Abstract
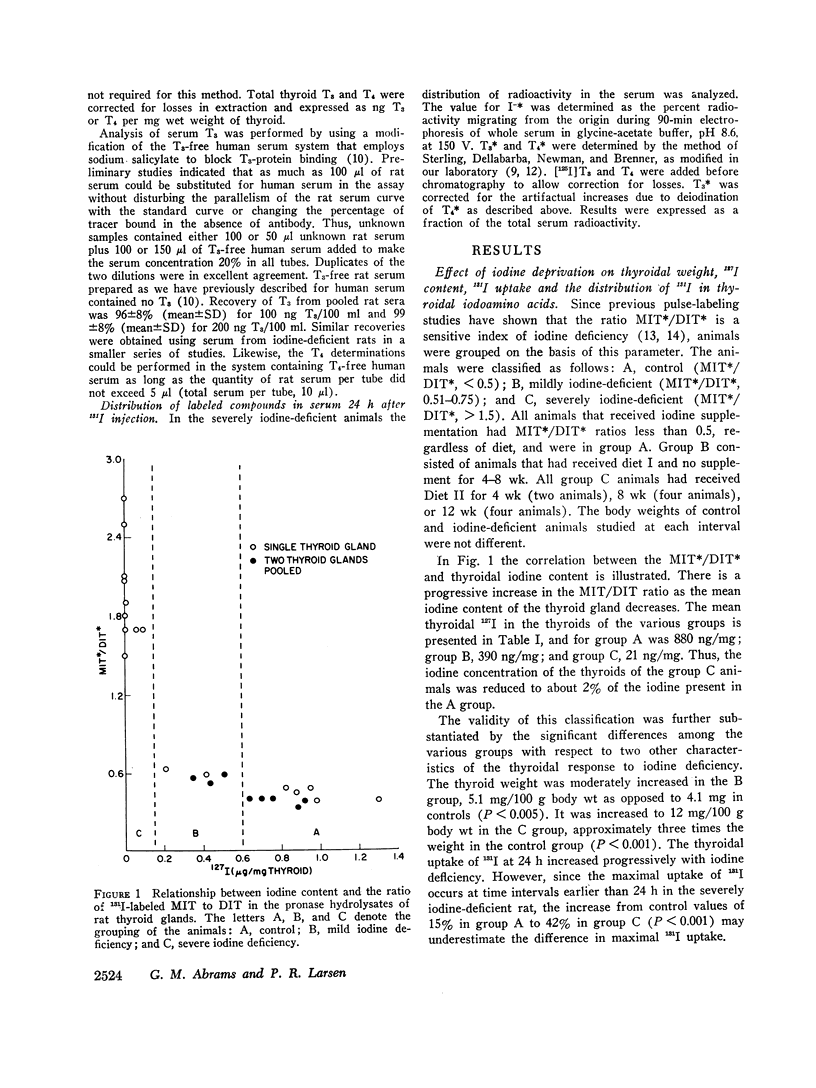
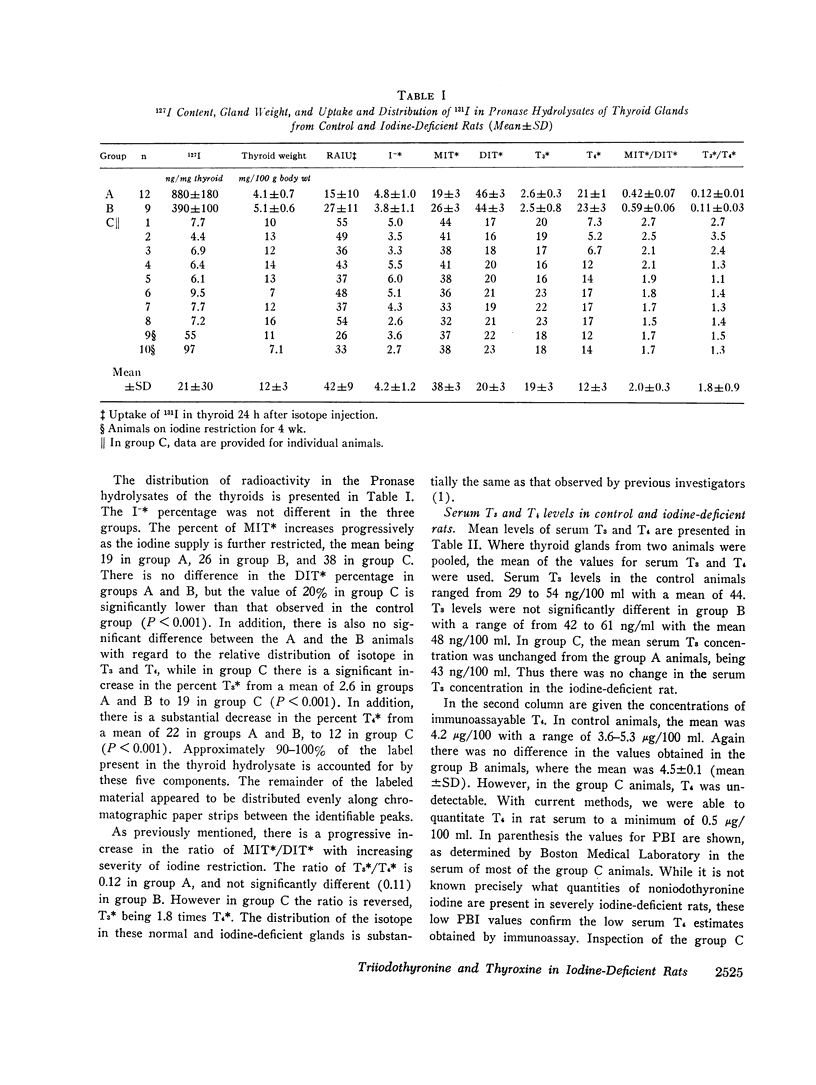
Triiodothyronine (T3) and thyroxine (T4) were measured by immunoassay in the serum and thyroid hydrolysates of control (group A), mildly iodine-deficient (group B), and severely iodine-deficient rats (group C). These results were correlated with changes in thyroidal weight, 131I uptake and 127I content as well as with the distribution of 131I in Pronase digests of the thyroid. There was a progressive increase in thyroid weight and 131I uptake at 24 h with decrease in iodine intake. The 127I content of the thyroids of the group B animals was 44% and that of the group C animals 2% of that in group A. The mean labeled monoiodotyrosine/diiodotyrosine (MIT/DIT) and T3/T4 ratios in group A were 0.42±0.07 (SD) and 0.12±0.01, 0.59±0.06 and 0.11±0.03 in group B, and 2.0±0.3 and 1.8±0.9 in the group thyroid digests.
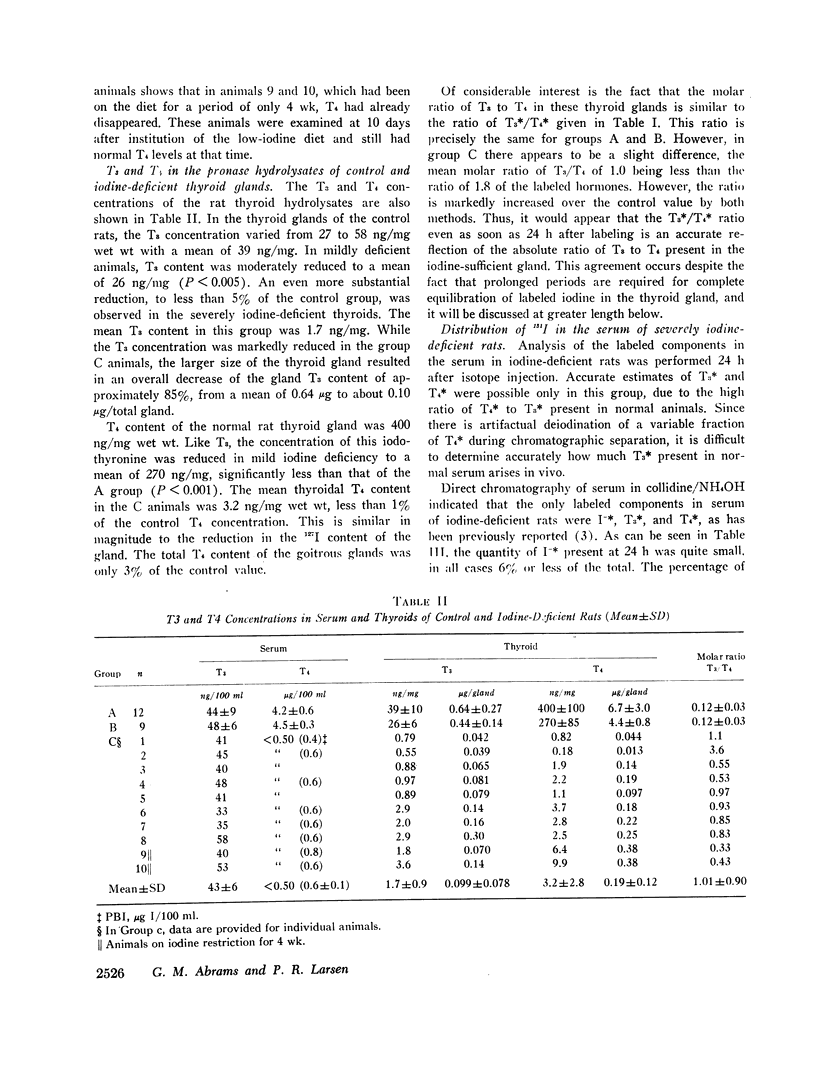
Mean serum T4 concentration in the control rats was 4.2±0.6 (SD) μg T4/100 ml, 4.5±0.3 μg/100 ml in group B animals, and undectectable (<0.5 μ4/100 ml) in group C animals. There was no effect of iodine deficiency on serum T3 concentrations, which were 44±9 (Mean±SD) ng/100 ml in A animals, 48±6 ng/100 ml n B animals, and 43±6 ng/100 ml in the C group. Thyroidal digest T3 and T4 concentrations were 39 and 400 ng/mg in group A animals and were reduced to 5 and 1% of this, respectively, in group C. The molar ratio of T3/T4 in the thyroid digests of the groups A and B animals was identical to the ratio of labeled T3/T4 and was slightly less (1.0±0.9) than the labeled T3/T4 ratio in the group C animals.
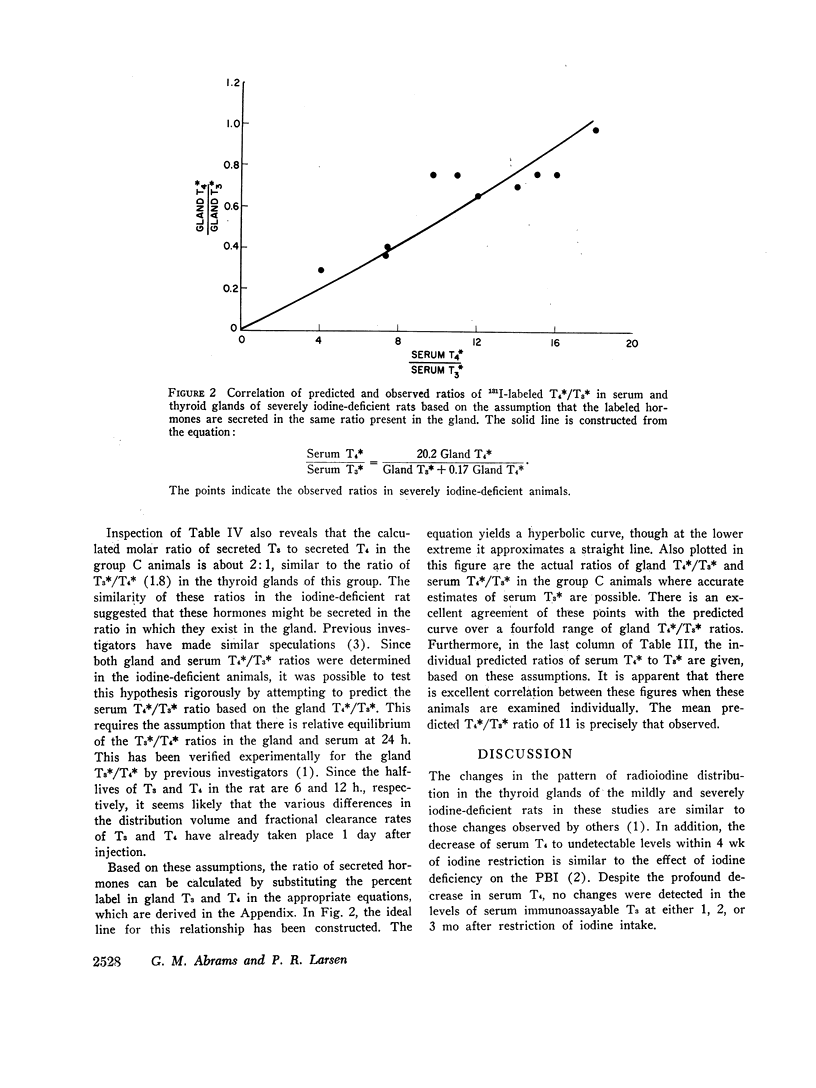
The mean ratio of labeled T4 to labeled T3 in the serum of the severely iodine-deficient animals 24 h after isotope injection was 11±1 (SEM). With previously published values, it was possible to correlate the ratio of labeled T4/T3 in the thyroid digest with the labeled T4/T3 ratio in the serum of each iodine-deficient animal. This analysis suggested that the labeled thyroid hormones in the severely iodine-deficient rat were secreted in the ratio in which they are present in the gland.
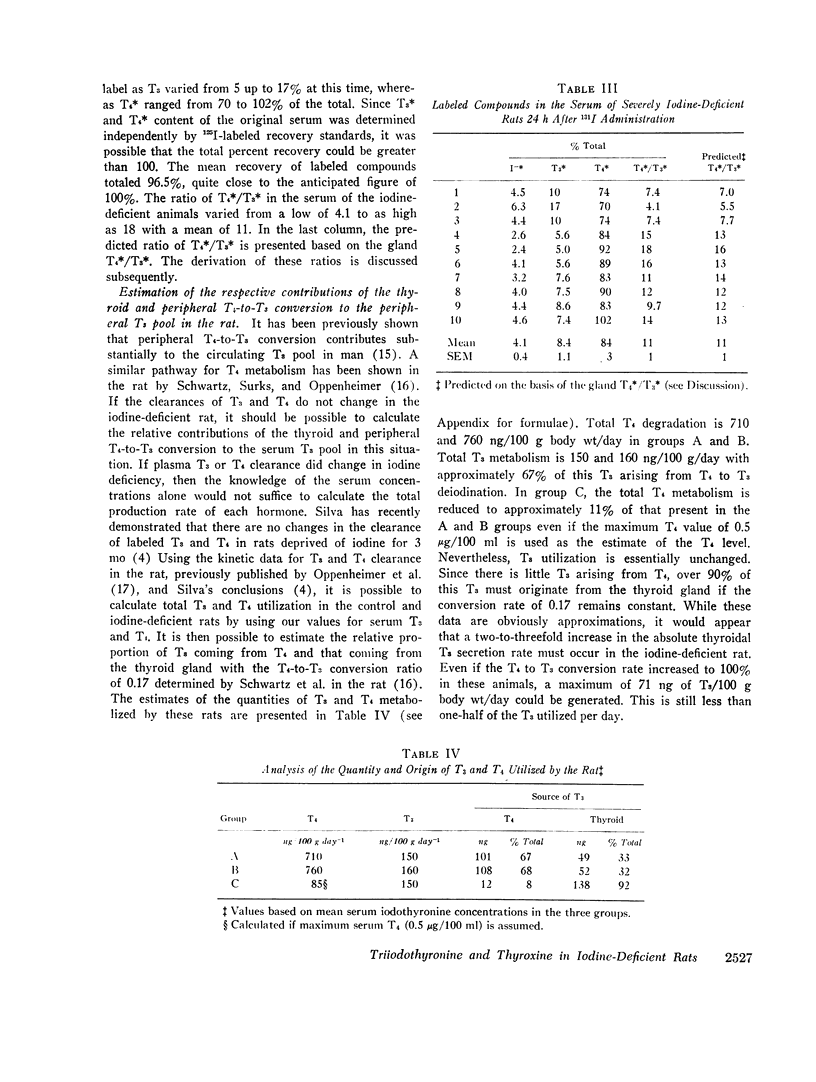
Kinetic analysis of total iodothyronine turnover indicated that two-thirds of the T3 utilized per day by the iodine-sufficient rat arises from T4. If the T4-T3 conversion ratio remains the same in iodine deficiency, then the analysis suggests that about 90% of the T3 arises directly from the thyroid. Therefore, it would appear that absolute T3 secretion by the thyroid increases severalfold during iodine deficiency. The fact that serum T3 remains constant and T4 decreases to extremely low levels, combined with previous observations that iodine-deficient animals appear to be euthyroid, is compatible with the hypothesis that T4 in the normal rat serves primarily as a precursor of T3.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benotti J., Benotti N., Pino S., Gardyna H. Determination of total iodine in urine, stool, diets, and tissue. Clin Chem. 1965 Oct;11(10):932–936. [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer M. A., Grimm Y., Studer H. Qualitative changes in the secretion of thyroid hormones induced by iodine deficiency. Endocrinology. 1968 Dec;83(6):1193–1198. doi: 10.1210/endo-83-6-1193. [DOI] [PubMed] [Google Scholar]
- Heninger R. W., Albright E. C. Effect of iodine deficiency on iodine-containing compounds of rat tissues. Endocrinology. 1966 Aug;79(2):309–315. doi: 10.1210/endo-79-2-309. [DOI] [PubMed] [Google Scholar]
- Inoue K., Taurog A. Acute and chronic effects of iodide on thyroid radioiodine metabolism in iodine-deficient rats. Endocrinology. 1968 Aug;83(2):279–290. doi: 10.1210/endo-83-2-279. [DOI] [PubMed] [Google Scholar]
- Inoue K., Taurog A. Digestion of 131I-labeled thyroid tissue with maximum recovery of 131I-iodothyronines. Endocrinology. 1967 Aug;81(2):319–332. doi: 10.1210/endo-81-2-319. [DOI] [PubMed] [Google Scholar]
- Lamas L., Morreale de Escobar G. Iodoamino acid distribution in the thyroid of rats on different iodine intakes and with normal plasma protein bound iodine. Acta Endocrinol (Copenh) 1972 Mar;69(3):473–487. doi: 10.1530/acta.0.0690473. [DOI] [PubMed] [Google Scholar]
- Larsen P. R. Direct immunoassay of triiodothyronine in human serum. J Clin Invest. 1972 Aug;51(8):1939–1949. doi: 10.1172/JCI107000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R. Technical aspects of the estimation of triiodothyronine in human serum: evidence of conversion of thyroxine to triiodothyronine during assay. Metabolism. 1971 Jun;20(6):609–624. doi: 10.1016/0026-0495(71)90009-6. [DOI] [PubMed] [Google Scholar]
- Larsen P. R. Triiodothyronine: review of recent studies of its physiology and pathophysiology in man. Metabolism. 1972 Nov;21(11):1073–1092. doi: 10.1016/0026-0495(72)90038-8. [DOI] [PubMed] [Google Scholar]
- Lieblich J., Utiger R. D. Triiodothyronine radioimmunoassay. J Clin Invest. 1972 Jan;51(1):157–166. doi: 10.1172/JCI106786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein J. E., Wollman S. H. Kinetics of equilibrium labeling of the rat thyroid gland with 125-I. Endocrinology. 1967 Nov;81(5):1063–1073. doi: 10.1210/endo-81-5-1063. [DOI] [PubMed] [Google Scholar]
- Nejad I. F., Bollinger J. A., Mitnick M., Reichlin S. Importance of T 3 (triiodothyronine) secretion in altered states of thyroid function in the rat: cold exposure, subtotal thyroidectomy, and hypophysectomy. Trans Assoc Am Physicians. 1972;85:295–308. [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Shapiro H. C., Bernstein G., Surks M. I. Differences in primary cellular factors influencing the metabolism and distribution of 3,5,3'-L-triiodothyronine and L-thyroxine. J Clin Invest. 1970 May;49(5):1016–1024. doi: 10.1172/JCI106301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest. 1972 Sep;51(9):2493–2497. doi: 10.1172/JCI107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDER H., GREER M. A. A STUDY OF THE MECHANISMS INVOLVED IN THE PRODUCTION OF IODINE-DEFICIENCY GOITER. Acta Endocrinol (Copenh) 1965 Aug;49:610–628. doi: 10.1530/acta.0.0490610. [DOI] [PubMed] [Google Scholar]
- Schadlow A. R., Surks M. I., Schwartz H. L., Oppenheimer J. H. Specific triiodothyronine binding sites in the anterior pituitary of the rat. Science. 1972 Jun 16;176(4040):1252–1254. doi: 10.1126/science.176.4040.1252. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Surks M. I., Oppenheimer J. H. Quantitation of extrathyroidal conversion of L-thyroxine to 3,5,3'-triiodo-L-thyronine in the rat. J Clin Invest. 1971 May;50(5):1124–1130. doi: 10.1172/JCI106584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E. Disposal rates of thyroxine and triiodothyronine in iodine-deficient rats. Endocrinology. 1972 Dec;91(6):1430–1435. doi: 10.1210/endo-91-6-1430. [DOI] [PubMed] [Google Scholar]
- Sterling K., Bellabarba D., Newman E. S., Brenner M. A. Determination of triiodothyronine concentration in human serum. J Clin Invest. 1969 Jun;48(6):1150–1158. doi: 10.1172/JCI106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling K., Brenner M. A., Newman E. S., Odell W. D., Bellabarba D. The significance of triiodothyronine (T3) in maintenance of euthyroid status after treatment of hyperthyroidism. J Clin Endocrinol Metab. 1971 Nov;33(5):729–731. doi: 10.1210/jcem-33-5-729. [DOI] [PubMed] [Google Scholar]


