Abstract
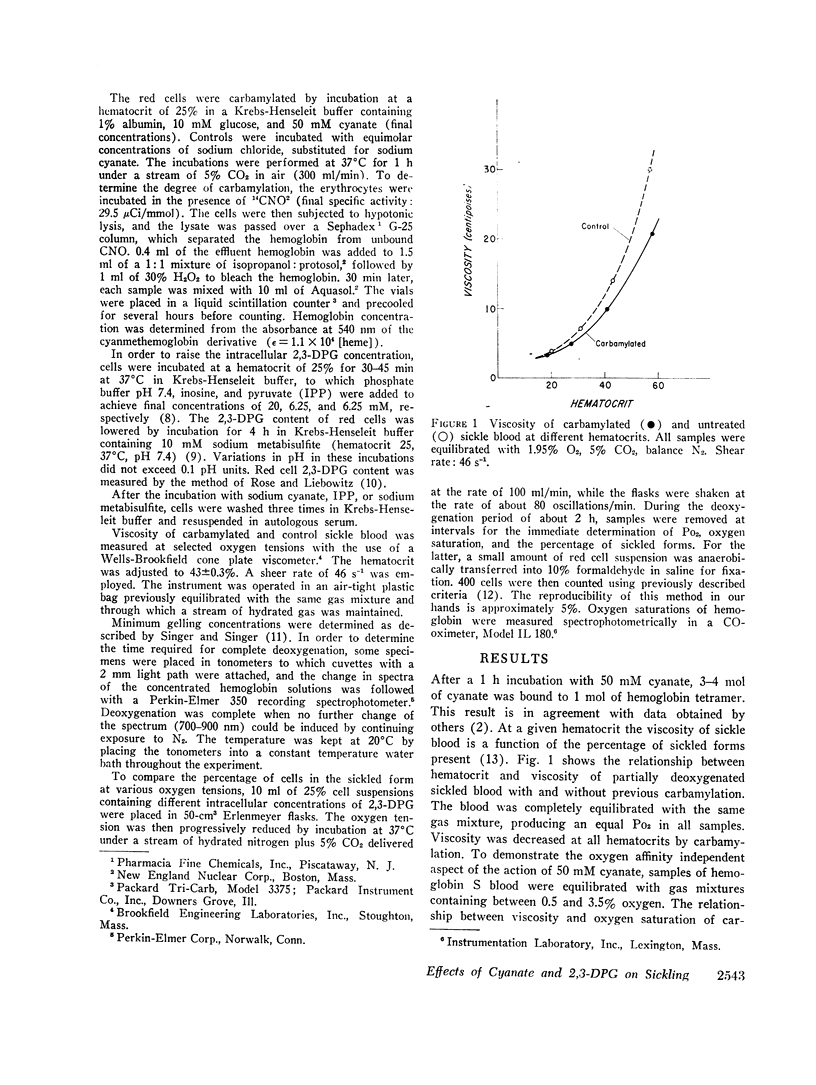
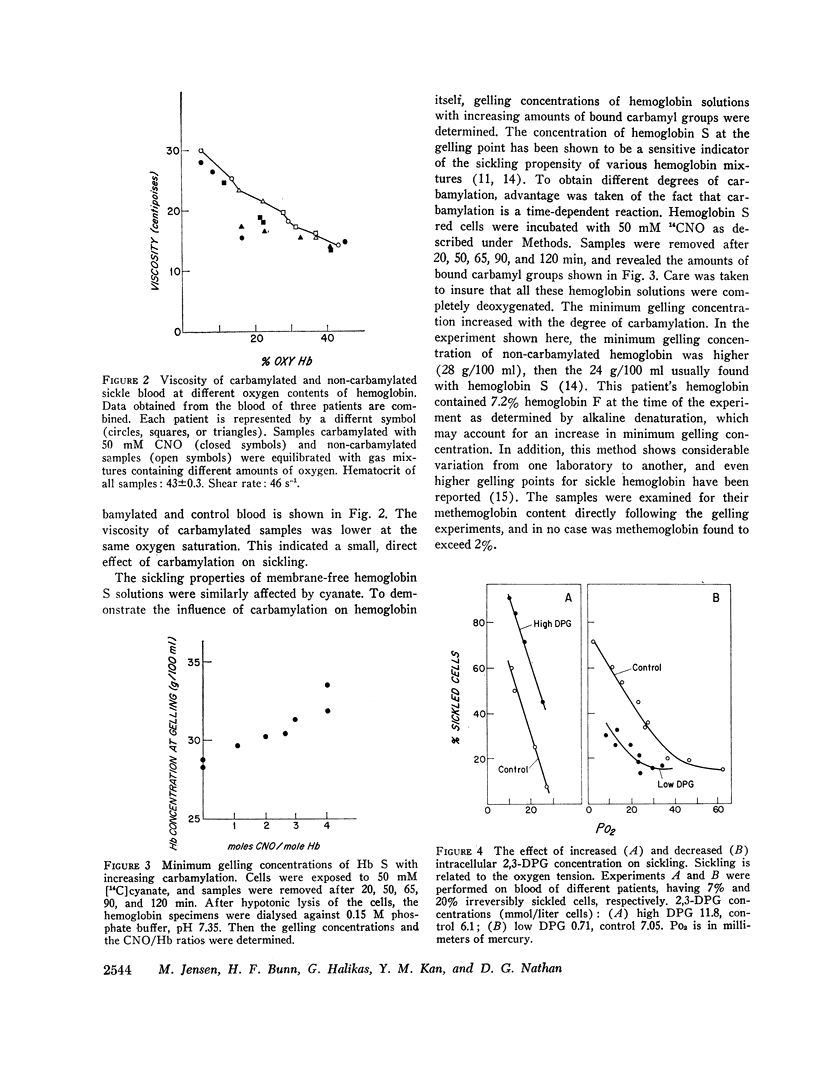
Cyanate and 2,3-diphosphoglycerate (2,3-DPG) both influence the oxygen affinity of hemoglobin. The studies presented here concern the effects of these compounds on the sickling phenomenon. The inhibitory effect of cyanate on sickling is largely due to the fact that it increases the percentage of oxyhemoglobin S at a given oxygen tension. In addition, cyanate inhibits sickling by a mechanism that is independent of oxygenation. In this paper, we have demonstrated that the viscosity of carbamylated sickle blood was lower than that of non-carbamylated controls at the same oxygen saturation. Furthermore, carbamylation resulted in an increase in the minimum concentration of deoxy-sickle hemoglobin required for gelation.
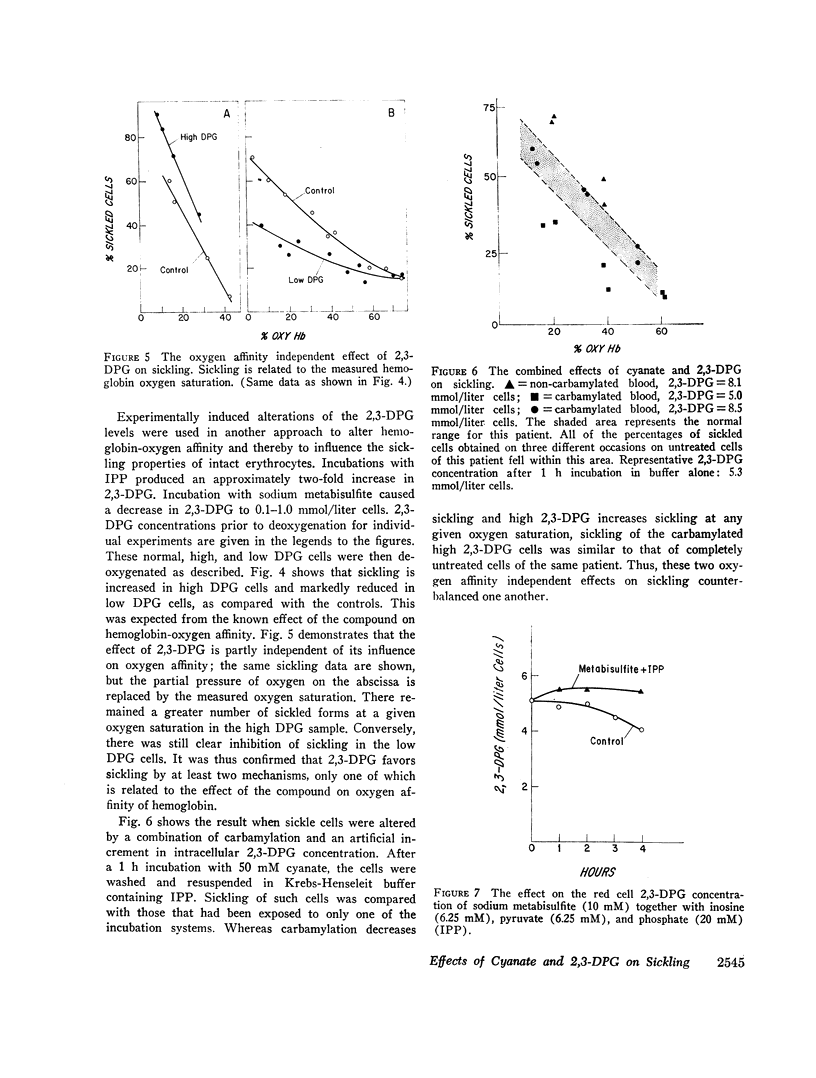
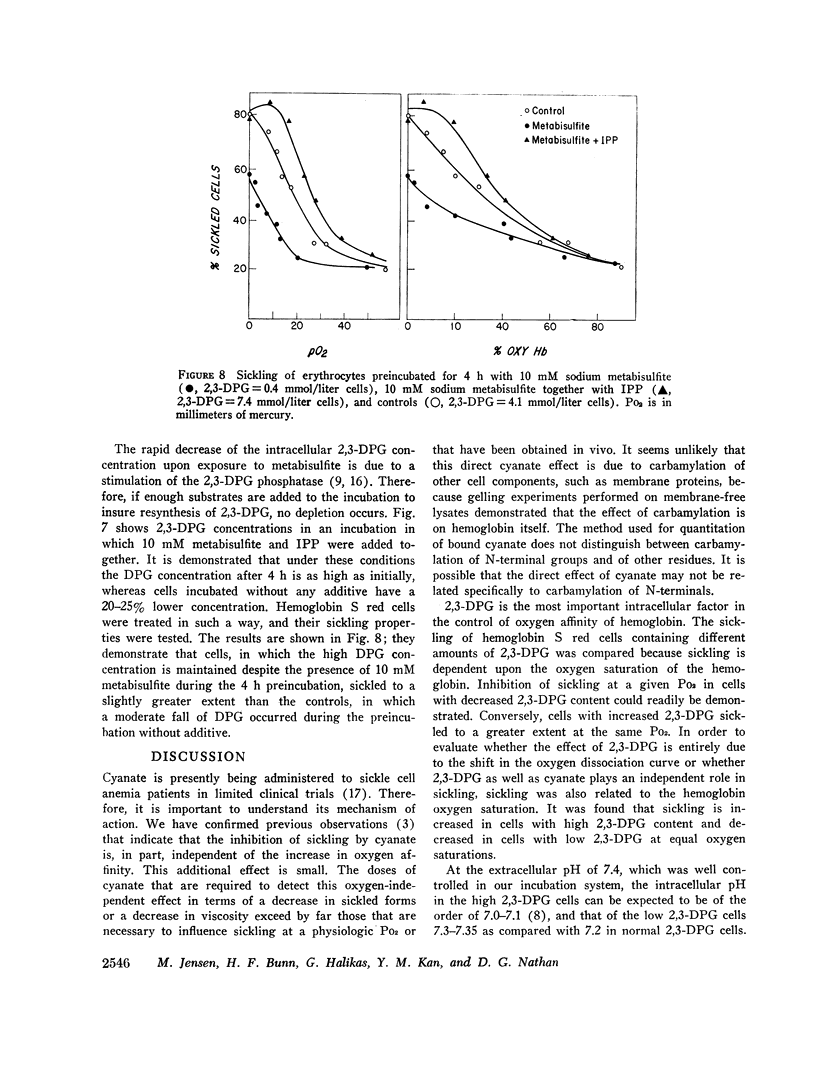
Like cyanate, 2,3-DPG affected sickling of intact erythrocytes by two mechanisms. Since 2,3-DPG decreases the percentage of oxyhemoglobin S at a given oxygen tension, sickling is enhanced. In addition, 2,3-DPG had a direct effect. When the intracellular 2,3-DPG concentration was increased in vitro, a greater percentage of cells were sickled at a given oxygen saturation. Conversely, sickling was inhibited in cells in which 2,3-DPG was artificially lowered. These data indicate that the enhancement of sickling by 2,3-DPG is in part independent of its influence on oxygen affinity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON R., CASSELL M., MULLINAX G. L., CHAPLIN H., Jr Effect of normal cells on viscosity of sickle-cell blood. In vitro studies and report of six years' experience with a prophylactic program of "partial exchange transfusion". Arch Intern Med. 1963 Mar;111:286–294. doi: 10.1001/archinte.1963.03620270012003. [DOI] [PubMed] [Google Scholar]
- BEUTLER E. The effect of methemoglobin formation in sickle cell disease. J Clin Invest. 1961 Oct;40:1856–1871. doi: 10.1172/JCI104410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L., Ranney H. M. Structure and properties of hemoglobin C-Harlem, a human hemoglobin variant with amino acid substitutions in 2 residues of the beta-polypeptide chain. J Biol Chem. 1967 Jan 25;242(2):248–255. [PubMed] [Google Scholar]
- Cerami A., Manning J. M. Potassium cyanate as an inhibitor of the sickling of erythrocytes in vitro. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1180–1183. doi: 10.1073/pnas.68.6.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Ostertag W., von Ehrenstein G. Clinical studies and physiological properties of Hopkins-2 haemoglobin. Nat New Biol. 1972 May 17;237(72):88–90. doi: 10.1038/newbio237088a0. [DOI] [PubMed] [Google Scholar]
- De Furia F. G., Miller D. R., Cerami A., Manning J. M. The effects of cyanate in vitro on red blood cell metabolism and function in sickle cell anemia. J Clin Invest. 1972 Mar;51(3):566–574. doi: 10.1172/JCI106845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich D. Relationship between the oxygen affinity and in vitro sickling propensity of carbamylated sickle erythrocytes. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1255–1261. doi: 10.1016/s0006-291x(72)80110-4. [DOI] [PubMed] [Google Scholar]
- Duhm J. Effects of 2,3-diphosphoglycerate and other organic phosphate compounds on oxygen affinity and intracellular pH of human erythrocytes. Pflugers Arch. 1971;326(4):341–356. doi: 10.1007/BF00586998. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Perutz M. F., Bertles J. F., Döbler J. Structure of sickled erythrocytes and of sickle-cell hemoglobin fibers. Proc Natl Acad Sci U S A. 1973 Mar;70(3):718–722. doi: 10.1073/pnas.70.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. L., Weissmann G., Gorman B. D., Cunningham-Rundles W. Sickle hemoglobin gelation--inhibition by tris (hydroxymethyl) aminomethane and sugars. Biochem Pharmacol. 1973 Mar 15;22(6):667–674. doi: 10.1016/0006-2952(73)90399-7. [DOI] [PubMed] [Google Scholar]
- Harkness D. R., Roth S. Purification and properties of 2, 3-diphosphoglyceric acid phosphatase from human erythrocytes. Biochem Biophys Res Commun. 1969 Mar 31;34(6):849–856. doi: 10.1016/0006-291x(69)90258-7. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. The binding of carbon dioxide by horse haemoglobin. Biochem J. 1971 Aug;124(1):31–45. doi: 10.1042/bj1240031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A., Bellingham A. J., Huehns E. R., Beaven G. H. Effect of cyanate on sickling. Lancet. 1972 Mar 25;1(7752):658–661. doi: 10.1016/s0140-6736(72)90462-x. [DOI] [PubMed] [Google Scholar]
- Paniker N. V., Ben-Bassat I., Beutler E. Evaluation of sickle hemoglobin and desickling agents by falling ball viscometry. J Lab Clin Med. 1972 Aug;80(2):282–290. [PubMed] [Google Scholar]
- Rose Z. B., Liebowitz J. Direct determination of 2,3-diphosphoglycerate. Anal Biochem. 1970 May;35(1):177–180. doi: 10.1016/0003-2697(70)90023-0. [DOI] [PubMed] [Google Scholar]
- SINGER K., SINGER L. Studies on abnormal hemoglobins. VIII. The gelling phenomenon of sickle cell hemoglobin: its biologic and diagnostic significance. Blood. 1953 Nov;8(11):1008–1023. [PubMed] [Google Scholar]



