Abstract
Background
Most cases of multiple endocrine neoplasia type 2B (MEN-2B) are attributable to a germline methionine to threonine mutation at codon 918 (M918T) of the RET proto-oncogene; very few cases of a germline alanine to phenylalanine mutation at codon 883 (A883F) are reported without a clear description of the clinical course. Nevertheless, RET-A883F is currently considered to be among the highest risk mutations, and prophylactic thyroidectomy is recommended as early as 6 months of life. Further characterization of the clinical behavior of RET-A883F mutation is warranted. We present the clinical data for a family with MEN-2B associated with RET-A883F mutation.
Summary
The proband, a 39-year-old woman, had multifocal medullary thyroid carcinoma (MTC) with cervical lymphadenopathy, but no evidence of distant metastases. She was disease free after surgical resection. She also had bilateral pheochromocytomas and mucosal neuromas leading to the clinical diagnosis of MEN-2B. Genetic testing showed that the woman and her three children (3–5 years old) had the RET-A883F mutation. The children had near-normal calcitonin levels, and none had sonographic evidence of suspicious thyroid nodules or cervical lymphadenopathy.
Conclusion
A family with MEN-2B due to RET-A883F mutation displayed a less aggressive form of MTC than what is usually seen in patients with RET-M918T mutation. RET-A883F mutation could be a lower-risk mutation than previously thought and the current recommendation of prophylactic thyroidectomy in the first year of life may not be warranted. Further reports will help clarify the natural history of MTC caused by this mutation.
Introduction
Activating mutations of the RET proto-oncogene on chromosome 10 q11.2 result in different distinct clinical phenotypes: familial medullary thyroid carcinoma (MTC), multiple endocrine neoplasia type 2A (MEN-2A), and MEN type 2B (MEN-2B). MEN-2A is associated with MTC, hyperparathyroidism, and pheochromocytoma and tends to behave in relatively indolent fashion. Patients affected with MEN-2B develop MTC; pheochromocytoma; neuromas of the lips, tongue, or conjunctiva; and ganglioneuromas of the intestines and often confront more aggressive MTC (1). MEN-2B is estimated to account for <10% of all cases of MEN-2 and is usually associated with higher mortality and morbidity than MEN-2A (2). Children with MEN-2B commonly develop microscopic MTC, with metastasis during the first year of life (2,3).
At least 95% of MEN-2B cases are due to germline methionine-to-threonine mutation at codon 918 (M918T) in exon 16 of the RET proto-oncogene, and very few cases have been reported to be associated with a germline alanine-to-phenylalanine RET mutation at codon 883 (A883F) in exon 15 (4–8).
The RET A883F mutation results from two base substitutions; this may account for its rarity relative to the RET M918T mutation, which results from a single-base substitution (6). MEN-2B patients with the RET A883F mutation have been reported to be indistinguishable clinically from those in whom the disease-causing mutation is RET M918T (6).
Despite the lack of clear clinical data about the natural history of MTC due to RET A883F mutation in the medical literature, it is currently classified among the highest risk mutations (class D) according to the American Thyroid Association updated guidelines for management of medullary thyroid cancer (9). Thus, in patients with this mutation, prophylactic thyroidectomy is recommended within the first 6–12 months of life (9,10).
Here, reported for the first time in the literature are the clinical data of a family with MEN-2B associated with the RET A883F. This report and future cases of this mutation will help to clarify the natural history of MTC associated with the RET A883F mutation and may affect future guidelines for optimal management of this condition.
Patients and Results
The proband is a 39-year-old white woman found to have an incidental thyroid enlargement on a magnetic resonance imaging scan obtained during an evaluation for upper back pain. Initial ultrasound-guided fine-needle aspiration at an outside institution had suggested the possibility of papillary thyroid carcinoma, and the patient underwent a total thyroidectomy with bilateral central neck dissection and limited left sided neck dissection. Pathology of surgical specimens revealed the presence of multifocal MTC involving both thyroid lobes; the largest focus measured 4.5 cm. No evidence of extrathyroidal extension was seen; there was evidence of lymphovascular invasion and C-cell hyperplasia. A total of 6 of 11 neck lymph nodes were positive for MTC. Retrospectively, the patient reported that for the year before her surgery she had experienced intermittent mild diarrhea, which disappeared post-thyroidectomy. Otherwise, her medical history was unremarkable, and she denied experiencing anxiety, hypertension, flushing, sweating, palpitations, or other complaints.
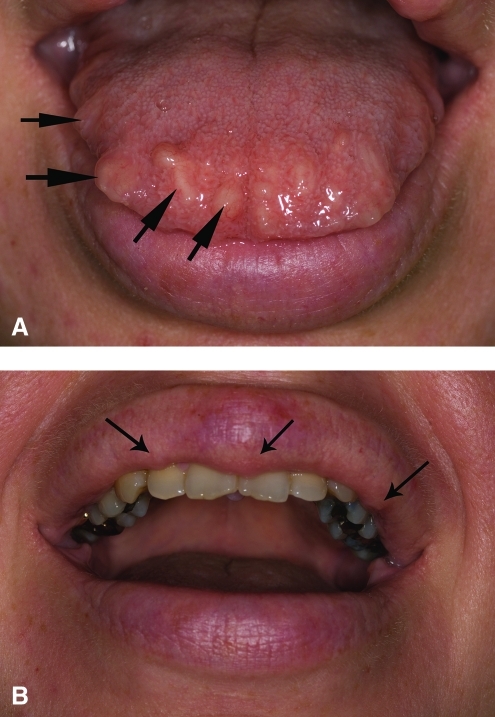
The patient was referred to The University of Texas M.D. Anderson Cancer Center for further evaluation post-thyroidectomy. She was noted to have facial features suggestive of MEN-2B, including mucosal neuromas on the lips, eyelids, and tongue (Fig. 1). Laboratory tests obtained 1 week after surgery showed the following serum levels: calcitonin 28 pg/mL (adult female reference range (RR) < 5 pg/mL), chromogranin-A 11.8 ng/mL (RR < 36.1 ng/mL), plasma free metanephrine 186 pg/mL (RR ≤ 57 pg/mL), plasma free normetanephrine 138 pg/mL (RR < 148 pg/mL), total metanephrine 324 pg/mL (RR < 205 pg/mL), serum calcium 9 mg/dL (RR, 8.6–10.2 mg/dL), and intact parathyroid hormone (PTH) of 37 pg/mL (RR, 10–65 pg/mL).
FIG. 1.
Multiple mucosal neuromas (black arrows) on the proband's tongue (A) and lips (B). Color images available online at www.liebertonline.com/thy.
An abdominal computed tomography (CT) scan revealed a 2.8 × 2.1 cm left adrenal mass with density >44 Hounsfield units on precontrast imaging and demonstrated heterogeneous enhancement on contrast enhanced images. In addition, a 1 cm nodule was seen in the right adrenal gland that had a precontrast density of 33 Hounsfield units. The kidneys demonstrated multiple cysts measuring up to 2.1 cm. No evidence of hepatic or bony metastases was seen.
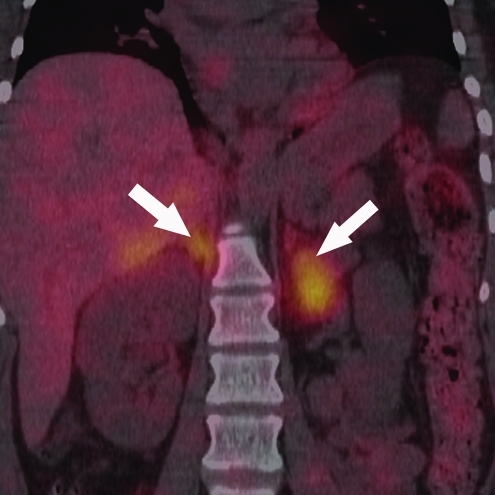
A [123I] metaiodobenzylguanidine (MIBG) scan with single-photon emission computed tomography/CT showed an MIBG-avid lesion in the left adrenal gland and another MIBG-avid lesion in the medial limb of the right adrenal gland, both suggestive of bilateral pheochromocytomas (Fig. 2). After alpha and beta adrenergic blockade, the patient underwent a right adrenalectomy and a left cortical sparing adrenalectomy, which confirmed the presence of bilateral pheochromocytomas.
FIG. 2.
Coronal view of [123I] metaiodobenzylguanidine scans with single-photon emission computed tomography/computed tomography, showing [123I] metaiodobenzylguanidine-avid lesions in both adrenal glands (white arrows). Color images available online at www.liebertonline.com/thy.
On subsequent follow-up, 8 months post-thyroidectomy, the patient had undetectable calcitonin levels, and her neck ultrasonography did not reveal any evidence of cervical lymphadenopathy or other suspicious nodules.
Genetic testing showed that the patient had two nucleotide substitutions in cis configuration in the RET proto-oncogene at codon 883 in exon 15: c.2647G > T (GCT > TCT) and c. 2648C > T (GCT > GTT). Subsequently, her three children also tested positive for the same RET A883F mutation.
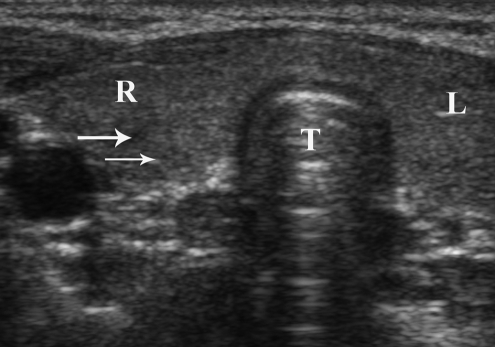
The patient's 5-year-old son had mucosal neuromas on his tongue without other evidence of thyroid nodules or cervical lymphadenopathy on physical examination. He had a calcitonin level of 7.6 pg/mL (adult male RR < 8.4 pg/mL) with normal levels of carcinoembryonic antigen, calcium, intact PTH, and plasma free metanephrines. His neck ultrasonography suggested the presence of small hypoechoic nodules measuring 0.2–0.3 cm, without increased vascular flow or calcification and with no evidence of pathologic cervical lymphadenopathy (Fig. 3). One year after initial evaluation, his calcitonin level remained stable and his neck ultrasonography appearance did not change.
FIG. 3.
Transverse view of neck ultrasonography of the patient's 5-year-old son showing very small hypoechoic nodules (white arrows) without increased vascular flow and no cervical lymphadenopathy (T, Trachea; R, Right thyroid lobe; L, Left thyroid lobe).
The patient's youngest children (3-year-old fraternal twin girls) were phenotypically normal with no clinical evidence of thyroid nodules, cervical lymphadenopathy, mucosal neuromas, or marfanoid features, and had calcitonin levels of 7.7 and 11.6 pg/mL (RR for children <3 years old <15 pg/mL), and normal carcinoembryonic antigen, calcium, intact PTH, and plasma free metanephrines. One year later, their calcitonin levels were 9.4 and 13.9 pg/mL, respectively (RR for age <15 pg/mL), without any obvious thyroid nodules or lymphadenopathy on ultrasonography.
Prophylactic thyroidectomy was extensively discussed with the family, who elected very close monitoring rather than proceeding with surgery.
Discussion
We reported a family that had MEN-2B secondary to the RET A883F mutation and displayed a less aggressive form of MTC than that reported in the literature of families with MEN-2B associated with the RET M918T mutation. MTC associated with MEN-2B has been reported to be the most aggressive form of MTC, with the worst prognosis compared with other forms MTC (11,12). Moreover, many patients display the clinical and phenotypic features of MEN-2B for many years before an accurate diagnosis is made, and this delay in diagnosis may affect the subsequent management and surgical approach (13). Current treatment guidelines are based on genotype–phenotype correlations in mutation carriers, and at present, children with MEN-2B (RET mutations at codon 918 or 883) are considered to have the highest risk mutations, and a total thyroidectomy is recommended within the first 6–12 months of life (9,10,14). This recommendation was based on many published cases about the RET M918T mutations, but no clinical information is available about the natural history and behavior of MTC associated with the RET A883F mutation. Because of the rarity of A883F mutation, it is unknown whether any early deaths have been associated with it or what the earliest age is at which MTC develops (14). On the basis of this report, we believe that the RET A883F mutation could be a lower-risk mutation than previously believed. Multiple factors validate our theory, including the incidental discovery of MTC in the proband at the age of 39 years, the absence of distant metastases despite the presence of cervical lymphadenopathy, and the apparent cure of the disease after one surgical resection that involved limited neck lymph node dissection. The presence of pheochromocytomas in this patient did not seem to be associated with a more aggressive MTC. In addition, the proband's three children, who also carry the RET A883F mutation, did not have suspicious findings on neck ultrasonography and had near-normal calcitonin levels based on age-specific pediatric RRs (15). Further, dry eyes, frequent constipation, and foot abnormalities, which have been reported as possible initial features of MEN-2B in early infancy (16), are missing in the patient's three children. Our report would be more complete if we had thyroid pathologic findings for the three children. However, after detailed discussion of the current treatment guidelines along with the theoretical morbidity of permanent hypothyroidism, potential postsurgical hypoparathyroidism, and other surgical morbidities, the family elected to pursue very close observation and monitoring rather than prophylactic thyroidectomy.
Future reports about more families with the RET A883F mutation will further clarify the natural history of MTC due to this mutation and guide future treatment recommendations in MEN-2B patients.
Acknowledgment
This article is supported in part by the National Institutes of Health through M. D. Anderson's Cancer Center Support Grant CA016672.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Eng C. Seminars in medicine of the Beth Israel Hospital, Boston. The RET proto-oncogene in multiple endocrine neoplasia type 2 and Hirschsprung's disease. N Engl J Med. 1996;335:943–951. doi: 10.1056/NEJM199609263351307. [DOI] [PubMed] [Google Scholar]
- 2.Skinner MA. DeBenedetti MK. Moley JF. Norton JA. Wells SA., Jr Medullary thyroid carcinoma in children with multiple endocrine neoplasia types 2A and 2B. J Pediatr Surg. 1996;31:177–181. doi: 10.1016/s0022-3468(96)90343-7. discussion 181–172. [DOI] [PubMed] [Google Scholar]
- 3.Gill JR. Reyes-Mugica M. Iyengar S. Kidd KK. Touloukian RJ. Smith C. Keller MS. Genel M. Early presentation of metastatic medullary carcinoma in multiple endocrine neoplasia, type IIA: implications for therapy. J Pediatr. 1996;129:459–464. doi: 10.1016/s0022-3476(96)70084-7. [DOI] [PubMed] [Google Scholar]
- 4.Mulligan LM. Marsh DJ. Robinson BG. Schuffenecker I. Zedenius J. Lips CJ. Gagel RF. Takai SI. Noll WW. Fink M. Raue F. Lacroix A. Thibodeau SN. Frilling BA. Ponder BAJ. Eng C. Genotype-phenotype correlation in multiple endocrine neoplasia type 2: report of the International RET Mutation Consortium. J Intern Med. 1995;238:343–346. doi: 10.1111/j.1365-2796.1995.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 5.Carlson KM. Dou S. Chi D. Scavarda N. Toshima K. Jackson CE. Wells SA., Jr. Goodfellow PJ. Donis-Keller H. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci USA. 1994;91:1579–1583. doi: 10.1073/pnas.91.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith DP. Houghton C. Ponder BA. Germline mutation of RET codon 883 in two cases of de novo MEN 2B. Oncogene. 1997;15:1213–1217. doi: 10.1038/sj.onc.1201481. [DOI] [PubMed] [Google Scholar]
- 7.Gimm O. Marsh DJ. Andrew SD. Frilling A. Dahia PL. Mulligan LM. Zajac JD. Robinson BG. Eng C. Germline dinucleotide mutation in codon 883 of the RET proto-oncogene in multiple endocrine neoplasia type 2B without codon 918 mutation. J Clin Endocrinol Metab. 1997;82:3902–3904. doi: 10.1210/jcem.82.11.4508. [DOI] [PubMed] [Google Scholar]
- 8.Rossel M. Schuffenecker I. Schlumberger M. Bonnardel C. Modigliani E. Gardet P. Navarro J. Luo Y. Billaud M. Romeo G. Lenoir G. Detection of a germline mutation at codon 918 of the RET proto-oncogene in French MEN 2B families. Hum Genet. 1995;95:403–406. doi: 10.1007/BF00208964. [DOI] [PubMed] [Google Scholar]
- 9.Kloos RT. Eng C. Evans DB. Francis GL. Gagel RF. Gharib H. Moley JF. Pacini F. Ringel MD. Schlumberger M. Wells SA., Jr Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 10.Brandi ML. Gagel RF. Angeli A. Bilezikian JP. Beck-Peccoz P. Bordi C. Conte-Devolx B. Falchetti A. Gheri RG. Libroia A. Lips CJ. Lombardi G. Mannelli M. Pacini F. Ponder BA. Raue F. Skogseid B. Tamburrano G. Thakker RV. Thompson NW. Tomassetti P. Tonelli F. Wells SA., Jr. Marx SJ. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 11.Massoll N. Mazzaferri EL. Diagnosis and management of medullary thyroid carcinoma. Clin Lab Med. 2004;24:49–83. doi: 10.1016/j.cll.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Brauckhoff M. Gimm O. Weiss CL. Ukkat J. Sekulla C. Brauckhoff K. Thanh PN. Dralle H. Multiple endocrine neoplasia 2B syndrome due to codon 918 mutation: clinical manifestation and course in early and late onset disease. World J Surg. 2004;28:1305–1311. doi: 10.1007/s00268-004-7637-4. [DOI] [PubMed] [Google Scholar]
- 13.Wray CJ. Rich TA. Waguespack SG. Lee JE. Perrier ND. Evans DB. Failure to recognize multiple endocrine neoplasia 2B: more common than we think? Ann Surg Oncol. 2008;15:293–301. doi: 10.1245/s10434-007-9665-4. [DOI] [PubMed] [Google Scholar]
- 14.Machens A. Niccoli-Sire P. Hoegel J. Frank-Raue K. van Vroonhoven TJ. Roeher HD. Wahl RA. Lamesch P. Raue F. Conte-Devolx B. Dralle H European Multiple Endocrine Neoplasia (EUROMEN) Study Group. Early malignant progression of hereditary medullary thyroid cancer. N Engl J Med. 2003;349:1517–1525. doi: 10.1056/NEJMoa012915. [DOI] [PubMed] [Google Scholar]
- 15.Basuyau J-P. Mallet E. Leroy M. Brunelle P. Reference intervals for serum calcitonin in men, women, and children. Clin chem. 2004;50:1828–1830. doi: 10.1373/clinchem.2003.026963. [DOI] [PubMed] [Google Scholar]
- 16.Brauckhoff M. Machens A. Hess S. Lorenz K. Gimm O. Brauckhoff K. Sekulla C. Dralle H. Premonitory symptoms preceding metastatic medullary thyroid cancer in MEN 2B: an exploratory analysis. Surgery. 2008;144:1044–1050. doi: 10.1016/j.surg.2008.08.028. discussion 1050–1043. [DOI] [PubMed] [Google Scholar]