Abstract
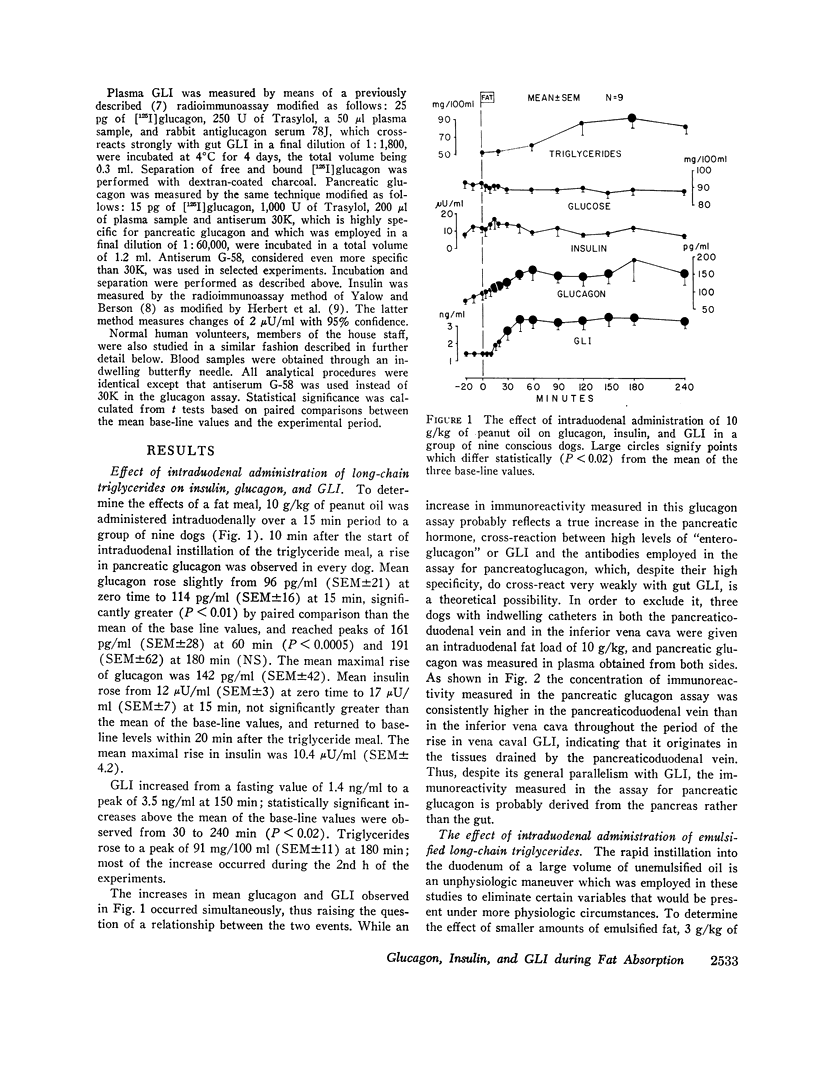
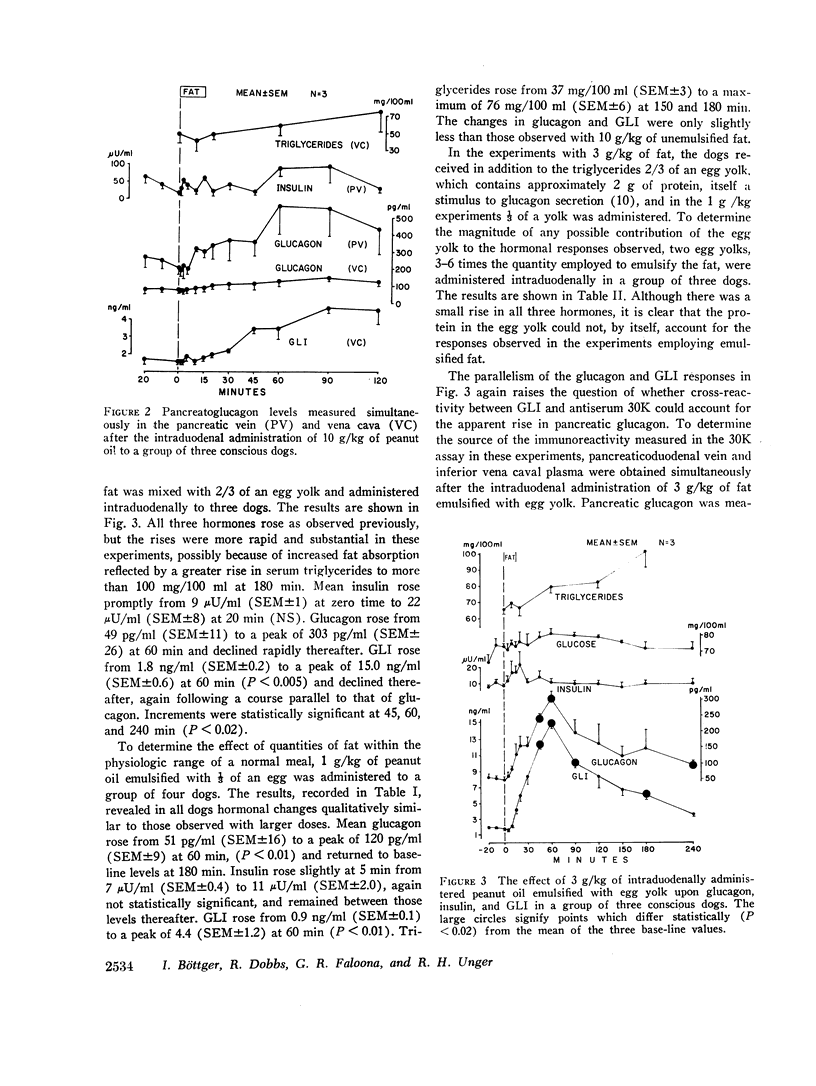
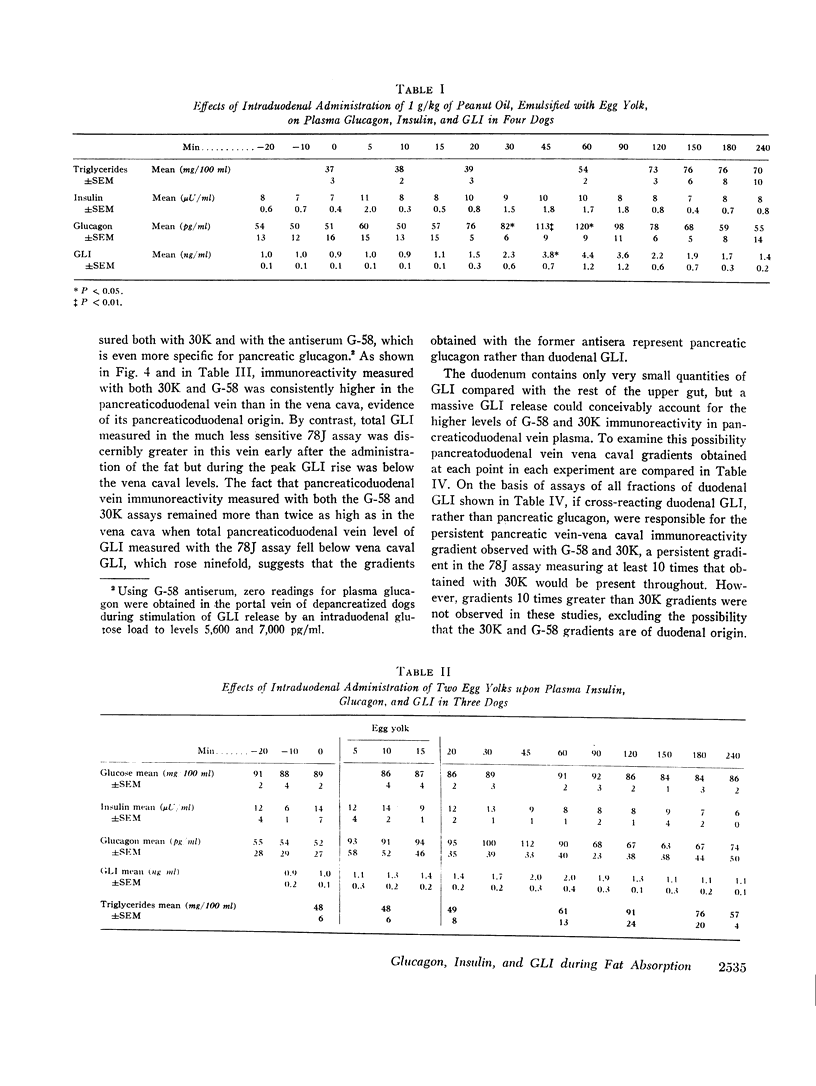
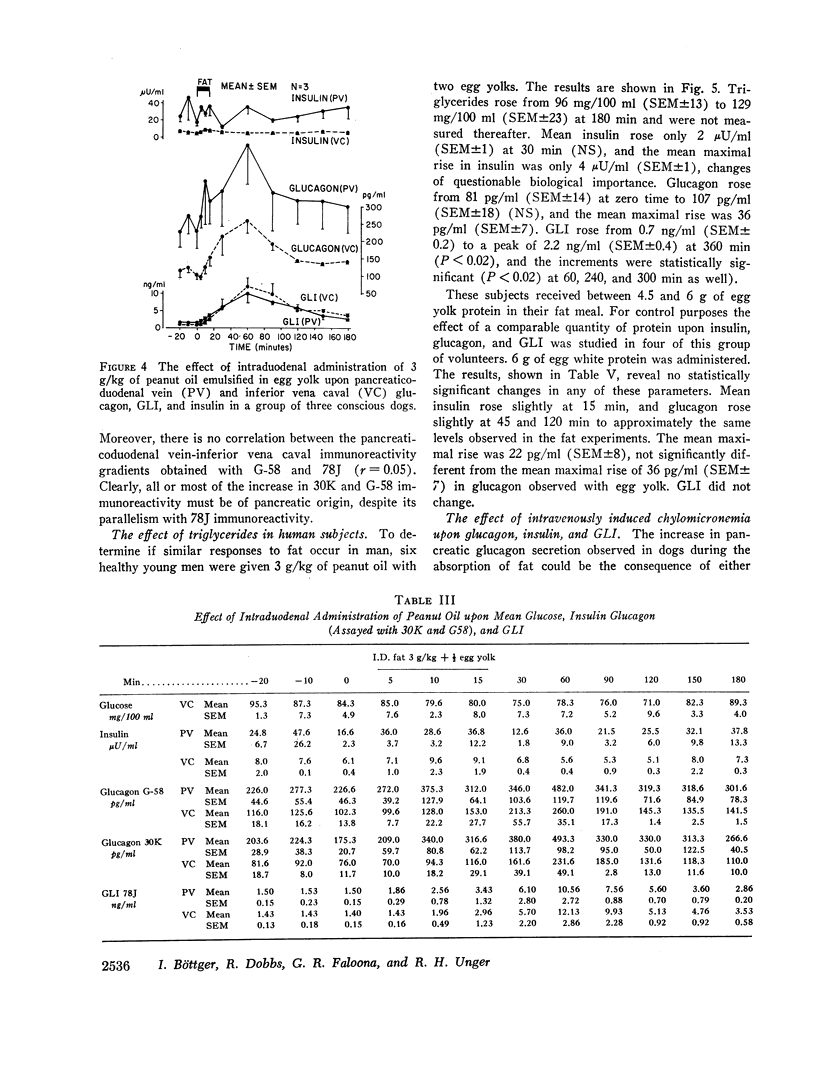
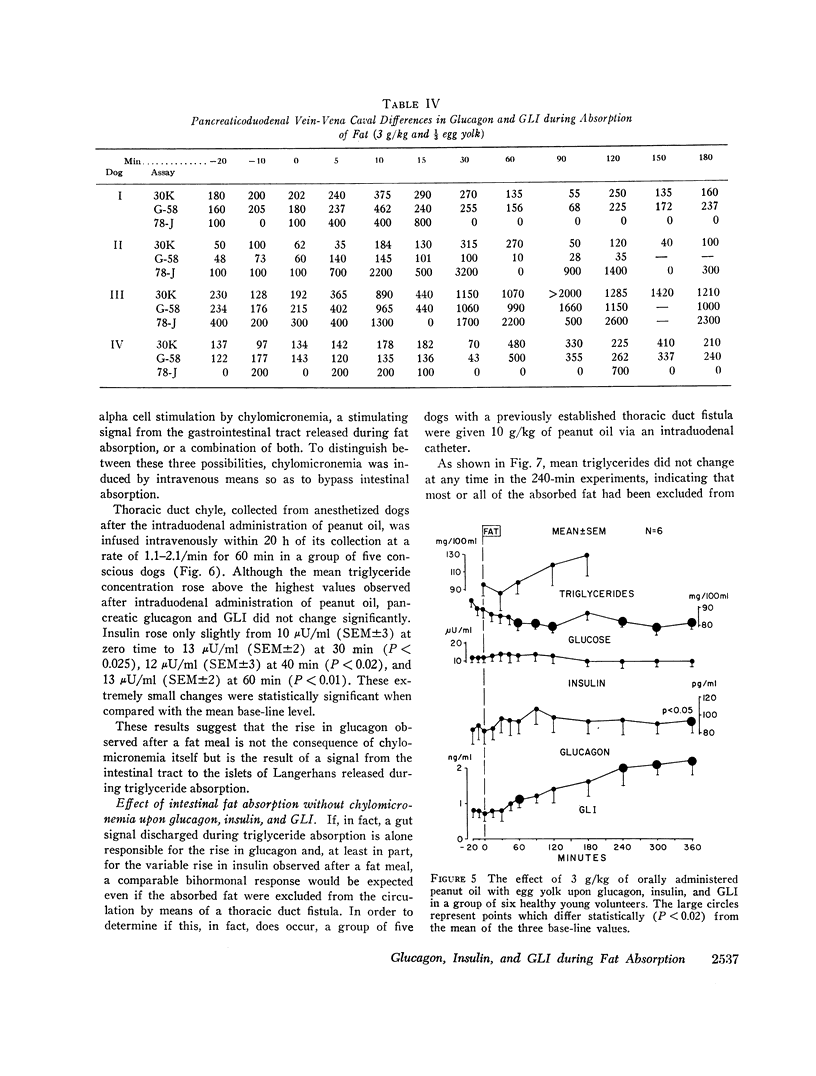
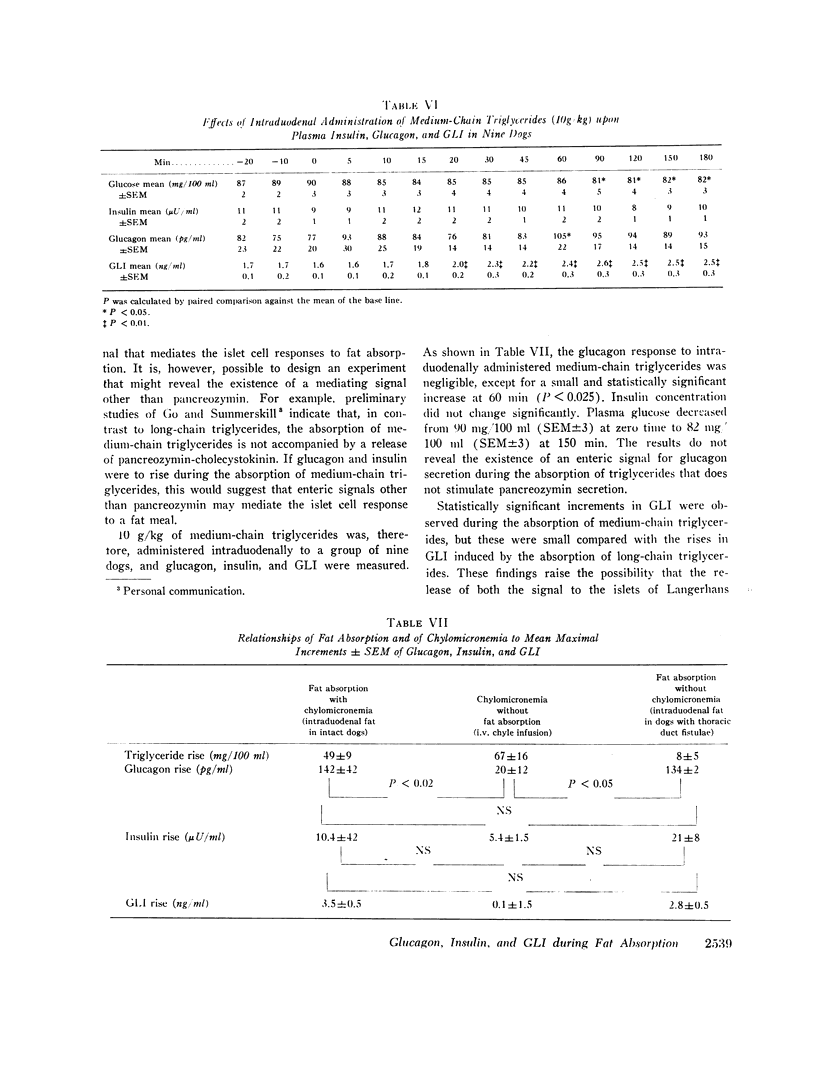
The effects of a fat meal upon plasma insulin, glucagon, and glucagon-like immunoreactivity (GLI) have been studied in conscious dogs and in human volunteers. In dogs the intraduodenal instillation of 10 g/kg of peanut oil was accompanied by increases in the mean plasma levels of all three polypeptides that averaged 5 μU/ml, 107 pg/ml, and 2.1 ng/ml, respectively. 3 g/kg of peanut oil, when emulsified with egg yolk, elicited a much greater response of the three hormones, and a physiologic dose of 1 g/kg in emulsified form also caused a significant rise in glucagon and GLI. The islet cell hormone response was not ascribable to chylomicronemia since intravenous infusion of canine chyle failed to stimulate glucagon secretion; moreover, in dogs with a thoracic duct fistula in which chyle was excluded from the circulation, the intraduodenal administration of a fat meal elicited the normal islet cell hormone response, as well as a rise in GLI. 10 g/kg of medium-chain triglycerides failed to elicit these same responses. In six human volunteers the oral administration of 3 g/kg peanut oil was accompanied by increments of 2 μU/ml, 26 pg/ml, and 1.5 ng/ml in the mean levels of insulin, glucagon, and GLI. The changes in insulin and glucagon in man were neither statistically significant nor biologically impressive.
It is concluded that in dogs fat absorption is accompanied by prompt and substantial increases in plasma glucagon and GLI and a small transient rise in insulin. The evidence favors an enterogenic signal to the islets of Langerhans rather than their stimulation by chylomicrons. Pancreozymin is qualified to serve as such a signal. The physiologic implications of this study are considered.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRINK M. J., FITZGERALD J. R., MAN E. B. Effect of glucagon on alimentary lipemia. Proc Soc Exp Biol Med. 1957 Aug-Sep;95(4):778–780. doi: 10.3181/00379727-95-23362. [DOI] [PubMed] [Google Scholar]
- AMATUZIO D. S., GRANDE F., WADA S. Effect of glucagon on the serum lipids in essential hyperlipemia and in hypercholesterolemia. Metabolism. 1962 Dec;11:1240–1249. [PubMed] [Google Scholar]
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Balasse E., Ooms H. A. Effet d'une élévation aiguë du taux des acides gras libres (nefa) sur la tolérance glucidique et la réponse insulinique a l'hyperglycémie chez l'homme normal. Rev Fr Etud Clin Biol. 1968 Jan;13(1):62–67. [PubMed] [Google Scholar]
- Böttger I., Faloona G. R., Unger R. H. The effect of calcium and other salts upon the release of glucagon-like immunoreactivity from the gut. J Clin Invest. 1972 Apr;51(4):831–836. doi: 10.1172/JCI106878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespin S. R., Greenough W. B., 3rd, Steinberg D. Stimulation of insulin secretion by infusion of free fatty acids. J Clin Invest. 1969 Oct;48(10):1934–1943. doi: 10.1172/JCI106160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oya M., Prigge W. F., Swenson D. E., Grande F. Role of glucagon on fatty liver production in birds. Am J Physiol. 1971 Jul;221(1):25–30. doi: 10.1152/ajplegacy.1971.221.1.25. [DOI] [PubMed] [Google Scholar]
- Eaton R. P., Kipnis D. M. Effect of glucose feeding on lipoprotein synthesis in the rat. Am J Physiol. 1969 Oct;217(4):1153–1159. doi: 10.1152/ajplegacy.1969.217.4.1153. [DOI] [PubMed] [Google Scholar]
- Eggstein M., Kreutz F. H. Eine neue Bestimmung der Neutralfette im Blutserum und Gewebe. I. Prinzip, Durchführung und Besprechung der Methode. Klin Wochenschr. 1966 Mar 1;44(5):262–267. doi: 10.1007/BF01747716. [DOI] [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., Kohout M. The effects of glucagon, dibutyryl cyclic adenosine 3',5'-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J Biol Chem. 1969 Oct 10;244(19):5131–5139. [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Aguilar-Parada E., Unger R. H. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970 Jul 16;283(3):109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- Ohneda A., Parada E., Eisentraut A. M., Unger R. H. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. J Clin Invest. 1968 Oct;47(10):2305–2322. doi: 10.1172/JCI105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALOYAN E., HARPER P. V., Jr Glucagon as a regulating factor of plasma lipids. Metabolism. 1961 Apr;10:315–323. [PubMed] [Google Scholar]
- Pi-Sunyer F. X., Hashim S. A., Van Itallie T. B. Insulin and ketone responses to ingestion of medium and long-chain triglycerides in man. Diabetes. 1969 Feb;18(2):96–100. doi: 10.2337/diab.18.2.96. [DOI] [PubMed] [Google Scholar]
- Schalch D. S., Kipnis D. M. Abnormalities in carbohydrate tolerance associated with elevated plasma nonesterified fatty acids. J Clin Invest. 1965 Dec;44(12):2010–2020. doi: 10.1172/JCI105308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Dupré J., Eisentraut A. M. The effects of secretin, pancreozymin, and gastrin on insulin and glucagon secretion in anesthetized dogs. J Clin Invest. 1967 Apr;46(4):630–645. doi: 10.1172/JCI105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C. C., GROSSMAN M. I. Physiological determination of release of secretin and pancreozymin from intestine of dogs with transplanted pancreas. Am J Physiol. 1951 Feb;164(2):527–545. doi: 10.1152/ajplegacy.1951.164.2.527. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]


