Abstract
The biochemical mechanism accounting for the connective tissue abnormalities in homocystinuria was explored by examining the effects of various amino acids known to accumulate in the plasma of patients with this disease on cross-link formation in collagen. Neutral salt solutions of purified, rat skin collagen, rich in cross-link precursor aldehydes, were polymerized to native type fibrils by incubating at 37°C in the presence of homocysteine, homocystine, or methionine. After the polymerization was completed, each sample was examined for the formation of covalent intermolecular cross-links, assessed indirectly by solubility tests and directly by measuring the cross-link compounds after reduction with tritiated sodium borohydride and hydrolysis.
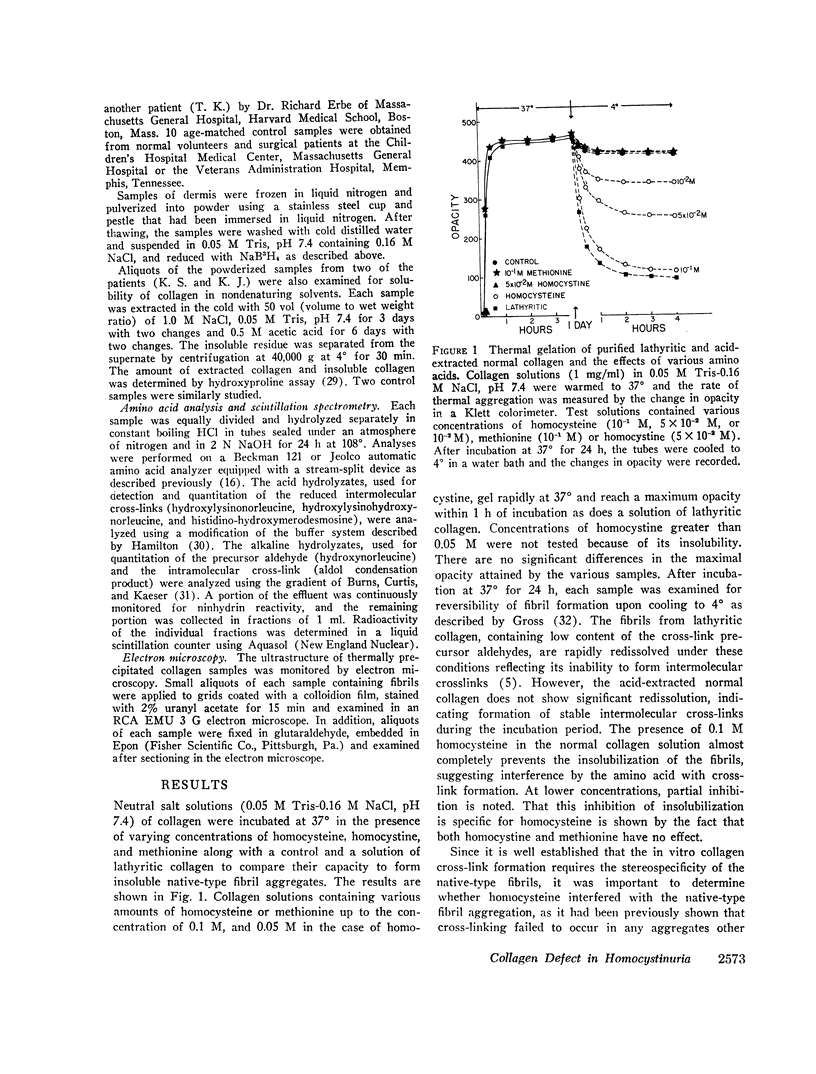
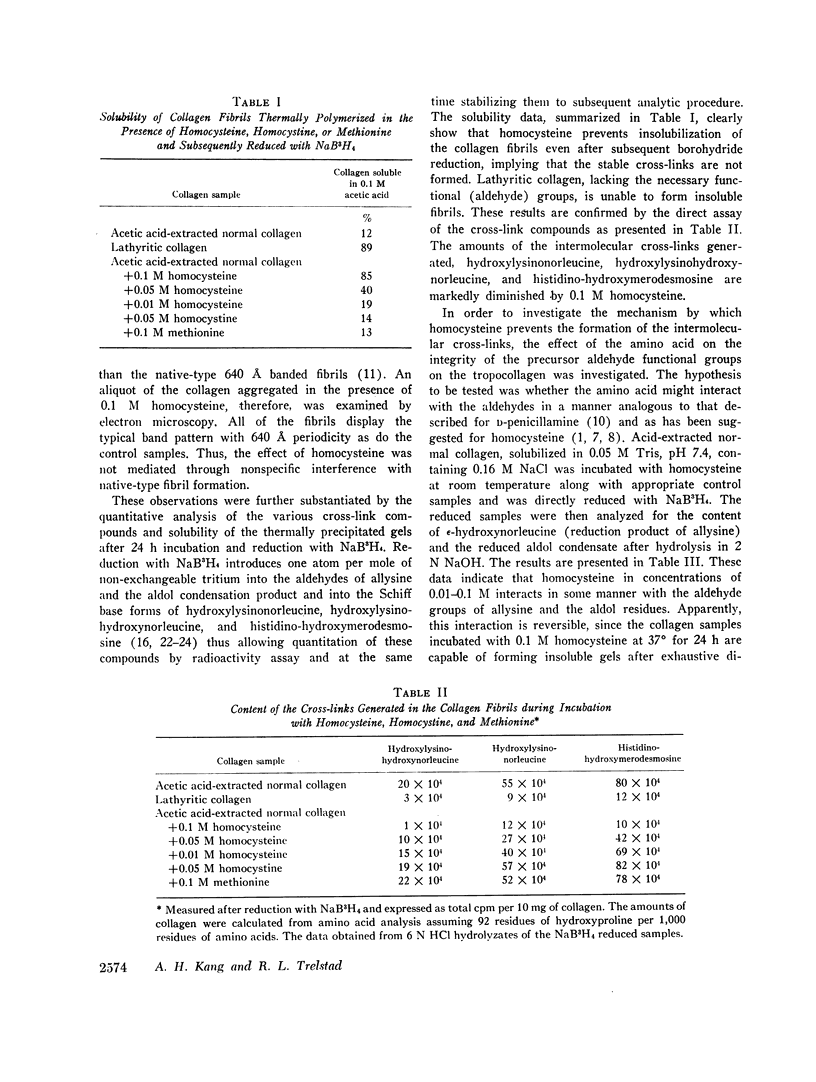
Collagen solutions containing homocysteine (0.01 M-0.1 M) failed to form insoluble fibrils. Furthermore, much less of the reducible cross-links, Δ6,7 dehydrohydroxylysinonorleucine, Δ6,7 dehydrohydroxylysinohydroxynorleucine, and histidino-dehydrohydroxymerodesmosine were formed in the preparations containing homocysteine as compared with the control and the samples containing methionine or homocystine. The content of the precursor aldehydes, α-aminoadipic-δ-semialdehyde (allysine) and the aldol condensation product, was also markedly diminished in tropocollagen incubated with homocysteine. It is concluded that homocysteine interferes with the formation of intermolecular cross-links that help stabilize the collagen macromolecular network via its reversible binding to the aldehydic functional groups.
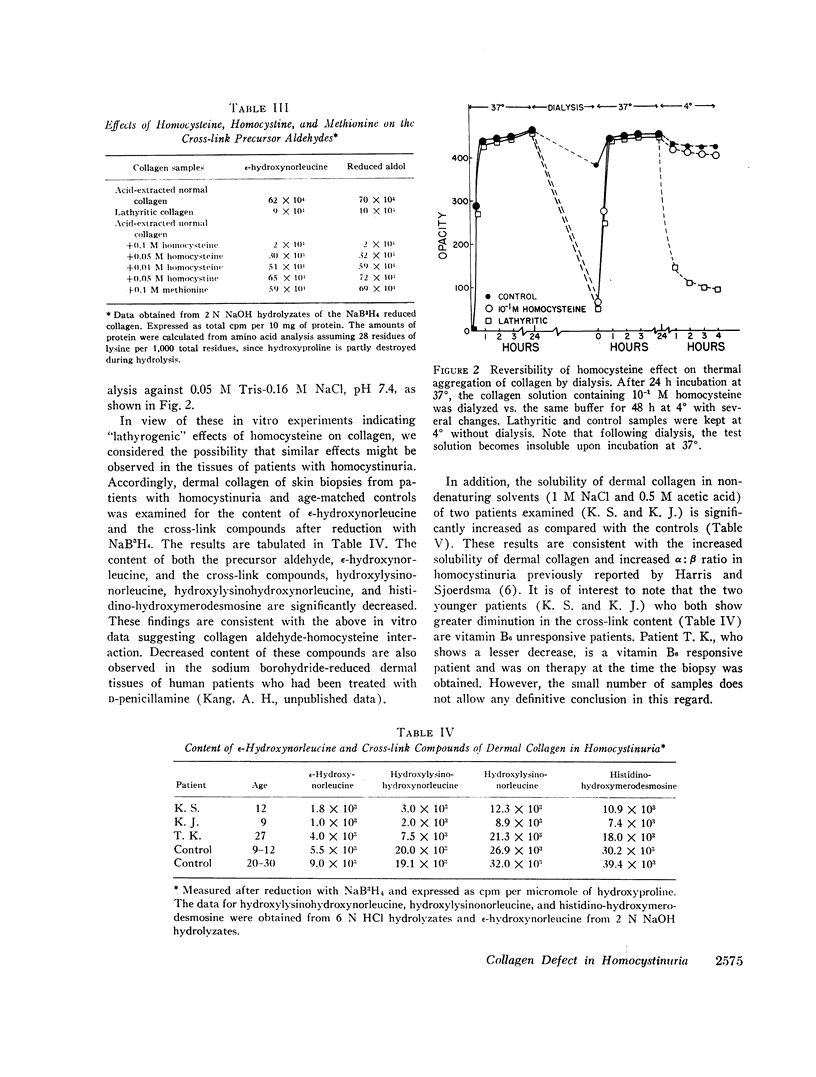
Analysis of the collagen cross-links in skin biopsy samples obtained from three patients with documented homocystinuria showed that the cross-links were significantly decreased as compared with the age-matched controls, supporting the tentative conclusions reached from the in vitro model studies. In addition, the solubility of dermal collagen in non-denaturing solvents was significantly increased in the two patients examined, reflecting a functional defect in collagen cross-linking. Although the concentration of homocysteine used in this study to demonstrate these effects in vitro is clearly higher than that which is observed in homocystinuric's plasma, the data do suggest a possible pathogenetic mechanism of connective tissue defect in homocystinuria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Peach C. M., Fowler L. J. Chemistry of the collagen cross-links. Isolation and characterization of two intermediate intermolecular cross-links in collagen. Biochem J. 1970 May;117(5):819–831. doi: 10.1042/bj1170819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A. J., Peach C. M. Isolation and structural identification of a labile intermolecular crosslink in collagen. Biochem Biophys Res Commun. 1968 Dec 9;33(5):812–819. doi: 10.1016/0006-291x(68)90233-7. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Kang A. H., Piez K. A. The nature and location of intramolecular cross-links in collagen. Proc Natl Acad Sci U S A. 1966 Feb;55(2):417–424. doi: 10.1073/pnas.55.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Piez K. A. The nature of the intramolecular cross-links in collagen. The separation and characterization of peptides from the cross-link region of rat skin collagen. Biochemistry. 1966 Nov;5(11):3460–3473. doi: 10.1021/bi00875a012. [DOI] [PubMed] [Google Scholar]
- Bornstein P. The cross-linking of collagen and elastin and its inhibition in osteolathyrism. Is there a relation to the aging process? Am J Med. 1970 Oct;49(4):429–435. doi: 10.1016/s0002-9343(70)80036-5. [DOI] [PubMed] [Google Scholar]
- Deshmukh K., Nimni M. E. A defect in the intramolecular and intermolecular cross-linking of collagen caused by penicillamine. II. Functional groups involved in the interaction process. J Biol Chem. 1969 Apr 10;244(7):1787–1795. [PubMed] [Google Scholar]
- Fairweather R. B., Tanzer M. L., Gallop P. M. Aldol-histidine, a new trifunctional collagen crosslink. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1311–1315. doi: 10.1016/0006-291x(72)90854-6. [DOI] [PubMed] [Google Scholar]
- Franzblau C., Kang A. H., Faris B. In vitro formation of intermolecular crosslinks in chick skin collagen. II. Kinetics. Biochem Biophys Res Commun. 1970 Jul 27;40(2):437–444. doi: 10.1016/0006-291x(70)91028-4. [DOI] [PubMed] [Google Scholar]
- GROSS J. An intermolecular defect of collagen in experimental lathyrism. Biochim Biophys Acta. 1963 Apr 2;71:250–252. doi: 10.1016/0006-3002(63)91079-5. [DOI] [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- GROSS J. [Studies on the formation of collagen. III. Time-dependent solubility changes of collagen in vitro]. J Exp Med. 1958 Aug 1;108(2):215–226. doi: 10.1084/jem.108.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallop P. M., Blumenfeld O. O., Henson E., Schneider A. L. Isolation and identification of alpha-amino aldehydes in collagen. Biochemistry. 1968 Jun;7(6):2409–2430. doi: 10.1021/bi00846a051. [DOI] [PubMed] [Google Scholar]
- Gallop P. M., Blumenfeld O. O., Seifter S. Structure and metabolism of connective 801 tissue proteins. Annu Rev Biochem. 1972;41:617–672. doi: 10.1146/annurev.bi.41.070172.003153. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 1. N Engl J Med. 1972 Jan 27;286(4):194–199. doi: 10.1056/NEJM197201272860406. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Sjoerdsma A. Collagen profile in various clinical conditions. Lancet. 1966 Oct 1;2(7466):707–711. doi: 10.1016/s0140-6736(66)92976-x. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Sjoerdsma A. Effect of penicillamine on human collagen and its possible application to treatment of scleroderma. Lancet. 1966 Nov 5;2(7471):996–999. doi: 10.1016/s0140-6736(66)92926-6. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Faris B., Franzblau C. The in vitro formation of intermolecular cross-links in chick skin collagen. Biochem Biophys Res Commun. 1970 Apr 8;39(1):175–182. doi: 10.1016/0006-291x(70)90774-6. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Gross J. Relationship between the intra and intermolecular cross-links of collagen. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1307–1314. doi: 10.1073/pnas.67.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang A. H., Piez K. A., Gross J. Characterization of the alpha-chains of chick skin collagen and the nature of the NH2-terminal cross-link region. Biochemistry. 1969 Sep;8(9):3648–3655. doi: 10.1021/bi00837a023. [DOI] [PubMed] [Google Scholar]
- LEVENE C. I., GROSS J. Alterations in state of molecular aggregation of collagen induced in chick embryos by beta-aminopropionitrile (lathyrus factor). J Exp Med. 1959 Nov 1;110:771–790. doi: 10.1084/jem.110.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDD S. H., FINKELSTEIN J. D., IRREVERRE F., LASTER L. HOMOCYSTINURIA: AN ENZYMATIC DEFECT. Science. 1964 Mar 27;143(3613):1443–1445. doi: 10.1126/science.143.3613.1443. [DOI] [PubMed] [Google Scholar]
- Mechanic G., Tanzer M. L. Biochemistry of collagen crosslinking. Isolation of a new crosslink, hydroxylysinohydroxynorleucine, and its reduced precursor, dihydroxynorleucine, from bovine tendon. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1597–1604. doi: 10.1016/0006-291x(70)90571-1. [DOI] [PubMed] [Google Scholar]
- Nimni M. E. A defect in the intramolecular and intermolecular cross-linking of collagen caused by penicillamine. I. Metabolic and functional abnormalities in soft tissues. J Biol Chem. 1968 Apr 10;243(7):1457–1466. [PubMed] [Google Scholar]
- PONSETI I. V. Lesions of the skeleton and of other mesodermal tissues in rats fed sweet-pea (Lathyrus odoratus) seeds. J Bone Joint Surg Am. 1954 Oct;36-A(5):1031–1058. [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Piez K. A. Cross-linking of collagen and elastin. Annu Rev Biochem. 1968;37:547–570. doi: 10.1146/annurev.bi.37.070168.002555. [DOI] [PubMed] [Google Scholar]
- Rojkind M., Gutierrez A. M., Zeichner M., Lent R. W. The nature of the intramolecular cross-link in collagen. Biochem Biophys Res Commun. 1969 Aug 7;36(3):350–356. doi: 10.1016/0006-291x(69)90571-3. [DOI] [PubMed] [Google Scholar]
- Rojkind M., Rhi L., Aguirre M. Biosynthesis of the intramolecular cross-links in rat skin collagen. J Biol Chem. 1968 May 10;243(9):2266–2272. [PubMed] [Google Scholar]
- Tanzer M. L. Collagen reduction by sodium borohydride: effects of reconstitution, maturation and lathyrism. Biochem Biophys Res Commun. 1968 Sep 6;32(5):885–892. doi: 10.1016/0006-291x(68)90324-0. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Housley T., Berube L., Fairweather R., Franzblau C., Gallop P. M. Structure of two histidine-containing crosslinks from collagen. J Biol Chem. 1973 Jan 25;248(2):393–402. [PubMed] [Google Scholar]
- Tanzer M. L. Intermolecular cross-links in reconstituted collagen fibrils. Evidence for the nature of the covalent bonds. J Biol Chem. 1968 Aug 10;243(15):4045–4054. [PubMed] [Google Scholar]
- Tanzer M. L., Mechanic G. Isolation of lysinonorleucine from collagen. Biochem Biophys Res Commun. 1970 Apr 8;39(1):183–189. doi: 10.1016/0006-291x(70)90775-8. [DOI] [PubMed] [Google Scholar]
- Uerre J. A., Miller C. H. Preparation of L-homocysteine from L-homocysteine thiolactone. Anal Biochem. 1966 Nov;17(2):310–315. doi: 10.1016/0003-2697(66)90209-0. [DOI] [PubMed] [Google Scholar]


