Abstract
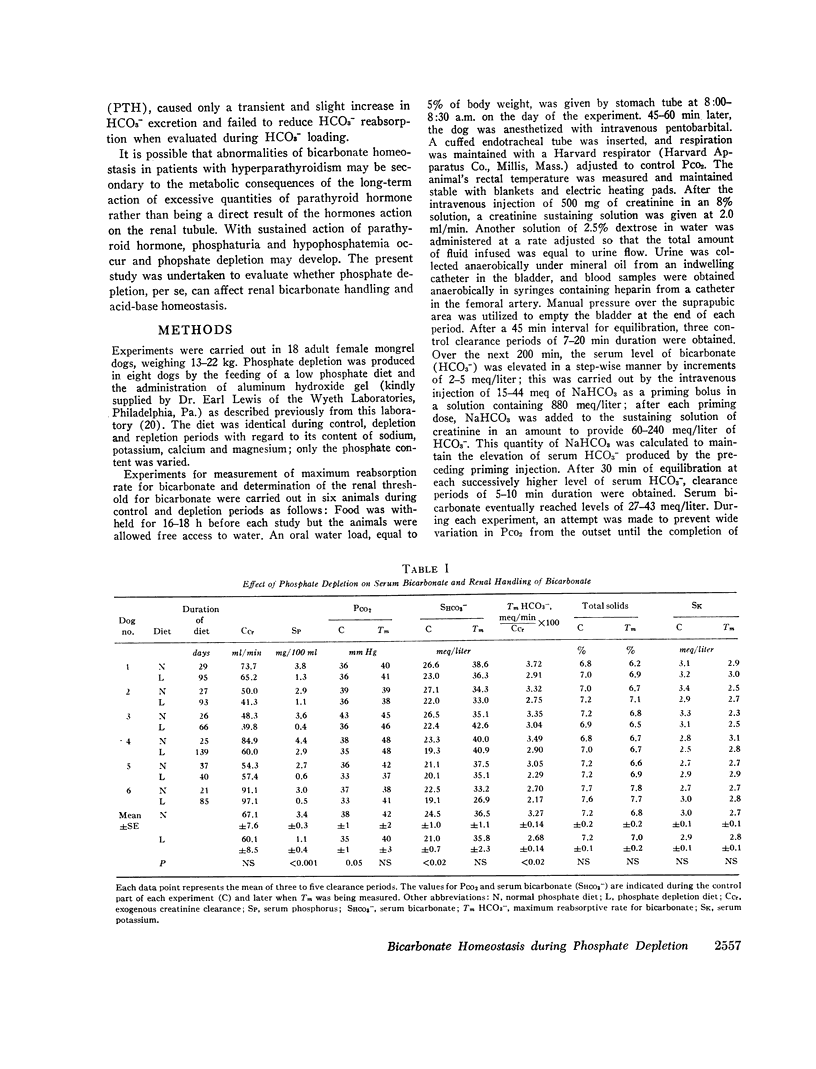
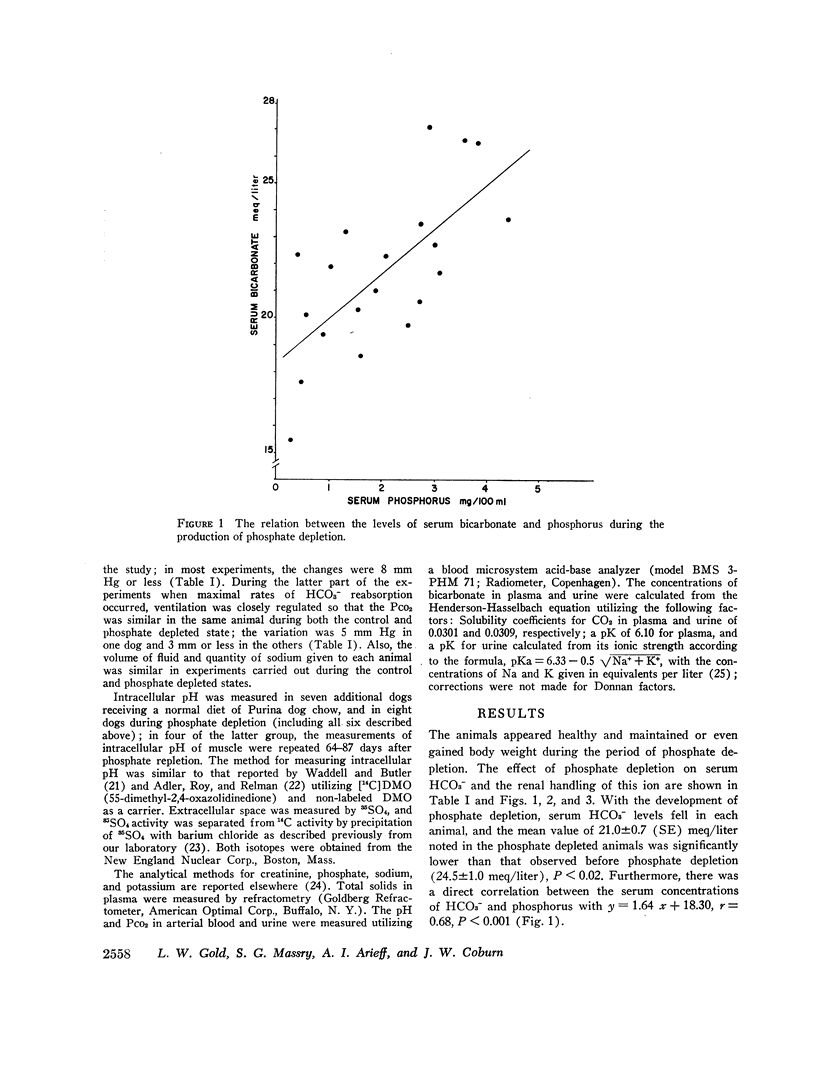
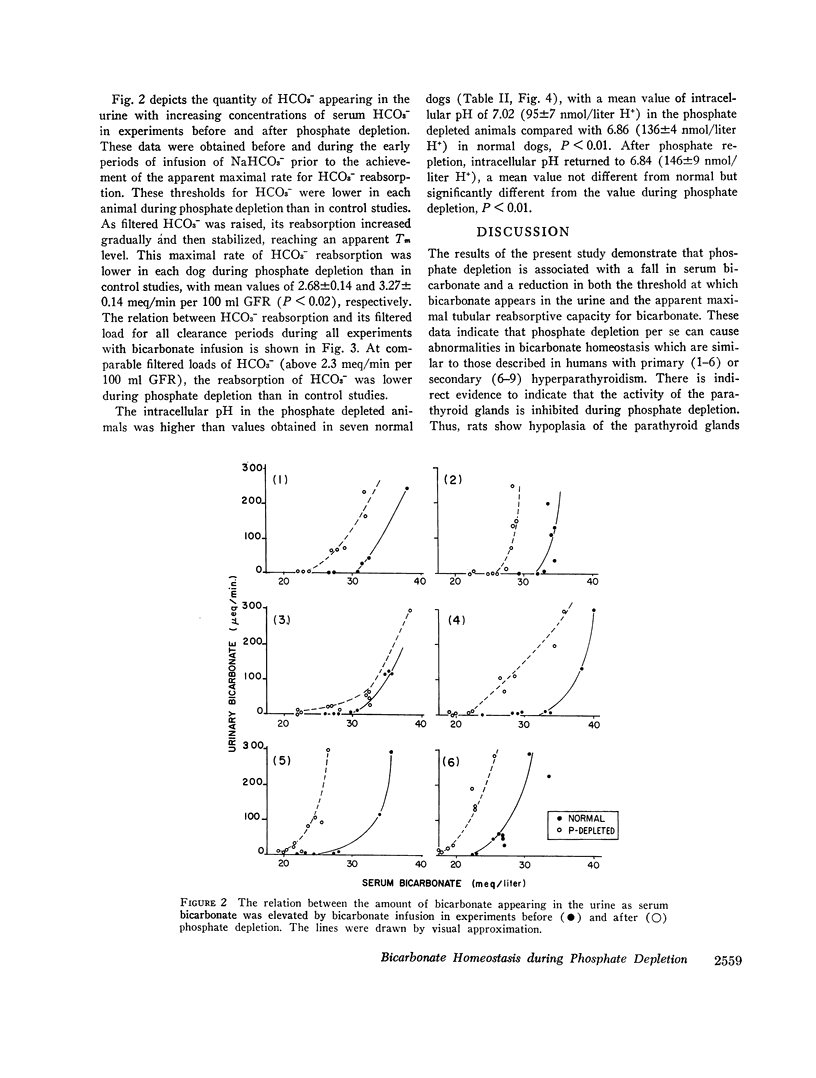
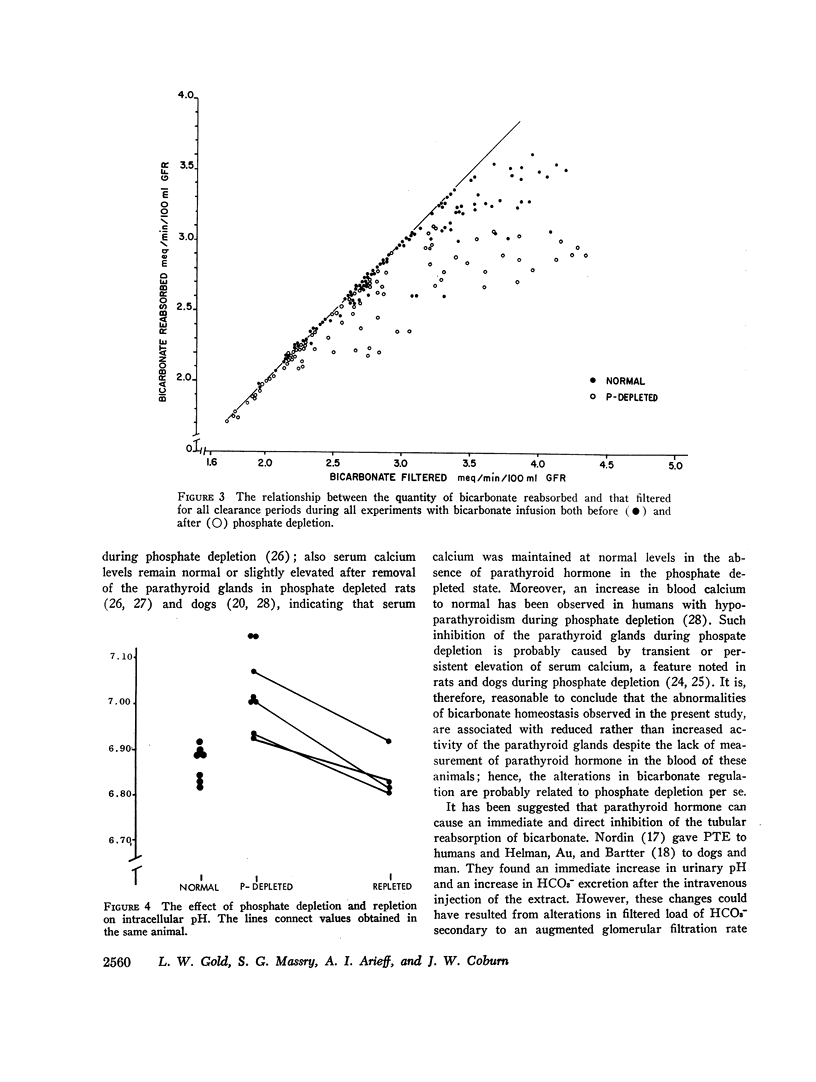
With hyperparathyroidism, serum bicarbonate (HCO3-) is low, urinary excretion of HCO3- is increased and the apparent Tm for HCO3- is reduced. These findings have been ascribed to a direct renal action of parathyroid hormone (PTH). Since hypophosphatemia and phosphate depletion may occur in hyperparathyroidism, it is possible that phosphate depletion could account for the abnormal renal HCO3- handling. To test this possibility, renal reabsorption of HCO3- was evaluated in dogs before and after phosphate depletion. Serum HCO3- was significantly lower in phosphate depleted dogs than in normal animals, and serum HCO3- was directly related to serum phosphorus. Both the threshold at which HCO3- appeared in the urine and the Tm for HCO3- were reduced during phosphate depletion. Intracellular pH of muscle was significantly higher in phosphate depleted dogs than in normals and the pH returned to normal after phosphate repletion. These data show that phosphate depleted dogs, which probably have physiological hypoparathyroidism, display abnormalities in both serum HCO3- and its renal handling which are similar to those seen in hyperparathyroidism, supporting the concept that the PTH-induced alterations in HCO3- homeostasis may be due to phosphate depletion. The latter could alter cell metabolism, resulting in reduced intracellular H+ concentration, which may then impair H+ secretion by the renal tubules and decrease their ability to reabsorb HCO3-. Consequently, Tm HCO3- and serum HCO3- fall.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. I. THE RESPONSE OF MUSCLE CELLS TO CHANGES IN CO2 TENSION OR EXTRACELLULAR BICARBONATE CONCENTRATION. J Clin Invest. 1965 Jan;44:8–20. doi: 10.1172/JCI105129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzel U. S. Parathyroid hormone, blood phosphorus, and acid-base metabolism. Lancet. 1971 Jun 26;1(7713):1329–1331. doi: 10.1016/s0140-6736(71)91888-5. [DOI] [PubMed] [Google Scholar]
- Barzel U. S. Systemic alkalosis in hypoparathyroidism. J Clin Endocrinol Metab. 1969 Jul;29(7):917–918. doi: 10.1210/jcem-29-7-917. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Villamil M., Kleeman C. R. Extracellular fluid, ionic distribution and exchange in isolated frog brain. Am J Physiol. 1968 Mar;214(3):643–651. doi: 10.1152/ajplegacy.1968.214.3.643. [DOI] [PubMed] [Google Scholar]
- Coburn J. W., Massry S. G. Changes in serum and urinary calcium during phosphate depletion: studies on mechanisms. J Clin Invest. 1970 Jun;49(6):1073–1087. doi: 10.1172/JCI106323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolman M., Barzel U. S. Primary hyperparathyroidism. Diagnosis and treatment. N Y State J Med. 1971 Jun 1;71(11):1201–1204. [PubMed] [Google Scholar]
- Ellsworth R., Nicholson W. M. FURTHER OBSERVATIONS UPON THE CHANGES IN THE ELECTROLYTES OF THE URINE FOLLOWING THE INJECTION OF PARATHYROID EXTRACT. J Clin Invest. 1935 Nov;14(6):823–827. doi: 10.1172/JCI100730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOURMAN P., McCONKEY B., SMITH J. W. Defects of water reabsorption and of hydrogen-ion excretion by the renal tubules in hyperparathyroidism. Lancet. 1960 Mar 19;1(7125):619–623. doi: 10.1016/s0140-6736(60)90503-1. [DOI] [PubMed] [Google Scholar]
- Heinemann H. O. Metabolic alkalosis in patients with hypercalcemia. Metabolism. 1965 Nov;14(11):1137–1152. doi: 10.1016/0026-0495(65)90086-7. [DOI] [PubMed] [Google Scholar]
- Hellman D. E., Au W. Y., Bartter F. C. Evidence for a direct effect of parathyroid hormone on urinary acidification. Am J Physiol. 1965 Sep;209(3):643–650. doi: 10.1152/ajplegacy.1965.209.3.643. [DOI] [PubMed] [Google Scholar]
- KLEEMAN C. R., COOKE R. E. The acute effects of parathyroid hormone on the metabolism of endogenous phosphate. J Lab Clin Med. 1951 Jul;38(1):112–127. [PubMed] [Google Scholar]
- Kurtzman N. A. Regulation of renal bicarbonate reabsorption by extracellular volume. J Clin Invest. 1970 Mar;49(3):586–595. doi: 10.1172/JCI106269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty F. W. Pseudohyperparathyroidism. Medicine (Baltimore) 1966 May;45(3):247–260. doi: 10.1097/00005792-196605000-00004. [DOI] [PubMed] [Google Scholar]
- Lotz M., Zisman E., Bartter F. C. Evidence for a phosphorus-depletion syndrome in man. N Engl J Med. 1968 Feb 22;278(8):409–415. doi: 10.1056/NEJM196802222780802. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Kleeman C. R. The influence of extracellular volume expansion on renal phosphate reabsorption in the dog. J Clin Invest. 1969 Jul;48(7):1237–1245. doi: 10.1172/JCI106088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldowney F. P., Carroll D. V., Donohoe J. F., Freaney R. Correction of renal bicarbonate wastage by parathyroidectomy. Implications in acid-base homeostasis. Q J Med. 1971 Oct;40(160):487–498. [PubMed] [Google Scholar]
- Muldowney F. P., Donohoe J. F., Freaney R., Kampff C., Swan M. Parathormone-induced renal bicarbonate wastage in intestinal malabsorption and in chronic renal failure. Ir J Med Sci. 1970 May;3(5):221–231. doi: 10.1007/BF02957057. [DOI] [PubMed] [Google Scholar]
- Muldowney F. P., Freaney R., Brennan P. A case of osteomalacia and renal tubular acidosis associated with occult idiopathic steatorrhoea: the effect of vitamin D on renal tubular hydrion transport. Ir J Med Sci. 1965 Dec;6(480):435–448. doi: 10.1007/BF02976590. [DOI] [PubMed] [Google Scholar]
- Muldowney F. P., Freaney R., McGeeney D. Renal tubular acidosis and amino-aciduria in osteomalacia of dietary or intestinal origin. Q J Med. 1968 Oct;37(148):517–539. [PubMed] [Google Scholar]
- NORDIN B. E. The effect of intravenous parathyroid extract on urinary pH, bicarbonate and electrolyte excretion. Clin Sci. 1960 May;19:311–319. [PubMed] [Google Scholar]
- Purkerson M. L., Lubowitz H., White R. W., Bricker N. S. On the influence of extracellular fluid volume expansion on bicarbonate reabsorption in the rat. J Clin Invest. 1969 Sep;48(9):1754–1760. doi: 10.1172/JCI106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIKITA M., TSURUFUJI S., ITO Y. Adaptation in renal phosphorus excretion under the influence of parathyroids; a study in ureterally catheterized rats. Endocrinol Jpn. 1962 Sep;9:171–180. doi: 10.1507/endocrj1954.9.171. [DOI] [PubMed] [Google Scholar]
- THOMAS W. C., Jr, CONNOR T. B., MORGAN H. G. Some observations on patients with hypercalcemia exemplifying problems in differential diagnosis, especially in hyperparathyroidism. J Lab Clin Med. 1958 Jul;52(1):11–19. [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLS M. R., MCGOWAN G. K. PLASMA-CHLORIDE LEVELS IN HYPERPARATHYROIDISM AND OTHER HYPERCALCAEMIC STATES. Br Med J. 1964 May 2;1(5391):1153–1156. doi: 10.1136/bmj.1.5391.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRONG O., DAVIES H. E. The excretion of acid in renal disease. Q J Med. 1959 Apr;28(110):259–313. [PubMed] [Google Scholar]
- York S. E., Yendt E. R. Osteomalacia associated with renal bicarbonate loss. Can Med Assoc J. 1966 Jun 25;94(26):1329–1342. [PMC free article] [PubMed] [Google Scholar]


