Abstract
Introduction
Recent clinical trials incorporating maintenance chemotherapy into the initial treatment of advanced non-small cell lung cancer (NSCLC) have highlighted the benefits of exposing patients to second-line therapies. We therefore determined the predictors and impact of second-line chemotherapy administration in a contemporary, diverse NSCLC population.
Methods
We performed a retrospective analysis of consecutive patients diagnosed with stage IV NSCLC from 2000 to 2007 at clinical facilities associated with the University of Texas Southwestern Medical Center. Demographic, disease, treatment, and outcome data were obtained from hospital tumor registries. The association between these variables was assessed using univariate analysis and multivariate logistic regression.
Results
A total of 406 patients in this cohort received first-line chemotherapy and were included in the analysis. Mean age was 59 years, 28% were women, and 59% were white. Among these patients, 197 (49%) received second-line chemotherapy. Among those patients who had not progressed after 4–6 cycles of first-line chemotherapy, 67% received second-line chemotherapy. Receipt of second-line chemotherapy was significantly associated with patient insurance type (P=0.007), number of cycles of first-line chemotherapy (P<0.001), and receipt of pre-chemotherapy palliative radiation therapy (P=0.005), but was not associated with patient age, gender, race, histology, or year of diagnosis. In a multivariate model, second-line chemotherapy administration remained associated with insurance type (P=0.003), number of cycles of first-line chemotherapy (P<0.001), and receipt of pre-chemotherapy palliative radiation therapy (P=0.008). The number of cycles of first-line chemotherapy and administration of second-line chemotherapy were associated with overall survival in both univariate and multivariate analyses.
Conclusions
In this unselected, contemporary and diverse cohort of patients with advanced NSCLC, 67% of individuals whose disease had not progressed after 4–6 cycles of first-line chemotherapy eventually received second-line chemotherapy. Markers of socioeconomic status, symptom burden, and response to and tolerance of first-line chemotherapy were associated with receipt of second-line chemotherapy. These factors may assist in the selection of patients most likely to benefit from maintenance chemotherapy.
INTRODUCTION
The role of second-line chemotherapy for advanced non-small cell lung cancer (NSCLC) has been highlighted by a number of recent clinical trials examining the role of “maintenance” therapy.1–6 Traditionally, patients with responsive or stable disease after four to six cycles of first-line platinum doublet chemotherapy have been monitored clinically and radiographically off therapy, with second-line chemotherapy initiated upon disease progression. Currently, three agents—docetaxel, pemetrexed, and erlotinib—are approved for this indication in the United States.7–9 With maintenance therapy, patients receive subsequent treatment immediately after completing first-line chemotherapy, either with a new agent (“switch maintenance”)2,4–5,10 or with an agent given during first-line therapy (“continuation maintenance”).1,5 Across studies, maintenance chemotherapy has been associated with prolongation of progression-free survival. Some trials have also demonstrated improvement in overall survival.2,10
Clinical trials of maintenance chemotherapy have been noteworthy for widely varying rates of second-line chemotherapy administration. Among patients randomized to observation after completion of first-line treatment, anywhere from 17–82% of patients received second-line therapy upon disease progression; 3–63% of patients received the same agent given in the maintenance arm.1,4–5 These discrepancies have confounded the interpretation of study results. It is not clear if maintenance chemotherapy provides a benefit because of its timing, or because it exposes more patients to additional, potentially effective therapies. That is, if there were a means to predict which patients would be fit to receive second-line therapy at the time of progression, it might not be necessary to offer these individuals maintenance regimens.
Outside the controlled environment of a clinical trial, little is known about administration of second-line chemotherapy. Large administrative databases do not routinely record this information. A recently published study from South Korea reported that 86% of patients received second-line treatment.11 This unusually high rate exceeds those of prospective, randomized maintenance chemotherapy trials and may reflect the young age and good performance status of the patient population. Indeed, multiple lung cancer studies have demonstrated substantial differences in treatment effects and overall prognosis between East Asian and western populations.12–16 To provide further insight into this issue, we examined the predictors and impact of second-line chemotherapy administration at a large North American medical center providing care to a diverse patient population within three different hospital systems.
METHODS
Study setting
The study cohort was captured from clinical facilities associated with the University of Texas Southwestern Medical Center (UT Southwestern), including Parkland Health and Hospital System (PHHS), University Hospital (which includes the freestanding Harold C. Simmons Cancer Center), and the Dallas Veterans Affairs (VA) Medical Center. PHHS consists of a 968-bed public hospital and outpatient clinics that provide health care to predominantly indigent and uninsured residents of Dallas County. Dallas County is the ninth most populous county in the United States, with an estimated 2.4 million residents, of whom 39 percent are Hispanic, 35 percent are white, and 21 percent are African-American.17 University Hospital (415 beds) is the principal medical and surgical referral hospital for UT Southwestern. The Dallas VA, a 289-bed hospital and outpatient clinics, serves as the principal tertiary care center for military veterans in a 40-county region of Northern Texas and Southern Oklahoma. It provides full medical, radiation, and surgical oncology services.
Data extraction
This study was approved by the UT Southwestern and the Veterans Affairs North Texas Health Care SystemInstitutional Review Boards. We identified patients diagnosed with stage IV NSCLC between January 1, 2000, and December 31, 2007, in the UT Southwestern, Parkland Health and Hospital System, and Dallas VA tumor registries. Additional information was obtained through electronic and paper medical records. The tumor registries identify cases through review of pathology records, clinic schedules, and hospital admission and discharge records. Certified tumor registrars extract data directly from medical records according to standards established by the American College of Surgeons Commission on Cancer, Surveillance Epidemiology and End Results (SEER)/National Cancer Institute (NCI) and the National Program of Cancer Registries (NPCR). Multiple data fields are collected per patient, including demographics, cancer diagnosis and stage, treatment, and follow-up. After initial cancer diagnosis and treatment, the tumor registries contact patients and their medical providers every six months for follow-up data. These data are then reported to the Texas State Cancer Registry and to the Commission on Cancer’s National Cancer Database.
We limited our study period to the years 2000–2007 for the following reasons: (1) randomized clinical trial data supporting the use of second-line chemotherapy for advanced NSCLC was first published in 20007; (2) adequate data were first recorded by UT Southwestern-associated tumor registries in 2000; (3) maintenance chemotherapy for advanced NSCLC was not incorporated into clinical practice during this period; and (4) the 2007 cutoff provides sufficient follow-up time for survival outcomes. We included only those patients who received platinum-based doublet chemotherapy as first-line treatment, as a survival benefit of second-line or maintenance chemotherapy has not been demonstrated for patients treated with single-agent first-line regimens.
Recording and Definition of Variables
For each patient, the following demographic data were recorded: age, gender, race/ethnicity, and insurance type. Race/ethnicity was categorized as white (non-Hispanic), Hispanic, African-American, or other. Insurance type was recorded as one of the following: no insurance, Medicaid (a federal/state health care program for low-income families), Medicare (a federal health care program for individuals age 65 years and older), VA, and private. The designation “no insurance” primarily includes individuals ultimately treated through a Dallas County public health plan that provides patients access to all standard diagnostic and treatment modalities. Disease variables recorded included tumor histology, date of diagnosis, and date of death or last known follow-up. Histology was categorized as adenocarcinoma, squamous cell carcinoma, or other. Overall survival was defined as the interval between date of diagnosis and date of death.
We recorded the following treatment variables: receipt of palliative radiotherapy prior to initiation of first-line chemotherapy (and site irradiated), number of cycles of first-line chemotherapy, disease status at end of first-line chemotherapy, and receipt of second-line chemotherapy. For patients who received at least four cycles of first-line chemotherapy, post-treatment disease status was characterized as progressive or non-progressive according to the overall radiographic and clinical impression in the medical record. We did not review imaging studies or employ formal scales, such as those of the World Health Organization (WHO) or Response Evaluation Criteria in Solid Tumors (RECIST) for this determination.
Statistical analysis
Descriptive statistics (medians/means for continuous variables and percentages for discrete variables) were generated for baseline demographic and clinical characteristics. Both univariate and multivariate logistic regression models were used to explore the association between demographic, disease, treatment characteristics, and receipt of second-line chemotherapy. In these analyses, age was dichotomized as < 65 years and ≥ 65 years; year of diagnosis was dichotomized as 2000–2003 and 2004–2007; race/ethnicity was characterized as white (non-Hispanic) or other. In the multivariate model, we included age, gender, race/ethnicity, insurance type, number of cycles of first-line chemotherapy, and pre-chemotherapy palliative radiation therapy. We analyzed the association between demographic, disease and treatment characteristics, receipt of second-line chemotherapy, and overall survival using univariate and multivariate Cox regression. Age, gender, race/ethnicity, insurance type, number of cycles of first-line chemotherapy, pre-chemotherapy palliative radiation therapy, and administration of second-line chemotherapy were included in the multivariate model. All reported P values are two-sided.
All statistical analyses were performed using SAS 9.2 Service Pack 4 for Windows (SAS Institute Inc., Cary, NC).
RESULTS
Study population
From the tumor registries, we identified a total of 472 patients who received first-line chemotherapy. Of these patients, 66 received single-agent first-line therapy (39 received a cytotoxic agent; 27 received an epidermal growth factor receptor [EGFR] tyrosine kinase inhibitor) and were excluded from the analysis. Within the remaining cohort of 406 patients, 186 (46%) were from Parkland Health and Hospital System, 153 (38%) were from the Dallas VA, and 67 (16%) were from University Hospital. Mean age was 59 years, 28% were women, and 59% were white. Additional patient characteristics are listed in Table 1. Median follow-up was 9.4months.
TABLE 1.
Baseline patient characteristics
| Characteristic | Mean (±SD) or number (%) |
|---|---|
| Total number | 406 |
| Age (y) | 59.1±10.6 |
| Gender | |
| Male | 294(72.4) |
| Female | 112 (27.6) |
| Race/ethnicity | |
| Non-Hispanic white | 238(58.6) |
| African-American | 127(31.3) |
| Hispanic | 32(7.9) |
| Other | 9(2.2) |
| Insurance type | |
| Private insurance | 91(22.9) |
| No insurance | 85(21.4) |
| Medicaid | 20(5.0) |
| Medicare | 47(11.8) |
| VA | 154(38.8) |
| Year of diagnosis | |
| 2000–2003 | 182(44.8) |
| 2004–2007 | 224(55.2) |
| Histology | |
| Adenocarcinoma | 168(41.4) |
| Squamous cell | 106(26.1) |
| Other | 132(32.5) |
| Number of cycles of first-line chemotherapy | |
| 1–3 | 202 (49.8) |
| ≥4 | 204 (50.2) |
| Non-progressor after ≥ 4 cycles of first-line chemotherapy | |
| Yes | 141(34.7) |
| No | 59(14.5) |
| Not applicable | 206 (50.7) |
| Pre-chemotherapy palliative radiation therapy | |
| Yes | 121(30.0) |
| No | 283(70.0) |
SD, Standard Deviation
Specific years of diagnosis were as follows: 2000 (32 patients), 2001 (48), 2002 (53), 2003 (50), 2004 (60), 2005 (48), 2006 (63), 2007 (52). Of the 132 patients listed as “other” histology, 3had large cell and 129 had NSCLC not otherwise specified. Among the 121 patients who received pre-chemotherapy palliative radiation therapy, the following sites were irradiated: brain (65 patients), lung (23 patients), bone (18 patients), brain and lung (9 patients), brain and bone (5 patients), lung and bone (1 patient).
Second-line therapy administration
Overall, 197of 406patients (49%) received second-line chemotherapy. Of the 142 patients with non-progressive disease after 4–6 cycles of first-line chemotherapy, 95 (67%) received second-line chemotherapy. For 149 patients (76%), second-line chemotherapy was a cytotoxic agent. Forty-eightpatients (24%) received an EGFR tyrosine kinase inhibitor as second-line therapy.
In univariate analysis, insurance type, number of cycles of first-line chemotherapy, and pre-chemotherapy palliative radiation therapy were significantly associated with receipt of second-line chemotherapy (see Table 2). In multivariate analysis, the following variables remained significantly associated with second-line chemotherapy administration: insurance type (P=0.003), number of cycles of first-line chemotherapy (OR for < 4 cycles 0.24; 95% CI, 0.16–0.38; P<0.001), and receipt of pre-chemotherapy palliative radiation therapy (OR 0.51; 95% CI, 0.31–0.84; P=0.008).
TABLE 2.
Association Between Baseline Characteristics and Administration of Second-Line Chemotherapy (Univariate Analysis)
| Characteristic | Number (%) Receiving Second-Line Chemotherapy | OR(95% CI) for Receiving Second-Line Chemotherapy | Overall P Value |
|---|---|---|---|
| Age | 0.73 | ||
| < 65 y | 137/287(47.7) | Reference | |
| ≥ 65 y | 59/119(49.6) | 1.08(0.70–1.65) | |
| Gender | 0.51 | ||
| Male | 139/294(47.3) | Reference | |
| Female | 57/112(50.9) | 1.16(0.75–1.79) | |
| Race/ethnicity | 0.82 | ||
| Non-Hispanic white | 116/238(48.7) | Reference | |
| Other | 80/168(47.6) | 0.96(0.64–1.42) | |
| Insurance type | 0.007 | ||
| Private insurance | 55/91(60.4) | Reference | |
| No insurance | 37/85(43.5) | 0.51(0.28–0.92) | |
| Medicaid | 7/20(35.0) | 0.35 (0.13–0.97) | |
| Medicare | 29/47(61.7) | 1.06(0.51–2.17) | |
| VA | 63/154(40.9) | 0.45(0.27–0.77) | |
| Year of diagnosis | 0.24 | ||
| 2000–2003 | 82/182(45.1) | 0.79(0.53–1.17) | |
| 2004–2007 | 114/224(50.9) | Reference | |
| Histology | 0.37 | ||
| Adenocarcinoma | 88/168(52.4) | Reference | |
| Squamous cell | 47/106(44.3) | 0.72(0.44–1.18) | |
| Other | 61/132(46.2) | 0.78(0.50–1.23) | |
| No.cycles of first-line chemotherapy | <0.001 | ||
| 1–3 | 64/202(32.0) | 0.26(0.17–0.40) | |
| ≥4 | 131/204(64.2) | Reference | |
| Pre-chemotherapy palliative radiation therapy | 0.005 | ||
| Yes | 45/121(37.2) | 0.53(0.34–0.82) | |
| No | 149/283(52.7) | Reference | |
CI, Confidence Interval; OR, Odds Ratio
Survival analysis
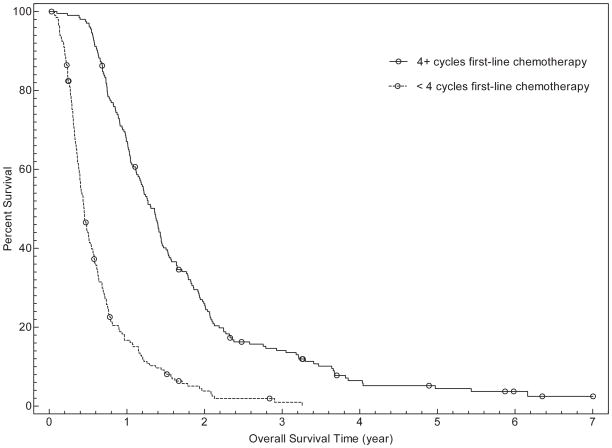
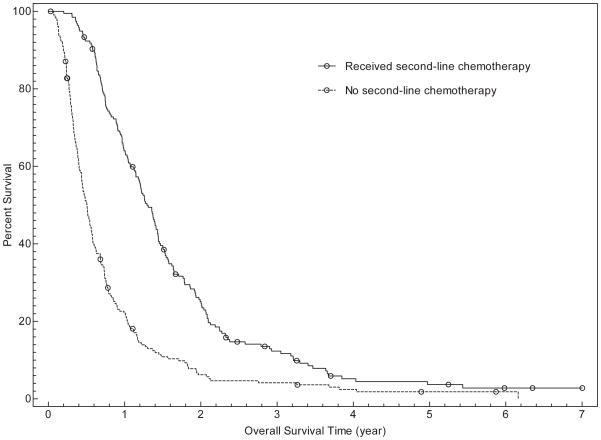
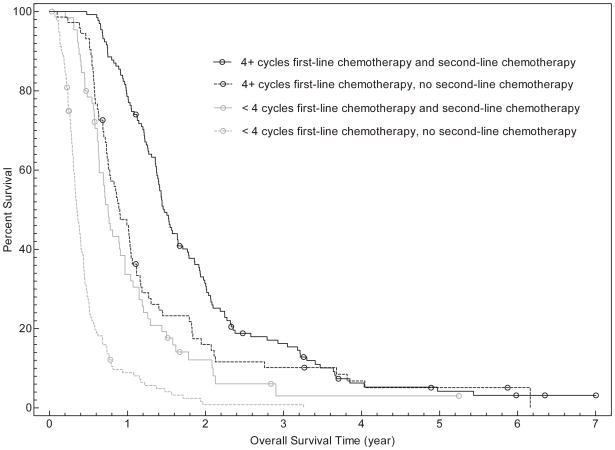
In univariate analysis, overall survival was associated with age (for ≥ 65years, HR for death 0.76; 95% CI, 0.61–0.95; P=0.02), number of cycles of first-line chemotherapy (for < 4 cycles, HR for death 3.50; 95% CI, 2.82–4.34; P<0.001), and administration of second line chemotherapy (if second-line chemotherapy received, HR for death 0.41; 95% CI, 0.34–0.51; P<0.001). Overall survival was not associated with gender, race/ethnicity, insurance type, or receipt of pre-chemotherapy palliative radiation therapy. Inmultivariate analysis, gender, number of cycles of first-line chemotherapy, and receipt of second-line chemotherapy were associated with overall survival (see Table 3 and Figure 1). For patients receiving fewer than 4 cycles of first-line chemotherapy, median survival was 164 days (95% CI, 146–185 days) compared to 495 days (95% CI, 431–522days) for patients receiving 4 or more cycles. Overall, median survival was 185 days (95% CI, 159–206 days) for patients who did not receive second-line chemotherapy, versus 472 days (95% CI, 419–522 days) for those who did receive second-line treatment. When both variables were considered, median overall survival was as follows: fewer than 4 cycles of first-line chemotherapy without second-line therapy (128 days; 95% CI, 117–146 days), fewer than 4 cycles of first-line chemotherapy with second-line therapy (274days; 95% CI, 231–353days), 4 or more cycles of first-line chemotherapy without second-line therapy (329 days; 95% CI, 274–382 days), 4 or more cycles of first-line chemotherapy with second-line therapy (537 days; 95% CI, 503–601 days).
TABLE 3.
Association Between Demographics, Treatment Characteristics, and Overall Survival (Multivariate Analysis)
| Characteristic | HR (95% CI) for Death | Overall P Value |
|---|---|---|
| Age | 0.14 | |
| < 65 y | Reference | |
| ≥ 65 y | 0.82(0.63–1.07) | |
| Gender | 0.03 | |
| Male | Reference | |
| Female | 0.75(0.57–0.97) | |
| Race/ethnicity | 0.25 | |
| Non-Hispanic white | Reference | |
| Other | 0.87(0.70–1.10) | |
| Insurance type | 0.46 | |
| Private insurance | Reference | |
| No insurance | 1.17(0.85–1.60) | |
| Medicaid | 1.38(0.83–2.30) | |
| Medicare | 1.40(0.93–2.09) | |
| VA | 1.13(0.82–1.55) | |
| Number of cycles of first-line chemotherapy | <0.001 | |
| 1–3 | 3.16(2.52–3.96) | |
| ≥4 | Reference | |
| Pre-chemotherapy palliative radiation therapy | 0.13 | |
| Yes | 0.83(0.65–1.05) | |
| No | Reference | |
| Receipt of second-line chemotherapy | <0.001 | |
| Yes | 0.46(0.37–0.58) | |
| No | Reference | |
CI, Confidence Interval
FIGURE 1.
Overall survival curves of patients categorized by number of cycles of first-line chemotherapy (Fig. 1a), receipt of second-line chemotherapy (Fig. 1b), and both the number of cycles of first-line chemotherapy and receipt of second-line chemotherapy (Fig. 1c).
DISCUSSION
The recent wave of clinical trials examining the role of maintenance chemotherapy for advanced NSCLC hasagain placed a spotlight on the benefits of second-line chemotherapy for this disease. Somewhat unexpectedly, these studies have revealed widely varying rates of second-line chemotherapy administration. In some studies, the likelihood of patients randomized to observation after first-line chemotherapy receiving chemotherapy at the time of progression is below 20%,1 raising the possibility that broader use of second-line therapies could mitigate some of the benefit attributed to a maintenance approach. The study of immediate (i.e., maintenance) or delayed docetaxel following 4 cycles of first-line therapy provides a prime example of this scenario; overall survival for patients who received immediate docetaxel and for the two-thirds of patients randomized to delayed docetaxel who ultimately received the assigned treatment was identical.4
The current study, which employs a contemporary, diverse, and unselected population, offers further insight into the real-world experience of second-line NSCLC treatment. In this cohort, 67% of individuals who had not progressed after receiving 4 cycles of first-line chemotherapy (i.e., those patients considered candidates for maintenance chemotherapy) ultimately received second-line treatment. While this rate itself is noteworthy for matching those reported in numerous maintenance therapy clinical trials,2,4,10 it must also be placed into context. Our population likely includes many individuals who, eitherdue to performance status, adherence to medical care, or comorbidities, would not be candidates for clinical trials. This study also examines second-line chemotherapy patterns among the larger population of all patients with advanced NSCLC receiving first-line treatment. Compared to the maintenance chemotherapy-eligible cohort, patients who—either because of disease progression, intolerable toxicities, or non-adherence—did not receive 4 cycles of first-line chemotherapy were substantially less likely to receive second-line chemotherapy (OR 0.26).
This and earlier studies raise numerous questions. Why is there such variation in rates of second-line chemotherapy administration? What are the reasons patients do not receive second-line therapy? Why does the rate of second-line chemotherapy use in our series of unselected patients treated in a relatively uncontrolled setting match or exceed that of several prospective, randomized clinical trials? While there are no precise explanations, features of these clinical trials may have contributed to these observations. One study—in which disease progression was cited as the predominant reason why one-third of patients in the non-maintenance arm did not receive second-line chemotherapy—employed a relatively long (three-month) inter-scan interval in the non-maintenance arm, during which symptomatic progression and associated clinical decline may have hindered administration of second-line therapy.4 Another study—conducted in over 80 centers in 20 countries, throughout which second-line practice patterns could vary considerably—left the administration and selection of post-progression treatment to the discretion of the investigator rather than mandating second-line therapy for patients in the non-maintenance arm.10 A third study included a high proportion of patients with poor performance status(>80% ECOG 2).1
We found the following variables to be associated with receipt of second-line chemotherapy: insurance type, number of cycles of first-line chemotherapy, and receipt of palliative radiation therapy prior to first-line chemotherapy administration. In a previous study of a similar patient cohort, we found that older patients with advanced NSCLC were less likely to receive first-line chemotherapy,18 presumably because older individuals tend to be more frail and have more medical comorbidities. It seems logical that age would not be associated with receipt of second-line chemotherapy in the same population because those older patients not fit for chemotherapy have already been selected out of the present study cohort. These observations echo those of a subset analysis of the phase III trial of second-line pemetrexed versus docetaxel, in which elderly patient participation was similar to rates observed in the first-line setting.19 By contrast, we found insurance type to predict receipt of both first-line18 and second-line treatment. While reasons for this ongoing association throughout the entire disease course are not evident from either study, it seems possible that insurance type—a surrogate marker of socioeconomic status—could be associated not only with performance status and comorbidities, but also with treatment preferences and adherence to medical care, factors that continue to impact populations well beyond first-line chemotherapy. Year of diagnosis was not associated with second-line chemotherapy administration, although we had expected to see an increase after 2004, when results of phase III trials of second-line erlotinib and pemetrexed, as well as second-line docetaxel quality of life data, were presented.8–9,20
Our use of pre-chemotherapy palliative radiation therapy as a predictive variable also merits comment. We selected this unconventional metric as a potential marker of disease burden and severity. It represents a diverse group of patients, including those with brain metastases; clinically significant hemoptysis or airway compromise; and refractory pain, neurologic sequelae, or skeletal instability from bony metastases. It is possible that these patients represent a population at subsequent risk for a more symptomatic, complex clinical course. It follows that these patients are substantially less likely to receive second-line chemotherapy (OR 0.53 in this study). It seems less likely that pre-chemotherapy palliative radiation therapy itself—either via the delay in initiation of systemic therapy or through radiation-associated toxicities—accounts for the reduced rate of second-line chemotherapy administration.
Both the number of cycles of first-line chemotherapy and the receipt of second-line chemotherapy were independently associated with overall survival. While no conclusions about the effect of these treatment factors on clinical endpoints can be drawn from this observational, non-randomized trial, these findings may provide insight into overall outcomes. We selected a cut-off of 4 cycles of first-line chemotherapy because this number implies clinical effect (as radiographic studies assessing response to therapy are typically performed every 2 cycles), acceptable toxicity profile, and patient adherence to treatment. Among patients who ultimately received second-line chemotherapy, median survival was 17.9 months for those who received 4 or more cycles of first-line chemotherapy, compared to 8.7 months for those who received fewer than 4 cycles of first-line chemotherapy. These findings echo those of earlier studies, in which response to first-line chemotherapy was an independent predictor of receipt of second-line chemotherapy11 and overall survival.21
Limitations of this study include its retrospective nature, its single academic center setting, and relatively small sample size. Despite the retrospective design, disease and treatment follow-up data were available until patient death for over 95% of the cohort. Due to the geographical setting and variety of UT Southwestern-affiliated clinical facilities, our patient cohort is racially and socioeconomically diverse. Nonetheless, certain patient populations, such as East Asians, are under-represented. Furthermore, the physicians caring for these individuals are predominantly academic thoracic oncologists, who may be more likely to employ second-line chemotherapy than are other practitioners. That stated, the ability of these physicians to deliver second-line chemotherapy to two-thirds of this largely socioeconomically challenged cohort suggests that it may be feasible in most other U.S. settings as well. Finally, reasons why second-line chemotherapy was not administered were not available.
In conclusion, in this unselected, diverse cohort of patients with advanced NSCLC, approximately 50% of patients who received first-line chemotherapy eventually received second-line chemotherapy. Limiting the analysis to those individuals whose disease did not progress after 4–6 cycles of first-line chemotherapy—the population eligible for maintenance chemotherapy—the rate rises to 67%, a figure that meets or exceeds those of numerous recent clinical trials. Markers of socioeconomic status, symptom burden, and response to and tolerance of first-line chemotherapy were associated with receipt of second-line chemotherapy. Maintenance chemotherapy trials have highlighted critical economic and quality of life issues. The cost per life-year gained from maintenance pemetrexed exceeds $120,000.22 While approved maintenance agents such as pemetrexed and erlotinib are generally well tolerated, there is clearly a subset of patients who maintain prolonged disease control after first-line chemotherapy with no subsequent treatment—and who then successfully receive second-line therapy at the time of progression. It follows that identifying those patients least likely to receive second-line chemotherapy might guide the selective use of maintenance chemotherapy, thereby limiting both costs and toxicities. Based on the findings in the present study, socioeconomically disadvantaged patients and patients with greater symptom burden—manifest by the need for pre-chemotherapy palliative radiation therapy—may represent such a target population.
Acknowledgments
Supported by American Cancer Society and Simmons Cancer Center Grant ACS-IRG-02-196 and the North and Central Texas Clinical and Translational Science Initiative (KL2RR024983) (to D.E.G.)
The authors thank Alejandra Madrigales from the UT Southwestern tumor registry, Joan Cox from the Parkland Health and Hospital System tumor registry, Debbie Munday and Deborah Rios from the Dallas Veterans Affairs Medical Center tumor registry, and Eileen Marley, PharmD, from the Parkland Health and Hospital System oncology pharmacy for providing patient data.
The authors acknowledge the assistance of the Biostatistics Shared Resource at the Harold C. Simmons Cancer Center, which is supported in part by a National Cancer Institute Cancer Center Support Grant, 1P30 CA142543-01. Key Wordsnon-small cell lung cancer; metastatic; second-line chemotherapy; maintenance chemotherapy; practice patterns; radiation therapy; insurance
References
- 1.Belani CP, Waterhouse DM, Ghazal H, et al. Phase III study of maintenance gemcitabine (G) and best supportive care (BSC) versus BSC, following standard combination therapy with gemcitabine-carboplatin (G-Cb) for patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28 abstr 7506. [Google Scholar]
- 2.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 3.Cappuzzo F, Coudert BP, Wierzbicki R, et al. Efficacy and safety of erlotinib as first-line maintenance in NSCLC following non-progression with chemotherapy: results from the phase III SATURN study. J Thorac Oncol. 2009;4:S289. (Abstract A2.1) [Google Scholar]
- 4.Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:591–8. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]
- 5.Perol M, Chouaid C, Milleron BJ, et al. Maintenance with either gemcitabine of erlotinib versus observation with predefined second-line treatment after cisplatin-gemcitabine induction chemotherapy in advanced NSCLC: IFCT-GFPC 0502 phase III study. J Clin Oncol. 2010;28 abstr 7507. [Google Scholar]
- 6.Grossi F, Aita M, Follador A, et al. Sequential, alternating, and maintenance/consolidation chemotherapy in advanced non-small cell lung cancer: a review of the literature. Oncologist. 2007;12:451–64. doi: 10.1634/theoncologist.12-4-451. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 8.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 10.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 11.Sun JM, Park JO, Won YW, et al. Who are less likely to receive subsequent chemotherapy beyond first-line therapy for advanced non-small cell lung cancer? Implications for selection of patients for maintenance therapy. J Thorac Oncol. 2010;5:540–5. doi: 10.1097/JTO.0b013e3181d3504d. [DOI] [PubMed] [Google Scholar]
- 12.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 13.Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–43. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 14.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 15.Ahn MJ, Lee J, Park YH, et al. Korean ethnicity as compared with white ethnicity is an independent favorable prognostic factor for overall survival in non-small cell lung cancer before and after the oral epidermal growth factor receptor tyrosine kinase inhibitor era. J Thorac Oncol. 2010;5:1185–96. doi: 10.1097/JTO.0b013e3181e2f624. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi T, Matsumura A, Fukai S, et al. Japanese ethnicity compared with Caucasian ethnicity and never-smoking status are independent favorable prognostic factors for overall survival in non-small cell lung cancer: a collaborative epidemiologic study of the National Hospital Organization Study Group for Lung Cancer (NHSGLC) in Japan and a Southern California Regional Cancer Registry databases. J Thorac Oncol. 2010;5:1001–10. doi: 10.1097/JTO.0b013e3181e2f607. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Census State and County QuickFacts: Dallas County, TX. [Accessed June 16, 2010]; Available at http://quickfacts.census.gov/qfd/states/48/48113.html.
- 18.Rasco DW, Yan J, Xie Y, Dowell JE, Gerber DE. Looking Beyond Surveillance, Epidemiology, and End Results: Patterns of Chemotherapy Administration for Advanced Non-small Cell Lung Cancer in a Contemporary, Diverse Population. J Thorac Oncol. 2010;5:1529–35. doi: 10.1097/JTO.0b013e3181e9a00f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss GJ, Langer C, Rosell R, et al. Elderly patients benefit from second-line cytotoxic chemotherapy: a subset analysis of a randomized phase III trial of pemetrexed compared with docetaxel in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:4405–11. doi: 10.1200/JCO.2006.06.7835. [DOI] [PubMed] [Google Scholar]
- 20.Dancey J, Shepherd FA, Gralla RJ, Kim YS. Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: results of a prospective, randomized phase III trial. Lung Cancer. 2004;43:183–94. doi: 10.1016/j.lungcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Weiss GJ, Rosell R, Fossella F, et al. The impact of induction chemotherapy on the outcome of second-line therapy with pemetrexed or docetaxel in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007;18:453–60. doi: 10.1093/annonc/mdl454. [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Wielage R, Muehlenbein C, et al. Cost-effectiveness of pemetrexed as first-line maintenance therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2010;5:1263–72. doi: 10.1097/JTO.0b013e3181e15d16. [DOI] [PubMed] [Google Scholar]