Abstract
BACKGROUND
Prostate cancer (PCa) risk-associated single nucleotide polymorphisms (SNPs) are continuously being discovered. Their ability to identify men at high risk and the impact of increasing numbers of SNPs on predictive performance are not well understood.
METHODS
Absolute risk for PCa was estimated in a population-based case-control study in Sweden (2,899 cases and 1,722 controls) using family history and three sets of sequentially discovered PCa risk-associated SNPs. Their performance in predicting PCa was assessed by positive predictive values (PPV) and sensitivity.
RESULTS
SNPs and family history were able to differentiate individual risk for PCa and identify men at higher risk; ~18% and ~8% of men in the study had 20-year (55–74 years) absolute risks that were two-fold (0.24) or three-fold (0.36) greater than the population median risk (0.12), respectively. When predictive performances were compared at absolute risk cutoffs of 0.12, 0.24 or 0.36, PPV increased considerably (~20%, ~30% and ~37%, respectively) while sensitivity decreased considerably (~55%, ~20% and ~10%, respectively). In contrast, when increasing numbers of SNPs (5, 11 and 28 SNPs) were used in risk prediction, PPV approached a constant value while sensitivity increased steadily.
CONCLUSIONS
SNPs discovered to date are suitable for risk prediction while additional SNPs discovered in the future may identify more subjects at higher risk. Men identified as high-risk by SNP-based testing may be targeted for PCa screening or chemoprevention. The clinical impact on improving the effectiveness of these interventions can be and should be assessed.
Keywords: Absolute risk, SNPs, association, screening, chemoprevention
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed non-cutaneous malignancy of men in developed countries,1 and is one of the most heritable forms of cancer.2 Recently, considerable progress has been made towards the identification of genetic risk factors for the disease using genome-wide association studies (GWAS), with more than two dozen PCa risk-associated SNPs having been discovered.3–13 Additional risk-associated SNPs are expected to be discovered from ongoing GWAS and/or from combined analyses of existing GWAS.
The risk-associated SNPs discovered to date from GWAS have several common features.14 The association of these established SNPs and PCa risk have been consistently replicated in multiple populations of European descent. The risk alleles of these SNPs are common in the general population, with a risk allele frequency of at least 5%, and over 50% for several SNPs. In addition, although each of these SNPs is only moderately associated with PCa risk, collectively, they have a stronger, dose-dependent association.15–16 Furthermore, these risk SNPs can be measured with high accuracy, at low cost and at any age (inherited SNPs do not change with time). These important characteristics make PCa risk-associated SNPs attractive candidates to predict an individual’s risk for PCa.
However, two major concerns about the utility of known risk-associated SNPs in risk prediction of PCa are widely shared among clinicians, researchers, and policy makers. It is argued that these SNPs perform poorly at discriminating cases from controls, as indicated by low area under curve (AUC) statistic of the receiver operating characteristic (ROC) of these SNPs.17–18 In addition, some argue that currently identified risk-associated SNPs only represent a small fraction of all risk-associated SNPs, and therefore fear that risk prediction based on these small numbers of SNPs may be unreliable and likely to change as more risk-associated SNPs are included in the model.19
Rather than attempting to discriminate PCa risk for all men, in this study, we focus primarily on identifying men at high risk for PCa. We evaluated the predictive performance at various cutoffs of absolute risk using positive predictive values (PPV) and sensitivity. We also assessed the change of discriminative performance with three sets of PCa risk-associated SNPs sequentially discovered over the past four years, as well as degree of reclassification of high risk using these evolving sets of SNPs.
PATIENTS AND METHODS
Study population
A large population-based PCa case–control study in Sweden named CAncer of the Prostate in Sweden (CAPS) was used to develop a risk prediction model. CAPS has been described in detail elsewhere (Table 1).15 Briefly, PCa patients in CAPS were identified and recruited from regional cancer registries in Sweden. The inclusion criterion for case subjects was pathologically or cytologically verified adenocarcinoma of the prostate. Tumor-node-metastasis (TNM) stage, Gleason grade (biopsy), and prostate-specific antigen (PSA) levels at diagnosis, as well as DNA samples from blood were available for 2,899 patients. Control subjects were recruited concurrently with case subjects. They were randomly selected from a Swedish Population Registry, and matched according to the expected age distribution of cases (groups of 5-year intervals) and geographic region. DNA samples from blood were available for 1,722 control subjects. Positive family history was defined as any first-degree relatives with a diagnosis of PCa. The research ethics committees at Wake Forest University School of Medicine and the Karolinska Institute approved the study.
Table 1.
Clinical and demographic characteristics of subjects in CAPS
| # (%) of cases | # (%) of controls | |
|---|---|---|
| Characteristics | cases (N=2,899) | (N=1,722) |
| Age at enrollment (Year) | ||
| Mean (sd) | 66.36 (7.13) | 67.15 (7.39) |
| Family History (first-degree relatives) | ||
| No | 2342 (80.95) | 1565 (90.57) |
| Yes | 551 (19.05) | 163 (9.43) |
| PSA levels at diagnosis for cases or at enrollment for controls (ng/ml) | ||
| ≤ 4 | 221 (7.85) | 1438 (83.56) |
| 4.01–9.99 | 926 (32.91) | 230 (13.36) |
| 10–19.99 | 654 (23.24) | 37 (2.15) |
| 20–49.99 | 467 (16.60) | 13 (0.76) |
| 50–99.99 | 229 (8.14) | 2 (0.12) |
| ≥ 100 | 317 (11.27) | 1 (0.06) |
| Missing | 85 | 1 |
| T-stage | ||
| T1 or lower | 1089 (38.62) | N/A |
| T2 | 904 (32.06) | N/A |
| T3 | 724 (25.67) | N/A |
| T4 | 103 (3.65) | N/A |
| TX | 79 | N/A |
| N-stage | ||
| N0 | 524 (84.65) | N/A |
| N1 | 95 (15.35) | N/A |
| NX | 2280 | N/A |
| M-stage | ||
| M0 | 1244 (81.95) | N/A |
| M1 | 274 (18.05) | N/A |
| MX | 1381 | N/A |
| Gleason (biopsy) | ||
| ≤ 6 | 1382 (52.39) | N/A |
| 7 | 788 (29.87) | N/A |
| ≥ 8 | 468 (17.74) | N/A |
| Missing | 261 | N/A |
Three sets of risk-associated SNPs
The three sets of SNPs were discovered sequentially from GWAS in the past four years, subtracting the SNPs that were initially reported in the CAPS population (Table 2). The first set of SNPs includes five SNPs discovered from two PCa GWAS prior to 2007.3–6 The second set of SNPs has 11 SNPs that were reported by end of 2008 from several PCa GWAS, including the 5 SNPs described in the first set of SNPs.3–10 The last set of SNPs contains 28 SNPs discovered in PCa GWAS to date (by December, 2009), including SNPs in the first and second sets of SNPs.3–13
Table 2.
Prostate cancer risk-associated SNPs discovered from GWAS
| Predictor | SNPs | Chr | Position | Alleles | Risk allele | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| Family History | 2.50 | 2.20 – 2.80 | |||||
| 5 SNPs | |||||||
| rs16901979 | 8 | 128,194,098 | A/C | A | 1.82 | 1.44 – 2.30 | |
| rs6983267 | 8 | 128,482,487 | T/G | G | 1.20 | 1.14 – 1.26 | |
| rs1447295 | 8 | 128,554,220 | A/C | A | 1.47 | 1.33 – 1.62 | |
| rs4430796 | 17 | 33,172,153 | G/A | A | 1.22 | 1.17 – 1.26 | |
| rs1859962 | 17 | 66,620,348 | C/T | C | 1.20 | 1.13 – 1.27 | |
| 11 SNPs(5 previous SNPs + 6 new SNPs) | |||||||
| rs2660753 | 3 | 87,193,364 | T/C | T | 1.24 | 1.04 – 1.48 | |
| rs10486567 | 7 | 27,943,088 | A/G | G | 1.16 | 1.10 – 1.23 | |
| rs6465657 | 7 | 97,654,263 | T/C | C | 1.14 | 1.05 – 1.23 | |
| rs10993994 | 10 | 51,219,502 | T/C | T | 1.25 | 1.12 – 1.40 | |
| rs10896449 | 11 | 68,751,243 | A/G | G | 1.16 | 1.11 – 1.22 | |
| rs5945619 | 23 | 51,258,412 | A/G | G | 1.27 | 1.12 – 1.43 | |
| 28 SNPs(11 previous SNPs + 17 new SNPs) | |||||||
| rs1465618 | 2 | 43,407,453 | T/C | T | 1.15 | 1.04 – 1.26 | |
| rs721048 | 2 | 62,985,235 | A/G | A | 1.16 | 1.11 – 1.22 | |
| rs12621278 | 2 | 173,019,799 | A/G | A | 1.35 | 1.27 – 1.44 | |
| rs10934853 | 3 | 129,521,063 | A/C | A | 1.12 | 1.06 – 1.18 | |
| rs17021918 | 4 | 95,781,900 | T/C | C | 1.14 | 1.10 – 1.18 | |
| rs7679673 | 4 | 106,280,983 | A/C | C | 1.13 | 1.10 – 1.17 | |
| rs9364554 | 6 | 160,753,654 | T/C | T | 1.17 | 1.06 – 1.29 | |
| rs2928679 | 8 | 23,494,920 | A/G | A | 1.13 | 1.02 – 1.25 | |
| rs1512268 | 8 | 23,582,408 | T/C | T | 1.17 | 1.14 – 1.21 | |
| rs16902094 | 8 | 128,389,528 | G/A | G | 1.20 | 1.12 – 1.30 | |
| rs620861 | 8 | 128,404,855 | A/G | G | 1.16 | 1.11 – 1.20 | |
| rs4962416 | 10 | 126,686,862 | C/T | C | 1.15 | 1.04 – 1.27 | |
| rs7127900 | 11 | 2,190,150 | A/G | A | 1.25 | 1.20 – 1.30 | |
| rs12418451 | 11 | 68,691,995 | A/G | A | 1.16 | 1.09 – 1.23 | |
| rs8102476 | 19 | 43,427,453 | T/C | C | 1.12 | 1.08 – 1.15 | |
| rs2735839 | 19 | 56,056,435 | A/G | G | 1.30 | 1.11 – 1.51 | |
| rs5759167 | 22 | 41,830,156 | T/G | G | 1.18 | 1.14 – 1.21 | |
Statistical analyses
The cumulative effect of these SNPs and family history was modeled by multiplying the OR of each risk SNP and family history, as briefly described in the following steps. The allelic OR for each SNP was obtained from a meta-analysis of external study populations. The allelic OR of each SNP is presented in Table 2. A multiplicative model was used to derive genotype relative risks from the allelic OR. For each of the three genotypes at each SNP, the genotype relative risk was converted to the risk relative to the population. The overall risk relative to the population was derived by combining the risks relative to the population of all SNPs as well as the family history of the individual by simple multiplication. Finally, the absolute risk for each man was then estimated based on the overall risk relative to the population, the incidence rate of PCa in the general population, and the all cause mortality rate excluding PCa in Sweden.20
To assess the performance of absolute risk estimates in discriminating cases and controls, we first estimated sensitivity and specificity at absolute risk cutoffs of one-, two- and three-fold of population median risk. We then estimated positive predictive value (PPV) and negative predictive value (NPV) based on sensitivity and specificity and population prevalence of PCa using Bayes’ Theorem. We also used the AUC statistic of the ROC to assess the overall performance of estimated absolute risk in discriminating PCa cases and controls. A nonparametric approach developed by Delong and colleagues was used to test for equality of the AUCs.21
RESULTS
The 20-year absolute risk for PCa among men aged 55–74 years was estimated for each subject using family history and each set of 5, 11, and 28 SNPs that have been sequentially discovered in GWAS over the past four years. Absolute risk measures the likelihood of an individual, of a given age, developing the tested condition over time, and therefore, provides a more meaningful measure of risk to clinicians and patients as compared with ORs. The distributions of absolute risk for PCa using family history and each set of SNPs are presented in Figure 1a–c. Compared to a uniform 20-year cumulative risk for PCa of 0.12 in the population when family history and SNPs were not considered, a wide spectrum of absolute risk estimates were found using these genetic markers. In addition, the variation of absolute risk distribution increased with increasing numbers of SNPs used in the risk prediction model. Consequently, more subjects were classified as high risk with increasing numbers of SNPs used. For example, at a cutoff of 0.24 (two-fold of population median risk), 12%, 14%, and 18% men were classified as high risk, using the 5, 11, and 28 SNP panels respectively. Similarly, at a cutoff of 0.36 (three-fold of population median risk), 4%, 5%, and 8% men were classified as high risk, using the sets of 5, 11, and 28 SNPs respectively.
Figure 1.
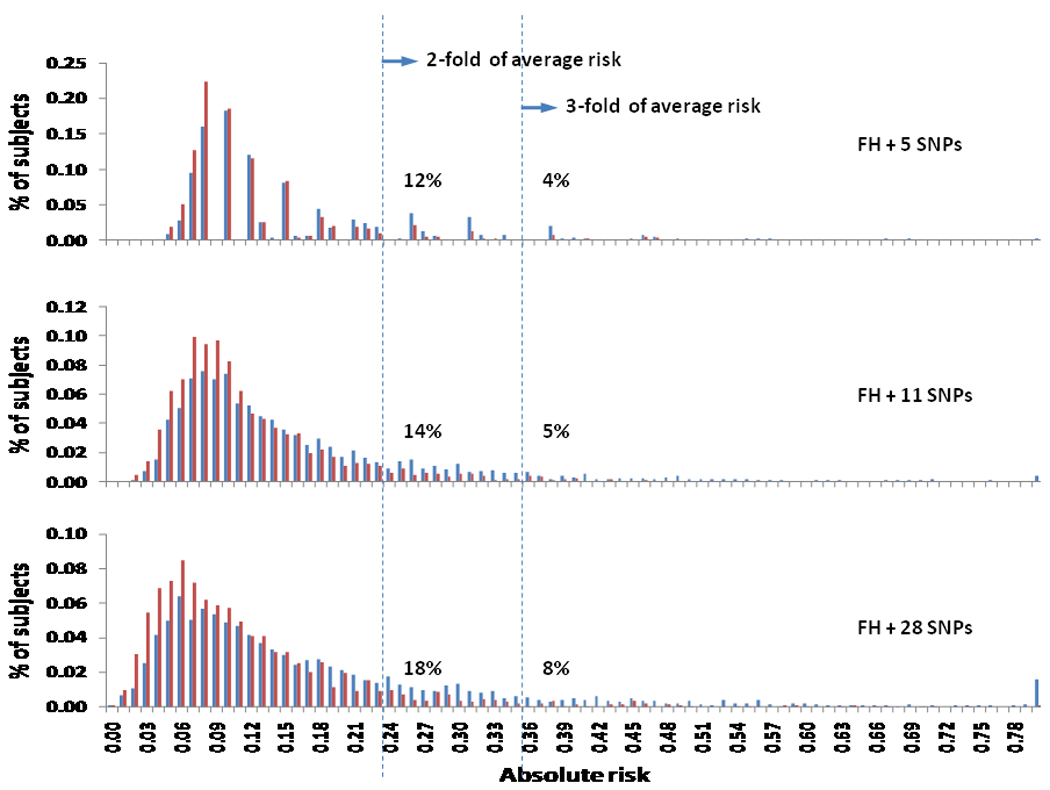
Distribution of estimated absolute risk using family history and three sets of PCa risk-associated SNPs in CAPS.
To examine the performance of absolute risk estimates in discriminating PCa status, we first calculated the AUC statistic, a measurement of overall discriminative performance. For the three sets of SNPs, the AUC was 0.60, 0.61, and 0.62 for the 5, 11, and 28 SNPs, respectively. Compared to the AUC of 5 SNPs, the AUC of 11 and 28 SNPs was significantly higher (P = 0.007 and 0.01, respectively).
However, because our primary purpose was to identify men at high risk, the more relevant statistics to measure predictive performance are sensitivity and PPV at higher cutoffs of absolute risk. Sensitivity measures the proportion of cases that are test positive (exceeding a certain cutoff) while PPV measures the proportion of test positive men that develop the disease. Sensitivities with absolute risk at cutoffs of 0.12, 0.24, and 0.36 (corresponding to one-, two- and three-fold of population median risk) are presented in Table 3 and Figure 2a. While sensitivity decreased with increasing cutoffs of absolute risk, it increased with increasing numbers of SNPs used in risk prediction at each cutoff, especially at higher cutoffs. For example, sensitivity for the 28 SNPs model was 55%, 23% and 11% at one-, two- and three-fold population median risk, respectively. In contrast, sensitivity at three-fold increased population median risk was 5%, 7% and 11%, respectively when 5, 11, and 28 SNPs were used.
Table 3.
Sensitivity and specificity at three cutoffs for three sets of SNPs
| Cutoff | High risk |
5 SNPs + FH # of subjects |
11 SNPs + FH # of subjects |
28 SNPs + FH # of subjects |
|||
|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | ||
| 1-fold median | Yes | 1528 | 679 | 1561 | 646 | 1580 | 650 |
| No | 1371 | 1042 | 1338 | 1075 | 1319 | 1071 | |
| Sen=0.53 | Spe=0.61 | Sen=0.54 | Spe=0.62 | Sen=0.55 | Spe=0.62 | ||
| 2-fold median | Yes | 454 | 112 | 532 | 131 | 673 | 161 |
| No | 2445 | 1609 | 2367 | 1590 | 2226 | 1560 | |
| Sen=0.16 | Spe=0.93 | Sen=0.18 | Spe=0.92 | Sen=0.23 | Spe=0.91 | ||
| 3-fold median | Yes | 158 | 33 | 205 | 37 | 314 | 55 |
| No | 2741 | 1688 | 2694 | 1684 | 2585 | 1666 | |
| Sen=0.05 | Spe=0.98 | Sen=0.07 | Spe=0.98 | Sen=0.11 | Spe=0.97 | ||
Figure 2.
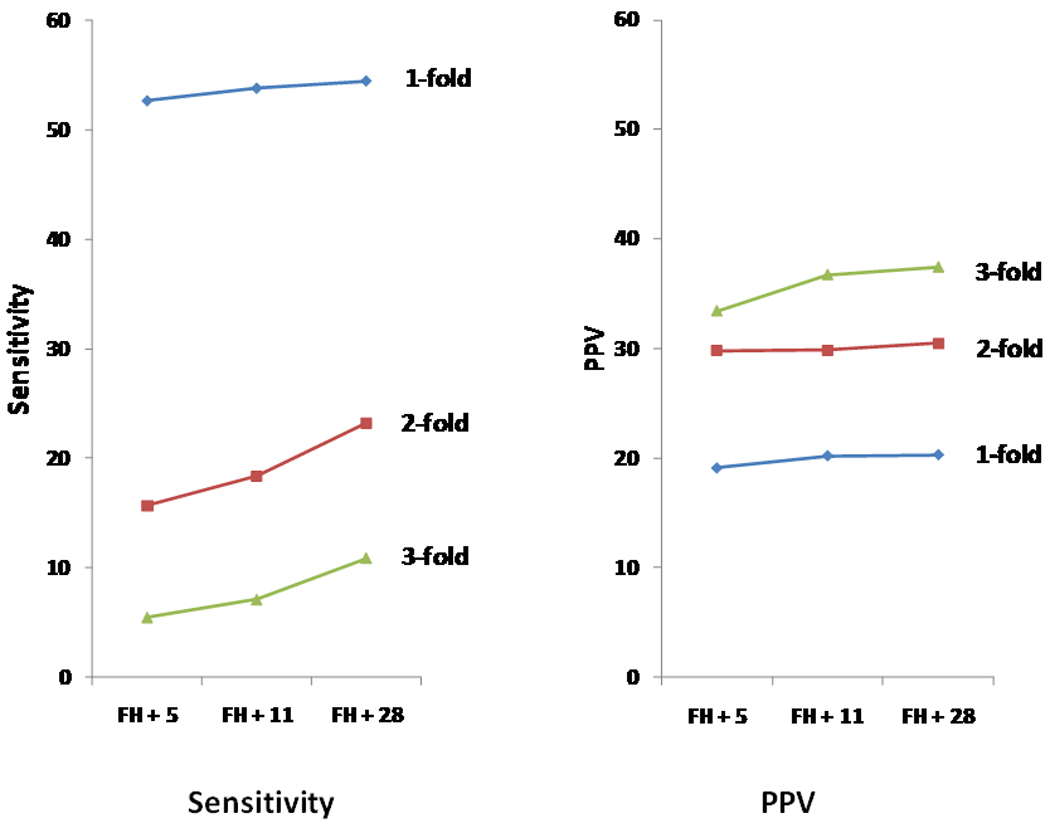
Predictive performance of absolute risk estimated from family history and three sets of PCa risk-associated SNPs in CAPS, measured by sensitivity (a) and PPV (b).
More importantly, the PPV increased with increasingly higher cutoffs of absolute risk, from ~20% to ~30% and ~37% for cutoffs at one-, two-, and three-fold of population median risk (Figure 2b). Interestingly, we found that at each cutoff of absolute risk, PPV did not change considerably and approached a constant value with the use of increasing numbers of SNPs. For example, at a cutoff of three-fold of population median risk, the PPV was 34%, 37%, and 37%, respectively when 5, 11, and 28 sequentially discovered PCa risk-associated SNPs were used. It is noted that the first 11 PCa risk-associated SNPs that were discovered generally have higher ORs for PCa than the SNPs discovered subsequently.
To further examine the impact of numbers of SNPs and effect of SNPs on sensitivity and PPV, we sorted the genetic risk factors (family history and each of the 28 risk-associated SNP) from the highest to lowest, based on their contribution to genetic variance estimated from their OR and frequency (Supplementary Table 1). We then added them one at a time to the risk prediction model and calculated sensitivity and PPV at cutoffs of one-, two- and three-fold of population median risk (Figure 3). Again, at each cutoff, PPV increased only slightly and approached a fixed value with increasing numbers of ordered SNPs. On the other hand, sensitivity continued to increase, albeit gradually, with increasing numbers of these ordered SNPs.
Figure 3.
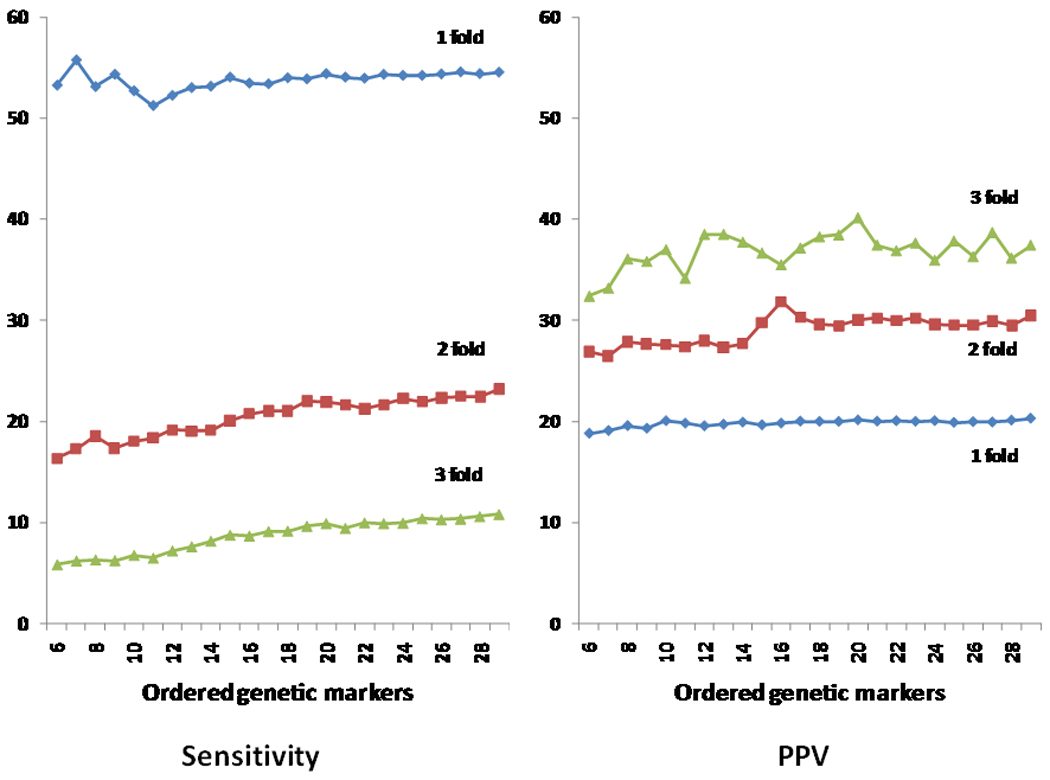
Predictive performance (sensitivity and PPV) of absolute risk estimated from genetic markers in CPAS, genetic markers were added to the risk prediction one at a time, from highest to lowest based on the genetic variance explained by the genetic marker. Note the PPV and sensitivity were presented starting from the first six genetic markers because results were unstable for the initial several genetic markers due to the small number of cases and controls who tested positive, especially at higher cutoff values of absolute risk.
DISCUSSION
In this study, we provide novel results to address the controversies surrounding PCa risk prediction based on newly discovered genetic markers. We have demonstrated that currently established PCa risk-associated SNPs and family history are informative in differentiating an individual’s risk for PCa and are able to identify men who have considerably elevated risk for PCa (two- and three-fold times the population median risk). We also established that these genetic markers have better discriminative performance, measured by PPV, for men at considerably elevated risk. Finally, we demonstrated that while the sensitivity of classifying men at considerably elevated risk continues to increase with increasing numbers of genetic risk factors, PPV does not follow this trend and reaches a plateau after the most important SNPs are used in the risk prediction model. Together, these results challenge the current pessimistic view held by some clinicians, researchers, and policy makers that these markers are too weak for risk prediction. It also suggests that the PCa risk-associated SNPs identified to date are suitable for risk prediction because additional SNPs that are weakly associated with PCa risk may not appreciably improve the PPV. Risk-associated SNPs discovered in the future may, however, further improve sensitivity for identifying additional men at high risk.
The major findings from this study are not limited to this Swedish CAPS population. Similar findings were observed in a U.S. study population. We applied the same methods to the National Cancer Institute Cancer Genetic Markers of Susceptibility Initiative (CGEMS) study population where family history and genotypes of known PCa risk-associated SNPs are available publicly for 1,172 PCa patients and 1,157 control subjects nested in the Prostate, Lung, Colon and Ovarian (PLCO) Cancer Screening Trial.5,8 The 20-year absolute risk for men aged 55–74 years based on family history and 27 SNPs were informative to differentiate an individual’s risk (Supplementary Figure 1, one of the 28 SNPs included in the CAPS study, rs16902094, was not genotyped and could not be imputed). Furthermore, the discriminative performance for men at high risk, measured by PPVs and sensitivity confirmed the trend observed in CAPS (Supplementary Figure 2).
It is important to note the difference between AUC and PPV, two measurements used for assessing discriminative performance. The former is widely used and is more appropriate if the purpose of risk prediction is to discriminate disease outcome for the entire study population. Very strong predictors, with ORs greater than several hundred, are needed to completely discriminate cases from controls.17 PPV, on the other hand, measures the predictive performance among men who exceed a certain cutoff of absolute risk. Therefore, PPV is a more appropriate measurement if the primary purpose of the risk prediction is to identify men at considerably elevated risk for PCa.
It is also important to note that risk prediction is not a diagnostic tool, but provides an estimate of likelihood of developing PCa in the future. Choosing an optimal cutoff to classify men at high risk for PCa depends on the purpose of the test and should be based on the balance of PPV and sensitivity. If the purpose of risk prediction is to identify high risk men for more intensive (earlier and more often) PCa screening with PSA and DRE and for chemoprevention, a relatively liberal cutoff of absolute risk (for example, 2-fold of population median risk) may be used to trade lower PPV for better sensitivity (i.e., identify more men for these intervention).
Identifying men at considerably high risk for PCa may have important implications for targeted PSA screening and chemoprevention. There is an ongoing debate surrounding the benefits of PSA screening for PCa. Results from two large randomized trials in Europe and the U.S. have provided evidence that PSA-based screening for PCa was associated with significant cost due to the numbers of men needed to screen to derive benefit.22–23 In the European trial, PSA screening was associated with decreased PCa related mortality but at a great cost; ~1,410 men needed to be screened and 48 additional PCa cases needed to be treated to prevent one death from PCa.22 The concept of targeted screening for men at high risk may emerge as an important alternative to achieve the goal of reducing mortality while limiting the numbers of individuals needed to screen. Results from this study suggest that family history and PCa-risk associated SNPs can be used to develop an important tool for targeted screening. The benefit of genomic-targeted PCa screening needs to be tested.
Similarly, targeting only high-risk men for chemoprevention may be an important alternative to the costly, widespread use of chemoprevention. Two large, randomized clinical trials, Prostate Cancer Prevention Trial (PCPT) and The Reduction by Dutasteride of Prostate Cancer Events (REDUCE), have demonstrated a 23–25% reduction in PCa risk with the use of 5-alpha reductase inhibitors (5ARIs), finasteride and dutasteride.24–25 This represents the single largest risk reduction by any chemopreventive agent in any malignancy in men. Despite this reduction rate, 5ARIs have not been widely adopted,26 in part due to the concern of cost.27 By only targeting high risk men for chemoprevention, the cost could be considerably reduced even if the PCa reduction rate of the chemoprevention is the same across risk category. The benefit/cost ratio would further improve if its PCa reduction rate is higher among men at higher risk. We are currently testing the potentially improved efficacy of genomic-targeted chemoprevention in the REDUCE study subjects.
Our study has several notable limitations. Firstly, the SNPs used for risk prediction in this study are not associated with aggressiveness of PCa,28–29 and therefore are not able to discriminate risk for aggressive or indolent PCa. Recently, a SNP that is significantly associated with more aggressive PCa risk but not indolent PCa was reported by our group.30 When a group of such SNPs is identified, they may be used to predict risk for the most important and potentially lethal forms of PCa. However, it is important to note that a potential reduction of PCa through genomic-targeted chemoprevention using the current risk prediction model, regardless of aggressive or indolent PCa, represents an important advance. Those men who are prevented from being diagnosed with PCa do not have to face the stress, exposure to cost and complications of treatment as well as insurance implications related to being a PCa patient. Secondly, the PPV estimates of this study were based on sensitivity and specificity derived from this case-control study and population prevalence data. This approach is different from the traditional approach which estimates PPV from cohort studies. However, our approach may be more efficient due to the oversampling of cases in this case-control study which leads to more accurate estimates of sensitivity. Finally, it is noted that these findings are limited to men of Caucasian race because these genetic markers were discovered in European populations.
In summary, for the primary purpose of identifying a subset of high risk men at an early, curable stage and reducing the burden of PCa through PSA screening and chemoprevention, we believe, based on these results, that it is time to critically consider genomic-targeted screening and chemoprevention of PCa. This approach may also help to address the significant issue of over-screening and over-diagnosis of PCa in developed countries.
STATEMENT OF TRANSLATIONAL RELEVANCE
This study suggests that PCa risk-associated SNPs discovered to date may be used to identify men at high risk for PCa for targeted PCa screening and/or chemoprevention.
Supplementary Material
Distribution of estimated absolute risk using family history and three sets of PCa risk-associated SNPs in CGEMS.
Predictive performance (sensitivity and PPV) of absolute risk estimated from genetic markers in CGEMS. Genetic markers were added to the risk prediction one at a time, from highest to lowest based on the genetic variance explained by the genetic marker.
Acknowledgments
The authors thank all of the study subjects who participated in the CAPS study and the urologists who provided their patients to the CAPS study. We acknowledge the contribution of multiple physicians and researchers in designing and recruiting study subjects, including Dr. Hans-Olov Adami. The authors also thank the National Cancer Institute Cancer Genetic Markers of Susceptibility Initiative (CGEMS) for making the data publicly available.
Funding: National Cancer Institute of the U.S., Department of Defense of the U.S., Swedish Cancer Society (Cancerfonden) and Swedish Academy of Sciences.
Footnotes
Disclaimers: None
Conflict of interest statement
There is no perceived conflict of interest relevant to this article.
Author contributions
Conception and design: Jianfeng Xu, Henrik Grönberg, William B. Isaacs
Study subject recruitment: Henrik Grönberg, Jan Adolfsson
Genotyping: S. Lilly Zheng
Analysis and interpretation of data: Jielin Sun, A. Karim Kader, Fang-Chi Hsu, Seong-Tae Kim, Yi Zhu, Aubrey R. Turner, Tao Jin, Zheng Zhang, Fredrik Wiklund, Jianfeng Xu
Drafting of the manuscript: Jianfeng Xu, Jielin Sun, A. Karim Kader
Obtained funding: Jianfeng Xu, Henrik Grönberg
Final approval of manuscript: Jielin Sun, A. Karim Kader, Fang-Chi Hsu, Seong-Tae Kim, Yi Zhu, Aubrey R. Turner, Tao Jin, Zheng Zhang, Jan Adolfsson, Fredrik Wiklund, S. Lilly Zheng, William B. Isaacs, Henrik Grönberg, Jianfeng Xu
REFERENCES
- 1.Jemal A, Siegel R, Ward E. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 3.Amundadottir LT, Sulem P, Gudmundsson J, Helgason A, Baker A, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Cazier JB, Sainz J, Jakobsdottir M, Kostic J, Magnusdottir DN, Ghosh S, Agnarsson K, Birgisdottir B, Le Roux L, Olafsdottir A, Blondal T, Andresdottir M, Gretarsdottir OS, Bergthorsson JT, Gudbjartsson D, Gylfason A, Thorleifsson G, Manolescu A, Kristjansson K, Geirsson G, Isaksson H, Douglas J, Johansson JE, Bälter K, Wiklund F, Montie JE, Yu X, Suarez BK, Ober C, Cooney KA, Gronberg H, Catalona WJ, Einarsson GV, Barkardottir RB, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. A common variant associated with prostate cancer in European and African populations. Nat Genet. 2006;38:652–658. doi: 10.1038/ng1808. [DOI] [PubMed] [Google Scholar]
- 4.Gudmundsson J, Sulem P, Manolescu A, Amundadottir LT, Gudbjartsson D, Helgason A, Rafnar T, Bergthorsson JT, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Xu J, Blondal T, Kostic J, Sun J, Ghosh S, Stacey SN, Mouy M, Saemundsdottir J, Backman VM, Kristjansson K, Tres A, Partin AW, Albers-Akkers MT, Godino-Ivan Marcos J, Walsh PC, Swinkels DW, Navarrete S, Isaacs SD, Aben KK, Graif T, Cashy J, Ruiz-Echarri M, Wiley KE, Suarez BK, Witjes JA, Frigge M, Ober C, Jonsson E, Einarsson GV, Mayordomo JI, Kiemeney LA, Isaacs WB, Catalona WJ, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet. 2007;39:631–637. doi: 10.1038/ng1999. [DOI] [PubMed] [Google Scholar]
- 5.Yeager M, Orr N, Hayes RB, Jacobs KB, Kraft P, Wacholder S, Minichiello MJ, Fearnhead P, Yu K, Chatterjee N, Wang Z, Welch R, Staats BJ, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Gelmann EP, Tucker M, Gerhard DS, Fraumeni JF, Jr, Hoover R, Hunter DJ, Chanock SJ, Thomas G. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39:645–649. doi: 10.1038/ng2022. [DOI] [PubMed] [Google Scholar]
- 6.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PC, Ng MC, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F, Ruiz-Echarri M, Asin L, Saez B, van Boven E, Klaver S, Swinkels DW, Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen Trip O, Frigge ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer CN, Rotimi C, Chan JC, Pedersen O, Sigurdsson G, Benediktsson R, Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39:977–983. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 7.Duggan D, Zheng SL, Knowlton M, Benitez D, Dimitrov L, Wiklund F, Robbins C, Isaacs SD, Cheng Y, Li G, Sun J, Chang BL, Marovich L, Wiley KE, Bälter K, Stattin P, Adami HO, Gielzak M, Yan G, Sauvageot J, Liu W, Kim JW, Bleecker ER, Meyers DA, Trock BJ, Partin AW, Walsh PC, Isaacs WB, Grönberg H, Xu J, Carpten JD. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. J Natl Cancer Inst. 2007;99:1836–1844. doi: 10.1093/jnci/djm250. [DOI] [PubMed] [Google Scholar]
- 8.Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, Yu K, Chatterjee N, Welch R, Hutchinson A, Crenshaw A, Cancel-Tassin G, Staats BJ, Wang Z, Gonzalez-Bosquet J, Fang J, Deng X, Berndt SI, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cussenot O, Valeri A, Andriole GL, Crawford ED, Tucker M, Gerhard DS, Fraumeni JF, Jr, Hoover R, Hayes RB, Hunter DJ, Chanock SJ. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40:310–315. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]
- 9.Gudmundsson J, Sulem P, Rafnar T, Bergthorsson JT, Manolescu A, Gudbjartsson D, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Blondal T, Jakobsdottir M, Stacey SN, Kostic J, Kristinsson KT, Birgisdottir B, Ghosh S, Magnusdottir DN, Thorlacius S, Thorleifsson G, Zheng SL, Sun J, Chang BL, Elmore JB, Breyer JP, McReynolds KM, Bradley KM, Yaspan BL, Wiklund F, Stattin P, Lindström S, Adami HO, McDonnell SK, Schaid DJ, Cunningham JM, Wang L, Cerhan JR, St Sauver JL, Isaacs SD, Wiley KE, Partin AW, Walsh PC, Polo S, Ruiz-Echarri M, Navarrete S, Fuertes F, Saez B, Godino J, Weijerman PC, Swinkels DW, Aben KK, Witjes JA, Suarez BK, Helfand BT, Frigge ML, Kristjansson K, Ober C, Jonsson E, Einarsson GV, Xu J, Gronberg H, Smith JR, Thibodeau SN, Isaacs WB, Catalona WJ, Mayordomo JI, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Common sequence variants on 2p15 and Xp11.22 confer susceptibility to prostate cancer. Nat Genet. 2008;40:281–283. doi: 10.1038/ng.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, Mulholland S, Leongamornlert DA, Edwards SM, Morrison J, Field HI, Southey MC, Severi G, Donovan JL, Hamdy FC, Dearnaley DP, Muir KR, Smith C, Bagnato M, Ardern-Jones AT, Hall AL, O'Brien LT, Gehr-Swain BN, Wilkinson RA, Cox A, Lewis S, Brown PM, Jhavar SG, Tymrakiewicz M, Lophatananon A, Bryant SL UK Genetic Prostate Cancer Study Collaborators; British Association of Urological Surgeons' Section of Oncology; UK ProtecT Study Collaborators. Horwich A, Huddart RA, Khoo VS, Parker CC, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Fisher C, Jamieson C, Cooper CS, English DR, Hopper JL, Neal DE, Easton DF. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40:316–321. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 11.Yeager M, Chatterjee N, Ciampa J, Jacobs KB, Gonzalez-Bosquet J, Hayes RB, Kraft P, Wacholder S, Orr N, Berndt S, Yu K, Hutchinson A, Wang Z, Amundadottir L, Feigelson HS, Thun MJ, Diver WR, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Crawford ED, Haiman CA, Henderson B, Kolonel L, Le Marchand L, Siddiq A, Riboli E, Key TJ, Kaaks R, Isaacs W, Isaacs S, Wiley KE, Gronberg H, Wiklund F, Stattin P, Xu J, Zheng SL, Sun J, Vatten LJ, Hveem K, Kumle M, Tucker M, Gerhard DS, Hoover RN, Fraumeni JF, Jr, Hunter DJ, Thomas G, Chanock SJ. Identification of a new prostate cancer susceptibility locus on chromosome 8q24. Nat Genet. 2009;41:1055–1057. doi: 10.1038/ng.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudmundsson J, Sulem P, Gudbjartsson DF, Blondal T, Gylfason A, Agnarsson BA, Benediktsdottir KR, Magnusdottir DN, Orlygsdottir G, Jakobsdottir M, Stacey SN, Sigurdsson A, Wahlfors T, Tammela T, Breyer JP, McReynolds KM, Bradley KM, Saez B, Godino J, Navarrete S, Fuertes F, Murillo L, Polo E, Aben KK, van Oort IM, Suarez BK, Helfand BT, Kan D, Zanon C, Frigge ML, Kristjansson K, Gulcher JR, Einarsson GV, Jonsson E, Catalona WJ, Mayordomo JI, Kiemeney LA, Smith JR, Schleutker J, Barkardottir RB, Kong A, Thorsteinsdottir U, Rafnar T, Stefansson K. Genome-wide association and replication studies identify four variants associated with prostate cancer susceptibility. Nat Genet. 2009;41:1122–1126. doi: 10.1038/ng.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, Muir K, Hopper JL, Henderson BE, Haiman CA, Schleutker J, Hamdy FC, Neal DE, Donovan JL, Stanford JL, Ostrander EA, Ingles SA, John EM, Thibodeau SN, Schaid D, Park JY, Spurdle A, Clements J, Dickinson JL, Maier C, Vogel W, Dörk T, Rebbeck TR, Cooney KA, Cannon-Albright L, Chappuis PO, Hutter P, Zeegers M, Kaneva R, Zhang HW, Lu YJ, Foulkes WD, English DR, Leongamornlert DA, Tymrakiewicz M, Morrison J, Ardern-Jones AT, Hall AL, O'Brien LT, Wilkinson RA, Saunders EJ, Page EC, Sawyer EJ, Edwards SM, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As N, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Cooper CS, Southey MC, Lophatananon A, Liu JF, Kolonel LN, Le Marchand L, Wahlfors T, Tammela TL, Auvinen A, Lewis SJ, Cox A, FitzGerald LM, Koopmeiners JS, Karyadi DM, Kwon EM, Stern MC, Corral R, Joshi AD, Shahabi A, McDonnell SK, Sellers TA, Pow-Sang J, Chambers S, Aitken J, Gardiner RA, Batra J, Kedda MA, Lose F, Polanowski A, Patterson B, Serth J, Meyer A, Luedeke M, Stefflova K, Ray AM, Lange EM, Farnham J, Khan H, Slavov C, Mitkova A, Cao G UK Genetic Prostate Cancer Study Collaborators/British Association of Urological Surgeons' Section of Oncology; UK ProtecT Study Collaborators, PRACTICAL Consortium. Easton DF. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet. 2009;41:1116–1121. doi: 10.1038/ng.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Easton DF, Eeles RA. Genome-wide association studies in cancer. Hum Mol Genet. 2008;17(R2):R109–R115. doi: 10.1093/hmg/ddn287. [DOI] [PubMed] [Google Scholar]
- 15.Zheng SL, Sun J, Wiklund F, Smith S, Stattin P, Li G, Adami HO, Hsu FC, Zhu Y, Bälter K, Kader AK, Turner AR, Liu W, Bleecker ER, Meyers DA, Duggan D, Carpten JD, Chang BL, Isaacs WB, Xu J, Grönberg BL. Cumulative association of five genetic variants with prostate cancer. N Engl J Med. 2008;358:910–919. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Sun J, Kader AK, Lindström S, Wiklund F, Hsu FC, Johansson JE, Zheng SL, Thomas G, Hayes RB, Kraft P, Hunter DJ, Chanock SJ, Isaacs WB, Grönberg H. Estimation of absolute risk for prostate cancer using genetic markers and family history. Prostate. 2009;69:1565–1572. doi: 10.1002/pros.21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 18.Ioannidis JP. Personalized genetic prediction. too limited, too expensive, or too soon? Ann Intern Med. 2009;150:139–141. doi: 10.7326/0003-4819-150-2-200901200-00012. [DOI] [PubMed] [Google Scholar]
- 19.Kraft P, Hunter DJ. Genetic risk prediction--are we there yet? N Engl J Med. 2009;360:1701–1703. doi: 10.1056/NEJMp0810107. [DOI] [PubMed] [Google Scholar]
- 20.The National Board of Health, Welfare of Sweden. Statistical databases. http.//www.socialstyrelsen.se/en/Statistics/Statistical_databases.htm.
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves. a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 22.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A. ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 23.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer A, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA., Jr The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 25.Andriole GL, Roehrborn C, Schulman C, Slawin KM, Somerville M, Rittmaster RS. Effect of dutasteride on the detection of prostate cancer in men with benign prostatic hyperplasia. Urology. 2004;64:537–541. doi: 10.1016/j.urology.2004.04.084. [DOI] [PubMed] [Google Scholar]
- 26.Kramer BS, Hagerty KL, Justman S, Somerfield MR, Albertsen PC, Blot WJ, Ballentine Carter H, Costantino JP, Epstein JI, Godley PA, Harris RP, Wilt TJ, Wittes J, Zon R, Schellhammer P American Society of Clinical Oncology Health Services Committee; American Urological Association Practice Guidelines Committee. Use of 5-alpha-reductase inhibitors for prostate cancer chemoprevention. American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. J Clin Oncol. 2009;27:1502–1516. doi: 10.1200/JCO.2008.16.9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svatek RS, Lee JJ, Roehrborn CG, Lippman SM, Lotan Y. Cost-effectiveness of prostate cancer chemoprevention. A quality of life-years analysis. Cancer. 2008;112:1058–1065. doi: 10.1002/cncr.23276. [DOI] [PubMed] [Google Scholar]
- 28.Kader AK, Sun J, Isaacs SD, Wiley KE, Yan G, Kim ST, Fedor H, DeMarzo AM, Epstein JI, Walsh PC, Partin AW, Trock B, Zheng SL, Xu J, Isaacs W. Individual and cumulative effect of prostate cancer risk-associated variants on clinicopathologic variables in 5,895 prostate cancer patients. Prostate. 2009;69:1195–1205. doi: 10.1002/pros.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald LM, Kwon EM, Koopmeiners JS, Salinas CA, Stanford JL, Ostrander EA. Analysis of recently identified prostate cancer susceptibility loci in a population-based study: associations with family history and clinical features. Clin Cancer Res. 2009;15:3231–3237. doi: 10.1158/1078-0432.CCR-08-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, Zheng SL, Isaacs SD, Wiley KE, Wiklund F, Sun J, Kader AK, Li G, Purcell LD, Kim ST, Hsu FC, Stattin P, Hugosson J, Adolfsson J, Walsh PC, Trent JM, Duggan D, Carpten J, Grönberg H, Isaacs WB. Inherited genetic variant predisposes to aggressive but not indolent prostate cancer. Proc Natl Acad Sci U S A. 2010;107:2136–2140. doi: 10.1073/pnas.0914061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of estimated absolute risk using family history and three sets of PCa risk-associated SNPs in CGEMS.
Predictive performance (sensitivity and PPV) of absolute risk estimated from genetic markers in CGEMS. Genetic markers were added to the risk prediction one at a time, from highest to lowest based on the genetic variance explained by the genetic marker.


