Abstract
Purpose
Congenital fibrosis of the extraocular muscles type I (CFEOM1), the most common CFEOM worldwide, is characterized by bilateral ptotic hypotropia, an inability to supraduct above the horizontal midline, horizontal strabismus (typically exotropia), and ophthalmoplegia with abnormal synkinesis. This distinct non-syndromic phenotype is considered autosomal dominant and is virtually always from heterozygous missense mutations in kinesin family member 21A (KIF21A). However, there are occasional KIF21A-negative cases, opening the possibility for a recessive cause. The objective of this study is to explore this possibility by assessing CFEOM1 patients exclusively from consanguineous families, who are the most likely to have recessive cause for their phenotype if a recessive cause exists.
Methods
Ophthalmic examination and candidate gene direct sequencing (KIF21A, paired-like homeobox 2A [PHOX2A], tubulin beta-3 [TUBB3]) of CFEOM1 patients from consanguineous families referred for counseling from 2005 to 2010.
Results
All 5 probands had classic CFEOM1 as defined above. Three had siblings with CFEOM. None of the probands had mutations in KIF21A, PHOX2A, or TUBB3.
Conclusions
The lack of KIF21A mutations in CFEOM1 patients exclusively from consanguineous families, most of whom had siblings with CFEOM, is strong evidence for a recessive form of CFEOM1. Further studies of such families will hopefully uncover the specific locus(loci).
Introduction
Congenital fibrosis of the extraocular muscles type 1 (CFEOM1, OMIM 135700) is the most common form of CFEOM reported worldwide [1]. It is a distinct non-syndromic congenital cranial dysinnervation disorder and is characterized by bilateral ptotic hypotropia, an inability to supraduct above the horizontal midline, horizontal strabismus (typically exotropia), and a variable degree of ophthalmoplegia with abnormal synkinesis [2-4]. The phenotype was first mapped as an autosomal dominant fully penetrant trait to the centromere on chromosome 12 [5]. Screening of transcripts in this region in several affected families lead to the discovery of heterozygous missense mutations in kinesin family member 21A (KIF21A) as the cause [2]. Since then further studies provide strong evidence that the classic CFEOM1 phenotype results from mutations in KIF21A and that sporadic cases are due to de novo mutations in the same gene [1-3,6-14]. A heterozygous KIF21A missense mutation has been found to underlie CFEOM1 patients across populations worldwide with most patients having mutations in exon 21 that affect arginine at position 954 of the protein (p.R954W, p.R954Q, or p.R954L) [1-3]. It has been suggested that methylation of CpG dinucleotides in exon 21 of KIF21A increases susceptibility to mutational events [13]. Other than exon 21, only 2 other exons in the 38-exon gene have been reported to harbor mutations: exon 8 (p.M356T) and exon 20 (p.E944Q, p.M947V, p.M947T, p.M947R) [3]. The lack of mutations in other exons of KIF21A and the lack of other types of mutations (other than missense) may be because such mutations are lethal or because they underlie a phenotype thus far not associated with the gene. The normal function of the KIF21A protein includes the transport of membranous organelles, protein complexes, and mRNAs to specific destinations within the cell in a microtubule- and ATP-dependent manner. These functions are essential for normal morphogenesis and functioning of the cell [15]; however, missense KIF21A mutations only appear to significantly affect the orbit, causing widespread orbital dysinnervation [16].
Other clinical forms of CFEOM with a known genetic basis are CFEOM2 (OMIM 602078) and CFEOM3 (OMIM 600638). CFEOM2, the rarest CFEOM phenotype, is a recessive disorder that was first mapped to 11q13 in consanguineous families [17] and later found to be secondary to homozygous mutations in the hindbrain transcription factor paired-like homeobox 2A (PHOX2A) [18]. PHOX2A knockout animal models reveal that the gene is responsible for development of the oculomotor and trochlear cranial nerve nuclei [19,20]. The CFEOM2 phenotype is characterized by bilateral large-angle exotropia, ptosis, miosis, and ophthalmoplegia with abnormal synkinesis [4,21]. CFEOM3 is CFEOM that does not meet the classic criteria for CFEOM1 or CFEOM2. CFEOM3 can be unilateral or bilateral, is often autosomal dominant, and can have variable penetrance [4,22,23]. A family with autosomal dominant CFEOM is considered to be a CFEOM3 pedigree even if one or more members has (have) classic CFEOM1 if at least one affected family member does not meet the criteria for CFEOM1 [4,23]. Most CFEOM3 families have mapped to 16qter [23-25] and are due to heterozygous mutation in tubulin beta-3 (TUBB3), a gene involved in microtubule dynamics, kinesin interactions, and axon guidance [22]. Unlike patients with CFEOM1 or CFEOM2, patients with TUBB3-related CFEOM3 can have extraorbital neurologic findings as well [2]. In some instances, CFEOM3 can be caused by heterozygous missense KIF21A mutations [23].
CFEOM1 is considered to be an autosomal dominant fully penetrant condition. Although KIF21A is the only gene associated with CFEOM1 to date, up to 40% of sporadic CFEOM1 cases do not have identifiable mutations in KIF21A [3]. Among the possibly genetic causes are mutations in a KIF21A promotor, mutations in PHOX2A and/or TUBB3, or dominant or recessive mutations at a different locus. If a recessive cause for CFEOM1 exists, one would expect it to occur more commonly in CFEOM1 patients from large consanguineous families [26]. In the current study, we perform candidate gene testing in CFEOM1 patients from consanguineous families to explore the possibility of a recessive cause for the CFEOM1 phenotype.
Methods
Institutional board approval was granted for this study. Only probands with CFEOM1 who were from consanguineous families were invited to participate in the study. Enrolled patients, who had no known relationship to each other, had complete orthoptic and ophthalmic examination as well as 5 ml venous blood sampling for candidate gene testing and were referred to one of the authors (A.O.K.) from 2005 to 2010. Cyclopentolate 1% was used for dilation and cycloplegic refraction. When affected relatives were available and willing, they were examined as well. The candidate genes KIF21A, PHOX2A, and TUBB3 were directly sequenced. Briefly, polymerase chair reaction products from all exons of KIF21A (NM_017641), PHOX2A (NM_005169), and TUBB3 (NM_006086.2) were sequenced using the ABI Prism Big Dye Terminator v3.1 Cycle Sequencing Kit as described by the manufacturer. Results were exported in one of several formats for visualization and sequence was analyzed using SeqMan 6.1 (Lasergene 6 software package) [22,27]. Primers used for KIF21A are shown in Table 1, for PHOX2A are shown in Table 2, and for TUBB3 are shown in Table 3 and are as previously published [22].
Table 1. Primers for KIF21A.
| Exon | 5′ to 3′ primer sequence | PCR conditions |
|---|---|---|
| Kfi21a_x1Fn |
ctgttggcttctccacagg |
52 °C/35 cycles |
| Kfi21a_x1Rn |
gggactcactgcctcagttt |
|
| Kfi21a_x2Fn |
tcatgattttgggggattgt |
53 °C/35 cycles |
| Kfi21a_x2Rn |
caaaaatgaaagcgcaactg |
|
| Kif21a_x3F |
tcagttgcgctttcatttttg |
53 °C/35 cycles |
| Kif21a_x3R |
ctccaacctgggtgacagaa |
|
| Kif21a _x4F |
tagcctcattcattttaatgtgtt |
59 °C/35 cycles |
| Kif21a _x4R |
gatcttaattccatgtcatgcttc |
|
| Kif21a _x5F |
tgcctgtaactgaactaataatgtga |
59 °C/35 cycles |
| Kif21a _x5R |
atggctgaccagcttcaact |
|
| Kif21a _x6F |
tttggctttatgcctgtttc |
59 °C/35 cycles |
| Kif21a _x6R |
tgaggagattggagattcagtg |
|
| Kif21a_x7F |
cttatttctgtttcaaagaattagta |
59 °C/35 cycles |
| Kif21a_x7R |
cctacacctcaagggatgct |
|
| Kif21a _x8F |
caggggcttttaaatttgct |
59 °C/35 cycles |
| Kif21a _x8R |
ctccaaaaggaaggaggaca |
|
| Kif21a_x9Fn |
tggtcttgaactcctgacctc |
59 °C/ 35 cycles |
| Kif21a_x9rn |
tgccctccagaagttaatcc |
|
| Kif21a_x10F |
tgtggtctgctcatgtaataaagg |
53 °C/35 cycles |
| Kif21a_x10R |
ggaatatgacatcaagggaaagg |
|
| Kif21a_x11Fn |
ccacagagaaaaatgctcccta |
59 °C/35 cycles |
| Kif21a_x11Rn |
tgaatggaatgcaaaagcag |
|
| Kif21a _x12F |
gcatccaagcatgcctaatc |
59 °C/35 cycles |
| Kif21a _x13R |
tttaggagcagcccagctta |
|
| Kif21a_x13Fn |
tgattggcaatttccattttt |
59 °C/35 cycles |
| Kif21a_x13Rn |
gactccccaacacaatgctt |
|
| Kif21a _x14F |
gttggggagtcaggggtaga |
56 °C/35 cycles |
| Kif21a _x14R |
taaagccttggaaggcaaatg |
|
| Kif21a _x15F |
cattcaccttttggttgttgg |
59 °C/35 cycles |
| Kif21a _x15R |
aggcacaaactttgacttgc |
|
| Kif21a _x16F |
gacaccctagtcttctgagatgtg |
59 °C/35 cycles |
| Kif21a _x16R |
ttgccaaaggaaattacatca |
|
| Kif21a _x17F |
taaacgtgcagcaaaactgc |
59 °C/35 cycles |
| Kif21a _x17R |
tgcttatctattgtccttaacctgc |
|
| Kif21a x18F |
tggccgttaatactgaatgttg |
56 °C/35cycles |
| Kif21a x18R |
aaagcaggttggattttaagaaa |
|
| Kif21a_x19F |
ccatttggaagaaaccttctg |
56 °C/35 cycles |
| Kif21a_x19R |
tgcactgccaaataatgagc |
|
| Kif21a _x20–21F |
ggcaacaaatggaaacaggt |
59 °C/35 cycles |
| Kif21a _x20–21R |
tggcatacatgtaaaacctaagc |
|
| Kif21a _x22F |
ccctatgtttcttggggtaatgat |
59 °C/35 cycles |
| Kif21a _x22R |
tccttattacaaagcaaagggtta |
|
| Kif21a _x23–24F |
ttactggaggagctgggatg |
59 °C/35 cycles |
| Kif21a _x23–24R |
tagtgtgtttgtgggcatgg |
|
| Kif21a _x25_ 26F |
actaaaaccatcgtgcccat |
59 °C/35 cycles |
| Kif21a _x25_ 26R |
gctttagtaaaaccatgccctc |
|
| Kif21a 26F |
tggcctagtgaatagcacttagaa |
59 °C/35 cycles |
| Kif21a 26R |
cagttaccacttaaagggaaatatga |
|
| Kif21a _x27F |
cacacctaggaaaagacacgct |
56 °C/35 cycles |
| Kif21a _x27R |
ggggagacaacacctagcaa |
|
| Kif21a_x28F |
caagtaataatctttctgaggttcca |
56 °C/35 cycles |
| Kif21a_x28R |
accacagcaccagcctaaat |
|
| Kif21a _x29F |
ttgttcagaatgcattttatcttaca |
59 °C/35 cycles |
| Kif21a _x29R |
gcatggttcctttcccatt |
|
| Kif21a _x30F |
agcagggcactatgaaggaa |
56 °C/35 cycles |
| Kif21a _x30R |
tttatctaaaaggtatgaccacaaaa |
|
| Kif21a_x31Fn |
tgtctcattccctttcacca |
56 °C/35 cycles |
| Kif21a_x31Rn |
caacagacttgatctgaaggaga |
|
| Kif21a _x32F |
gcttaaaagagagcagtfctgga |
59 °C/35 cycles |
| Kif21a _x32R |
ggttgaaccagattatccga |
|
| Kif21a _x33F |
tgaagttaggatccttgtggtatg |
59 °C/35 cycles |
| Kif21a _x33R |
tgggaagtggacaggtatacaa |
|
| Kif21a _x34F |
tgtgttaggtgctgtgctagg |
56 °C/35 cycles |
| Kif21a _x34R |
aaggacacaagagacatttagagg |
|
| Kif21a _x35F |
gcccaagatcccatctctaa |
56 °C/35 cycles |
| Kif21a _x35R |
ccactaactatgaatgaaggaaaaga |
|
| Kif21a_x36Fn |
ctccagcctgggaaacatag |
59 °C/35 cycles |
| Kif21a_x36Rn |
ggcctgattaatattatctgtaaatga |
|
| Kif21a _x37F |
ctttctccagccaattccaa |
59 °C/35cycles |
| Kif21a _x37R |
aacctggggtgcctaaattc |
|
| Kif21a _x38F |
tgtaaagggcacatggtaacaa |
59 °C/35 cycles |
| Kif21a _x38R | gcagttgaattcagatatattttcca |
Table 2. Primers for PHOX2A.
| Exon | 5′ to 3′ primer sequence | PCR conditions |
|---|---|---|
| Phox2ax1.1Fn |
tccacacctctgagccctaagacgg |
63 °C/DMSO10% |
| Phox2ax1.1Rn |
gccgcagggggctgtattggaagc |
|
| Phox2ax1.2fn |
ccccgggccgatggactact |
63 °C/DMSO10% |
| Phox2ax1.2Rn |
agcgggcccagggattc |
|
| Phox2ax2fn |
tcactcccccatcctttttgc |
57 °C/35 cycles |
| Phox2ax2Rn |
gctcccacacctccttcca |
|
| Phox2ax3.1fn |
gatctcactcgagccttgc |
57 °C/35 cycles |
| Phox2ax3.1Rn |
ctgcacgtggactccttgga |
|
| Phox2ax3.2fn |
cgggccaagttccgcaaacaggag |
57 °C/35 cycles |
| Phox2ax3.2Rn | ggacgtctctgggggcaggctcgga |
Table 3. List of primers for TUBB3.
| Exon | 5’ to 3’ sequence | Product size | Temperature/cycles |
|---|---|---|---|
| TUBB3X1F |
ggccgcggctataagag |
272 |
56 °C /35 |
| TUBB3X1R |
catccctttgttgcaggttc |
|
|
| TUBB3X2F |
tgggtcaaaagccctaatttt |
317 |
56 °C /35 |
| TUBB3X2R |
ctgagagctggtgagtccag |
|
|
| TUBB3X3F |
gctcttaggatgtgagcagga |
323 |
56 °C /35 |
| TUBB3X3R |
ggagctgaccattccttgtt |
|
|
| TUBB3X4-1F |
atgagaaggggtgctcagtg |
489 |
56 °C /35 |
| TUBB3X4-1R |
ctcgttgtcgatgcagtagg |
|
|
| TUBB3X4-2F |
cgcatcatgaacaccttcag |
498 |
56 °C/35 |
| TUBB3X4-2R |
gtccacctccttcatggaca |
|
|
| TUBB3X4-3F |
agctcacccagcagatgttc |
594 |
56 °C /35 |
| TUBB3X4-3R | gaggggaaagcagggtgt |
Sequences in Table are based on [22].
Results
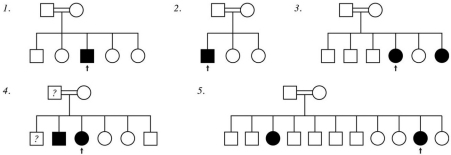
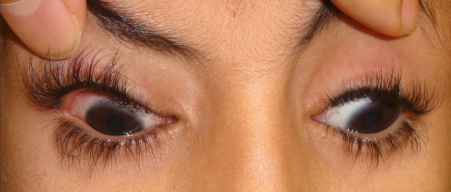
Pedigrees for the 5 patients are shown in Figure 1. All 5 probands had classic CFEOM1 without pupillary abnormality as defined above. One proband had an affected sibling with CFEOM1 (family 3 in Figure 1) and 2 probands each had an affected sibling with CFEOM3 (unilateral CFEOM ; families 4 and 5 in Figure 1). Clinical features of the probands are summarized in Table 4 and the typical proband phenotype is shown in Figure 2 (patient 1 from Table 4). No patient had significant extra-orbital disease.
Figure 1.
Pedigrees for the five CFEOM probands (arrow indicates proband). All individuals indicated as affected were confirmed to be affected to have CFEOM by examination. Question mark indicates that the individual was described as having strabismus but was not available for confirmatory ophthalmic examination.
Table 4. Summary of clinical features.
| ID | Age | Sex | Total siblings | Family history | BCVA | Primary | AB/AD | UP/DN | CycloRef | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
7 |
M |
5 |
none |
20/20 |
xt/hypo |
−4/-2 |
−6/-1 |
+0.50 |
attempt up=ad |
| |
|
|
|
|
20/30 |
xt/hypo |
−4/-2 |
−6/-1 |
+0.50 |
attempt up=ad |
| 2 |
7 |
M |
3 |
maternal uncle with bilateral ptosis |
20/40 |
xt/hypo |
−4/-2 |
−6/-1 |
+2.00–2.75x015 |
attempt up=ad |
| |
|
|
|
|
20/400 |
xt/hypo |
−4/-2 |
−6/-1 |
−5.00–2.00x150 |
attempt up=ad |
| 3 |
9 |
F |
6 |
Younger sister with CFEOM1 |
20/70 |
xt/hypo |
−1/-1 |
−5/0 |
+2.50–2.00x180 |
attempt ad=dn; attempt up=ad |
| |
|
|
|
|
20/60 |
xt/hypo |
−1/-1 |
−5/0 |
+3.50–3.25x180 |
attempt ad=dn; attempt up=ad |
| 4 |
13 |
F |
6 |
Older brother with CFEOM3 in left eye |
20/30 |
et/hypo |
−2/0 |
−5/0 |
+2.00 |
attempt ab=dn; attempt up=ad; torsional nystagmus |
| |
|
|
|
|
20/25 |
et/hypo |
−3/0 |
−5/0 |
+2.00 |
attempt up=ad; torsional nystagmus |
| 5 |
17 |
F |
11 |
Older sister with CFEOM3 in left eye |
20/40 |
xt/hypo |
−2/-3 |
−5/-3 |
+2.00 |
attempt up=dn&ad |
| 20/60 | xt/hypo | −2/-1 | −5/-3 | +8.00 | attempt up=ab |
For each patient where relevant, first row represents right eye data and second row represents left eye data; AGE:age in years; F:female; M:male; BCVA:best-corrected visual acuity; CSM:central steady maintained; et:esotropia; xt:exotropia; hypo: hypotropia;ab/ad:limitation of abduction/adduction on a scale of 0 to −4 (−5=eye cannot reach primary); CycloRef:cycloplegic refraction; up/down:limitation of supraduction/infraduction on a scale of 0 to −4 (−5=eye cannot reach primary); attempted x=y: when x attempted, y inappropriately occurs (dysinnervation).
Figure 2.
Typical CFEOM1 phenotype. Patient 1 is shown in forced primary position with his eyelids held upward. He has bilateral blepharoptosis, exotropia, hypotropia, and almost complete ophthalmoloplegia. When released, he assumes a chin up position with a left face turn (because of preference for the right eye).
No proband had mutations or polymorphic variations in KIF21A, PHOX2A, or TUBB3.
Discussion
Five CFEOM1 probands from consanguineous families were assessed in this study, none of whom had significant extra-orbital disease. Two were sporadic cases, one had a sibling with CFEOM1, and 2 each had a sibling with CFEOM3. No proband from this unique CFEOM1 cohort harbored mutations in KIF21A, TUBB3, or PHOX2A. Rather than being an autosomal dominant phenotype, CFEOM1 in our cohort was almost certainly related to homozygous mutations in a locus (or in loci) that to date has (or have) not been associated with the condition.
The 3 previously-reported CFEOM1 families from Saudi Arabia were not consanguineous and all harbored heterozygous missense KIF21A mutations [6,9]. Two families had autosomal dominant inheritance and both harbored the most common KIF21A mutation reported worldwide, p.R954W [9]. The third family, of Jordanian ancestry, exhibited apparent autosomal recessive inheritance with atypical abnormal pupils but in fact harbored heterozygous p.R954L KIF21A mutation with parental germline mosaicism [6]. In the current series, none of the 5 CFEOM1 patients harbored mutations in known CFEOM genes. Two cases were sporadic and 3 had affected siblings. For one, the sibling also had CFEOM1 (family 3 from Figure 1). For the other 2, the each had an affected sibling with CFEOM3 (families 4 and 5 from Figure 1). These latter 2 families would be considered by some authors as CFEOM3 pedigrees [4,23].
Studies of consanguineous families are more likely to uncover recessive cause for a given phenotype if a recessive cause exists because of parental shared recent ancestry. Although every individual is a heterozygous carrier for mutated alleles that would potentially cause recessive disease in the homozygous (or compound heterozygous) state, it is unlikely that the individual's spouse will carry the same disorder unless they are related [28]. Thus studies of exclusively consanguineous families with a specific phenotype offer a unique opportunity to uncover a recessive cause for the phenotype if a recessive cause exists. Our study confirms the existence of recessive CFEOM1. There may be one or more such loci, each of which may be a separate gene or locus that regulates pathways in known genes associated with CFEOM. Whether the 2 families that included a sibling with CFEOM3 (families 4 and 5 from Figure 1) are considered CFEOM3 families or families with CFEOM1 probands, the observed phenotype is likely related to a recessive cause that has not yet been described.
In summary, although most CFEOM1 is due to heterozygous missense KIF21A mutations, there exists at least one additional autosomal recessive cause for the phenotype. This information is useful in the genetic counseling of sporadic KIF21A-negative CFEOM1 patients. It is hoped that further ascertainment and study of CFEOM1 patients from consanguineous families will uncover the novel locus(loci).
Acknowledgments
Funding for this study was from the Department of Genetics, King Faisal Hospital & Research Center; Research Department, King Khaled Eye Specialist Hospital
References
- 1.Traboulsi EI, Engle EC. Mutations in KIF21A are responsible for CFEOM1 worldwide. Ophthalmic Genet. 2004;25:237–9. doi: 10.1080/13816810490911684. [DOI] [PubMed] [Google Scholar]
- 2.Yamada K, Andrews C, Chan WM, McKeown CA, Magli A, de Berardinis T, Loewenstein A, Lazar M, O'Keefe M, Letson R, London A, Ruttum M, Matsumoto N, Saito N, Morris L, Del Monte M, Johnson RH, Uyama E, Houtman WA, de Vries B, Carlow TJ, Hart BL, Krawiecki N, Shoffner J, Vogel MC, Katowitz J, Goldstein SM, Levin AV, Sener EC, Ozturk BT, Akarsu AN, Brodsky MC, Hanisch F, Cruse RP, Zubcov AA, Robb RM, Roggenkäemper P, Gottlob I, Kowal L, Battu R, Traboulsi EI, Franceschini P, Newlin A, Demer JL, Engle EC. Heterozygous mutations of the kinesin KIF21A in congenital fibrosis of the extraocular muscles type 1 (CFEOM1). Nat Genet. 2003;35:318–21. doi: 10.1038/ng1261. [DOI] [PubMed] [Google Scholar]
- 3.Chan WM, Andrews C, Dragan L, Fredrick D, Armstrong L, Lyons C, Geraghty MT, Hunter DG, Yazdani A, Traboulsi EI, Pott JW, Gutowski NJ, Ellard S, Young E, Hanisch F, Koc F, Schnall B, Engle EC. Three novel mutations in KIF21A highlight the importance of the third coiled-coil stalk domain in the etiology of CFEOM1. BMC Genet. 2007;8:26. doi: 10.1186/1471-2156-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutowski NJ, Bosley TM, Engle EC. 110th ENMC international workshop: The congenital cranial dysinnervation disorders (CCDDs). Naarden, The Netherlands, 25–27 October, 2002. Neuromuscul Disord. 2003;13:573–8. doi: 10.1016/s0960-8966(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 5.Engle EC, Kunkel LM, Specht LA, Beggs AH. Mapping a gene for congenital fibrosis of the extraocular muscles to the centromeric region of chromosome 12. Nat Genet. 1994;7:69–73. doi: 10.1038/ng0594-69. [DOI] [PubMed] [Google Scholar]
- 6.Khan AO, Khalil DS, Al Sharif LJ, Al-Ghadhfan FE, Al Tassan NA. Germline mosaicism for KIF21A mutation (p.R954L) mimicking recessive inheritance for congenital fibrosis of the extraocular muscles. Ophthalmology. 2010;117:154–8. doi: 10.1016/j.ophtha.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Yamada K, Katz B, Guan H, Wang L, Andrews C, Zhao G, Engle EC, Chen H, Tong Z, Kong J, Hu C, Kong Q, Fan G, Wang Z, Ning M, Zhang S, Xu J, Zhang K. KIF21A mutations in two Chinese families with congenital fibrosis of the extraocular muscles (CFEOM). Mol Vis. 2010;16:2062–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Rudolph G, Nentwich M, Hellebrand H, Pollack K, Gordes R, Bau V, Kampik A, Meindl A. KIF21A variant R954W in familial or sporadic cases of CFEOM1. Eur J Ophthalmol. 2009;19:667–74. doi: 10.1177/112067210901900423. [DOI] [PubMed] [Google Scholar]
- 9.Khan AO, Khalil DS, Al-Tassan NA. Congential fibrosis of the extraocular muscles type I (CFEOM1) on the Arabian Peninsula. Ophthalmic Genet. 2008;29:25–8. doi: 10.1080/13816810701850058. [DOI] [PubMed] [Google Scholar]
- 10.Lu S, Zhao C, Zhao K, Li N, Larsson C. Novel and recurrent KIF21A mutations in congenital fibrosis of the extraocular muscles type 1 and 3. Arch Ophthalmol. 2008;126:388–94. doi: 10.1001/archopht.126.3.388. [DOI] [PubMed] [Google Scholar]
- 11.Zhang XQ, Peng JH, Tang ZH, Xu CQ, Zhou X, Gong SX, Liu JY, Wang Q, Liu MG. Mutation p.Arg954Trp of KIF21A causes congenital fibrosis of the extraocular muscles in a Chinese family. Yi Chuan Xue Bao. 2006;33:685–91. doi: 10.1016/S0379-4172(06)60100-5. [DOI] [PubMed] [Google Scholar]
- 12.Lin LK, Chien YH, Wu JY, Wang AH, Chiang SC, Hwu WL. KIF21A gene c.2860C>T mutation in congenital fibrosis of extraocular muscles type 1 and 3. Mol Vis. 2005;11:245–8. [PubMed] [Google Scholar]
- 13.Ali M, Venkatesh C, Ragunath A, Kumar A. Mutation analysis of the KIF21A gene in an Indian family with CFEOM1: Implication of CpG methylation for most frequent mutations. Ophthalmic Genet. 2004;25:247–55. doi: 10.1080/13816810490498198. [DOI] [PubMed] [Google Scholar]
- 14.Tiab L, d'Alleves Manzi V, Borruat FX, Munier F, Schorderet D. Mutation analysis of KIF21A in congenital fibrosis of the extraocular muscles (CFEOM) patients. Ophthalmic Genet. 2004;25:241–6. doi: 10.1080/13816810490902828. [DOI] [PubMed] [Google Scholar]
- 15.Miki H, Setou M, Kaneshiro K, Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci USA. 2001;98:7004–11. doi: 10.1073/pnas.111145398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–9. doi: 10.1167/iovs.04-1125. [DOI] [PubMed] [Google Scholar]
- 17.Wang SM, Zwaan J, Mullaney PB, Jabak MH, Al-Awad A, Beggs AH, Engle EC. Congenital fibrosis of the extraocular muscles type 2, an inherited exotropic strabismus fixus, maps to distal 11q13. Am J Hum Genet. 1998;63:517–25. doi: 10.1086/301980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano M, Yamada K, Fain J, Sener EC, Selleck CJ, Awad AH, Zwaan J, Mullaney PB, Bosley TM, Engle EC. Homozygous mutations in ARIX(PHOX2A) result in congenital fibrosis of the extraocular muscles type 2. Nat Genet. 2001;29:315–20. doi: 10.1038/ng744. [DOI] [PubMed] [Google Scholar]
- 19.Guo S, Brush J, Teraoka H, Goddard A, Wilson SW, Mullins MC, Rosenthal A. Development of noradrenergic neurons in the zebrafish hindbrain requires BMP, FGF8, and the homeodomain protein soulless/Phox2a. Neuron. 1999;24:555–66. doi: 10.1016/s0896-6273(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 20.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–75. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- 21.Bosley TM, Oystreck DT, Robertson RL, al Awad A, Abu-Amero K, Engle EC. Neurological features of congenital fibrosis of the extraocular muscles type 2 with mutations in PHOX2A. Brain. 2006;129:2363–74. doi: 10.1093/brain/awl161. [DOI] [PubMed] [Google Scholar]
- 22.Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, Chan WM, Andrews C, Demer JL, Robertson RL, Mackey DA, Ruddle JB, Bird TD, Gottlob I, Pieh C, Traboulsi EI, Pomeroy SL, Hunter DG, Soul JS, Newlin A, Sabol LJ, Doherty EJ, de Uzcátegui CE, de Uzcátegui N, Collins ML, Sener EC, Wabbels B, Hellebrand H, Meitinger T, de Berardinis T, Magli A, Schiavi C, Pastore-Trossello M, Koc F, Wong AM, Levin AV, Geraghty MT, Descartes M, Flaherty M, Jamieson RV, Møller HU, Meuthen I, Callen DF, Kerwin J, Lindsay S, Meindl A, Gupta ML, Jr, Pellman D, Engle EC. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada K, Chan WM, Andrews C, Bosley TM, Sener EC, Zwaan JT, Mullaney PB, Oztürk BT, Akarsu AN, Sabol LJ, Demer JL, Sullivan TJ, Gottlob I, Roggenkäemper P, Mackey DA, De Uzcategui CE, Uzcategui N, Ben-Zeev B, Traboulsi EI, Magli A, de Berardinis T, Gagliardi V, Awasthi-Patney S, Vogel MC, Rizzo JF, 3rd, Engle EC. Identification of KIF21A mutations as a rare cause of congenital fibrosis of the extraocular muscles type 3 (CFEOM3). Invest Ophthalmol Vis Sci. 2004;45:2218–23. doi: 10.1167/iovs.03-1413. [DOI] [PubMed] [Google Scholar]
- 24.Doherty EJ, Macy ME, Wang SM, Dykeman CP, Melanson MT, Engle EC. CFEOM3: A new extraocular congenital fibrosis syndrome that maps to 16q24.2-q24.3. Invest Ophthalmol Vis Sci. 1999;40:1687–94. [PubMed] [Google Scholar]
- 25.Mackey DA, Chan WM, Chan C, Gillies WE, Brooks AM, O'Day J, Engle EC. Congenital fibrosis of the vertically acting extraocular muscles maps to the FEOM3 locus. Hum Genet. 2002;110:510–2. doi: 10.1007/s00439-002-0707-5. [DOI] [PubMed] [Google Scholar]
- 26.Woods CG, Cox J, Springell K, Hampshire DJ, Mohamed MD, McKibbin M, Stern R, Raymond FL, Sandford R, Malik Sharif S, Karbani G, Ahmed M, Bond J, Clayton D, Inglehearn CF. Quantification of homozygosity in consanguineous individuals with autosomal recessive disease. Am J Hum Genet. 2006;78:889–96. doi: 10.1086/503875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan AO, Khalil DS, Al-Sharif LJ, Al-Tassan NA. Mutations in KIF21A and PHOX2A are absent in 16 patients with congenital vertical incomitant strabismus. Ophthalmic Genet. 2009;30:206–7. doi: 10.3109/13816810903183613. [DOI] [PubMed] [Google Scholar]
- 28.Modell B, Darr A. Science and society: Genetic counselling and customary consanguineous marriage. Nat Rev Genet. 2002;3:225–9. doi: 10.1038/nrg754. [DOI] [PubMed] [Google Scholar]