Abstract
Our understanding of how natural selection should shape sex allocation is perhaps more developed than for any other trait. However, this understanding is not matched by our knowledge of the genetic basis of sex allocation. Here, we examine the genetic basis of sex ratio variation in the parasitoid wasp Nasonia vitripennis, a species well known for its response to local mate competition (LMC). We identified a quantitative trait locus (QTL) for sex ratio on chromosome 2 and three weaker QTL on chromosomes 3 and 5. We tested predictions that genes associated with sex ratio should be pleiotropic for other traits by seeing if sex ratio QTL co-occurred with clutch size QTL. We found one clutch size QTL on chromosome 1, and six weaker QTL across chromosomes 2, 3 and 5, with some overlap to regions associated with sex ratio. The results suggest rather limited scope for pleiotropy between these traits.
Keywords: brood size, clutch size, linkage map, local mate competition, Nasonia, oviposition, parasitoid wasp, sex allocation
Introduction
The study of sex allocation and sex ratios has produced many of the best tests of evolutionary theory to date (Charnov, 1982; Herre, 1987; West, 2009). This is in part attributed to a rigorous and well-developed theory base, originating from the ideas of frequency-dependent selection on sex allocation proposed by Düsing and Fisher (Fisher, 1930; Edwards, 2000) through to the ground-breaking work of Hamilton and Trivers (e.g. Hamilton, 1967; Trivers & Willard, 1973) and the many refinements of sex allocation theory developed by later evolutionary ecologists (reviewed by Charnov, 1982; Frank, 1998; Hardy, 2002; West, 2009). It is also partly attributed to the simplicity of the sex allocation trait itself. When considered in terms of the proportion of offspring that are male (sex ratio), the underlying trade-off is simply the production of either sons or daughters. As a result, patterns of sex ratios have been successfully predicted across an extremely broad range of organisms, from single-celled organisms such as Plasmodium, through to many plants and animals, largely regardless of the genetic system of sex determination (reviewed by West et al., 2005; West, 2009). In contrast, we know much less about the genetic basis of sex ratio, and as such we have only half of the evolutionary story (Pannebakker et al., 2008). For instance, understanding how sex ratios should evolve under natural selection is of limited use if genetic architecture constrains how sex ratio behaviour actually can evolve.
The genetic basis of sex ratio behaviour is important for three reasons. First, for all the successes of sex allocation theory, there still remains variation around predicted sex ratios (West & Herre, 1998; Orzack, 2002). To know how biologically meaningful this variation is we need to ascertain whether it is environmental or genetic in origin, and if the latter is the case, we need to assess whether variation around (or away from) the predicted optimum sex ratio represents a genetic constraint. Genetic constraints could arise through a lack of appropriate mutations (Blows & Hoffmann, 2005; but see Pannebakker et al., 2008) or through particular genetic architectures, for instance if there are negative genetic correlations between fitness traits including sex ratio (Barton & Partridge, 2000). Second, identifying the genetic basis of sex ratio would allow us to explore the genetics of a well-characterized adaptation, including the molecular evolution of the genes associated with the trait. Third, that facultative sex allocation remains controversial in some taxa (particularly vertebrates with chromosomal sex determination) is not least because the proximate mechanisms underlying sex allocation are somewhat obscure (Krackow, 2002; West & Sheldon, 2002; Pike & Petrie, 2003; West et al., 2005; Uller & Badyaev, 2009). Identifying the genetic basis of sex ratio traits in other taxa, such as parasitoid wasps, may provide another starting point for exploring sex allocation in species with less understood mechanisms of sex allocation.
To date, studies of sex ratio genetics have focused on quantitative genetic analyses, including studies on Drosophila (Toro & Charlesworth, 1982; Carvalho et al., 1998), fish (Vandeputte et al., 2007), turtles (Janzen, 1992), pigs (Toro et al., 2006) and humans (Gellatly, 2009). However, an important group for sex ratio studies has been the parasitoid wasps. Genetic variation for sex ratio has been found in both Trichogramma and Heterospilus (Wajnberg, 1994; Kobayashi et al., 2003), although most work has been carried out in Nasonia vitripennis, a species well known for its facultative sex ratio behaviour. Work by Orzack and colleagues in the 1980s and 1990s showed additive genetic variation in N. vitripennis sex ratios, using a mixture of artificial selection and quantitative genetic techniques (Parker & Orzack, 1985; Orzack & Parker, 1986, 1990; Orzack et al., 1991; Orzack & Gladstone, 1994). More recently, we have explored how mutation inputs genetic variation in sex ratio, through the first mutation accumulation study of sex ratio (Pannebakker et al., 2008). We found that in N. vitripennis one mutation occurs every 5–60 generations, which shifts the sex ratio by approximately 1% and that mutational heritabilities for sex ratio in N. vitripennis (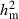 = 0.0008–0.0019) were similar to those for other life-history traits in species such as Drosophila melanogaster and Caenorhabditis elegans. Moreover, by estimating the strength of stabilizing selection on sex ratio in Nasonia, we estimated the degree of standing genetic variation for sex ratio we would expect to see in a population and compared this to our own estimate of sex ratio heritability and those of Orzack and colleagues. We predicted greater genetic variation in sex ratio than any study has so far observed, suggesting that sex ratio genes (i.e. genes that influence variation for sex allocation) are pleiotropic, influencing other fitness-related traits (and thus facing stronger purifying selection, leading to less variation at mutation-selection balance: Turelli, 1984).
= 0.0008–0.0019) were similar to those for other life-history traits in species such as Drosophila melanogaster and Caenorhabditis elegans. Moreover, by estimating the strength of stabilizing selection on sex ratio in Nasonia, we estimated the degree of standing genetic variation for sex ratio we would expect to see in a population and compared this to our own estimate of sex ratio heritability and those of Orzack and colleagues. We predicted greater genetic variation in sex ratio than any study has so far observed, suggesting that sex ratio genes (i.e. genes that influence variation for sex allocation) are pleiotropic, influencing other fitness-related traits (and thus facing stronger purifying selection, leading to less variation at mutation-selection balance: Turelli, 1984).
In this study, we consider the genetic basis of sex ratio in N. vitripennis using a quantitative trait locus (QTL) approach. We chose two wasp strains that diverged in their patterns of sex ratio (specifically in their sex ratios when a single female lays eggs on a host) and performed a QTL study to identify regions of the genome associated with this intra-specific variation. Given that our previous work made the prediction that at least some sex ratio genes should be pleiotropic, influencing other fitness-related traits in N. vitripennis, we also looked for QTL for clutch size in crosses between the same two lines to see whether or not sex ratio and clutch size QTL overlapped. Theory suggests that clutch size and sex ratio should be simultaneously optimized by natural selection when females lay eggs across multiple patches and kin-related processes such as local mate competition (LMC) influence sex ratio (Werren, 1980; Godfray, 1986; Nagelkerke, 1994; Greeff, 1997; West et al., 1997). More practically, clutch size is a straightforward trait to analyse alongside sex ratio. Whilst the prediction of pleiotropic gene action can only be confirmed once individual genes are identified and cloned, we could at least ascertain whether such pleiotropy was plausible.
Materials and methods
Study organism
The parasitoid wasp N. vitripennis (Hymenoptera: Chalcidoidea) is a generalist gregarious parasitoid of large dipteran pupae (including Sarcophagidae and Calliphoridae: Whiting, 1967; Werren et al., 2010). Depending on the host species and whether or not a host has been already parasitized, females can oviposit more than 50 eggs on an individual host. Eggs are laid between the puparium wall and the host pupa, with larvae hatching and attaching themselves to the fly pupa to feed. On the blowfly Calliphora vomitoria egg-to-adult development takes approximately 14 days at 25 °C, with males eclosing and emerging from the host puparium before females. Nasonia vitripennis males are brachypterous and are unable to fly, remaining close to the emergence patch where they compete with each other for matings with emerging females. Females are fully winged and typically mate only once before dispersing to find new hosts. This life history creates extremely localized mating populations, selecting for female-biased sex ratios as envisaged by LMC theory (Hamilton, 1967; Taylor & Bulmer, 1980; Herre, 1985; Frank, 1986). LMC theory predicts that sex ratios should become increasingly female biased as LMC among related males increases. The extent of LMC will depend on how many females (or ‘foundresses’) contribute eggs to a patch, specifically in terms of the relative clutch sizes of the different foundresses (Werren, 1980; Stubblefield & Seger, 1990). The most intense LMC, and the most female-biased sex ratios, occurs if only one female oviposits on a patch. Repeated tests of N. vitripennis have generally confirmed predictions from both general and specific models of LMC in the laboratory (Werren, 1980, 1983; Orzack & Parker, 1986; Flanagan et al., 1998; Shuker & West, 2004; Shuker et al., 2005, 2006, 2007; West et al., 2005) and in the wild (Molbo & Parker, 1996; Burton-Chellew et al., 2008; Grillenberger et al., 2008).
Experimental strains
We used two iso-female N. vitripennis strains that were previously collected from a single population (De Hoge Veluwe, the Netherlands). The low sex ratio strain C222a (denoted L) was collected in 2003 by L.W. Beukeboom, and the high sex ratio strain HV06 (denoted H) was collected in 2004 by T. Koevoets, M.N. Burton-Chellew and E.M. Sykes (for collection details see Burton-Chellew et al., 2008 and Grillenberger et al., 2008). These strains were chosen from a panel of strains that was previously screened for sex ratio variation on the basis of their different single foundress sex ratios behaviour (Table 1, Pannebakker et al., 2008). Strains were maintained on Calliphora vomitoria pupae as hosts at 25 °C, 16L : 8D light conditions.
Table 1.
The experimental set-up and sample sizes for the genotyping and phenotyping for sex ratio and clutch size. Two hundred and ninety-nine clonal sibships of recombinant males were generated across two sets of crosses. Recombinant F2 males were derived from virgin F1 females, and sibships were created by crossing these F2 males to females from the high and low sex ratio lines. The F3 clonal sibships of these crosses were then phenotyped for single foundress sex ratio and clutch size.
| Recombinant F2 males | |||
|---|---|---|---|
| Cross | Genotyped | Phenotyped | F3 broods analysed |
| 1 | 276 | 156 | 2057 |
| 2 | 143 | 143 | 1657 |
| Total | 419 | 299 | 3714 |
We took advantage of haplodiploid genetics to generate large numbers of genetically near-identical females with recombinant genotypes, following methods outlined in Velthuis et al. (2005). An L male was crossed to a virgin H female (generation P) to produce genetically identical F1 hybrid females (Fig. 1). The F1 females were allowed to oviposit as virgins, generating F2 males with recombinant genotypes between the L and H lines. Because Nasonia males are haploid, they produce genetically identical sperm. Each F2 male was then backcrossed to females from both the H and L strains, thus generating so-called clonal sibships. In two separate experimental blocks, we generated clonal sibships in both H and L backgrounds from a total of 299 recombinant males (Table 1). This set-up takes advantages of haplodiploid genetics in two ways: (i) it allows for replicate phenotyping of each recombinant haploid genotype expressed in both H and L backgrounds; (ii) it allows for the estimation of additive and dominance effects.
Fig. 1.
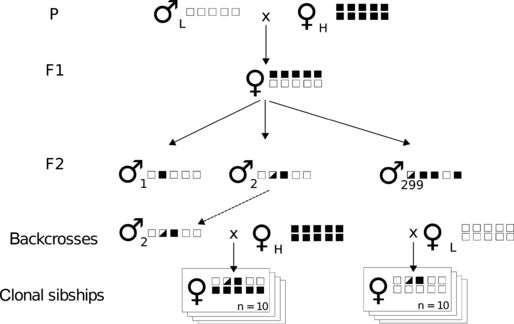
The experimental crossing procedure, used to generate clonal sibships. Virgin LxH F1 females produced recombinant F2 males, which were then backcrossed to both the H and the L parental line, to produce females in clonal sibships. These females were assayed for single foundress sex ratio and clutch size in each genetic background (N = 10 females per recombinant male genotype per background). In total, 299 F2 recombinant male genotypes from two sets of crosses were combined in a single quantitative trait locus analysis, with a further 120 male genotypes used for the linkage mapping. Squares represent the five different chromosomes of Nasonia vitripennis.
Phenotyping
For each of the sibships, we isolated approximately ten 2-day mated old females in glass vials and provided them with a single host for 24 h as pretreatment to facilitate egg development. We then discarded the pretreatment hosts and gave each female a piece of chromatography paper soaked in honey solution for a further 24 h. We then provided these females with a single host for oviposition. After 3 h, we placed one-way escape tubes on the glass vials to allow females to disperse away from the patches, to limit superparasitism (Godfray, 1994; Shuker et al., 2005). After 24 h, we removed the females and incubated the parasitized hosts at 25 °C. We randomly distributed these vials across racks to ensure that hosts from the same sibship were distributed across racks and shelves in the incubator. We allowed the resulting adult wasps to emerge and die prior to counting and sexing the brood, resulting in a total of 3714 clutches comprising 106 646 wasps (Table 1).
Genotyping
We determined the genotypes of the L and H parental strains and of a total of 419 F2 recombinant males (Table 1). DNA from adult males was extracted using 5% Chelex 100 solution (Bio-Rad, Hercules, CA, USA). For genotyping, we chose 48 markers from the collection of microsatellite markers derived in silico from the Nasonia genome sequence (Release 1.0) by Pannebakker et al. (2010). We performed PCRs of microsatellite markers (Appendix 1) in a 10.0 μL volume (1× NH4 Buffer, 10 mm MgCl2, 1 mm dNTP mix, 4 mm of each primer, 0.5 units BIOTAQ DNA polymerase [Bioline Ltd, London, UK), 2 μL DNA (20 ng)]. The PCR temperature profile started with 4-min denaturation step at 94 °C followed by 39 cycles of 30 s at 94 °C, 30 s at the annealing temperature (Ta) specified in Appendix 1, and 1 min at 72 °C, followed by 10 min at 72 °C. We determined length differences of the PCR products on 4% agarose gels stained with EtBr.
We generated an intra-specific N. vitripennis linkage map using a population of 419 recombinant F2 males, scored for 48 microsatellite markers. We used MapDisto software (version 1.7; Lorieux, 2007) to determine linkage and evaluate the order of markers on each of the five linkage groups. Marker order was confirmed using the N. vitripennis genome sequence (Werren et al., 2010). We used Kosambi’s mapping function (Kosambi, 1944) to translate recombination fractions into map distances (cM). We visualized linkage groups using MapChart (version 2.1; Voorrips, 2002). The map generated in this study is an extension of the intra-specific N. vitripennis map presented in Beukeboom et al. (2010).
QTL analysis
We identified QTL using Haley–Knott regressions (Haley & Knott, 1992) in the r language (version 2.9.0, Ihaka & Gentleman, 1996). We performed multiple regressions using a general linear mixed-effect model implemented using the ‘lme’ function in the nlme package (Pinheiro & Bates, 2000). To obtain the minimal adequate model, we first fitted the maximal model, containing sibship background (H/L), Parental Female ID and their interaction as fixed effects and F2 recombinant genotype as a random effect. Model simplification allowed us to drop Parental Female ID and the interaction between sibship background and Parental Female ID as a fixed effects (likelihood-ratio test; sibship background : Parental Female ID, 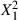 = 0.38, P = 0.54; Parental Female ID,
= 0.38, P = 0.54; Parental Female ID, 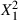 = 0.01, P = 0.91). Next, for each F2 male, we calculated the probabilities of the two alternative genotypes at every centiMorgan position along the chromosomes, conditional on the available marker data, using the R/QTL package (Broman et al., 2003). R/QTL uses a hidden Markov model to calculate the probabilities of the true underlying genotypes given the observed multipoint marker data, with allowance for genotyping errors. Next, we tested for an association between sex ratio and genotype at each centiMorgan location using multiple regression. Sex ratios were calculated as the proportion of offspring that are male, and we arcsine-square-root transformed the data to remove the dependency of the variance on the mean. We regressed the transformed data on the conditional probability of a F2 male carrying the high sex ratio line (H) allele. The strength of the association between genotype at a location and transformed sex ratio was described as an F-statistic. We tested the association between clutch size and genotype in a similar way, where clutch size was transformed using sqrt(clutch size + 0.5).
= 0.01, P = 0.91). Next, for each F2 male, we calculated the probabilities of the two alternative genotypes at every centiMorgan position along the chromosomes, conditional on the available marker data, using the R/QTL package (Broman et al., 2003). R/QTL uses a hidden Markov model to calculate the probabilities of the true underlying genotypes given the observed multipoint marker data, with allowance for genotyping errors. Next, we tested for an association between sex ratio and genotype at each centiMorgan location using multiple regression. Sex ratios were calculated as the proportion of offspring that are male, and we arcsine-square-root transformed the data to remove the dependency of the variance on the mean. We regressed the transformed data on the conditional probability of a F2 male carrying the high sex ratio line (H) allele. The strength of the association between genotype at a location and transformed sex ratio was described as an F-statistic. We tested the association between clutch size and genotype in a similar way, where clutch size was transformed using sqrt(clutch size + 0.5).
We estimated significance thresholds for the association between marker genotype and phenotype by permutation (Churchill & Doerge, 1994). The phenotype data were permuted over the F2 genotypes, and the maximum F-statistic across all locations was recorded. One thousand permutations were performed to generate a null distribution of the F-statistic and the following thresholds obtained: the genome-wide significance threshold (P = 0.05) and chromosome-wide significance threshold (P = 0.05) levels as suggested by Lander & Kruglyak (1995). We determined the confidence intervals on the QTL locations by bootstrapping, for which we repeated the analysis on 1000 different data sets generated by re-sampling the F2 genotypes and associated phenotypes with replacement. We determined the location of the maximum F-statistic in each round of bootstrapping and obtained empirical 95% confidence intervals from the resulting distribution (Visscher et al., 1996). We determined the percentage phenotypic variance explained as 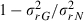 , where
, where 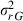 is the residual variance in the model with all genetic terms, and
is the residual variance in the model with all genetic terms, and 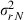 is the residual variance in a null model in which the genetic terms are omitted (Borevitz et al., 2002). The percentage genetic variance explained was determined by
is the residual variance in a null model in which the genetic terms are omitted (Borevitz et al., 2002). The percentage genetic variance explained was determined by 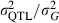 , where
, where 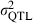 is the variance explained by the QTL
is the variance explained by the QTL 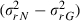 , and
, and 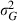 is the genetic variance in the F2 recombinant males. The effect of the QTL on sex ratio was determined by back-transforming the arsine-square-root transformed data obtained from the regression of each of the two genotypes for both backgrounds (H/L). Effects are expressed as (i) the difference between the two values, scaled to the mean sex ratio in that background (normalized QTL effect) and (ii) separated out as the genotypic additive (a) and dominance (d) effects. We did a similar calculation for the effect of QTLs for clutch size. We obtained the approximate confidence limits on QTL effects using the ‘intervals’ function of lme (Pinheiro & Bates, 2000) and back-transformed in a similar manner.
is the genetic variance in the F2 recombinant males. The effect of the QTL on sex ratio was determined by back-transforming the arsine-square-root transformed data obtained from the regression of each of the two genotypes for both backgrounds (H/L). Effects are expressed as (i) the difference between the two values, scaled to the mean sex ratio in that background (normalized QTL effect) and (ii) separated out as the genotypic additive (a) and dominance (d) effects. We did a similar calculation for the effect of QTLs for clutch size. We obtained the approximate confidence limits on QTL effects using the ‘intervals’ function of lme (Pinheiro & Bates, 2000) and back-transformed in a similar manner.
Evidence for pleiotropic QTLs for sex ratio and clutch size of QTLs was tested for by comparing the genome-wide test statistics for correlation (Keightley & Knott, 1999). Critical values were obtained by applying a Bonferroni correction for multiple comparisons with the equivalent number of independent tests determined from the QTL threshold F-statistics at P = 0.05.
Broad-sense heritability
We estimated the broad-sense heritability H2 of sex ratio and clutch size in our data using linear mixed-effect models on the transformed data. F2 recombinant males were fitted as a random effect and variance components were estimated by REML. Linear mixed-effect models were implemented using the ‘lme’ function in the nlme package (Pinheiro & Bates, 2000) in the r language (ver. 2.10.1; Ihaka & Gentleman, 1996).
Results
Descriptive statistics
We analysed the sex ratios associated with recombinant F2 male genotypes produced by a cross between the low sex ratio line C222a (L) and the high sex ratio line HV06 (H) when then back-crossed into the H or L background. The recombinant genotypes produced intermediate sex ratios (Table 2), when measured in either the L or the H backgrounds. The recombinant F2 males showed a broad-sense heritability of H2 = 9.5% for sex ratio (likelihood-ratio test; 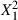 = 38.40, P < 0.0001), and H2 = 15.3% for clutch size (likelihood-ratio test;
= 38.40, P < 0.0001), and H2 = 15.3% for clutch size (likelihood-ratio test; 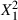 = 84.75, P < 0.0001). Our data showed a weak but significant positive correlation between sex ratio and brood size (binomial logistic regression: b = 0.005,
= 84.75, P < 0.0001). Our data showed a weak but significant positive correlation between sex ratio and brood size (binomial logistic regression: b = 0.005, 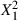 = 39.9, P < 0.0001).
= 39.9, P < 0.0001).
Table 2.
Descriptive statistics for the sex ratios (proportion male) and clutch sizes of Nasonia vitripennis lines C222A (L) and HV06 (H) From Pannebakker et al. (2008) and for the recombinant male genotypes from the cross C222A × HV06 when backcrossed into both the H and L backgrounds. Data are presented as means (standard error).
| Line/cross | Sex ratio | Clutch size |
|---|---|---|
| C222A (L) | 0.132 (0.008) | 35.91 (1.75) |
| HV06 (H) | 0.231 (0.027) | 35.27 (1.80) |
| Recombinants (L) | 0.151 (0.002) | 29.16 (0.26) |
| Recombinants (H) | 0.168 (0.003) | 28.25 (0.27) |
Intra-specific linkage map
Our linkage analysis resulted in a linkage map for N. vitripennis (Fig. 2) with five linkage groups (representing the five N. vitripennis chromosomes), spanning a total of 477.5 cM, and with an average marker distance of 11.11 cM.
Fig. 2.
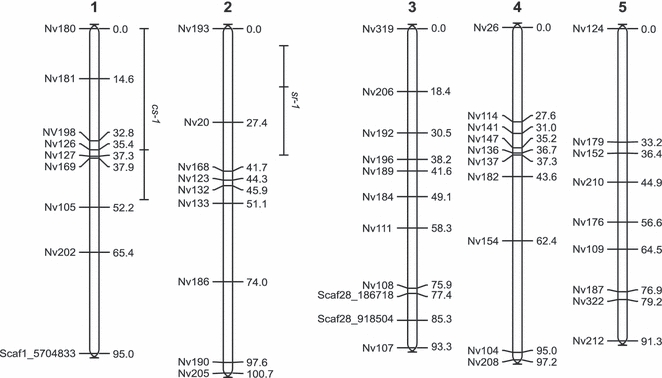
Intra-specific linkage map for Nasonia vitripennis. Map distances are calculated using Kosambi’s mapping function, with a total map length of 477.5 cM. The map is based on 419 recombinant F2 males genotyped for 48 microsatellite markers. Genome-wide significant quantitative trait locus (QTLs) for sex ratio (sr-1) and clutch size (cs-1) are indicated; the error bars represent 95% confidence interval for the QTL determined by bootstrapping.
QTL for sex ratio and clutch size
There was one QTL for sex ratio significant at the genome-wide level (P<0.05) located on linkage group 2 at 17.0 cM (F1,297 = 12.12; Fig. 3). This QTL was associated with a change in sex ratio of 15% in the high background and 6% in the low background (Table 3). The low allele showed partial dominance over the high allele (degree of dominance d/a = −0.5). As expected from the low heritability of sex ratio (H2 = 9.5%), the QTL explained only 0.16% of the phenotypic variance, and 1.56% of the genetic variance in our cross-population. In addition, three further suggestive sex ratio QTL (significant at the chromosome-wide level, P<0.05) were identified on linkage group 3 (at 53.0 cM), and on linkage group 5 (at 39.0 cM and at 79.2 cM; Table 4). The QTL on chromosome 3 is particularly close to genome-wide significance (Fig. 3).
Fig. 3.
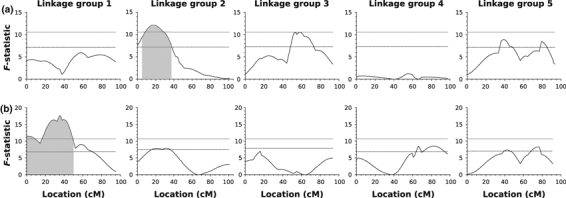
Locations of (a) sex ratio and (b) clutch size quantitative trait locus (QTL) in Nasonia vitripennis. F-statistic obtained by regressing genotype against sex ratio or clutch size. The grey, solid line is the 5% genome-wide significance threshold from permutation tests, and the black, dashed line is the 5% chromosome-wide significance threshold. The shaded region is the 95% confidence interval for the two QTL significant at the genome-wide level (obtained by bootstrapping; see main text for details).
Table 3.
Summary of genome-wide significant QTL identified for sex ratio and brood size in Nasonia vitripennis. CIs are confidence intervals.
| Variance explained (%) | Background | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Chr* | Location† (95% CI) | Phenotypic | Genetic | QTL effect‡ (95% CI) | Normalized QTL effect§ | μ¶ | a** | d†† | |
| Sex ratio | 2 | 17.0 (5.0–37.0) | 0.16 | 1.56 | High | 0.0251 (0.0249 to 0.0253) | +14.9% | 0.168 | 0.016 | −0.008 |
| Low | 0.0083 (0.0078 to 0.0089) | +5.5% | 0.136 | |||||||
| Brood size | 1 | 35.4 (0.0–50.0) | 0.13 | 0.76 | High | −2.27 (−3.64 to −0.90) | −8.0% | 25.92 | −1.588 | −0.798 |
| Low | −2.39 (−3.74 to −1.02) | −8.2% | 29.09 | |||||||
QTL, quantitative trait locus.
Chromosome.
Location in centiMorgans.
Estimated effect size of the QTL, calculated as the difference in sex ratio (or brood size) between sibships inheriting the high vs. the low allele from the recombinant F2 male.
Change relative to the mean in the high and low genetic backgrounds.
Estimated genotypic mean in the high and low backgrounds.
Estimated additive effect of the QTL in the sibships. Positive values indicate that the H allele (from the high sex ratio line) confers greater sex ratio/brood size.
Estimated dominance effect of the QTL in the sibships.
Table 4.
Summary of QTL identified for sex ratio and clutch size in Nasonia vitripennis. The most likely genomic locations are given as position along the chromosome. Significant QTLs at the 5% genome-wide level are given in bold type, and significant QTLs at the 5% chromosome-wide level are given in italic. Locations were determined in individual single-QTL models, whilst effect sizes were determined by simultaneously fitting all chromosome-wide significant QTL in one multiple-QTL model.
| Effect‡ | Normalized QTL effect§ | |||||
|---|---|---|---|---|---|---|
| Trait | Chr* | Location† | High background | Low background | High background (%) | Low background (%) |
| Sex ratio | 2 | 17.0 | 0.0147 | 0.0141 | +8.8 | +9.4 |
| 3 | 53.0 | 0.0133 | 0.0127 | +7.9 | +8.4 | |
| 5 | 39.0 | −0.0089 | −0.0086 | −5.3 | −5.7 | |
| 5 | 79.2 | −0.0059 | −0.0056 | −3.5 | −3.7 | |
| Clutch size | 1 | 35.4 | −2.16 | −2.19 | −7.6 | −7.5 |
| 2 | 21.0 | 1.77 | 1.80 | +6.2 | +6.2 | |
| 2 | 32.0 | 0.06 | 0.06 | +0.2 | +0.2 | |
| 4 | 66.2 | 0.83 | 0.84 | +2.9 | +2.9 | |
| 4 | 83.0 | 1.30 | 1.32 | +4.6 | +4.5 | |
| 5 | 43.0 | −1.05 | −1.07 | −3.7 | −3.7 | |
| 5 | 76.0 | −0.53 | −0.54 | −1.9 | −1.9 | |
QTL, quantitative trait locus.
Chromosome.
Location in centiMorgans.
Estimated effect size of the QTL, calculated as the difference in sex ratio (or brood size) between sibships inheriting the high vs. the low allele from the recombinant F2 male.
Change relative to the mean in the high and low genetic backgrounds.
There was one QTL for clutch size significant at the genome-wide level (P<0.05). This QTL was located on linkage group 1 at 35.4 cM (at marker Nv126: F1,297 = 17.65; Fig. 3) and was associated with a reduction in clutch size of 8% in both high and low backgrounds (Table 3). The high allele showed partial dominance over the low allele (d/a = 0.5). This QTL explained 0.13% of the phenotypic variance in clutch size, and 0.76% of the genetic variance. At the weaker chromosome-wide significance level, six further suggestive QTL were identified (Table 4), located on linkage group 2 at 21.0 and 32.0 cM, on linkage group 4 at 66.2 and 83.0 cM and on linkage group 5 at 43.0 and 76.0 cM. When considered together to test for pleiotropy, the genome-wide test statistics for sex ratio and clutch size did not reveal a significant correlation (r = 0.06, Pearson’s product–moment correlation, t522 = 1.44, P = 0.15).
Because additional QTL could only be found that were significant on a chromosome-wide level, we refrained from a formal analysis of epistasis between the different loci. However, a preliminary exploration of possible interactions between the loci did not reveal significant interactions between the genome-wide QTL and the suggestive QTL (data not shown). A more complete analysis of the genetic architecture of sex ratio in Nasonia will have to await the identification of further loci.
Discussion
Here, we have described the first QTL for sex ratio variation in any organism. Differences in single foundress sex ratio between two wild-caught strains from the same population of N. vitripennis are associated with a region near the end of chromosome 2. Additional, weaker QTLs map to chromosomes 3 and 5. Intra-specific genetic variation in sex ratio in N. vitripennis is limited (Pannebakker et al., 2008), and we required a large sample size to reveal these putative QTL (299 recombinants assayed in two backgrounds, using more than 3700 test females). However, we have been able to identify with confidence one QTL segregating in a natural population of wasps, with reasonable confidence in another. In addition, we have identified a genome-wide QTL influencing clutch size, as well as a number of lesser putative QTL. We have less idea of the underlying heritability of clutch size, and the two strains used were not chosen to maximize differences in clutch size, but our experiment was still able to resolve an intra-population QTL for this important life-history trait.
The QTL identified at the 5% genome-wide level span a large part of the genome, i.e. 32 cM for sex ratio variation corresponding to ∼21.3 Mb, and 50 cM for clutch size variation corresponding to ∼33.3 Mb assuming a genome-wide recombination rate of 1.5 cM Mb−1 (Niehuis et al., 2010). These figures correspond to approximately 1257 candidate genes for sex ratio and 1965 for clutch size, assuming ∼59 genes per Mb (Niehuis et al., 2010). Our results provide the starting point in the search for possible candidate loci for sex ratio and brood size, but given the size of these genomic regions and the number of possible candidates, future studies will require a much higher resolution before we can start to screen the genome for candidate loci. Alternatively, a combined approach taking advantage of studies of the genes expressed during oviposition and sex allocation decision-making may help generate candidate genes in the QTL regions of interest; we are currently attempting this.
Our weaker sex ratio QTL on chromosome 3 is close to genome-wide significance, but the QTL suggested for chromosome 5 are only convincingly significant at the chromosome-wide threshold level. However, recent work exploring inter-specific differences in sex ratio among the Nasonia genus has identified a region on chromosome 5 that influences sex ratio when introgressed from Nasonia giraulti into N. vitripennis (Werren et al., 2010). This region includes the well-known red eye mutant locus STDR. Whilst neither of our QTL is centred on this region, our second QTL (centred on 79 cM) is close. This raises the possibility that genes associated with differences in sex ratio between Nasonia species are also segregating within populations, unlike the case for courtship song differences within and between Drosophila species (Gleason & Ritchie, 2004; see also Arbuthnott, 2009). The differences between the Nasonia species in single foundress sex ratios are thought to be arising from differences in the extent of so-called within-host mating (mating that occurs before males and females emerge from the host puparium: Drapeau & Werren, 1999). Within-host mating leads to very extreme LMC if all the brood are relatives, and N. giraulti displays the greatest extent of within-host mating, and N. vitripennis the least (see also Leonard & Boake, 2006). As such, it would also be useful to identify genome regions associated with differences between the species in within-host mating, and see whether they co-occurred with putative sex ratio QTL.
Even though we found one single genome-wide significant QTL for sex ratio, it only explains a small part of the phenotypic (0.16%) and genetic variance (1.56%). This suggests that many other genes are segregating for sex ratio. In line with this, we found several minor QTL segregating for sex ratio, and our estimate of QTL number is likely to be an underestimate (Beavis, 1994). A genetic architecture of multiple loci underlying sex ratio is compatible with recent theoretical predictions from mutation accumulation experiments. Based on estimates of mutational parameters and on the natural variation for sex ratio, Pannebakker et al. (2008) predicted that the genetic variation for sex ratio in Nasonia is maintained by selection on pleiotropic loci with effects on other fitness-related traits. Pleiotropy as the primary cause of genetic variation in sex ratio is more plausible when more loci are involved (Barton & Turelli, 1989).
Our data allow a weak test of the prediction of Pannebakker et al. (2008). Here, we of course have only considered one other fitness-related trait: clutch size. The genome-wide QTL for clutch size and sex ratio were clearly independent of each other, occurring on different chromosomes. There was also no hint of a QTL for sex ratio on chromosome 1 that corresponded to the clutch size QTL (Fig. 3). When tested for concordance between the test statistics of sex ratio and clutch size as an indication for the existence of pleiotropic QTL, we could not detect a significant correlation. There was, however, a weakly suggestive QTL for clutch size on chromosome 2 that overlapped with our major sex ratio region. In addition, the weak QTL for sex ratio and clutch size located on chromosome 5 potentially overlap. That said these particular QTL are at best preliminary and of course cover very many possible candidate loci; as such the conclusion we draw at the moment is that loci pleiotropic for clutch size and sex ratio remain possible. A more detailed multitrait QTL analysis should be carried out before pleiotropy can be formally excluded. Of course, analysis of different fitness-related traits may yet throw up other associations with sex ratio QTL, and various aspects of Nasonia behaviour and life history are currently the focus of genetic study (Werren et al., 2010).
The genetic basis of sex ratio is of prime importance if we want to understand sex ratio evolution. The number of loci and alleles underlying the trait will influence its dynamics in populations (Basolo, 1994). Even though the present study offers a first insight, we are currently only at the start of uncovering the genetic basis of sex ratio in Nasonia. Our limited knowledge of the genetic basis of sex ratio contrasts with our increasingly sophisticated knowledge of how sex is determined, both in Nasonia (Verhulst et al., 2010; Werren et al., 2010) and across other taxa more generally (Bull, 1983). We now have a very good idea of key aspects in the sex determination cascade, and which components tend to vary across species and which tend to be conserved (Sanchez, 2008). The extent to which behavioural sex allocation mechanisms interact with the underlying machinery is unknown and they may well be totally separate. However, the increasing awareness that there may be genomic conflicts over sex allocation has meant that a renewed focus has been placed on how and when sex is determined, and what genetic entities (such as males and females) may be trying to influence it (e.g. Shuker et al., 2007, 2009; Uller et al., 2007; Wild & West, 2009). As such, sex ratio control and sex determination may be a joint evolutionary battleground worth considering together (Werren & Beukeboom, 1998).
The success of phenotypic models of sex allocation could be taken to suggest that genetic constraints are unimportant in the evolution of adaptive sex ratios, but that same success has perhaps kept focus away from the genetics underlying sex ratio. Perhaps just as important has been the apparent contradiction between chromosomal sex determination and adaptive sex allocation, especially in vertebrates (e.g. what is the mechanism?), or the low heritability often associated with sex ratio (as seen in Nasonia). However, by identifying QTL in a trait that typically has low heritability within populations, we can now begin to isolate regions of interest for further study. Combined with insights from inter-specific differences between the four different Nasonia species in sex ratio, and potentially any other traits genetically associated with sex ratio (Werren et al., 2010), we can also begin to explore the genetic architecture of sex ratio. Whilst we may perhaps not expect to find genetic constraints per se to sex ratio adaptation in Nasonia (female Nasonia are rather good at solving sex ratio problems after all), we can turn the question around and ask what the genetic architecture of a well-adapted phenotypic trait actually looks like. We may then begin to consider how natural selection shapes the genome from the phenotype inwards, rather than from the genotype outwards. Given the scepticism about the role of natural selection in shaping aspects of the genome more generally (e.g. Lynch, 2007), it may prove useful to let well-understood phenotypes guide us to the evolutionary processes occurring at the molecular level, in addition to considering sequence evolution itself.
Acknowledgments
We are extremely grateful to Max Burton, Aleta Graham, Sven Lumsden, Anna Moynihan, Claire Smillie and Steve Spencer for all their help with setting up and processing the sex ratio and clutch size assays. We are also grateful to Frank Jiggins for his help with the QTL analysis, Oliver Niehuis for sharing unpublished marker information, and the Groningen EvolGen discussion group for useful comments on the manuscript. Finally, we also thank the Natural Environment Research Council, the Royal Society, Research Councils UK, and the Netherlands Organization for Scientific Research (NWO) for funding.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Appendix S1 Microsatellite markers.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be recognized for online delivey, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Arbuthnott D. The genetic architecture of insect courtship behavior and premating isolation. Heredity. 2009;103:15–22. doi: 10.1038/hdy.2009.22. [DOI] [PubMed] [Google Scholar]
- Barton NH, Partridge L. Limits to natural selection. Bioessays. 2000;22:1075–1084. doi: 10.1002/1521-1878(200012)22:12<1075::AID-BIES5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Barton NH, Turelli M. Evolutionary quantitative genetics: how little do we know? Annu. Rev. Genet. 1989;23:337–370. doi: 10.1146/annurev.ge.23.120189.002005. [DOI] [PubMed] [Google Scholar]
- Basolo AL. The dynamics of Fisherian sex ratio evolution: theoretical and experimental investigations. Am. Nat. 1994;144:473–490. [Google Scholar]
- Beavis WD. Proceedings of the Forty-Ninth Annual Corn & Sorghum Industry Research Conference. Washington, DC: American Seed Trade Association; 1994. The power and deceit of QTL experiments: lessons from comparative QTL studies; pp. 250–256. [Google Scholar]
- Beukeboom LW, Niehuis O, Pannebakker BA, Koevoets T, Gibson JD, Shuker DM, et al. A comparison of recombination frequencies in intraspecific versus interspecific mapping populations of Nasonia. Heredity. 2010;104:302–309. doi: 10.1038/hdy.2009.185. [DOI] [PubMed] [Google Scholar]
- Blows M, Hoffmann A. A reassessment of genetic limits to evolutionary change. Ecology. 2005;86:1371–1384. [Google Scholar]
- Borevitz J, Maloof J, Lutes J, Dabi T, Redfern J, Trainer G, et al. Quantitative trait loci controlling light and hormone response in two accessions of Arabidopsis thaliana. Genetics. 2002;160:683–696. doi: 10.1093/genetics/160.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- Bull JJ. Evolution of Sex Determining Mechanisms. Menlo Park, CA: Benjamin Cummings Publishing Company; 1983. [Google Scholar]
- Burton-Chellew MN, Koevoets T, Grillenberger B, Sykes E, Underwood S, Bijlsma K, et al. Facultative sex ratio adjustment in natural populations of wasps: cues of local mate competition and the precision of adaptation. Am. Nat. 2008;172:393–404. doi: 10.1086/589895. [DOI] [PubMed] [Google Scholar]
- Carvalho AB, Sampaio MC, Varandas FR, Klaczko LB. An experimental demonstration of Fisher’s principle: evolution of sexual proportion by natural selection. Genetics. 1998;148:719–731. doi: 10.1093/genetics/148.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov E. The Theory of Sex Allocation. Princeton, NJ: Princeton University Press; 1982. [PubMed] [Google Scholar]
- Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau MD, Werren JH. Differences in mating behaviour and sex ratio between three sibling species of Nasonia. Evol. Ecol. Res. 1999;1:223–234. [Google Scholar]
- Edwards A. Carl Düsing (1884) on the regulation of the sex-ratio. Theor. Popul. Biol. 2000;58:255–257. doi: 10.1006/tpbi.2000.1482. [DOI] [PubMed] [Google Scholar]
- Fisher R. The Genetical Theory of Natural Selection. Oxford, UK: Clarendon; 1930. [Google Scholar]
- Flanagan KE, West SA, Godfray HC. Local mate competition, variable fecundity and information use in a parasitoid. Anim. Behav. 1998;56:191–198. doi: 10.1006/anbe.1998.0768. [DOI] [PubMed] [Google Scholar]
- Frank SA. Hierarchical selection theory and sex ratios. I. General solutions for structured populations. Theor. Popul. Biol. 1986;29:312–342. doi: 10.1016/0040-5809(86)90013-4. [DOI] [PubMed] [Google Scholar]
- Frank SA. Foundations of Social Evolution. Princeton, NJ: Princeton University Press; 1998. [Google Scholar]
- Gellatly C. Trends in population sex ratios may be explained by changes in the frequencies of polymorphic alleles of a sex ratio gene. Evol. Biol. 2009;36:190–200. [Google Scholar]
- Gleason JM, Ritchie MG. Do quantitative trait loci (QTL) for a courtship song difference between Drosophila simulans and D. sechellia coincide with candidate genes and intraspecific QTL? Genetics. 2004;166:1303–1311. doi: 10.1534/genetics.166.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfray HC. Models for clutch size and sex-ratio with sibling interaction. Theor. Popul. Biol. 1986;30:215–231. [Google Scholar]
- Godfray HC. Parasitoids: Behavioral and Evolutionary Ecology. Princeton, NJ: Princeton University Press; 1994. [Google Scholar]
- Greeff JM. Offspring allocation in externally ovipositing fig wasps with varying clutch size and sex ratio. Behav. Ecol. 1997;8:500–505. [Google Scholar]
- Grillenberger BK, Koevoets T, Burton-Chellew MN, Sykes EM, Shuker DM, de Zande L, et al. Genetic structure of natural Nasonia vitripennis populations: validating assumptions of sex-ratio theory. Mol. Ecol. 2008;17:2854–2864. doi: 10.1111/j.1365-294X.2008.03800.x. [DOI] [PubMed] [Google Scholar]
- Haley C, Knott SA. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity. 1992;69:315–324. doi: 10.1038/hdy.1992.131. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. Extraordinary sex ratios. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- Hardy IC. Sex Ratios: Concepts and Research Methods. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- Herre E. Sex ratio adjustment in fig wasps. Science. 1985;228:896–898. doi: 10.1126/science.228.4701.896. [DOI] [PubMed] [Google Scholar]
- Herre EA. Optimality, plasticity and selective regime in fig wasp sex ratios. Nature. 1987;329:627–629. [Google Scholar]
- Ihaka R, Gentleman R. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 1996;5:299–314. [Google Scholar]
- Janzen FJ. Heritable variation for sex ratio under environmental sex determination in the common snapping turtle (Chelydra serpentina) Genetics. 1992;131:155–161. doi: 10.1093/genetics/131.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley PD, Knott SA. Testing the correspondence between map positions of quantitative trait loci. Genet. Res. Camb. 1999;74:323–328. doi: 10.1017/s0016672399004176. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Tanaka Y, Shimada M. Genetic variation of sex allocation in the parasitoid wasp Heterospilus prosopidis. Evolution. 2003;57:2659–2664. doi: 10.1111/j.0014-3820.2003.tb01510.x. [DOI] [PubMed] [Google Scholar]
- Kosambi D. The estimation of map distances from recombination values. Ann Eugen. 1944;12:172–175. [Google Scholar]
- Krackow S. Why parental sex ratio manipulation is rare in higher vertebrates. Ethology. 2002;108:1041–1056. [Google Scholar]
- Lander ES, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat. Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Leonard JE, Boake CR. Site-dependent aggression and mating behaviour in three species of Nasonia (Hymenoptera : Pteromalidae) Anim. Behav. 2006;71:641–647. [Google Scholar]
- Lorieux M. San Diego, CA, USA: Plant and animal genome XV conference; 2007. MapDisto: a free user-friendly program for computing genetic maps. Computer demonstration (P958) See http://mapdisto.free.fr/ [Google Scholar]
- Lynch M. The Origins of Genome Architecture. Sunderland, MA: Sinauer Associates; 2007. [Google Scholar]
- Molbo D, Parker ED. Mating structure and sex ratio variation in a natural population of Nasonia vitripennis. Proc. R. Soc. Lond. B Biol. Sci. 1996;263:1703–1709. [Google Scholar]
- Nagelkerke CJ. Simultaneous optimization of egg distribution and sex allocation in a patch-structured population. Am. Nat. 1994;144:262–284. [Google Scholar]
- Niehuis O, Gibson J, Rosenberg M, Pannebakker BA, Koevoets T, Judson A, et al. Recombination and its impact on the genome of the haplodiploid parasitoid wasp Nasonia. PLoS ONE. 2010;5:e8597. doi: 10.1371/journal.pone.0008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzack SH. Using sex ratios: the past and the future. In: Hardy IC, editor. Sex Ratios: Concepts and Research Methods. Cambridge, UK: Cambridge University Press; 2002. pp. 383–398. [Google Scholar]
- Orzack SH, Gladstone J. Quantitative genetics of sex ratio traits in the parasitic wasp, Nasonia vitripennis. Genetics. 1994;137:211–220. doi: 10.1093/genetics/137.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzack SH, Parker ED. Sex-ratio control in a parasitic wasp, Nasonia vitripennis. I. Genetic variation in facultative sex-ratio adjustment. Evolution. 1986;40:331–340. doi: 10.1111/j.1558-5646.1986.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Orzack SH, Parker ED. Genetic variation for sex ratio traits within a natural population of a parasitic wasp, Nasonia vitripennis. Genetics. 1990;124:373–384. doi: 10.1093/genetics/124.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzack SH, Parker ED, Gladstone J. The comparative biology of genetic variation for conditional sex ratio behavior in a parasitic wasp, Nasonia vitripennis. Genetics. 1991;127:583–599. doi: 10.1093/genetics/127.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannebakker BA, Halligan D, Reynolds K, Shuker DM, Ballantyne G, Barton NH, et al. Effects of spontaneous mutation accumulation on sex ratio traits in a parasitoid wasp. Evolution. 2008;62:1921–1935. doi: 10.1111/j.1558-5646.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- Pannebakker BA, Niehuis O, Hedley A, Gadau J, Shuker DM. The distribution of microsatellites in the Nasonia parasitoid wasp genome. Insect Mol. Biol. 2010;19(Suppl. 1):91–98. doi: 10.1111/j.1365-2583.2009.00915.x. [DOI] [PubMed] [Google Scholar]
- Parker ED, Orzack SH. Genetic variation for the sex ratio in Nasonia vitripennis. Genetics. 1985;110:93–105. doi: 10.1093/genetics/110.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike TW, Petrie M. Potential mechanisms of avian sex manipulation. Biol. Rev. 2003;78:553–574. doi: 10.1017/s1464793103006146. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D. Mixed-Effects Models in S and S-PLUS. Berlin: Springer Verlag; 2000. [Google Scholar]
- Sanchez L. Sex-determining mechanisms in insects. Int. J. Dev. Biol. 2008;52:837–856. doi: 10.1387/ijdb.072396ls. [DOI] [PubMed] [Google Scholar]
- Shuker DM, West SA. Information constraints and the precision of adaptation: sex ratio manipulation in wasps. Proc. Natl. Acad. Sci. USA. 2004;101:10363–10367. doi: 10.1073/pnas.0308034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuker DM, Pen I, Duncan A, Reece S, West SA. Sex ratios under asymmetrical local mate competition: theory and a test with parasitoid wasps. Am. Nat. 2005;166:301–316. doi: 10.1086/432562. [DOI] [PubMed] [Google Scholar]
- Shuker DM, Sykes E, Browning L, Beukeboom LW, West SA. Male influence on sex allocation in the parasitoid wasp Nasonia vitripennis. Behav. Ecol. Sociobiol. 2006;59:829–835. [Google Scholar]
- Shuker DM, Reece S, Lee A, Graham A, Duncan A, West SA. Information use in space and time: sex allocation behaviour in the parasitoid wasp Nasonia vitripennis. Anim. Behav. 2007;73:971–977. doi: 10.1016/j.anbehav.2006.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuker DM, Moynihan AM, Ross L. Sexual conflict, sex allocation and the genetic system. Biol. Lett. 2009;5:682–685. doi: 10.1098/rsbl.2009.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield JW, Seger J. Local mate competition with variable fecundity: dependence of offspring sex ratios on information utilization and mode of male production. Behav. Ecol. 1990;1:68–80. [Google Scholar]
- Taylor P, Bulmer M. Local mate competition and the sex ratio. J. Theor. Biol. 1980;86:409–419. doi: 10.1016/0022-5193(80)90342-2. [DOI] [PubMed] [Google Scholar]
- Toro MA, Charlesworth B. An attempt to detect genetic variation in sex ratio in Drosophila melanogaster. Heredity. 1982;49:199–209. [Google Scholar]
- Toro MA, Fernandez A, Garcia-Cortes LA, Rodriganez J, Silio L. Sex ratio variation in Iberian pigs. Genetics. 2006;173:911–917. doi: 10.1534/genetics.106.055939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R, Willard D. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Turelli M. Heritable genetic variation via mutation-selection balance: Lerch’s zeta meets the abdominal bristle. Theor. Popul. Biol. 1984;25:138–193. doi: 10.1016/0040-5809(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Uller T, Badyaev AV. Evolution of “determinants” in sex-determination: a novel hypothesis for the origin of environmental contingencies in avian sex-bias. Semin. Cell Dev. Biol. 2009;20:304–312. doi: 10.1016/j.semcdb.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Uller T, Pen I, Wapstra E, Beukeboom LW, Komdeur J. The evolution of sex ratios and sex-determining systems. Trends Ecol. Evol. 2007;22:292–297. doi: 10.1016/j.tree.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Vandeputte M, Dupont-Nivet M, Merdy O, Haffray P, Chavanne H, Chatain B. Quantitative genetic determinism of sex-ratio in the European sea bass (Dicentrarchus labrax L.) Aquaculture. 2007;272:S315–S321. [Google Scholar]
- Velthuis B, Yang W, Werren JH, van Opijnen T. Genetics of female mate discrimination of heterospecific males in Nasonia (Hymenoptera, Pteromalidae) Anim. Behav. 2005;69:1107–1120. [Google Scholar]
- Verhulst EC, Beukeboom LW, van de Zande L. Maternal control of haplodiploid sex determination in the wasp Nasonia. Science. 2010;328:620–623. doi: 10.1126/science.1185805. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Thompson R, Haley C. Confidence intervals in QTL mapping by bootstrapping. Genetics. 1996;143:1013–1020. doi: 10.1093/genetics/143.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorrips R. MapChart: software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002;93:77–78. doi: 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]
- Wajnberg E. Intra-population genetic variation in Trichogramma. In: Wajnberg E, Hassan SA, editors. Biological Control with Egg Parasitoids. Wallingford, UK: CAB International; 1994. pp. 245–271. [Google Scholar]
- Werren JH. Sex ratio adaptations to local mate competition in a parasitic wasp. Science. 1980;208:1157–1159. doi: 10.1126/science.208.4448.1157. [DOI] [PubMed] [Google Scholar]
- Werren JH. Sex ratio evolution under local mate competition in a parasitic wasp. Evolution. 1983;37:116–124. doi: 10.1111/j.1558-5646.1983.tb05520.x. [DOI] [PubMed] [Google Scholar]
- Werren JH, Beukeboom LW. Sex determination, sex ratios, and genetic conflict. Annu. Rev. Ecol. Syst. 1998;29:233–261. [Google Scholar]
- Werren JH, Richards S, Desjardins CA, Niehuis O, Gadau J, Colbourne JK, et al. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science. 2010;327:343–348. doi: 10.1126/science.1178028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SA. Sex Allocation. Princeton, NJ: Princeton University Press; 2009. [Google Scholar]
- West SA, Herre EA. Stabilizing selection and variance in fig wasp sex ratios. Evolution. 1998;52:475–485. doi: 10.1111/j.1558-5646.1998.tb01647.x. [DOI] [PubMed] [Google Scholar]
- West SA, Sheldon B. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]
- West SA, Herre EA, Compton SG, Godfray HC, Cook JM. A comparative study of virginity in fig wasps. Anim. Behav. 1997;54:437–450. doi: 10.1006/anbe.1996.0494. [DOI] [PubMed] [Google Scholar]
- West SA, Shuker DM, Sheldon B. Sex-ratio adjustment when relatives interact: a test of constraints on adaptation. Evolution. 2005;59:1211–1228. [PubMed] [Google Scholar]
- Whiting A. The biology of the parasitic wasp Mormoniella vitripennis (Walker) Q. Rev. Biol. 1967;42:333–406. [Google Scholar]
- Wild G, West SA. Genomic imprinting and sex allocation. Am. Nat. 2009;173:E1–E14. doi: 10.1086/593305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


