Abstract
Antimicrobial peptides such as human β-defensins (hBDs) and cathelicidins are critical for protection against infection and can be induced by activation of TLRs, a pathway that also activates cyclooxygenase(Cox)-2 expression. We hypothesized that Cox-2 is induced by TLR activation and is necessary for optimal AMP production, and that inhibitors of Cox-2 may therefore inhibit antimicrobial action. Normal human keratinocytes (NHEKs) stimulated with a TLR2/6 ligand, macrophage-activating lipo-peptide-2, or a TLR3 ligand, polyinosinic-polycytidylic acid, increased Cox-2 mRNA and protein and increased PGE2, a product of Cox-2. Treatment with a Cox-2 selective inhibitor (SC-58125) or Cox-2 small interfering RNA attenuated hBD2 and hBD3 production in NHEKs when stimulated with macrophage-activating lipopeptide-2, polyinosinic-polycytidylic acid, or UVB (15 mJ/cm2), but it did not attenuate vitamin D3-induced cathelicidin. SC-58125 also inhibited TLR-dependent NF-κB activation. Conversely, treatment with Cox-derived prostanoids PGD2 or 15-deoxy-Δ12,14-PGJ2 induced hBD3 or hBD2 and hBD3, respectively. The functional significance of these observations was seen in NHEKs that showed reduced anti-staphylococcal activity when treated with a Cox-2 inhibitor. These findings demonstrate a critical role for Cox-2 in hBD production and suggest that the use of Cox-2 inhibitors may adversely influence the risk for bacterial infection.
Skin is the largest organ of the body and our first line of defense against infection from invading pathogens. In addition to providing a physical barrier, epidermal keratinocytes are also key elements of the immune system that act both as a detection system and as effectors of inflammatory responses. For example, keratinocytes express several TLRs (1, 2). TLR activation in keratinocytes initiates the release of several cytokines and chemokines that stimulate and recruit NK cells and T lymphocytes to the site of infection (3) and can also enhance the ability of Langerhans cells to present Ag (4). Furthermore, keratinocytes function directly as effectors against microbial invasion by producing a wide variety of antimicrobial peptides (AMPs) that are essential for immune defense against microbial infection (5).
The production of AMPs belonging to the β-defensin and cathelicidin families have been previously shown to be directly responsible for the capacity of keratinocytes to inhibit or kill pathogenic microbes such as some species of bacteria and viruses (5–7). Of the three β-defensins produced, the constitutively expressed human β-defensin (hBD)-1 and the inducible hBD2 are more active against Gram-negative bacteria, whereas the inducible hBD3 is active against Gram-positive bacteria (8). These amphipathic molecules have an overall net positive charge, which allows them to directly interact and penetrate the negatively charged cell membrane of microbes. Although AMPs can directly kill pathogens, they also exhibit cross-talk between the innate and adaptive immune system by stimulating the release of cytokines and chemokines, recruiting other immune cells, and acting as adjuvants for Ab production (9, 10). The physiological significance of these observations has been seen in studies of abnormal AMP regulation that leads to the pathogenesis of human diseases (11–14). However, even as the knowledge of AMP expression and function advances, these natural antibiotics are still regulated by an incompletely understood process.
Cyclooxygenase (Cox) enzymes are well known to influence inflammatory events, but their participation in antimicrobial events in the skin has not been fully elucidated. Cox enzymes regulate the inflammatory response by generating prostanoids, which include PGs, prostacyclin, and thromboxane and exist in two isoforms. The constitutively expressed Cox-1 promotes tissue homeostasis, while the inducible isoform Cox-2 is upregulated during inflammation and cancer (15). Cox-2 can be constitutively expressed in some tissues, including the kidney, bladder, lung, heart, and human skin. While Cox-2 was thought to mostly reside in structural cells, it is abundant in many immune cells such as T cells, macrophages, and B cells (16, 17). Cox activity has been shown to have both positive and negative immune regulatory roles. PGE2 was shown to inhibit alveolar macrophage phagocytosis of Escherichia coli and Kleb-siella pneumoniae (18), and misoprostol, a PGE2 pharmacomimetic, was shown to impair TNF-α–induced hBD2 and hBD4 production in human uterine epithelial cells (19). Recently, it was discovered that nonsteroidal anti-inflammatory drugs (NSAIDs) that exert their effects through the inhibition of the Cox enzymes attenuate Ab production, suggesting that Cox activity is necessary for optimal adaptive immune responses (20). It has also been speculated that NSAID use may be associated with infectious disease (21–25). However, determining a causal relationship between NSAIDs and infectious disease has been problematic because retrospective studies often lack proper controls and NSAIDs can mollify symptoms, which may mask disease severity (21, 26). Thus, despite some suggestive evidence, the role of Cox-2 in the antimicrobial response, or the role of NSAIDs in promoting infectious disease in the skin, remains unclear.
In the present study, we investigated the in vitro effects of Cox inhibitors on AMP expression and bacteriocidal activity in normal human keratinocytes. We found that TLR3 and TLR2/6 ligands induce Cox-2, and that inhibiting Cox-2 abrogates TLR ligand-induced hBD2 and hBD3 induction and attenuates anti-staphylococcal activity. Cox-2 small interfering RNA (siRNA) also attenuated TLR ligand- and UVB-induced hBD2 and hBD3. Furthermore, we demonstrated that two Cox-derived lipid mediators, PGD2 and 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2), stimulate hBD expression in keratinocytes, suggesting a new role for PGs in enhancing AMP production and the subsequent innate immune response.
Materials and Methods
Reagents
The following reagents were purchased from Cayman Chemical (Ann Arbor, MI): PGE2, PGF2a, iloprost, U-46619, PGD2, 15d-PGJ2, aspirin, SC-560, SC-58125, NS-398, BAY-11-7082 (BAY), and CAY10470 (CAY). Polyinosinic-polycytidylic acid [poly(I:C)] (a synthetic analog of dsRNA) was purchased from InvivoGen (San Diego, CA). Macrophage-activating lipopeptide-2 (MALP-2) was purchased from Enzo Life Sciences (Ply-mouth Meeting, PA). 1α,25-dihydroxyvitamin D3 (1,25-D3) was purchased from Sigma-Aldrich (St. Louis, MO). Cox-2 ON-TARGETplus SMARTpool siRNA constructs were purchased from Thermo Fisher Scientific (Chicago, IL).
Cell culture conditions
Normal human epidermal keratinocytes (NHEKs) were grown in serum-free EpiLife cell culture media (Cascade Biologics, Portland, OR) containing 0.06 mM Ca2+ and 1× EpiLife defined growth supplement at 37°C under standard tissue culture conditions. The cultures were maintained for up to four passages in this media with the addition of 50 U/ml penicillin and 50 μg/ml streptomycin. Cells were treated at 70–80% confluence.
Cox inhibitor experiments
NHEKs were preincubated with the Cox inhibitors aspirin (100 μM), SC-58125 (10 μM), NS-398 (10 μM), or SC-560 (10 μM) for 0.5–1 h prior to stimulation. NHEKs were stimulated with poly(I:C) (10 μg/ml), MALP-2 (500 ng/ml), or 1,25-D3 (100 nM) or were treated with prostanoids, PGE2 (10 μM), PGF2a (10 μM), iloprost (10 μM), U-46619 (10 μM), PGD2 (1 μM), or 15d-PGJ2 (500 nM) for 24 h. NHEKs were irradiated by UVB at 15 mJ/cm2.
Cox-2 siRNA
NHEKs were transfected with 3 μM Cox-2 siRNA constructs using DharmaFECT (Thermo Fisher Scientific) transfection reagent. Cells were incubated for 24 h and the transfection was repeated. Twenty-four hours after the last transfection, cells were treated with TLR ligands or UVB as previously described.
Real-time quantitative RT-PCR
Total RNA was extracted by using TRIzol Reagent (Invitrogen, Carlsbad, CA). One microgram of total RNA was used for cDNA synthesis by the iSCRIPT cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Real-time RT-PCR was conducted in an ABI PRISM 7000 sequence detector (Applied Biosystems, Carlsbad, CA). The primers and probes used for real-time RT-PCR were purchased from Applied Biosystems. RNA analysis was performed using the TaqMan Master Mix reagents kit (Applied Biosystems). The quantification of gene expression was determined by the comparative ΔΔCT method. The target gene expression in the test samples was normalized to the endogenous reference GAPDH level and was reported as the fold difference relative to the GAPDH gene expression. All of the assays were performed in triplicate and repeated at least three times.
Western blotting
NHEKs were stimulated with poly(I:C) (10 μg/ml), MALP-2 (500 ng/ml), or MALP-2 (500 ng/ml) plus 1,25-D3 (100 nM) for 24 h to analyze Cox-1 and Cox-2 protein. Cells were lysed in 1× RIPA buffer with protease inhibitors. NHEKs were preincubated with SC-58125 (10 μM) and then treated with poly(I:C) or MALP-2 for 60 min to analyze RelA/p65 and lamin B1 protein. Nuclear lysates were separated from cytoplasmic lysates using a PARIS kit (Ambion, Austin, TX). Ten percent gels were run and transferred onto polyvinylidene difluoride transfer membranes (Millipore, Bedford, MA). Membranes were blocked with the Odyssey infrared imaging system blocking buffer (LI-COR, Lincoln, NE) then incubated with anti–Cox-1 (Cayman Chemical), anti–Cox-2 (Cayman Chemical), anti-RelA/p65 (Santa Cruz Biotechnology, Santa Cruz, CA), or anti-lamin B1 (Abcam, Cambridge, MA) primary Abs in 5% blocking buffer for 2 h at room temperature. Membranes were washed and incubated with goat anti-rabbit IRDye 680 or goat anti-mouse IRDye 800CW secondary Abs (LI-COR) for 30 min at room temperature. Membranes were washed and fluorescence was detected using the Odyssey infrared imaging system (LI-COR). Western blotting for each protein was repeated at least three times.
Enzyme immunoassay
Supernatants were assayed by the PGE2 or PGD2 enzyme immunoassay (EIA) from Cayman Chemical according to the manufacturer’s instructions. This assay was performed in triplicate and repeated three times.
Fluorescence microscopy
NHEKs were grown on chamber slides and treated as described in Results. Cells were fixed in 2% formaldehyde for 15 min, washed with 1× PBS, blocked in 3% BSA for 30 min, and stained with a rabbit anti-p65 Ab (Santa Cruz Biotechnology) (1/200) or rabbit IgG for 2 h at room temperature. Cells were then washed with 1× PBS stained with Alexa Fluor 568 goat anti-rabbit IgG (1/1000) for 1 h at room temperature. Cells were washed with 1× PBS, mounted in ProLong anti-fade reagent containing DAPI (Molecular Probes, Eugene, OR) and evaluated with an Olympus BX41 microscope (Olympus, Mellville, NY) at original magnification of × 400. Levels of mean fluorescence intensity (MFI) were measured with ImageJ (National Institutes of Health, Bethesda, MD).
Bacterial extract preparation and bacterial killing assay
Staphylococcus aureus strain ΔmprF was a gift from A. Peschel (Microbial Genetics, University of Tubingen, Tubingen, Germany), and strain Sa113 was a gift from Dr. Victor Nizet (University of California, San Diego, CA). Bacteria were grown in tryptic soy broth at 37°C for 15–16 h and collected in the log phase. The number of S. aureus was enumerated by applying a conversion factor (2.0 × 108 bacteria/ml = 0.6 OD U at 600 nm). Bacteria (CFU) was diluted in 3% tryptic soy broth in DMEM (Invitrogen) and sonicated keratinocytes were added at a multiplicity of infection (MOI) of 20 and incubated at 37°C for 1, 3, and 5 h. After incubation, 10-fold dilutions were prepared and plated on tryptic soy broth agar and the plates were incubated for 1 d at 37°C. All assays were performed in duplicate and repeated at least three times.
Statistical analysis
To determine statistical significance between groups, comparisons were made using two-tailed t tests. Analyses of multiple groups were done by one-way or two-way ANOVA using GraphPad Prism version 4 (GraphPad Software, San Diego, CA). For all statistical tests, a p value of <0.05 was accepted for statistical significance.
Results
Cox-2 inhibitors attenuate β-defensin production
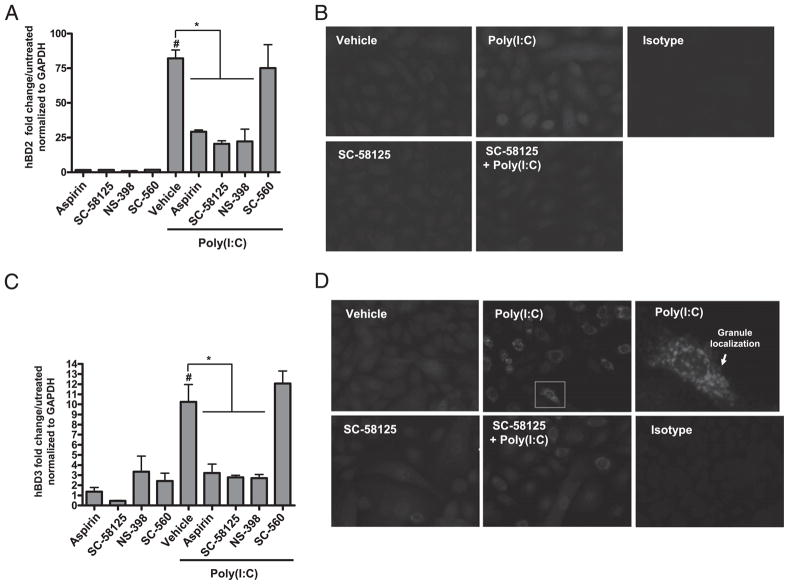
The essential role of keratinocytes in innate immune responses (27), and prior observations that Cox-2 influences immunity (17, 28), led us to the hypothesis that Cox-2 plays a role in hBD production by keratinocytes. NHEKs were preincubated for 30 min with inhibitors of either Cox-1, Cox-2, or Cox-1 and Cox-2, then treated with the TLR3 ligand poly(I:C), a potent stimulus of hBD2 and hBD3 (29). After 24 h, hBD2 and hBD3 mRNA expression was analyzed by quantitative RT-PCR. Aspirin (a Cox-1/ Cox-2 dual inhibitor), NS-398 (a Cox-2 selective inhibitor), and SC-58125 (a Cox-2 selective inhibitor) attenuated poly(I:C)-induced hBD2 mRNA expression, whereas SC-560 (a Cox-1 selective inhibitor) failed to attenuate hBD2 (Fig. 1A). NHEKs treated with the Cox-2 selective inhibitor SC-58125 also showed decreased poly(I:C)-induced hBD2 protein expression as evaluated by fluorescence microscopy at 24 h (Fig. 1B). A similar dependence on Cox-2 activity was also seen for hBD3 mRNA and protein expression induced by poly(I:C) (Fig. 1C, 1D).
FIGURE 1.
Cox inhibitors attenuate poly(I:C)-induced β-defensins. A, NHEKs were pretreated with aspirin (100 μM), SC-58125 (10 μM), NS-398 (10 μM), or SC-560 (10 μM) for 30 min and then treated with poly(I:C) (10 μg/ml) for 24 h. Poly(I:C) induces hBD2 mRNA expression ~75-fold over untreated when normalized to GAPDH levels (#p < 0.05). Aspirin, SC-58125, and NS-398 significantly attenuated poly(I:C)-induced hBD2 mRNA expression (*p < 0.05). SC-560 failed to attenuate poly(I:C)-induced hBD2 mRNA expression. B, Poly(I:C)-induced hBD2 protein expression was attenuated by pretreating NHEKs with SC-58125 (10 μM) as demonstrated by fluorescence microscopy (original magnification ×400): vehicle (MFI = 0.3), poly(I:C) (MFI = 13.0), SC-58125 (MFI = 0.8), SC-58125 plus poly(I:C) (MFI = 13.9). C, NHEKs were pretreated with aspirin (100 μM), SC-58125 (10 μM), NS-398 (10 μM), or SC-560 (10 μM) for 30 min and then treated with poly(I:C) (10 μg/ml) for 24 h. Poly(I:C) induced hBD3 mRNA expression ~10-fold over untreated when normalized to GAPDH levels (#p < 0.05). Aspirin, SC-58125, and NS-398 significantly attenuated poly(I:C)-induced hBD3 mRNA expression (*p < 0.05). SC-560 failed to attenuate poly(I:C)-induced hBD3 mRNA expression. D, hBD3 protein expression was attenuated by pretreating NHEKs with SC-58125 (10 μM) as demonstrated by fluorescence microscopy (original magnification ×400): vehicle (MFI = 106), poly(I:C) (MFI = 104), SC-58125 (MFI = 34), SC-58125 plus poly(I:C) (MFI = 54). A single NHEK stained for hBD3 is highlighted by the white box in the poly(I:C) panel and amplified to demostrate hBD3 localization. Cells in B and D were stained with defensin primary Abs and an Alexa Fluor 568 secondary Ab.
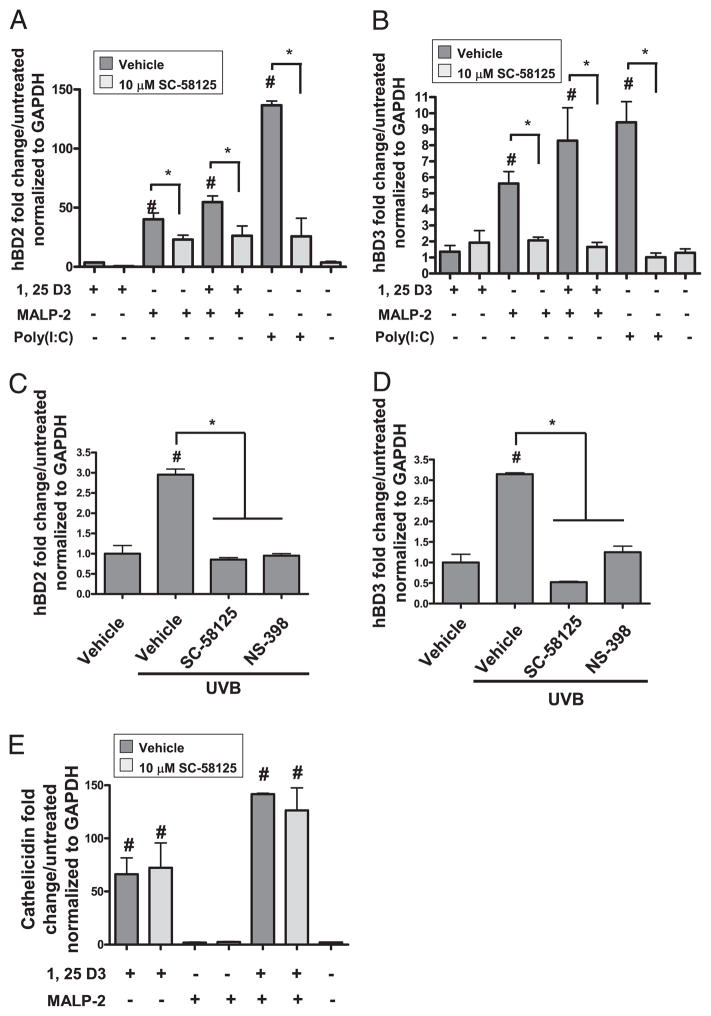
To determine whether Cox-2 activity was necessary for optimal AMP production in response to other AMP-inducing stimuli, we next tested the effect of Cox-2 inhibition on AMP expression by 1,25-D3, a potent inducer of cathelicidin (CAMP), a TLR2/6 ligand MALP-2, and UVB. UVB induces hBD2 and hBD3 (30, 31) and was previously shown to induce Cox-2 and PGE2 in human keratinocytes (32, 33). Twenty-four hours following stimulation, hBD2, hBD3, and cathelicidin mRNA expression were analyzed by quantitative RT-PCR. Inhibition of Cox-2 by SC-58125 attenuated hBD2 and hBD3 mRNA induced by MALP-2 or by MALP-2 plus 1,25-D3 (Fig. 2A, 2B). hBD2 and hBD3 mRNA induced in NHEKs stimulated with 15 mJ/cm2 UVB was suppressed by either Cox-2 inhibitor NS-398 or SC-58125 (Fig. 2C, 2D). In contrast to hBD-inducing stimuli, SC-58125 failed to inhibit 1,25-D3–induced cathelicidin mRNA expression (Fig. 2E).
FIGURE 2.
Cox-2 inhibitors attenuate TLR ligand-induced β-defensins. A, NHEKs were pre-treated with SC-58125 (10 μM) and then treated with 1,25-D3 (500 nM), MALP-2 (500 ng/ml), 1,25-D3 (500 nM) plus MALP-2 (500 ng/ml), or poly(I:C) (10 μg/ml) for 24 h. MALP-2, MALP-2 plus 1,25-D3, and poly(I:C) significantly induced hBD2 (#p < 0.05), and SC-58125 significantly attenuated this induction (*p < 0.05). B, MALP-2, MALP-2 plus 1,25-D3, and poly(I:C) significantly induced hBD3 (#p < 0.05), and SC-58125 significantly attenuated this induction (*p < 0.05). C, NHEKs were pre-treated with SC-58125 (10 μM) or NS-398 (10 μM) and then exposed to UVB (15 mJ/cm2). hBD mRNA expression was measured after 24 h. UVB induced hBD2 mRNA ~3-fold (#p < 0.05). Cox-2 selective inhibitors significantly attenuated UVB-induced hBD2 mRNA (*p < 0.05). D, UVB induced hBD3 mRNA ~3-fold (#p < 0.05). Cox-2 selective inhibitors significantly attenuated UVB-induced hBD3 mRNA (*p < 0.05). E, 1,25-D3, 1,25-D3 plus SC-58125, MALP-2 plus 1,25-D3, and MALP-2 plus 1,25-D3 plus SC-58125 significantly induced cathelicidin mRNA expression (#p < 0.05). SC-58125 failed to inhibit 1,25-D3 or 1,25-D3 plus MALP-2–induced cathelicidin mRNA expression.
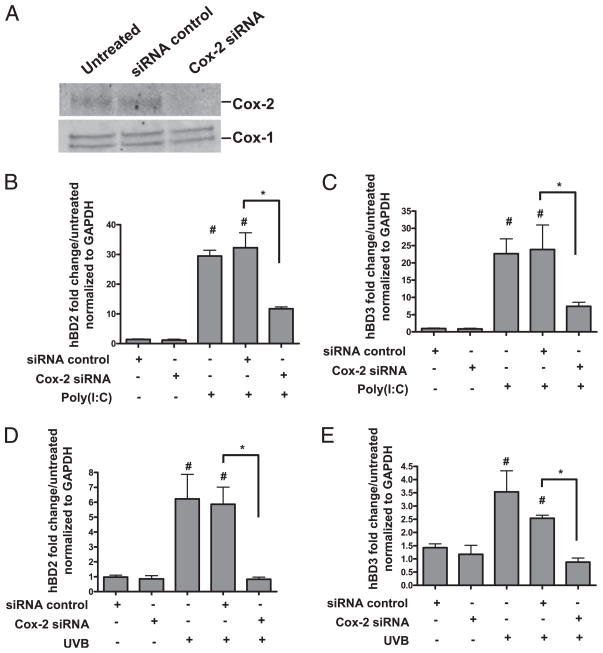
In addition to pharmacological inhibition of Cox-2, Cox-2 was reduced by targeted knockdown by RNA interference (Fig. 3A). NHEKs that were thus made deficient in Cox-2 protein demonstrated a reduced ability to induce hBD2 (Fig. 3B) and hBD3 (Fig. 3C) in response to poly(I:C). Consistent with these findings, Cox-2–deficient NHEKs also demonstrated a reduced ability to induce hBD2 (Fig. 3D) and hBD3 (Fig. 3E) in response to UVB.
FIGURE 3.
Cox-2 siRNA attenuates TLR ligand-induced β-defensins. NHEKs were transfected with 3 μM Cox-2 siRNA at 0 h and then again at 24 h. Twenty-fours after the last tranfection cells were harvested for Western blotting or treated with AMP-inducing stimuli for 24 h. A, Transfection of Cox siRNA constructs knocked-down Cox-2 protein by >99%. B, Poly(I:C) significantly induced hBD2 mRNA ~30-fold (#p < 0.05). Cox-2 siRNA significantly attenuated poly(I:C)-induced hBD2 mRNA (*p < 0.05). C, Poly(I:C) significanlty induced hBD3 mRNA ~20-fold (#p < 0.05). Cox-2 siRNA significantly attenuated poly(I:C)-induced hBD3 mRNA (*p < 0.05). D, UVB induced hBD2 mRNA ~6-fold (#p < 0.05). Cox-2 siRNA significantly attenuated UVB-induced hBD2 mRNA (*p < 0.05). E, UVB induced hBD3 mRNA ~3-fold (#p < 0.05). Cox-2 siRNA significantly attenuated UVB-induced hBD3 mRNA (*p < 0.05).
TLR ligands induce Cox-2 mRNA protein and activity
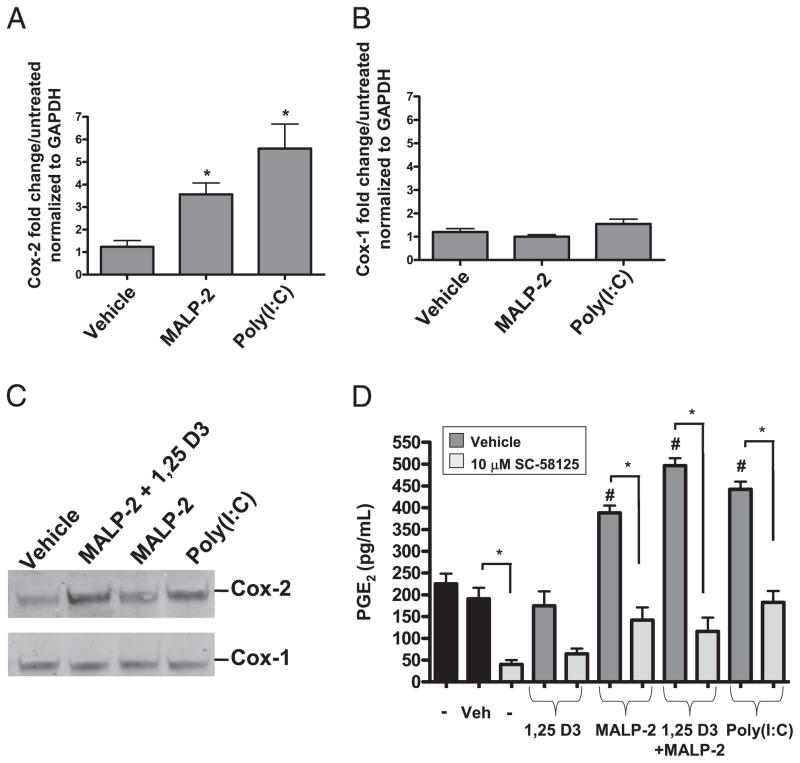
Because inhibition of Cox-2 suppressed optimal hBD2 and hBD3 production, we hypothesized that TLR ligands could influence Cox-2 mRNA and protein expression in keratinocytes. Cox-2 and Cox-1 mRNA expression was measured by quantitative RT-PCR after 24 h of MALP-2 or poly(I:C) stimulation. MALP-2 induced Cox-2 mRNA ~4-fold and poly(I:C) induced Cox-2 mRNA ~6-fold (Fig. 4A). Cox-1 mRNA was not induced by MALP-2 or poly(I:C) treatment (Fig. 4B). The increase in Cox-2 mRNA expression corresponded with an increase in Cox-2 protein abundance as observed by Western blotting, while Cox-1 protein levels remained unchanged under these conditions (Fig. 4C).
FIGURE 4.
TLR ligands induce Cox-2 mRNA protein and activity. A, NHEKs were treated with MALP-2 (500 ng/ml) or poly(I:C) (10 μg/ml) for 24 h. MALP-2 and poly(I:C) significantly induced Cox-2 mRNA (*p < 0.05). B, MALP-2 and poly (I:C) failed to induce Cox-1 mRNA. C, MALP-2 (500 ng/ml) plus 1,25-D3 (500 nM), MALP-2 (500 ng/ml), and poly(I:C) (10 μg/ml) induced Cox-2 protein, but not Cox-1 protein. Western blot is representative of three separate experiments. D, NHEKs were pretreated with SC-58125 (10 μM) followed by treatment with 1,25-D3 (500 nM), MALP-2 (500 ng/ml), 1,25-D3 (500 nM) plus MALP-2 (500 ng/ml), or poly(I:C) (10 μg/ml) for 24 h. Supernatants were collected and PGE2 was assayed by EIA for a measure of Cox activity. MALP-2 (500 ng/ml) plus 1,25-D3 (500 nM) plus MALP-2 (500 ng/ml) and poly(I:C) (10 μg/ml) induced PGE2 release (#p < 0.05). This release is attenuated with SC-58125 treatment (*p < 0.05). SC-58125 significantly attenuated basal level PGE2 release as shown comparing the first two bars (black) with the third bar (gray) (*p < 0.05).
We next evaluated PGE2, a product of Cox-2 enzymatic activity, in NHEKs activated by ligands that enhanced Cox-2 mRNA and protein abundance. Unstimulated NHEKs released ~225 pg/ml PGE2. MALP-2, MALP-2 plus 1,25-D3, and poly(I:C), but not 1,25-D3, all significantly induced PGE2 release when compared with both untreated and vehicle-treated NHEKs, and this induction was abrogated upon preincubation with SC-58125 (Fig. 4D). Collectively, these data suggest that Cox-2 is necessary for keratinocytes to respond to UVB, TLR3, and TLR2/6 ligands by increasing β-defensin expression, but not cathelicidin expression.
Cox-2–derived PGs induce β-defensins
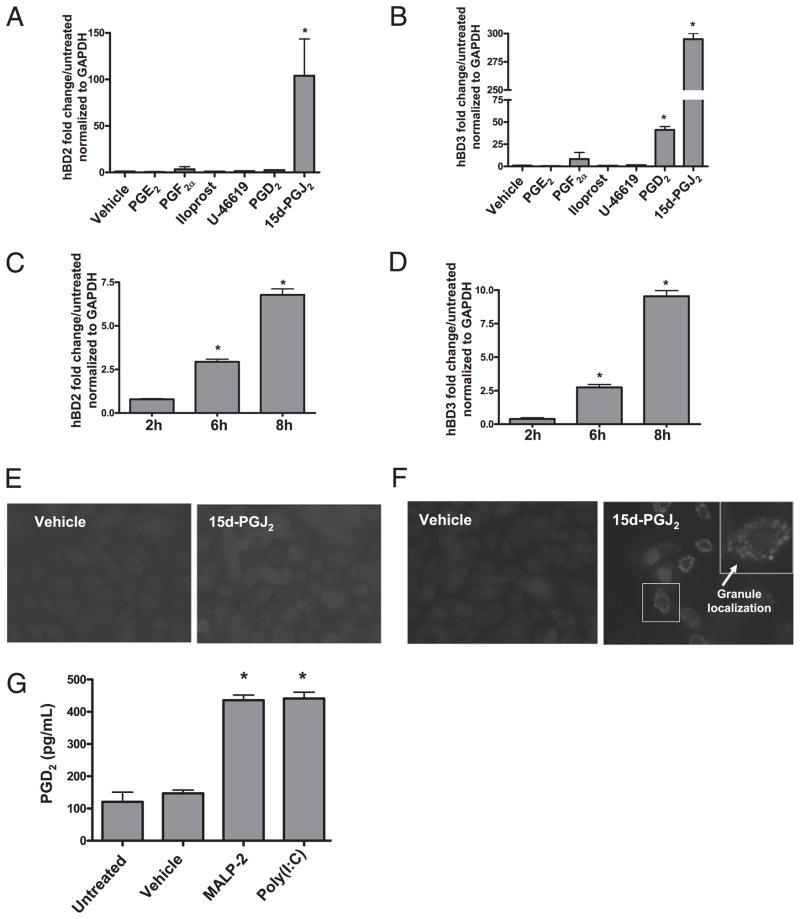
Because Cox-2 inhibitors significantly attenuate hBD2 and hBD3 induction, we next investigated whether prostanoid products of Cox-2 could induce AMP expression. NHEKs were stimulated with PGE2, PGF2α, iloprost (a stable analog of PGI2), U-46619 (a thromboxane A2 receptor agonist), PGD2, or 15d-PGJ2. Concentrations of these prostanoids were selected that had no apparent effect on cell viability as evaluated by visual observation of growth in culture. PGE2, PGF2α, iloprost, and U-46619 failed to induce hBD2 and hBD3 at concentrations of 100 nM, 500 nM, 1 μM (data not shown), and 10 μM (Fig. 5A, 5B). 15d-PGJ2 induced hBD2 mRNA expression ~100-fold at 24 h (Fig. 5A). PGD2 and 15d-PGJ2 induced hBD3 mRNA expression ~40-fold and 300-fold at 24 h (Fig. 5B). A time course experiment demonstrated that 15d-PGJ2 significantly induced hBD2 (Fig. 5C) and hBD3 (Fig. 5D) mRNA between 2 and 6 h. Additionally, 15d-PGJ2 in-creased the expression of hBD2 (Fig. 5E) and hBD3 (Fig. 5F) protein after 24 h.
FIGURE 5.
Cox-2–derived PGs induce β-defensins. NHEKs were stimulated with PGE2, PGF2a, iloprost (a stable analog of PGI2), U-46619 (a thromboxane A2 receptor agonist), PGD2, or 15d-PGJ2 for 24 h. A, 15d-PGJ2 induced hBD2 mRNA expression ~100-fold (*p < 0.05). B, PGD2 and 15d-PGJ2 induced hBD3mRNA expression ~40-fold and 300-fold (*p < 0.05). C, 15d-PGJ2 significantly induced hBD2 mRNA between 2 and 6 h (*p < 0.05). D, 15d-PGJ2 significantly induced hBD3 mRNA between 2 and 6 h (*p < 0.05). E, Fluorescence microscopy of hBD2 immunostaining in vehicle-treated (MFI = 0.3) and 15d-PGJ2–treated (MFI = 157) NHEKs. Cells were stained with a defensin primary Ab and an Alexa Fluor 568 secondary Ab. Original magnification ×400. Data are representative of three separate experiments. F, Fluorescence microscopy of hBD3 immunostaining in vehicle-treated (MFI = 107) and 15d-PGJ2–treated (MFI = 167) NHEKs. Cells were stained with a defensin primary Ab and an Alexa Fluor 568 secondary Ab. Original magnification ×400. Data are representative of three separate experiments. Arrows point to granule localization of hBD3. G, NHEKs were treated with MALP-2 (500 ng/ml) or poly(I:C) (10 μg/ml) for 24 h. Supernatants were collected and PGD2 was assayed by EIA. MALP-2 (500 ng/ml) and poly(I:C) (10 μg/ml) induced PGD2 release ~4-fold (*p < 0.05).
To examine whether keratinocytes induce PGD2 in response to TLR ligands, NHEKs were stimulated with poly(I:C) or MALP-2 for 24 h and PGD2 release was measure in the supernatant by EIA. Both poly(I:C) and MALP-2 induced PGD2 ~4-fold (Fig. 5G).
Inhibition of Cox-2 attenuates TLR ligand-induced NF-κB activation
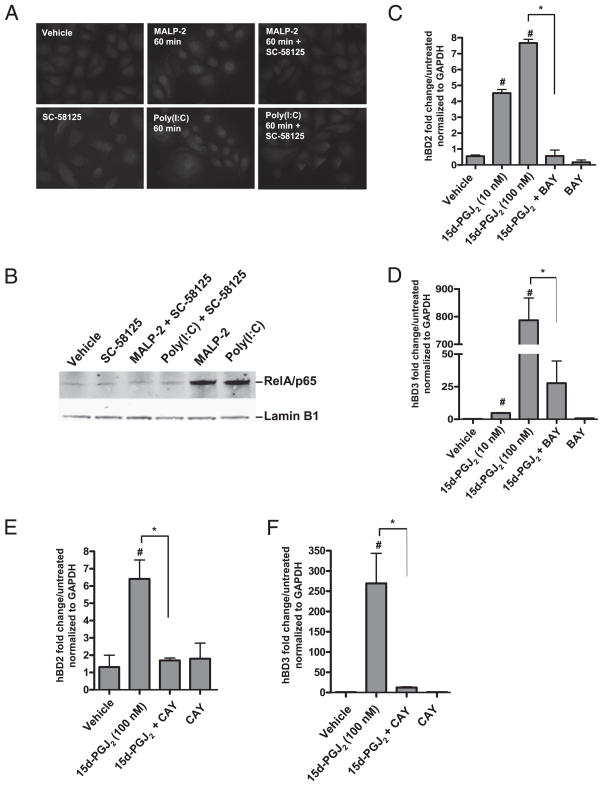
NF-κB activity is involved in the regulation of hBDs (34, 35). To determine whether Cox-2 inhibition influences NF-κB activation in keratinocytes, NHEKs were preincubated with SC-58125, then treated with poly(I:C) or MALP-2 for 5, 15, 30, or 60 min, at which time cells were stained for RelA/p65. Nuclear localization of RelA/p65 was analyzed by both fluorescence microscopy and Western blotting. As expected, MALP-2 and poly(I:C) promoted the nuclear translocation of RelA/p65 by 30 min (data not shown) and RelA/p65 remained in the nucleus at 60 min (Fig. 6A). Pre-incubation with SC-58125 attenuated both MALP-2– and poly(I:C)-induced nuclear translocation of RelA/p65 at 60 min (Fig. 6A). Western blotting-purified nuclear lysates revealed a similar effect and demonstrated that preincubation with SC-58125 inhibited MALP-2– and poly(I:C)-induced RelA/p65 nuclear localization at 60 min (Fig. 6B).
FIGURE 6.
Inhibition of Cox-2 attenuates TLR ligand-induced NF-κB activation. A, Fluorescence microscopy shows RelA/ p65 localization. NHEKs were pretreated with SC-58125 and then treated with MALP-2 or poly(I:C) for 60 min. SC-58125 attenuated RelA/p65 nuclear localization. Cells were stained with a p65 primary Ab and an Alexa Fluor 568 secondary Ab. Orignal magnification ×400. Results are representative of three separate experiments. B, Western blot of nuclear lysates of NHEKs pretreated with SC58125 for 30 min followed by treatment with MALP-2 or poly (I:C) for 60 min. MALP-2– and poly(I:C)-induced nuclear translocation of RelA/p65 was attenuated with SC58125. Lamin B1 was used as a nuclear lysate loading control. C, NHEKs were pretreated with BAY (1 μM) for 30 min followed by treatment with 15d-PGJ2. 15d-PGJ2 dose-dependently induced hBD2 mRNA (#p < 0.05). BAY significantly attenuated 15d-PGJ2–induced hBD2 mRNA (*p < 0.05). D, NHEKs were pretreated with BAY (1 μM) for 30 min followed by treatment with 15d-PGJ2. 15d-PGJ2 dose-dependently induced hBD3 mRNA (#p < 0.05). BAY significantly attenuated 15d-PGJ2–induced hBD3 mRNA (*p <0.05). E, NHEKs were pretreated with CAY (1 μM) for 30 min followed by treatment with 15d-PGJ2. 15d-PGJ2 dose-dependently induced hBD2 mRNA (#p < 0.05). CAY significantly attenuated 15d-PGJ2–induced hBD2 mRNA (*p < 0.05). F, NHEKs were pretreated with CAY (1 μM) for 30 min followed by treatment with 15d-PGJ2. 15d-PGJ2 dose-dependently induced hBD3 mRNA (#p < 0.05). CAY significantly attenuated 15d-PGJ2–induced hBD3 mRNA (*p < 0.05).
Cox-2 inhibition reduced RelA/p65 nuclear localization and, therefore, we next wanted to test whether the ability of 15d-PGJ2 to induce hBD2 and hBD3 was dependent on NF-κB activity. 15d-PGJ2 induced RelA/p65 nuclear localization by 30 min in NHEKs (Supplemental Fig. 1). NHEKs were pretreated with the NF-κB inhibitors BAY (1 μM) or CAY (1 μM) for 30 min prior to 15d-PGJ2 addition. BAY and CAY both block IκB-α phosphorylation and inhibited 15d-PGJ2–induced RelA/p65 nuclear translocation (data not shown). Pretreatment with BAY attenuated 15d-PGJ2–induced hBD2 (Fig. 6C) and hBD3 (Fig. 6D) mRNA expression, and pretreatment with CAY attenuated 15d-PGJ2–induced hBD2 (Fig. 6E) and hBD3 (Fig. 6F) mRNA expression, suggesting that the mechanism of hBD induction was partially dependent on NF-κB activity.
Cox-2 enhances S. aureus killing
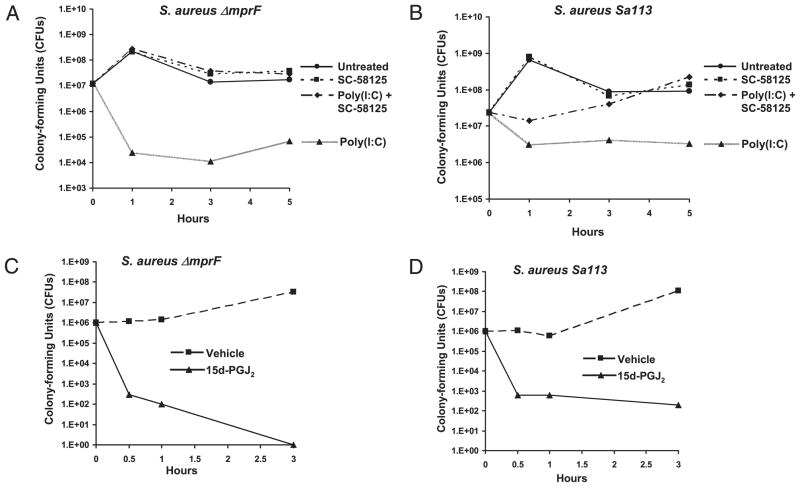
Staphylococci are prevalent bacterial skin inhabitants and are resistant to AMPs in part due to the expression of mprF, which modifies anionic membrane lipids (36). However, the expression of hBD3 is a critical determinant of the anti-staphylococcal activity of keratinocytes (37). Therefore, we next examined the anti-staphylococcal activity of keratinocytes with inhibited Cox-2 activity to determine whether the prior observations were functionally relevant to the capacity of keratinocytes to act as effectors of innate immune defense. Two different strains of S. aureus were used: strain ΔmprF, a well-defined strain selected for its sensitivity to cationic AMPs because it cannot modify the membrane, and the more resistant Sa113. NHEKs were preincubated with SC-58125, treated with poly(I:C) for 24 h, and then cells were harvested in 1× PBS and sonicated to isolate total soluble antimicrobial factors. These NHEK lysates were then incubated with S. aureus. Assays were done at an MOI of 20 for 1, 3, and 5 h. At 1 h, SC-58125 reduced the anti-staphylococcal activity of poly(I:C)-treated NHEKs by >3 logs in the AMP-sensitive ΔmprF strain, and at 5 h it inhibited the antimicrobial effect by >2 logs (Fig. 7A). A similar, but lesser, magnitude of effect was also seen on anti-staphylococcal activity against the more AMP-resistant Sa113 strain at 3 and 5 h (Fig. 7B).
FIGURE 7.
Cox-2 enhances S. aureus killing. NHEK lysates were incubated with 106 bacteria at an MOI of 20 for 1, 3, or 5 h. Bacteria was quantified after 24 h. NHEKs pretreated with SC-58125 (10 μM) and then treated with poly(I:C) (10 μg/ml) showed a reduced ability to kill S. aureus strains (A) ΔmprF and (B) Sa113 when compared with NHEKs treated with poly(I:C) in the absence of SC-58125. NHEK lysates were incubated with 107 bacteria at an MOI of 20 for 0.5, 1, or 3 h. Bacteria was quantified after 24 h. NHEKs treated with 15d-PGJ2 (500 nM) showed a reduced ability to kill S. aureus strains (C) ΔmprF and (D) Sa113 when compared with vehicle-treated NHEKs. Results are representative of three separate experiments.
Because 15d-PGJ2 stimulated hBD2 and hBD3 in keratinocytes, we next investigated whether 15d-PGJ2 treatment of NHEKs could enhance their bacteriocidal activity. NHEKs were treated with 15d-PGJ2 for 24 h, at which time cells were harvested in 1× PBS and sonicated. NHEK-sonicated lysates were then incubated with S. aureus strains ΔmprF and Sa113 at a MOI of 20 for 0.5, 1, and 3 h. 15d-PGJ2 enhanced the anti-staphylococcal activity of NHEKs by >3 logs in the ΔmprF strain at 0.5 h and completely inhibited bacteria survival at 3 h (Fig. 7C). 15d-PGJ2 enhanced the anti-staphylococcal activity of NHEKs by >3 logs in the Sa113 strain at 0.5 and 1 h and by >5 logs at 3 h (Fig. 7D).
Discussion
Keratinocytes are sentinel to epidermal protection as they are the first barrier against invading organisms and possess potent antimicrobial activity by their release of several molecules, including AMPs such as cathelicidins and β-defensins. Prior work has shown that the production of AMPs by many tissues is essential to defense against infectious microbial pathogens (38). Thus, the mechanisms that regulate AMP expression are an important area of investigation that may impact human diseases such as skin infections, atopic dermatitis, rosacea, and psoriasis (6). In this study, we examined the role of Cox-2 in keratinocyte AMP expression. We found that Cox-2 activity is important for optimal hBD2 and hBD3 production after a variety of stimuli. Moreover, we demonstrated that 15d-PGJ2, a small lipid molecule derived from Cox activity, increases hBD2 and hBD3 production in keratinocytes and enhances antimicrobial action. These uncover a previously unknown role for Cox-2 in controlling the innate immune response of the epidermis and suggest that compounds that influence this pathway may be useful for controlling innate immunity.
Support for a role for Cox-2 in hBD2 and hBD3 expression comes from several independent experimental approaches. We demonstrated that Cox-2 selective inhibitors and knockdown of Cox-2 by siRNA attenuate TLR ligand-induced hBD2 and hBD3 expression (Figs. 1–3) and that TLR3 and TLR2/6 ligands induce Cox-2 to and its product PGE2 in human keratinocytes (Fig. 4). Moreover, we demonstrated that Cox-2 selective inhibitors and Cox-2 siRNA also attenuate UVB-induced hBD2 and hBD3 in human keratinocytes (Figs. 2, 3). UVB was known to induce Cox-2 activity and PGE2 (32, 33), and recently to induce β-defensins (30, 31). These data suggest that Cox-2 induction is necessary for optimal hBD expression after UVB and demonstrate that the suppressive effect of this Cox-2 inhibitor applies to more than TLR ligands. However, the suppressive effect of Cox-2 inhibitors on hBD production reaches a plateau between the 10 and 20 μM dose (data not shown), and Cox siRNA failed to maintain hBDs at the uninduced level, suggesting a partial Cox-2–independent mechanism for hBD induction. Furthermore, we did not see an attenuation of vitamin D-induced human cath-elicidin expression with Cox-2 inhibitors (Fig. 2E). This provides further evidence that human cathelicidin and β-defensins are differentially regulated and that the effects of Cox-2 cannot be generalized to all AMPs. These data also suggest that Cox-2 inhibition would not inhibit UVB-mediated induction of cathelicidin in the skin, as recent data suggests that this is mediated, at least in part, by local synthesis of hormonally active vitamin D.
The addition of the Cox-2 product 15d-PGJ2 dose- (Fig. 6C, 6D) and time-dependently (Fig. 5C, 5D) induced hBD2 and hBD3 expression. Recently, Kanda et al. (39) demonstrated that PGD2 induced a small increase in hBD3 in human keratinocytes. Interestingly, PGD2 and 15d-PGJ2 induce β-defensins more potently than do the TLR ligands, suggesting a differential role for exogenously added versus endogenously generated PGs. 15d-PGJ2 is a dehydration product of PGD2 that, in our experiments, induced hBD3 mRNA >10-fold more than PGD2. 15d-PGJ2 has been shown to have both pro- and anti-inflammatory properties in keratinocytes (40) and is a well-established ligand for the perox-isome proliferator-activated receptor γ (PPARγ) and for inhibiting inflammation at nanomolar and low micromolar doses. However, rosiglitazone, a thiazolidinedione and potent PPARγ activator, failed to induced hBD2 and hBD3 in NHEKs (data not shown). PPARγ did not appear to be the mechanism by which 15d-PGJ2 mediated hBD induction. However, we demonstrated that 15d-PGJ2 induced RelA/p65 nuclear translocation and that inhibiting this event with pharmacological inhibitors of NF-κB abrogated hBD expression, suggesting a greater role for NF-κB. Several reports have described that 15d-PGJ2 induces intracellular oxidative stress independently of PPARγ (41, 42). Therefore, 15d-PGJ2 may induce NF-κB activation by promoting the formation of reactive oxygen species in NHEKs.
Interestingly, although keratinocytes have the capacity to produce both PGD2 and 15d-PGJ2, most of these PGs are released by mast cells in the skin. Mast cells are a potent source of AMPs and can provide protection against infection. Future studies will examine whether mast cell-derived PGs act to cooperate in the epidermal innate immune defense strategy and enhance skin antimicrobial activity.
To better understand the mechanism of Cox-2 on hBD expression, we examined downstream signaling events known to influence expression of these genes. TLR ligand-induced epithelial hBD gene expression has been reported to be mediated, in part, by NF-κB signaling, and NF-κB sites are active in the hBD2 promoter (34, 35). SC-58125 attenuated both poly(I:C)- and MALP-2–induced RelA/p65 nuclear localization (Fig. 6A), suggesting that Cox-2 inhibitors abrogate NF-κB activation. We also found that SC-58125 had no effect on p44/42 MAPK (ERK1/2), p38 MAPK (MapK), or JNK activation (data not shown), as these signaling intermediates represent other reporter regulators of defensin expression (43–46). Our observations with NF-κB suggest this is the most likely canonical pathway from TLR activation that is influenced by Cox-2 products.
We also demonstrated that a Cox-2 selective inhibitor significantly attenuated poly(I:C)-induced keratinocyte S. aureus killing (Fig. 7A, 7B), and that the addition of a prostanoid product of Cox-2, 15d-PGJ2, increased functional antimicrobial action (Fig. 7C, 7D). These functional effects could be the result of multiple antimicrobial effector molecules that are produced by keratinocytes. However, the robust decrease in antimicrobial action after the inhibition of Cox-2 and the increase in antimicrobial action after the addition of a Cox-2 product are evidences consistent with the induction of hBD3, as Kisich et al. (47) showed that secreted keratinocyte hBD3 promotes significant bacteriocidal activity against S. aureus.
Prior to our study, little mechanistic information was known about how Cox-2 inhibitors might influence the innate immune response. With the present observations, there are broad potential clinical applications to consider. NSAIDs and Cox-2 selective inhibitors are widely used to treat both acute and chronic inflammation, and some evidence suggests that NSAID use is linked to bacterial infection (21–25). For example, Factor et al. (23) observed a connection between new NSAID use and group A β-hemolytic streptococcal infection. However, no major effect has clearly been observed on enhanced susceptibility to infection. This lack of a clearly evident change in infection risk is consistent with a selective inhibition of some but not all of the AMPs produced by keratinocytes. Carefully controlled animal and in vitro observations have been necessary to uncover the essential role of AMPs in mammalian immunity (48), but their relative effects in mammals are less obvious compared with the profound defects seen when severe alterations in cellular and humoral immunity occur. Thus, although the anti-inflammatory benefits of Cox inhibition may outweigh risks, these data suggest that NSAID use must be carefully monitored for subtle influences on infection, or under circumstances of compromised immunity or bacterial infection.
Another implication of the present findings is in revealing an alternative system for increasing AMP responses. Infectious and inflammatory diseases are a major health care burden. Although pharmaceutically generated antibiotics can be an effective treatment for infections, the continual emergence of drug-resistant organisms has limited the effectiveness of this approach. Antibiotic resistance poses a tremendous threat to human health, which is best evidenced by methicillin-resistant S. aureus (49). Additionally, background levels of antibiotic-resistant genes continue to rise in soil (50). An alternative interventional strategy for treatment of infection is to enhance native host cell antimicrobial defense. Our results reveal a new mechanism for the involvement of Cox-2 in hBD induction by TLR ligands and indicate that the induction of a specific Cox-2 product, 15d-PGJ2, could be exploited as a new approach for the treatment of infection.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01 AR052728 and R01 AI052453 and a Veterans Affairs Merit Award (to R.L.G.).
Abbreviations used in this paper
- AMP
antimicrobial peptide
- BAY
BAY-11-7082
- CAY
CAY10470
- Cox
cyclooxygenase
- 15d-PGJ2
15-deoxy-Δ12,14-PGJ2
- 1,25-D3
1α,25-dihydroxyvitamin D3
- EIA
enzyme immunoassay
- hBD
human β-defensin
- MALP-2
macrophage-activating lipopeptide-2
- MFI
mean fluorescence intensity
- MOI
multiplicity of infection
- NHEK
normal human epidermal keratinocyte
- NSAID
nonsteroidal anti-inflammatory drug
- poly(I:C)
polyinosinic-polycytidylic acid
- PPARγ
peroxisome proliferator-activated receptor γ
- siRNA
small interfering RNA
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, de Jong EC. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 2.Kawai K, Shimura H, Minagawa M, Ito A, Tomiyama K, Ito M. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J Dermatol Sci. 2002;30:185–194. doi: 10.1016/s0923-1811(02)00105-6. [DOI] [PubMed] [Google Scholar]
- 3.Pivarcsi A, Kemény L, Dobozy A. Innate immune functions of the keratinocytes: a review. Acta Microbiol Immunol Hung. 2004;51:303–310. doi: 10.1556/AMicr.51.2004.3.8. [DOI] [PubMed] [Google Scholar]
- 4.Sugita K, Kabashima K, Atarashi K, Shimauchi T, Kobayashi M, Tokura Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clin Exp Immunol. 2007;147:176–183. doi: 10.1111/j.1365-2249.2006.03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamasaki K, Gallo RL. Antimicrobial peptides in human skin disease. Eur J Dermatol. 2008;18:11–21. doi: 10.1684/ejd.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- 8.Scott MG, Hancock RE. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit Rev Immunol. 2000;20:407–431. [PubMed] [Google Scholar]
- 9.Tani K, Murphy WJ, Chertov O, Salcedo R, Koh CY, Utsunomiya I, Funakoshi S, Asai O, Herrmann SH, Wang JM, et al. Defensins act as potent adjuvants that promote cellular and humoral immune responses in mice to a lymphoma idiotype and carrier antigens. Int Immunol. 2000;12:691–700. doi: 10.1093/intimm/12.5.691. [DOI] [PubMed] [Google Scholar]
- 10.Gallo RL, Huttner KM. Antimicrobial peptides: an emerging concept in cutaneous biology. J Invest Dermatol. 1998;111:739–743. doi: 10.1046/j.1523-1747.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- 11.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2009;124(Suppl 2):R13–R18. doi: 10.1016/j.jaci.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 13.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P, Hovnanian A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 14.Wehkamp J, Fellermann K, Herrlinger KR, Baxmann S, Schmidt K, Schwind B, Duchrow M, Wohlschläger C, Feller AC, Stange EF. Human β-defensin 2 but not β-defensin 1 is expressed preferentially in colonic mucosa of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2002;14:745–752. doi: 10.1097/00042737-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Rajakariar R, Yaqoob MM, Gilroy DW. COX-2 in inflammation and resolution. Mol Interv. 2006;6:199–207. doi: 10.1124/mi.6.4.6. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Zhang J, Moore SA, Ballas ZK, Portanova JP, Krieg AM, Berg DJ. CpG DNA induces cyclooxygenase-2 expression and prostaglandin production. Int Immunol. 2001;13:1013–1020. doi: 10.1093/intimm/13.8.1013. [DOI] [PubMed] [Google Scholar]
- 17.Iñiguez MA, Punzón C, Fresno M. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J Immunol. 1999;163:111–119. [PubMed] [Google Scholar]
- 18.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 19.Aronoff DM, Hao Y, Chung J, Coleman N, Lewis C, Peres CM, Serezani CH, Chen GH, Flamand N, Brock TG, Peters-Golden M. Misoprostol impairs female reproductive tract innate immunity against. Clostridium sordellii. J Immunol. 2008;180:8222–8230. doi: 10.4049/jimmunol.180.12.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernard MP, Bancos S, Chapman TJ, Ryan EP, Treanor JJ, Rose RC, Topham DJ, Phipps RP. Chronic inhibition of cyclooxygenase-2 attenuates antibody responses against vaccinia infection. Vaccine. 2010;28:1363–1372. doi: 10.1016/j.vaccine.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulla ZD, Gibbs SG. Invasive group A streptococcal disease: race, hypotension, and immunogenetics. Ethn Dis. 2005;15(Suppl 5):S5-63–S5-66. [PubMed] [Google Scholar]
- 22.Stevens DL. Could nonsteroidal antiinflammatory drugs (NSAIDs) enhance the progression of bacterial infections to toxic shock syndrome? Clin Infect Dis. 1995;21:977–980. doi: 10.1093/clinids/21.4.977. [DOI] [PubMed] [Google Scholar]
- 23.Factor SH, Levine OS, Harrison LH, Farley MM, McGeer A, Skoff T, Wright C, Schwartz B, Schuchat A. Risk factors for pediatric invasive group A streptococcal disease. Emerg Infect Dis. 2005;11:1062–1066. doi: 10.3201/eid1107.040900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez BE, Martinez-Aguilar G, Hulten KG, Hammerman WA, Coss-Bu J, Avalos-Mishaan A, Mason EO, Jr, Kaplan SL. Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant. Staphylococcus aureus. Pediatrics. 2005;115:642–648. doi: 10.1542/peds.2004-2300. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Ruiz N, Pentland A, Caparon M. Keratinocyte proinflammatory responses to adherent and nonadherent group A streptococci. Infect Immun. 1997;65:2119–2126. doi: 10.1128/iai.65.6.2119-2126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barton LL. Nonsteroidal anti-inflammatory drugs and invasive staphylococcal infections: the cart or the horse? Pediatrics. 2005;115:1790. doi: 10.1542/peds.2005-0558. [DOI] [PubMed] [Google Scholar]
- 27.Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy-Szabo A, Szell M, Dobozy A, Kemeny L. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human β-defensin-2 in vaginal epithelial cells. Microbes Infect. 2005;7:1117–1127. doi: 10.1016/j.micinf.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Ryan EP, Pollock SJ, Pollock SJ, Murant TI, Bernstein SH, Felgar RE, Phipps RP. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol. 2005;174:2619–2626. doi: 10.4049/jimmunol.174.5.2619. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 30.Gläser R, Navid F, Schuller W, Jantschitsch C, Harder J, Schröder JM, Schwarz A, Schwarz T. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J Allergy Clin Immunol. 2009;123:1117–1123. doi: 10.1016/j.jaci.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 31.Hong SP, Kim MJ, Jung MY, Jeon H, Goo J, Ahn SK, Lee SH, Elias PM, Choi EH. Biopositive effects of low-dose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J Invest Dermatol. 2008;128:2880–2887. doi: 10.1038/jid.2008.169. [DOI] [PubMed] [Google Scholar]
- 32.Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, Pentland AP. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19:723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 33.Chun KS, Akunda JK, Langenbach R. Cyclooxygenase-2 inhibits UVB-induced apoptosis in mouse skin by activating the prostaglandin E2 receptors, EP2 and EP4. Cancer Res. 2007;67:2015–2021. doi: 10.1158/0008-5472.CAN-06-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L, Wang L, Jia HP, Zhao C, Heng HH, Schutte BC, McCray PB, Jr, Ganz T. Structure and mapping of the human β-defensin HBD-2 gene and its expression at sites of inflammation. Gene. 1998;222:237–244. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 35.Wehkamp K, Schwichtenberg L, Schröder JM, Harder J. Pseudomonas aeruginosa- and IL-1β-mediated induction of human β-defensin-2 in keratinocytes is controlled by NF-κB and AP-1. J Invest Dermatol. 2006;126:121–127. doi: 10.1038/sj.jid.5700020. [DOI] [PubMed] [Google Scholar]
- 36.Peschel A, Collins LV. Staphylococcal resistance to antimicrobial peptides of mammalian and bacterial origin. Peptides. 2001;22:1651–1659. doi: 10.1016/s0196-9781(01)00500-9. [DOI] [PubMed] [Google Scholar]
- 37.Menzies BE, Kenoyer A. Signal transduction and nuclear responses in Staphylococcus aureus-induced expression of human β-defensin 3 in skin keratinocytes. Infect Immun. 2006;74:6847–6854. doi: 10.1128/IAI.00389-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallo RL. Sounding the alarm: multiple functions of host defense peptides. J Invest Dermatol. 2008;128:5–6. doi: 10.1038/sj.jid.5701073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanda N, Ishikawa T, Watanabe S. Prostaglandin D2 induces the production of human β-defensin-3 in human keratinocytes. Biochem Pharmacol. 2010;79:982–989. doi: 10.1016/j.bcp.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Kim YI, Lee JW, Lee MH, Park SW, Cho BN, Lee HK. Effects of 15-deoxy-Δ12,14-prostaglandin J2 on the production of IL-8 and the expression of Toll-like receptor 2 in human primary keratinocytes stimulated with lipopolysaccharide. Mol Biol Rep. 2010 doi: 10.1007/s11033-010-9993-5. [DOI] [PubMed] [Google Scholar]
- 41.Kondo M, Shibata T, Kumagai T, Osawa T, Shibata N, Kobayashi M, Sasaki S, Iwata M, Noguchi N, Uchida K. 15-Deoxy-Δ12,14-prostaglandin J2: the endogenous electrophile that induces neuronal apoptosis. Proc Natl Acad Sci USA. 2002;99:7367–7372. doi: 10.1073/pnas.112212599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata T, Yamada T, Ishii T, Kumazawa S, Nakamura H, Masutani H, Yodoi J, Uchida K. Thioredoxin as a molecular target of cyclo-pentenone prostaglandins. J Biol Chem. 2003;278:26046–26054. doi: 10.1074/jbc.M303690200. [DOI] [PubMed] [Google Scholar]
- 43.Kanda N, Watanabe S. Leptin enhances human β-defensin-2 production in human keratinocytes. Endocrinology. 2008;149:5189–5198. doi: 10.1210/en.2008-0343. [DOI] [PubMed] [Google Scholar]
- 44.Scharf S, Hippenstiel S, Flieger A, Suttorp N, N’Guessan PD. Induction of human β-defensin-2 in pulmonary epithelial cells by Legionella pneumophila: involvement of TLR2 and TLR5, p38 MAPK, JNK, NF-κB and AP-1. Am J Physiol Lung Cell Mol Physiol. 2010;298:L687–L695. doi: 10.1152/ajplung.00365.2009. [DOI] [PubMed] [Google Scholar]
- 45.Jang BC, Lim KJ, Paik JH, Kwon YK, Shin SW, Kim SC, Jung TY, Kwon TK, Cho JW, Baek WK, et al. Up-regulation of human β-defensin 2 by interleukin-1β in A549 cells: involvement of PI3K, PKC, p38 MAPK, JNK, and NF-κB. Biochem Biophys Res Commun. 2004;320:1026–1033. doi: 10.1016/j.bbrc.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 46.Krisanaprakornkit S, Kimball JR, Dale BA. Regulation of human β-defensin-2 in gingival epithelial cells: the involvement of mitogen-activated protein kinase pathways, but not the NF-κB transcription factor family. J Immunol. 2002;168:316–324. doi: 10.4049/jimmunol.168.1.316. [DOI] [PubMed] [Google Scholar]
- 47.Kisich KO, Howell MD, Boguniewicz M, Heizer HR, Watson NU, Leung DY. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on β-defensin 3. J Invest Dermatol. 2007;127:2368–2380. doi: 10.1038/sj.jid.5700861. [DOI] [PubMed] [Google Scholar]
- 48.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 49.Reygaert W. Methicillin-resistant Staphylococcus aureus (MRSA): molecular aspects of antimicrobial resistance and virulence. Clin Lab Sci. 2009;22:115–119. [PubMed] [Google Scholar]
- 50.Cantón R. Antibiotic resistance genes from the environment: a perspective through newly identified antibiotic resistance mechanisms in the clinical setting. Clin Microbiol Infect. 2009;15(Suppl 1):20–25. doi: 10.1111/j.1469-0691.2008.02679.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.