scholar]
- HUGUES J. [Binding of platelets to collagen]. C R Seances Soc Biol Fil. 1960;154:866–868. [PubMed] [Google Scholar]
- Harper E., Gross J. Separation of collagenase and peptidase activities of tadpole tissues in culture. Biochim Biophys Acta. 1970 Feb 11;198(2):286–292. doi: 10.1016/0005-2744(70)90061-6. [DOI] [PubMed] [Google Scholar]
- Hermans J., Jr, Puett D. Relative effects of primary and tertiary structure on helix formation in myoglobin and alpha-lactalbumin. Biopolymers. 1971;10(5):895–914. doi: 10.1002/bip.360100512. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Doery J. C. Platelet function in health and disease. Prog Hematol. 1971;7:185–234. [PubMed] [Google Scholar]
- Houck J., Chang C. Platelet aggregation produced by collagenolysis product peptides. Proc Soc Exp Biol Med. 1971 Oct;138(1):342–344. doi: 10.3181/00379727-138-35892. [DOI] [PubMed] [Google Scholar]
- Houck J., Chang C. The chemotactic properties of the products of collagenolysis. Proc Soc Exp Biol Med. 1971 Oct;138(1):69–75. doi: 10.3181/00379727-138-35834. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Urban C. L., Barber A. J. Enzymatic basis for platelet: collagen adhesion as the primary step in haemostasis. Nat New Biol. 1971 Nov 3;234(44):5–7. doi: 10.1038/newbio234005a0. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Bornstein P., Piez K. A. The amino acid sequence of peptides from the cross-linking region of rat skin collagen. Biochemistry. 1967 Mar;6(3):788–795. doi: 10.1021/bi00855a019. [DOI] [PubMed] [Google Scholar]
- Katzman R. L., Kang A. H., Beachey E. H. Collagen-induced platelet aggregation: involement of an active glycopeptide fragment (alpha1-CB5). Science. 1973 Aug 17;181(4100):670–672. doi: 10.1126/science.181.4100.670. [DOI] [PubMed] [Google Scholar]
- Lehnhardt W. F., Winzler R. J. Determination of neutral sugars in glycoproteins by gas-liquid chromatography. J Chromatogr. 1968 May 7;34(4):471–479. doi: 10.1016/0021-9673(68)80091-3. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Matukas V. J. Chick cartilage collagen: a new type of alpha 1 chain not present in bone or skin of the species. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1264–1268. doi: 10.1073/pnas.64.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972 Dec 19;11(26):4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Morgan P. H., Jacobs H. G., Segrest J. P., Cunningham L. W. A comparative study of glycopeptides derived from selected vertebrate collagens. A possible role of the carbohydrate in fibril formation. J Biol Chem. 1970 Oct 10;245(19):5042–5048. [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- Packham M. A., Mustard J. F. Platelet reactions. Semin Hematol. 1971 Jan;8(1):30–64. [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Rossi E. C. The function of platelets in hemostasis. Med Clin North Am. 1972 Jan;56(1):25–33. doi: 10.1016/s0025-7125(16)32420-8. [DOI] [PubMed] [Google Scholar]
- SPAET T. H., ZUCKER M. B. MECHANISM OF PLATELET PLUG FORMATION AND ROLE OF ADENOSINE DIPHOSPHATE. Am J Physiol. 1964 Jun;206:1267–1274. doi: 10.1152/ajplegacy.1964.206.6.1267. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Studies on the renal glomerular basement membrane. Nature of the carbohydrate units and their attachment to the peptide portion. J Biol Chem. 1967 Apr 25;242(8):1923–1932. [PubMed] [Google Scholar]
- Spiro R. G. The structure of the disaccharide unit of the renal glomerular basement membrane. J Biol Chem. 1967 Oct 25;242(20):4813–4823. [PubMed] [Google Scholar]
- Strawich E., Nimni M. E. Properties of a collagen molecule containing three identical components extracted from bovine articular cartilage. Biochemistry. 1971 Oct 12;10(21):3905–3911. doi: 10.1021/bi00797a017. [DOI] [PubMed] [Google Scholar]
- Wilner G. D., Nossel H. L., LeRoy E. C. Aggregation of platelets by collagen. J Clin Invest. 1968 Dec;47(12):2616–2621. doi: 10.1172/JCI105944. [DOI] [PMC free article] [PubMed] [Google Scholar]


