Abstract
Mitochondria are at the center of cellular energy metabolism and regulate cell life and death. The cell biological aspect of mitochondria, especially mitochondrial dynamics, has drawn much attention through implications in human pathology, including neurological disorders and metabolic diseases. Mitochondrial fission and fusion are the main processes governing the morphological plasticity and are controlled by multiple factors, including mechanochemical enzymes and accessory proteins. Emerging evidence suggests that mitochondrial dynamics plays an important role in metabolism–secretion coupling in pancreatic β-cells as well as complications of diabetes. This review describes an overview of mechanistic and functional aspects of mitochondrial fission and fusion, and comments on the recent advances connecting mitochondrial dynamics with diabetes and diabetic complications. Antioxid. Redox Signal. 14, 439–457.
Introduction
Oxidative stress has been identified as a major culprit for hyperglycemic complications arising in diabetes. Increased production of reactive oxygen species (ROS) is observed in hyperglycemia causing imbalance in the redox state. Mitochondria are one of several cellular sources generating ROS, and hyperglycemic conditions increase mitochondrial ROS production, contributing to oxidative stress and organ injury. Additionally, an increase in levels of superoxide generated during mitochondrial electron transport under hyperglycemic conditions is implicated as a major cause that exacerbates multiple pathological pathways, leading to diabetic complications (23). These aspects suggest that mitochondria play a critical role in diabetic pathophysiology.
Biology textbooks frequently depict mitochondria as granular oval-shaped organelles, likely stemming from modern electron micrographic images. However, when these organelles were first observed in cells, they must have appeared to be a mixture of threads and grains and thus named “mitochondrion” from “mitos-khondros” for “thread-grain” in Greek. More recent microscopic observations of cells have re-established the original view of mitochondria as filamentous tubular organelles, and additionally observed that mitochondria are not static, but highly dynamic, constantly changing shape and location in cells (11, 12). The morphological plasticity of mitochondria includes changes in size, tubule branching, extension/retraction, looping, rolling/unrolling, and so on (11, 12, 183). The molecular mechanisms mediating these shape changes are largely undefined. However, in the past decades, the molecular machineries mediating fission and fusion of the mitochondrial tubule have been identified, allowing mechanistic studies of some of these processes. One enticing question regarding mitochondrial dynamics is, why cells change mitochondrial shapes? To rephrase, what is the form–function relationship of mitochondria? Do different shapes merely reflect varying functional states of mitochondria, or do they have an active role in modulating bioenergetic activities of mitochondria?
Mitochondrial morphology change is associated with many pathological conditions, notably neurodegeneration and aging. In addition, apoptosis is almost inevitably accompanied by fragmentation/granulation of mitochondria. A growing number of studies have begun to investigate mitochondrial morphology as an important parameter for many disease-related disorders. Metabolic diseases are not an exception and recently attention has been drawn to the role of mitochondrial fission/fusion in pancreatic β-cell function, as well as hyperglycemic complications. In this review, we will first describe mitochondrial fission and fusion machineries and their presumed mechanisms mediating mitochondrial membrane remodeling. In the second part, the functional/physiological significance of mitochondrial fission and fusion will be discussed and finally highlighting where mitochondrial dynamics stands in the context of diabetic and hyperglycemic complications.
Mitochondrial Shape Change by Fission and Fusion
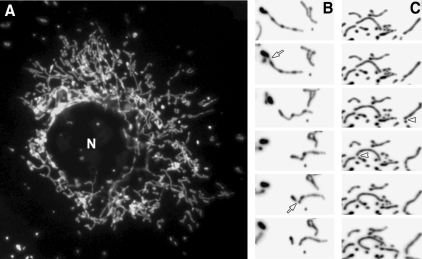
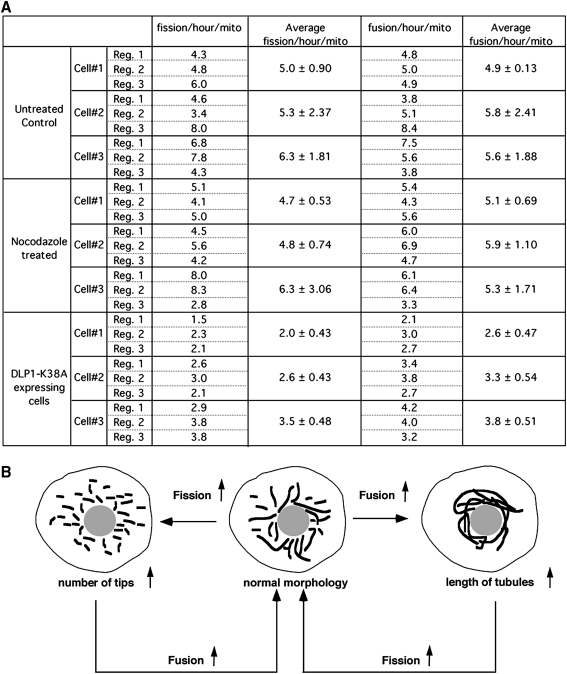
In typical cultured mammalian cells, mitochondria form reticular networks composed of elemental tubules. While the bulk of the mitochondrial networks crowd the perinuclear region, various sized mitochondria ranging from small spheres to rods to elongated tubules are readily found in peripheral cytoplasmic regions (Fig. 1A). Time-lapse imaging of mitochondria reveals fission and fusion, changing the sizes of mitochondrial tubules (Fig. 1B and C). Studies of yeast mitochondria indicated that mitochondrial fission and fusion occurred at a relatively balanced frequency (123, 151). We quantified mitochondrial fission and fusion, and confirmed the balanced fission and fusion in mammalian cells as well (Fig. 2A). Interestingly, against the notion that cytoskeleton may provide a scaffold for pulling mitochondria apart or bringing them together for fission and fusion (184), disruption of microtubule networks did not affect the frequency of fission and fusion (Fig. 2A).
FIG. 1.
Dynamic mitochondria. (A) Network organization of mitochondria. Mitochondria are labeled with the matrix-targeted green fluorescent protein. (B, C) Individual images from time-lapse imaging of mitochondria showing fission and fusion. Images are inverted for better observation of fission and fusion. Images in (B) show two successive fission events in one mitochondrial tubule (arrows). Arrowheads in (C) denote two fusion events. N, nucleus.
FIG. 2.
Control of fission/fusion balance. (A) Quantification of fission and fusion frequencies from time-lapse sequences acquired in every 10 s for 100 frames. Time-lapse images from normal, nocodazole-treated, and dynamin-like protein 1 (DLP1)-K38A mutant cells were subjected to the quantification. Fission events were counted when a single mitochondrion became two by a spatial separation that sustained at least two consecutive frames. Similarly, fusion was counted when separate mitochondria became converged and the connection sustained at least for two consecutive frames. Total number of fission and fusion were divided by the number of mitochondria that were present at the initial frame of the time-lapse sequence, and the frequency of fission and fusion was expressed as the events/hour/mitochondrion. Three regions were selected within a cell and the average fission and fusion were calculated. The quantification was repeated in a total of three cells for each treatment. The frequency of fission is similar to that of fusion within each region (∼5–6 events/hour/mito), demonstrating a balance between fission and fusion. Nocodazole treatment did not change the fission and fusion frequency, indicating that microtubules are not essential for these processes. Expression of DLP1-K38A resulted in a twofold decrease in both fission and fusion. (B) A proposed feedback mechanism to maintain a balance between mitochondrial fission and fusion. Frequency of mitochondrial fusion is predicted to be proportional to the number of mitochondrial tips. Likewise, the number of fission events is likely to increase as tubule length increases. In cells with reduced fission (e.g., DLP1-K38A expressing cells), fusion frequency also decreased due to the decrease of the number of mitochondrial tips available for fusion (A). In healthy cells with properly working fission and fusion machineries, the elongation of mitochondrial tubules due to increased fusion would increase fission frequency, which rapidly restores normal mitochondrial shape.
A shift of the fission–fusion balance alters mitochondrial morphology: more fission leads to fragmented mitochondria, whereas excess fusion results in elongated mitochondrial tubules (Fig. 2B). The quantification of fission and fusion frequency in cells containing excessively fused mitochondria via reduced fission (by a dominant-negative [DN] fission mutant: dynamin-like protein 1 [DLP1]-K38A) showed decreases in both fission and fusion (Fig. 2A). The extent of the fusion decrease is similar to that of fission, indicating that a new fission–fusion balance is established in these cells after the initial excess fusion that has produced elongated mitochondria. This observation brings up an interesting notion that normal mitochondrial morphology is maintained by a simple feedback control governing the fission–fusion balance. This notion is based on the predictions that the probability of mitochondrial fission is proportional to the length of mitochondrial tubules. Additionally, we observed in live cell imaging that the majority of fusion events occurred from one tip of a mitochondrial tubule to either another tip or side of other mitochondria with few fusion events occurring between the two sides of mitochondrial tubules. This suggests that mitochondrial fusion requires at least one mitochondrial tip and predicts that the mitochondrial fusion probability is proportional to the number of tips of mitochondria. As illustrated in Figure 2B, upon the increase of mitochondrial fusion, mitochondrial tubules become longer, which would increase the fission frequency and subsequently the normal morphology is regained. In the same manner, increased fission generates more mitochondrial tips, allowing the restoration of normal morphology through the increased fusion probability. Mechanistically, this feedback control predicts a close connection between fission and fusion, perhaps through direct and indirect communications among machineries mediating mitochondrial fission and fusion.
Abnormal mitochondrial morphology via the faulty feedback control is often associated with pathological conditions, suggesting that dysregulation of fission and fusion can cause disease states or vice versa. While this functional aspect of mitochondrial morphology will be discussed later, we will first describe the proteins and mechanisms that mediate mitochondrial fission and fusion. Although fission and fusion machineries have been identified in phylogenetically diverse organisms, this review will not cover beyond those in the mammalian system unless necessary.
Mitochondrial fission machinery in mammals
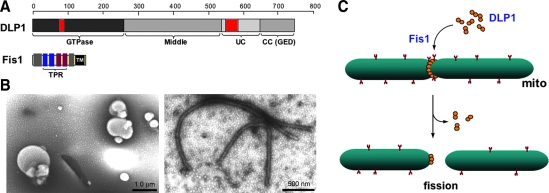
The DLP1/dynamin-related protein 1 (Drp1) mediates mitochondrial fission in mammals (133, 156) (Fig. 3A). DLP1 was identified as an immunologically related protein to dynamin (186). DLP1 is expressed in multiple different spliced variants, ranging 705–761 amino acids (81, 186). While these splicing variants are expressed ubiquitously in all tissues, their relative levels among tissues vary, most prominently in brain (186). DLP1 is a member of dynamin family proteins that are known to mechanically squeeze cellular membranes in a guanosine triphosphate (GTP)-dependent manner. Like conventional dynamin, DLP1 assembles into a ring-like structure (156, 187) and has a capacity to constrict synthetic liposomes into tubules in vitro (187) (Fig. 3B). DN mutants of DLP1 cause excessive elongation of mitochondria in cells, supporting the role of DLP1 in mitochondrial fission (133, 156). Subcellular fractionation experiments showed that most of the cellular DLP1 is in the cytosol, whereas only a small amount is associated with the mitochondrial fraction (153, 156, 186). Immunolocalization of DLP1 in cells revealed DLP1 forming distinct spots on the mitochondrial tubules (133, 156). These observations suggest that DLP1 shuttles between the cytosol and specific sites of mitochondria, but the period that DLP1 resides on the mitochondrial surface is relatively short. The current model for mitochondrial fission is that DLP1 binds to the mitochondrial surface where it assembles into a ring-like structure that squeezes and severs the mitochondrial tubule via its GTPase activity (Fig. 3C). In addition to the fact that DLP1 is abundant in the cytosol, increasing the level of DLP1 in cells does not enhance mitochondrial fission (133), suggesting the presence of limiting factors or regulatory mechanisms in mitochondrial fission. However, one report indicated that extreme overexpression could induce mitochondrial fragmentation (164). Given that DLP1 can directly bind to the membrane and tubulate them in vitro (187), it is possible that excess DLP1 in cells indiscreetly assembles onto and pinches mitochondrial tubules.
FIG. 3.
Mitochondrial fission. (A) Mitochondrial fission proteins DLP1 and fission protein 1 (Fis1). The guanosine triphosphate (GTP)ase domain of DLP1 is highly conserved to that of conventional dynamin, while the middle and coiled-coil (CC) domain are moderately conserved. The CC domain is also known as GTPase effector domain (GED). Unconserved (UC) domain shows no homology to other dynamin proteins. Two red-colored boxes in N- and C-termini denote alternative splicing regions. Fis1 is a small protein containing a single transmembrane (TM) domain. The middle four helices form tetratricopeptide repeat (TPR) motifs. (B) Liposome tubulation by DLP1. Incubation of spherical phosphatidylserine liposomes (left) with purified recombinant DLP1 in the presence of GTPγS induces the tubule formation (right). (C) Mitochondrial fission: DLP1 forms a ring-like structure around the mitochondrial tubule with or without the aid of Fis1. DLP1 constricts and divides the mitochondrial tubule through GTP hydrolysis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Fission protein 1 (Fis1), a mitochondrial outer membrane protein, is another protein participating in the mitochondrial fission process (Fig. 3A). Overexpression of the human protein hFis1 alone fragmented mitochondria by increasing fission, and this mitochondrial fragmentation required intact DLP1 function (160, 185), indicating that hFis1 is a limiting factor in the DLP1-mediated mitochondrial fission. The protein structure and membrane topology of hFis1 imply that it may function as a receptor recruiting DLP1 onto the mitochondrial surface. Fis1 is a small helix-rich protein (17 kDa) anchored in the mitochondrial outer membrane through a C-terminal single transmembrane domain, allowing the bulk of the protein to be exposed to the cytosol (160, 185). The cytosolic domain of Fis1 contains six α-helices of which the core four helices form two tandem tetratricopeptide repeat (TPR) motifs that are known to participate in protein–protein interactions (50, 163) (Fig. 3A). DLP1 was shown to interact transiently with the hFis1 TPR, which is regulated by the N-terminal first α1-helix of hFis1 (188). Mutations of the hFis1 TPR not only disrupted the DLP1 interaction but also exerted a DN effect on mitochondrial fission (188). Subsequent studies indicated that hFis1 forms homo-oligomers on the mitochondrial surface that are likely the functional configuration of this protein for the DLP1 interaction during mitochondrial fission (150). Whether Fis1 is a bona fide DLP1 receptor in mitochondrial fission is unclear. Fis1 has been shown to localize evenly throughout the mitochondrial surface (78, 163) and changes of Fis1 levels did not affect the DLP1 distribution/level in mitochondria (96, 163). On the contrary, knockdown of Fis1 prevented the formation of DLP1 puncta on mitochondria in metabolically noxious conditions that lead to mitochondrial fragmentation (115). In addition, phosphorylation of DLP1 has been shown to increase the Fis1-DLP1 interaction in vitro (69) (see below). It is likely that other factors, including signal-mediated modifications in participating proteins, and local lipid molecules, play a role in regulating DLP1-mediated mitochondrial fission.
Several studies showed that DLP1 is phosphorylated by different protein kinases to modulate mitochondrial fission. Cyclic adenosine monophosphate-dependent protein kinase (PKA) was demonstrated to phosphorylate DLP1 at a C-terminal serine residue (28, 37), which was dephosphorylated by the calcium-dependent protein phosphatase calcineurin (37). PKA-mediated DLP1 phosphorylation decreases the GTPase activity, thus attenuating mitochondrial fission (28). The same serine residue was reported to be phosphorylated by the calmodulin-dependent kinase Iα (CaMKIα) in neurons (69). However, in opposition to the PKA-mediated phosphorylation, the CaMKIα-mediated DLP1 phosphorylation increased mitochondrial fission. DLP1 phosphorylated by CaMKIα showed an increase of both mitochondrial translocation of DLP1 in cells and binding to Fis1 in vitro (69). Cyclin-dependent kinase 1 also phosphorylates DLP1 at a different serine residue of DLP1 to induce mitochondrial fragmentation in mitosis (165). In addition to phosphorylation, DLP1 undergoes sumoylation and ubiquitylation that may affect the DLP1 association to mitochondria and protein stability (18, 57, 70, 117, 177, 181, 194). Recently, it has been shown that increased nitric oxide induces mitochondrial fission through S-nitrosylation (SNO) of DLP1 (SNO-Drp1) (33). The SNO occurred at the cysteine residue within the C-terminal GTPase effector domain, and is necessary for the DLP1 dimer formation and the GTPase activity. An increase of SNO-Drp1 was observed in Alzheimer's brain, but not in Parkinson's, and β-amyloid protein can induce the DLP1 SNO in cultured neurons, suggesting the involvement of the SNO-Drp1-mediated mitochondrial fission in the pathogenesis of Alzheimer's disease (33).
Mitochondrial fusion machinery in mammals
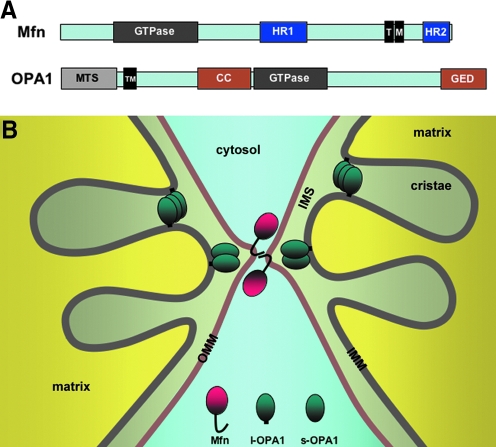
Mitochondria are double-membrane-bound organelles. Therefore, unlike the fission process that can conceivably be achieved by simple pinching from the outer surface of mitochondria, the completion of mitochondrial fusion is predicted to occur sequentially: fusion of the outer membrane followed by that of the inner membrane. Indeed, two different proteins, mitofusin (Mfn) and optic atrophy 1 (OPA1), associated with the outer and inner membranes, respectively, mediate mitochondrial fusion. Interestingly, like the fission protein DLP1/Drp1, both fusion proteins, Mfn and OPA1, also belong to the dynamin GTPase family (Fig. 4A). Although fusion of two separate membranes is considered a topologically opposite process to the membrane fission, the final steps of fission and fusion are thermodynamically identical processes in which lipid molecules of two contacting membranes mix and rearrange to form new bilayers (Fig. 5). Therefore, regardless of fission or fusion, a main task of dynamin proteins is likely to generate mechanical force that brings two membranes in close contact to allow lipid molecule mixing. Consistent with this idea, mitochondrial fusion is initiated by tethering of two opposing mitochondria mediated by the Mfn protein (91). Mfn is an 85-kD protein anchored at the outer mitochondrial membrane. Mammalian cells have two Mfn isoforms, Mfn1 and Mfn2. Both isoforms share a conserved structure containing the N-terminal GTPase domain, two heptad-repeat regions (HR1 and HR2), and two transmembrane domains near the C-terminus (146) (Fig. 4A). HR1 and HR2 lie at opposite sides of the transmembrane region that spans the outer membrane twice, exposing the GTPase, HR1, and HR2 domains to the cytosol (91, 142). Studies with Mfn1 showed that the C-terminal HR2 region interacts with the HR2 of another Mfn molecule in the adjacent mitochondrion through an antiparallel interaction, which allows tethering of the two membranes (Fig. 4B). The distance between the two outer membranes upon the HR2-mediated tethering was estimated to be ∼160 Å (91). Subsequently, the GTP-mediated conformational change of Mfn proteins would further draw two membranes closer for the outer membrane fusion.
FIG. 4.
Mitochondrial fusion. (A) Mitochondrial fusion proteins mitofusin (Mfn) and optic atrophy 1 (OPA1). See text for the structural description. MTS, mitochondrial targeting sequence. (B) Mitochondrial tethering and fusion. The interaction between C-terminal heptad repeat 2 (HR2) domains of Mfn in adjacent mitochondria tethers mitochondria, and membrane fusion ensues. Inner membrane fusion requires both long and short forms of OPA1 (l- and s-OPA1). OPA1 also regulates the size of the cristae junction. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
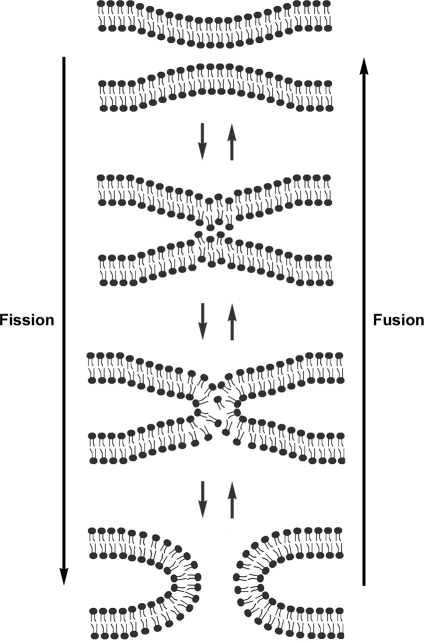
FIG. 5.
Fission and fusion of membrane bilayers. Mixing of lipid molecules in the two contacting bilayers form a hemifusion state before complete reorganization into new bilayers.
The inner membrane-associated OPA1 is presumed to mediate the fusion of the inner membranes. The human OPA1 gene expresses eight alternatively spliced variants (43). The OPA1 protein is further processed by differential proteolytic cleavages, producing long and short forms of OPA1 (74). Long forms are integrated in the mitochondrial inner membrane through the N-terminal transmembrane domain (Fig. 4). The OPA1 cleavage occurs within the membrane, producing short forms that remain associated with the inner membrane at the inter-membrane space. Different proteases, including presenilin-associated rhomboid-like, i-AAA (Yme1L), m-AAA (paraplegin and AFG3L1 and 2) proteases, and the inner membrane metalloprotease OMA1, were found to participate in the cleavage of OPA1 (36, 53, 54, 64, 71, 74, 157). It has been shown that the successful fusion requires the presence of both long and short forms, whereas either long forms or short forms alone do not support mitochondrial fusion (157). Although partial outer membrane fusion was observed in the absence of the inner membrane fusion in OPA1-null cells (158), OPA1 knockout or knockdown generally caused fragmentation of mitochondria even with intact Mfn proteins in the outer membrane. This suggests that functional coordination of Mfn and OPA1 is necessary for successful fusion of mitochondria (35, 41, 65, 124). In yeast, the physical connection between Fzo1p (yeast Mfn orthologue) and Mgm1p (yeast OPA1 ortholog), mediated by the transmembrane linker Ugo1p, may coordinate outer and inner membrane fusion. To date, however, no such linker protein has been identified in mammals. In mammalian cells, OPA1 appears to function with the Mfn1 isoform for mitochondrial fusion, suggesting a functional distinction between the two Mfn isoforms (35). Interestingly, Mfn2 has an additional function in tethering the mitochondria to the endoplasmic reticulum (42). OPA1 also has an additional function in regulating cristae junction opening for cytochrome c release during apoptosis (59) (Fig. 4B).
Other proteins in mitochondrial morphogenesis
While DLP1, Fis1, Mfn, and OPA1 are the most studied proteins for mitochondrial fission and fusion, additional proteins have been identified and implicated in mitochondrial morphogenesis. MTP18 is an 18-kDa mitochondrial inter-membrane space protein whose expression is regulated by phosphatidylinositol 3-kinase signaling (170). Knockdown of MTP18 induced interconnected mitochondrial tubules and it was suggested to act downstream of Fis1 (168, 170). Two outer membrane proteins, ganglioside-induced differentiation associated protein 1 (120) and mitochondrial fission factor, were also implicated in mitochondrial fission (60). A Bin-Amphiphysin-Rvs domain-containing protein, endophilin B1, has been shown to change mitochondrial morphology upon its downregulation, presumably by changing membrane curvature (82). Although these proteins affect mitochondrial morphology in the DLP1/Fis1-dependent fashion one way or another, no physical interactions between these proteins and DLP1 or Fis1 has been identified. More directly involved in the DLP1/Fis1-mediated process, a mitochondrial outer membrane-associated ubiquitin E3 ligase, MARCH-V, also referred to as MITOL, has been shown to bind to DLP1 and Fis1 as well as the fusion protein Mfn2 subsequently ubiquitinating DLP1 and Fis1 for degradation (117, 181). Accordingly, knockdown of MARCH-V led to mitochondrial fragmentation through decreasing the degradation of fission proteins (117, 181). However, a contradictory report indicated that MARCH-V inhibition results in mitochondrial elongation and net formation, possibly by inhibiting DLP1 assembly or postfission turnover at the fission site (83).
Mitochondrial phospholipase D was implicated in mitochondrial fusion, possibly downstream of Mfn-mediated tethering (34). Mitochondrial phospholipase D binds to the outer surface of mitochondria, where it hydrolyzes cadiolipin of an adjacent mitochondrial outer membrane to generate a fusogenic lipid phosphatidic acid, which would promote transmitochondrial membrane adherence (34). Several Mfn-binding proteins have been identified that regulate mitochondrial morphology. MIB, Mfn-binding protein, is a member of the medium-chain dehydrogenase/reductase superfamily, localized in the cytosol (56). Knockdown of MIB elongated mitochondria, indicating that it is a negative regulator of the Mfn function (56). Stoml2 or stomatin-like protein 2 interacts with Mfn2 and prohibitin 1 and 2 (39, 67), and is associated with the mitochondrial inner membrane and faces the intermembrane space (67). A recent study showed that stomatin-like protein 2 participates in the stress-induced mitochondrial hyperfusion through the OPA1 and Mfn1 functions but independently of Mfn2 and prohibitins (169). In addition, pro- and antiapoptotic proteins Bax, Bak, Bcl-2, and Bcl-xL have been shown to interact with the Mfn protein and affect mitochondrial morphology (see below) (22, 45). Locations and functions of proteins implicated in mitochondrial fission and fusion along with their associated mitochondrial phenotypes are summarized in Tables 1 and 2.
Table 1.
Location and Function of Proteins Implicated in Mitochondrial Fission and Fusion
| Component | Localization | Function |
|---|---|---|
| DLP1/Drp1 | Cytosol; mitochondrial outer membrane | Fission (mitochondrial membrane severing) |
| Fis1 | Mitochondrial outer membrane | Fission (possible receptor for DLP1 or other factors) |
| Mfn1 | Outer mitochondrial membrane | Outer membrane fusion (mitochondrial tethering) |
| Mfn2 | Outer mitochondrial membrane | Outer membrane fusion; ER-mitochondria tethering |
| OPA1 | Inner mitochondrial membrane; inter membrane space | Inner membrane fusion; cristae junction regulation |
| MTP18 | Inner mitochondrial membrane | Fission regulation |
| Endophilin B1 | Cytosol | Fission (membrane curvature) |
| GDAP1 | Outer mitochondrial membrane | Fission promotion |
| Mff | Outer mitochondrial membrane | Fission promotion |
| MARCH-V | Outer mitochondrial membrane | Ubiquitin E3 Ligase modifying DLP1, Fis1 and Mfn2 |
| MitoPLD | Outer mitochondrial membrane | Fusion promotion |
| MIB | Cytosol/microsomes/mitochondria | Negative regulator of Mfn |
| SLP-2 | Inner mitochondrial membrane | Stress-induced fusion |
DLP1, dynamin-like protein 1; Drp1, dynamin-related protein 1; Fis1, fission protein 1; GDAP1, ganglioside-induced differentiation associated protein 1; MARCH-V, membrane-associated RING-CH-V; Mfn, mitofusin; MIB, mitofusin-binding protein; MitoPLD, mitochondrial phospholipase D; MTP18, mitochondrial protein 18 kDa; OPA1, optic atrophy 1; SLP-2, stomatin-like protein 2.
Table 2.
Mitochondrial Phenotypes Associated with Alterations of Fission/Fusion Protein Expression
| Component | Alteration in expression | Mitochondrial morphology |
|---|---|---|
| DLP1/Drp1 | Overexpression | No change; fragmentation |
| Knockdown/knockout | Elongation | |
| Fis1 | Overexpression | Fragmentation |
| Knockdown | Elongation | |
| Mfn1 | Overexpression | Clustering (fragmentation); elongation |
| Knockdown/knockout | Fragmentation/short tubules | |
| Mfn2 | Overexpression | Clustering (fragmentation); elongation |
| Knockdown/knockout | Fragmentation/short tubules | |
| OPA1 | Overexpression | Fragmentation; elongation |
| Knockdown/knockout | Fragmentation | |
| MTP18 | Overexpression | Fragmentation |
| Knockdown | Interconnected networks | |
| Endophilin B1 | Knockdown | Dissociation of the outer membrane from the matrix compartment |
| MIB | Overexpression | Fragmentation |
| Knockdown | Elongation | |
| GDAP1 | Overexpression | Fragmentation |
| Knockdown | Elongation |
Functional Significance of Mitochondrial Fission and Fusion
One obvious question regarding mitochondrial dynamics is why cells bother changing mitochondrial morphology as it consumes a great deal of cellular energy. Clearly, mutations in mitochondrial fission and fusion genes cause human disease or death, indicating their roles in pathophysiology. Abnormal regulation of apoptosis is destined for developing disease conditions. Mitochondria are the central regulator of apoptosis, and mitochondrial morphology undergoes a drastic change during apoptosis, implying mitochondrial fission/fusion in apoptotic regulation.
Mitochondrial fission and fusion determine the continuity or separation of mitochondrial membranes and matrix in which bioenergetic/biosynthetic activities take place. Aberrations in the regulation of mitochondrial continuity are pathologic as mentioned above. Despite the clear link between fission/fusion and activity/function of mitochondria, knowledge regarding the mechanistic correlation of these two components is only rudimentary. In this section, the apoptotic role of mitochondrial fission/fusion and recent efforts to identify mechanistic links between mitochondrial morphology and function will be discussed.
Mitochondrial fission and fusion in apoptosis
Mitochondrial morphology changes during apoptosis. More specifically, tubular mitochondria disintegrate and become fragmented when the mitochondrial outer membrane is permeabilized for cytochrome c release. The time frame for mitochondrial fragmentation and the mitochondrial outer membrane permeabilization (MOMP) is almost simultaneous, suggesting a close correlation between the two processes. The fission proteins DLP1 and Fis1 mediate apoptosis-associated mitochondrial fragmentation, as the inhibition of DLP1 or Fis1 by knockdown or DN approach maintained tubular mitochondria upon apoptotic stimulation (58, 96). Further, cytochrome c release, caspase activation, and cell death are delayed upon inhibition of mitochondrial fission (3, 21, 22, 58, 96). Overexpression of the fusion proteins Mfn1 and Mfn2, which increases connected mitochondria, also delayed cytochrome c release and Bax/Bak activation upon apoptotic stimulation (77, 119, 162). In addition, Fis1 overexpression-induced mitochondrial fragmentation caused cytochrome c release and apoptosis (78, 188). Knockdown of the fusion proteins Mfn1, Mfn2, or OPA1 fragmented mitochondria and increased the sensitivity to apoptotic stimuli (96, 124, 162). These results suggest that mitochondrial fragmentation actively participates in promoting the MOMP (22, 58, 78, 96, 188). However, others reported contradicting results in which overexpression of Mfn1, Mfn2, or the DN DLP1-K38A had little effect on apoptosis (152). Additionally, it has been shown that the exodus of mitochondrial factors such as Smac/DIABLO, Omi, Tim8a, and adenylate kinase 2 upon MOMP still occurs with normal kinetics when mitochondrial fission is inhibited (55, 129). This indicates that MOMP and mitochondrial fragmentation are separate events and that DLP1-mediated fission is specific only for the release of cytochrome c. Moreover, an apoptotic induction leads to cell death in DLP1-knockout cells that maintain tubular mitochondria through the cytochrome c release phase, albeit with delayed apoptotic progression, demonstrating that mitochondrial fission is dispensable for apoptosis (76). Therefore, the significance of mitochondrial fission in apoptosis is unclear based on the nonexisting or delaying effect rather than a strong blocking effect presented by the absence of DLP1 function. Interestingly, in DLP1 knockout cells, DLP1-independent fragmentation of mitochondria was observed in the later stage of apoptosis after cytochrome c release (76).
Whether mitochondrial fragmentation is an active process or a coincidental phenomenon in apoptosis is still a matter of debate. Mitochondrial fragmentation appears neither sufficient nor necessary for apoptosis. However, given that almost all apoptosis is accompanied by mitochondrial fragmentation, there ought to be a reason for this phenomenon. A clue may be found in the recent finding that the MOMP-directing pro- and antiapoptotic Bcl-2 proteins interact with the fission and fusion machineries influencing mitochondrial morphology. The pro-apoptotic protein Bak has been shown to be responsible for mitochondrial fragmentation during apoptosis, potentially by differential interactions with the two Mfn isoforms, Mfn1 and Mfn2 (22). Mfn proteins were also shown to interact with Bax in the same study (22). Karbowski et al. reported that Bax altered Mfn2 activity and localization to increase mitochondrial fusion in healthy, nonapoptotic conditions (84). Mfn2 was also reported to interact with antiapoptotic Bcl-xL and Bcl-2 (45). Bcl-xL was found to interact with the Fis1 under overexpressing conditions. This interaction prevented the Fis1-induced cytochrome c release and apoptosis without inhibiting the Fis1-induced mitochondrial fragmentation (78). Fis1 was also shown to interact with Bcl-2, protecting cells from apoptosis by increasing mitochondrial calcium retention capacity (90). In addition, Bcl-xL has been shown to increase DLP1-mediated mitochondrial fission in neurons (14, 100). Although these studies indicate that there are physical interactions between fission/fusion proteins and Bcl-2 family proteins, morphological manifestations resulting from these interactions are not consistent with the fragmentation-MOMP axis, again bringing up skepticism regarding the active role of mitochondrial fragmentation in apoptosis. An emerging theory that reconciles this inconsistent effect is that mitochondrial fragmentation is the result of a regulatory function of Bcl-2 proteins in mitochondrial morphogenesis rather than having a direct role in MOMP/apoptosis (4). Bcl-2 proteins are critical regulators of cell survival and have a broad role in regulating cellular processes throughout the cell including calcium metabolism, autophagy, insulin secretion, and cell cycle control (40, 131, 149, 191). Because proper control of mitochondrial morphology is important for cell survival, cells may have developed a surveillance mechanism for mitochondrial morphology by employing Bcl-2 proteins in mitochondrial morphogenesis. Whether morphological change of mitochondria has a direct role in apoptosis or not, it is evident that there are physical and functional associations of Bcl-2 proteins that alter mitochondrial morphology. Further studies will define how these interactions differentially regulate mitochondrial fission and fusion in normal and apoptotic conditions.
Mitochondrial morphology, diseases, and bioenergetics
The functional importance of mitochondrial fission/fusion is reflected in the human diseases caused by mutations in mitochondrial fission and fusion genes. The mitochondrial fusion protein OPA1 is named after a disease, optic atrophy type 1, the most prevalent hereditary optic neuropathy that results in progressive loss of visual acuity (1, 44). Mutations in another fusion protein Mfn2 cause Charcot-Marie-Tooth type 2A, a peripheral neuropathy (193). One report identified a mutation in the DLP1 gene as a direct cause of the premature death of a newborn (178). In addition, Charcot-Marie-Tooth type 4A was reported to be associated with mutations in ganglioside-induced differentiation associated protein 1, a protein implicated in mitochondrial fission (8, 38, 118, 120). Prominent neurodegenerative diseases, Alzheimer's, Huntington's, and Parkinson's are also associated with dysregulation of mitochondrial morphology. Readers can refer to recent review articles dealing with this subject (30, 88, 89, 103, 161, 174, 175). In addition, genetic knockout of Mfn1, Mfn2, OPA1, or DLP1 causes embryonic lethality in mice, demonstrating that mitochondrial fission and fusion are required for embryonic development (32, 41, 76).
These disease states are the physiological consequences of dysregulation of mitochondrial morphogenesis, presenting circumstantial evidence for the significance of the mitochondrial morphology control. Plausibly, failure to maintain normal mitochondrial morphology would be detrimental for the mitochondrial bioenergetic activity. Several studies have shown that this is the case by manipulating mitochondrial morphology through overexpression or knockdown/knockout of fission and fusion proteins. Embryonic fibroblasts from Mfn1 and 2 double-knockout mice contained completely fragmented mitochondria. These morphologically altered mitochondria resulted in slower cell growth, loss and heterogeneity of the mitochondrial inner membrane potential, and decreased respiration, indicating the reduced mitochondrial function upon mitochondrial fragmentation (31). OPA1 knockdown also induced mitochondrial fragmentation and resulted in similar functional defects of mitochondria. In the same study, mitochondrial fragmentation was also observed upon overexpression of OPA1; however, these fragmented mitochondria were fusion competent and showed no defects in mitochondrial function (31). This suggests that mitochondrial fusion, not the fragmentation per se, is necessary to maintain the normal function of mitochondria. Conversely, mitochondrial fusion has been shown to require the mitochondrial inner membrane potential (75, 97, 112). Disruption of the membrane potential by p-trifluoromethoxyphenylhydrazone/carbonylcyanide m-chlorophenylhydrazone resulted in fragmented mitochondria by excess fission. The inner membrane fusion protein OPA1 seems to be a key mediator in this process, as the loss of mitochondrial membrane potential increases OPA1 cleavage and prevents fusion (52, 74). This observation raises an intriguing possibility that the OPA1 processing is a bioenergetic sensor, linking the functional state of mitochondria to their morphology. The membrane potential-dependent cleavage of OPA1 would determine whether given mitochondria undergo fusion or remain separated depending on their energetic capacity.
Inhibiting mitochondrial fission was also reported to impair mitochondrial function. Downregulation of DLP1 by RNA interference induced highly fused and interconnected mitochondria. This morphological deformation was accompanied by a multitude of anomalies in mitochondrial function, showing decreases in mitochondrial membrane potential, respiration, adenosine triphosphate (ATP) content, and cell proliferation as well as increases in ROS levels and oxidative damage, along with mitochondrial DNA loss and autophagic activation (9, 128). In contrast, embryonic fibroblasts from DLP1 knockout mice appeared normal in these aspects (76). Mitochondrial membrane potential, respiration, ATP levels, mitochondrial DNA levels, and autophagy were unaffected in DLP1-knockout fibroblasts, suggesting that DLP1 is dispensable for maintaining mitochondrial function in embryonic fibroblasts. In the DLP1 knockdown study, the functional defects of mitochondria were observed in HeLa cells derived from malignant tumor. Cancer cells are known to have different mitochondrial properties (143, 176), which might have contributed to the discrepancy between the two different experimental systems.
The experimental results described thus far clearly indicate that there is an intricate form–function relationship of mitochondria. One intriguing question is how the large-scale morphological change of mitochondria through fission and fusion influences mitochondrial bioenergetic activity. Mitochondria become completely fragmented when they are isolated away from the context of cells or tissues. However, these isolated mitochondria retain all the necessary bioenergetic components and maintain normal respiratory capacity. Interestingly, isolated mitochondria from DLP1-depleted cells revealed decreased respiration along with marked uncoupling of oxidative phosphorylation (OXPHOS) compared to those from normal cells (128), despite their identically fragmented morphology resulting from the isolation process. This observation suggests that the functional defect of mitochondria in DLP1-depleted cells is due to an intrinsic change in respiratory capacity. Related to this notion, Benard et al. proposed that an increase of mitochondrial membrane fluidity upon DLP1 depletion would decrease the constraint exerted on the OXPHOS supercomplexes within the inner membrane, causing functional defects through alterations in complex organization and substrate channeling (9, 10).
Mitochondrial Dynamics in Diabetes and Hyperglycemic Complications
Mitochondria play an important role in insulin secretion, and mitochondrial DNA mutation is a known etiology of a subset of diabetes. As a source of ROS and as a regulator of apoptosis, mitochondria also participate in the development of diabetic complications. Given the presence of the intricate form–function relationship of mitochondria, mitochondrial dynamics is likely an important contributing factor to pathology of diabetes.
Mitochondrial dysfunction in diabetes
Insulin is synthesized in the pancreatic β-cells and stored in the secretory vesicles. The secretion of insulin is controlled by blood glucose levels, amino acids, and other circulating hormones. An increase of glucose uptake by β-cells following an elevation of blood glucose concentration augments OXPHOS by β-cell mitochondria. The rise in ATP/ADP ratio inhibits the ATP-sensitive potassium channels on the cell membrane, which depolarizes the plasma membrane, leading to calcium influx into cells. Elevated cytosolic calcium stimulates the exocytosis of insulin-containing vesicles for insulin secretion into the circulation (13, 72, 105–108). This sequence of events leading to the insulin secretion requires normal bioenergetic activity of mitochondria. A critical role of mitochondria in insulin secretion is evident from the mitochondrial diabetes, for maternally inherited diabetes and deafness (MIDD). MIDD is a hereditary disorder accounting for ∼1% of total diabetes cases. The etiology of MIDD is the impairment of β-cell function caused mostly by A3243G mutation in the tRNALeu gene of the mitochondrial DNA. Patients carrying this mutation have almost 100% chance to gradually develop diabetes upon aging, which is associated with pancreatic β-cell dysfunction and a marked reduction in both first- and second-phase insulin secretion (104). Knockout of the nuclear gene encoding mitochondrial transcription factor A in pancreatic β-cells resulted in diabetes in mice (154). Islets from the mitochondrial transcription factor A knockout mice had mitochondrial dysfunction, including mitochondrial DNA depletion and deficient OXPHOS. The mutant islets displayed impairments in calcium signaling and insulin release in response to glucose stimulation, demonstrating a critical role of mitochondrial function in insulin secretion.
In addition to mitochondrial diabetes, the most common type of diabetes (type 2 diabetes) is also associated with mitochondrial dysfunction (102). Defects in insulin secretion are well documented in type 2 diabetes (80, 95, 141) and mitochondrial dysfunction impairs the insulin secretion as just described. The most notable characteristic of type 2 diabetes is insulin resistance, the decreased ability of target tissues to respond properly to normal circulating concentrations of insulin. Decreases in mitochondrial oxidative enzyme activity and lipid oxidation were observed in skeletal muscles of insulin-resistant and obese patients (85–87, 155). Type 2 diabetics also displayed downregulation of genes for mitochondrial biogenesis and OXPHOS (116, 130). The development of insulin resistance has been shown to occur concomitantly with mitochondrial dysfunction in humans and rodents (16, 19, 73, 139, 159). Several intervention studies also showed that both mitochondrial function and insulin sensitivity are improved upon exercise training, calorie restriction, and resveratrol treatment (92, 93, 125, 140, 167). However, some studies indicated that the improvement of insulin sensitivity occurs without enhancement of mitochondrial function (148, 166). In addition, genetic disruption of mitochondrial function in mice did not affect insulin sensitivity in skeletal muscle (135, 180). Although mitochondrial dysfunction is widely recognized as a causal factor for insulin resistance in type 2 diabetes, more mechanistic studies are necessary to further delineate the cause-and-effect relationship between these two pathological phenomena.
Mitochondrial dynamics in pancreatic β-cells and insulin secretion
Normal mitochondrial function is required for proper regulation of insulin secretion in pancreatic β-cells. Considering the intricate relationship between mitochondrial function and morphology, it is likely that mitochondrial dynamics plays a role in insulin secretion in β-cells. Type 2 diabetic patients exhibit both functional and morphological alterations of mitochondria in the pancreatic islets, including the reduced mitochondrial membrane potential, decreased ATP production, and high density of swollen mitochondria, along with impaired insulin secretion (2, 46). Shorter and swollen mitochondria were also reported in the β-cells from Zucker diabetic fatty rat model (15). These observations suggest potential disruption of mitochondrial dynamics in diabetic individuals and animals.
While the dysfunction of mitochondria with respect to their bioenergetic activity has been studied extensively in the context of diabetes, it is only recently that β-cell mitochondrial dynamics and its role in insulin secretion are being investigated. Using a mitochondrial matrix-targeted photoactivatable green fluorescent protein, Molina et al. examined mitochondrial morphology and dynamics in pancreatic islets and cultured β-cells (115). Photoactivation image analyses for mitochondrial connectivity indicated that densely organized mitochondria in primary mouse β-cells consist of individual tubules mostly at the length shorter than 2.5 μm, suggesting that β-cell mitochondria are shorter than those in other cell types such as fibroblasts. This result is somewhat in contrast to other reports in which mitochondria in human and rat primary islet cells formed tubular networks throughout the cytoplasm (15, 127). Elegant electron tomograms of intact islet revealed tubular and branched mitochondria in β-cells with the length ranging 0.5–5 μm (122). Recently, 4Pi microscopy, which has higher axial resolution, was employed to examine the mitochondrial morphology of pancreatic β-cells. Image analyses revealed that mitochondria of rat islet β-cells form highly interconnected reticulum similar to those observed in the insulinoma β-cell line (49, 134). In contrast, disintegration of tubular mitochondrial network was observed in islet β-cells from diabetic Goto Kakizaki rats (49). Individual mitochondria in healthy β-cells are dynamic, actively communicating with one another and with the rest of the network through frequent fusion and fission events, as evidenced by the observation that locally photoactivated mitochondrial matrix fluorescence rapidly becomes dispersed throughout the entire cell (115).
Decreased islet size and β-cell mass caused by β-cell apoptosis are also established features of type 2 diabetes (25, 51, 137, 145, 182), contributing to reduced circulating insulin levels. In a cultured β-cell model, high glucose concentrations induced apoptosis (114). Inhibiting mitochondrial fission prevented the high-glucose-induced β-cell apoptosis (114), suggesting that mitochondrial fission may regulate the circulating insulin levels by participating in pancreatic β-cell apoptosis in diabetes. Under apoptosis-inducing glucolipotoxic conditions, namely, high glucose and high fatty acid concentrations mimicking the type 2 diabetic milieu, β-cell mitochondria became fragmented by decreased fusion, and the glucose-stimulated insulin secretion was significantly reduced (115). Downregulation of Fis1 under the glucolipotoxic conditions rescued mitochondrial morphology and protected these cells from apoptosis, but was not able to restore the insulin secretion capacity (115). These results suggest that, despite the restoration of normal mitochondrial appearance by inhibiting fission under the fusion-limiting conditions, mitochondrial bioenergetic dysfunction is sustained in these cells. These observations support the notion that the dynamic nature of mitochondrial morphology by continuous fission and fusion, but not the static normal shape, is necessary to maintain proper mitochondrial function and activity.
The initial indication for the involvement of mitochondrial dynamics in insulin secretion was that downregulation of the mitochondrial Fis1 in β-cells significantly reduced glucose-induced insulin secretion presumably through decreased respiration (171). To directly study the role of mitochondrial dynamics in insulin secretion, mitochondrial morphology was manipulated in pancreatic β-cells, and the effect on the metabolism–secretion coupling was examined (127). Fis1 overexpression in the β-cell line induced extensive mitochondrial fragmentation and impaired the glucose-stimulated insulin secretion via decreased cellular ATP levels. A similar perturbation of metabolism–secretion coupling was observed upon overexpression of the fusion protein Mfn1, despite the apparently opposite mitochondrial morphology induced by this manipulation. However, overexpression of the DN-Mfn1 that caused mitochondrial fragmentation affected neither the cellular ATP level nor the insulin secretion. Because Fis1 and DN-Mfn1 cause almost identical mitochondrial fragmentation upon overexpression but have different effects on the metabolism–secretion coupling, it is unlikely that mitochondrial fragmentation itself is the determining factor for mitochondrial energy metabolism and insulin secretion in pancreatic β-cells (127). Further, overexpression of wild-type Mfn1 adversely affected the metabolism–secretion coupling similar to Fis1 overexpression despite forming the seemingly opposite mitochondrial morphology (127). These observations seem contradictory to the general notion that mitochondrial fusion is a requisite for maintaining normal mitochondrial function (29, 31, 32, 147, 179, 184); hence, it would be predicted that DN-Mfn1 inhibits mitochondrial fusion and resulting fragmentation would be associated with mitochondrial dysfunction and disruption of insulin secretion in β-cells. Instead, it was found that the metabolism–secretion coupling in β-cells is closely associated with cytoplasmic mitochondrial volume rather than the mitochondrial morphology (127). Overexpression of hFis1 and Mfn1 greatly decreased mitochondrial volume, whereas DN-Mfn1 overexpression had no effect on it. Mitochondrial flux through biogenesis and autophagic removal determines cellular mitochondrial contents. Mitochondrial fission and fusion are believed to be closely linked to autophagy (7, 171, 172). In this regard, inhibiting mitochondrial fission decreases glucose-stimulated insulin secretion in β-cells by accumulating dysfunctional mitochondria resulting from dysregulated autophagy (171). It has been reported that autophagy is associated with diabetes (62, 68, 110, 111, 113). It is evident that mitochondrial fission and fusion play an important role in metabolism–secretion coupling of pancreatic β-cells by regulating bioenergetics and homeostasis of mitochondria. In addition, mitochondrial dynamics is also likely a contributing factor for circulating insulin levels in diabetes by participating in β-cell apoptosis.
Mitochondrial dynamics in hyperglycemia, ROS production, and diabetic complications
Aside from pancreatic defects, deformation of mitochondria is frequently observed in other organs of hyperglycemic patients and diabetic animal models. Mitochondria in hepatocytes from hyperglycemic patients are swollen, and show disarrayed cristae and reduced electron density of matrix (173). In skeletal muscles from obese and type 2 diabetic subjects, mitochondria are smaller and have less defined internal structure with some vacuolization (85). Reduction of Mfn2 in both mRNA and protein levels were observed in skeletal muscles of obese Zucker rats and obese human subjects (5, 6). The reduction in Mfn2 levels was correlated with a smaller mitochondrial size and higher density, indicative of a fragmented mitochondrial network in obese Zucker rat skeletal muscle (6). In cultured cells, high glucose concentrations induce mitochondrial fragmentation in cell lines derived from liver, heart, endothelium, pancreatic islets, as well as in primary cell cultures of neuronal and the cardiovascular system (98, 114, 126, 189, 190). Mitochondrial fragmentation in high-glucose or high-glucose/high-fat conditions requires mitochondrial fission machinery (114, 115, 189, 190). Treatment of cultured dorsal root ganglia (DRG) neurons with high glucose concentrations caused mitochondrial fragmentation and increased expression and mitochondrial localization of the fission protein DLP1, indicating the activation of mitochondrial fission (98). Increases of expression, activation, and mitochondrial localization of pro-apoptotic proteins Bim and Bax were also observed in high-glucose treatment, indicating that high-glucose-induced mitochondrial fragmentation in DRG is associated with apoptosis. An increased DLP1 expression was also observed in DRG from streptozotocin-induced diabetic rats, pancreatic β-cells incubated in high glucose concentrations, and 3T3-L1 adipocytes in high free fatty acids (61, 98, 114).
ROS are a group of small molecules including superoxide anion, hydroxyl radical, and hydrogen peroxide. While a low level of ROS is an important cellular signaling component, high levels of ROS cause protein oxidation, lipid peroxidation, and DNA damage referred to altogether as oxidative stress that contributes to the development of the multitude of pathological conditions, including diabetic complications. Of multiple ROS sources in cells, nicotinamide adenine dinucleotide phosphate oxidase and mitochondria have been implicated in diabetic vascular injury in hyperglycemic conditions (23, 27, 48, 63, 121, 136). Mitochondria utilize the electrochemical gradient generated by the electron transport chain (ETC) to produce ATP. During the electron transfer, the ETC also produces ROS as a byproduct that, under normal conditions, are kept below harmful levels by antioxidant mechanisms. In hyperglycemic conditions, increased metabolic input overwhelms the ETC and causes electron backup within the ETC, allowing increased slippage of electrons resulting in excess formation of superoxide anions (Fig. 6). Brownlee postulated that superoxide anions produced from the mitochondria under hyperglycemic conditions inhibit the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase, diverting glycolytic intermediates into other harmful pathways implicated in the development of diabetic complications (23, 24, 121). These pathways include increased polyol pathway flux, increased formation of advanced glycation end products, activation of protein kinase C isoforms, and increased hexosamine pathway flux (23, 24) (Fig. 6). Several reports also indicate that increased ROS in hyperglycemia directly induce apoptosis and oxidative stress, causing organ damage in diabetes (26, 47, 144) (Fig. 6).
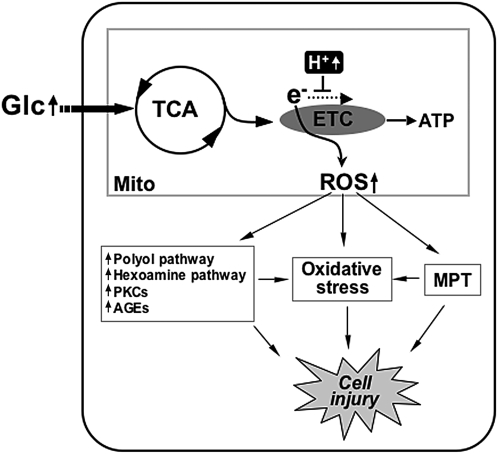
FIG. 6.
Mitochondrial reactive oxygen species (ROS) generation in hyperglycemia. High glucose concentrations increase metabolic input into mitochondria. Hyperpolarized mitochondria slow down electron flow through the electron transport chain (ETC) and increase superoxide (ROS) generation. High ROS levels cause the mitochondrial permeability transition (MPT), oxidative stresses, and the increase of other harmful metabolic/signaling pathways, leading to cell injury.
Our studies have identified the involvement of mitochondrial dynamics in high-glucose-induced ROS production (189). Acute exposure of cells to high glucose concentrations induced rapid fragmentation and recovery of mitochondria that occur concomitantly with an increase and decrease of ROS levels (189). Inhibiting mitochondrial fission or increasing fusion normalized ROS levels in the high-glucose incubation (189). An important contribution of this study is that the morphological change of mitochondria is an upstream causal factor for the ROS production under acute hyperglycemic stimulation, defining mitochondrial dynamics as a controlling factor regulating mitochondrial activity (189). Another report also showed that downregulation of Fis1 decreased the ROS level in acute high-glucose stimulation (171). As briefly discussed in the previous section, the underlying mechanisms of how alterations in mitochondrial morphology perturb mitochondrial bioenergetic activity to overproduce ROS remain unknown. Hackenbrock's classical observations indicate that active mitochondria are more condensed and have an electron dense matrix (66). Possibly, small short mitochondria formed upon high-glucose stimulation represent contracted and condensed mitochondria. The reversibility of mitochondrial fragmentation in the early high-glucose incubation suggests that the high-glucose-induced mitochondrial fragmentation is a physiological event, as opposed to the pathological fragmentation sustained in apoptosis. It is possible that morphological change of mitochondria in high-glucose conditions is a cellular response to increased metabolic substrate to facilitate metabolic input into mitochondria by increasing total surface area. In addition, recent electron tomographic analyses indicate that the dynamic change of the mitochondrial internal structure is closely associated with functional states of mitochondria (109). Ultrastructural change of the mitochondrial inner membrane was observed in nitric-oxide-induced mitochondrial fragmentation (7). Manipulations of the levels of Mfn2 have been shown to alter expression of mitochondrial respiratory chain complexes, leading to different glucose oxidation activities and conversion between orthodox and condensed mitochondria (132). Components of the mitochondrial ETC are arranged in an orderly fashion within the mitochondrial membrane for efficient transfer of electrons. Mitochondrial shape change in hyperglycemic exposure may alter structural organization and arrangement of respiratory chain complexes within the membrane, leading to perturbation of ETC activity or its coupling to ATP synthesis conducive to ROS overproduction. Changes in metabolic channeling, ETC complex organization, OXPHOS coupling efficiency, and membrane fluidity are plausible mechanisms to alter the mitochondrial activity upon hyperglycemia-induced mitochondrial fragmentation to increase ROS production.
Pathologic complications of hyperglycemia are associated with apoptosis and oxidative stress arising from increased levels of ROS, and accompanied by fragmentation of mitochondria (114, 173, 190). Under the pathological conditions, it is still debatable whether the elevated ROS cause mitochondrial fragmentation or vice versa. Inhibition of mitochondrial complex II in neuronal cells induced an ROS increase and mitochondrial fragmentation. The antioxidant N-acetyl-L-cysteine significantly decreased mitochondrial fragmentation under this condition, indicating that ROS is responsible for mitochondrial fragmentation (101). Treatment of cells with hydrogen peroxide results in an increase of ROS inside the cells that causes transient changes in the mitochondrial morphology (79). In a model of nitric-oxide-mediated neuronal injury, Barsoum et al. reported that nitrosative and oxidative stress induced DLP1-mediated mitochondrial fragmentation, which preceded neuronal cell death (7). In contrast to these observations, it has been reported that blocking mitochondrial fragmentation in hyperglycemic incubation decreases ROS levels, mitochondrial permeability transition (MPT), cytochrome c release, and apoptotic cell death, indicating that mitochondrial fragmentation in hyperglycemic insult is causal to the ROS increase (114, 190). High levels of ROS can induce the MPT, followed by MOMP and apoptosis (17, 94, 99, 192). The MPT allows leakage of mitochondrial glutathione resulting in accumulation of more ROS due to the diminished glutathione level (20). Dislocation of cytochrome c upon the MOMP would inhibit the ETC and further enhance ROS generation (20). During apoptosis, activated caspase cleaves the p75 subunit of mitochondrial complex I, causing ETC dysfunction and ROS increase (138). It is likely that ROS, MPT, MOMP, and caspase activation constitute a cyclic event that amplifies ROS accumulation, accelerating apoptotic cell death (Fig. 7). Mitochondrial fragmentation in hyperglycemic insult could be either the cause or consequence of the ROS increase, as discussed. However, due to the cyclic nature of the hyperglycemia-induced mitochondrial fragmentation, ROS increase, and apoptosis, blocking mitochondrial fission would be effective in preventing the ROS amplification and cell injury during hyperglycemic complications, regardless of where the hyperglycemia-induced mitochondrial fragmentation acts (Fig. 7). In this regard, mitochondrial dynamics could be a novel therapeutic target to decrease the pathological effects of increased ROS production in hyperglycemia-associated disorders.
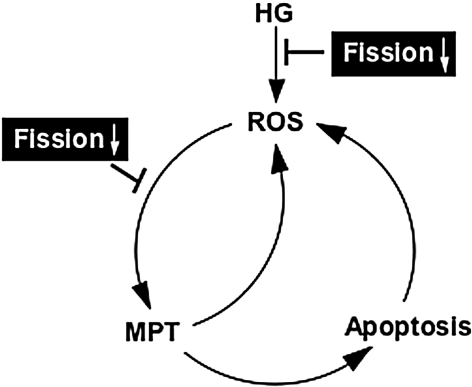
FIG. 7.
A vicious cycle of ROS and apoptosis during hyperglycemic insult. Hyperglycemia (HG) increases ROS through mitochondrial fragmentation. Increased ROS induces MPT, initiating apoptotic cascade. Cytochrome c dislocation and caspase activation further enhance ROS production through ETC dysfunction. We postulate that mitochondrial fission participates in both HG-induced mitochondrial fragmentation and ROS-induced MPT/apoptosis. Therefore, inhibiting mitochondrial fission would diminish ROS toxicity by acting at initial ROS generation and/or ROS amplification in HG.
Perspectives
Abnormal mitochondrial morphology is frequently seen in many pathological conditions. These include prominent neurological disorders and the focus of this review, diabetes and its associated complications. Defining the form–function relationship of mitochondria will be crucial in our understanding of pathologies of the disease states associated with their dysfunction. Given the long-standing ascribed function of mitochondria as metabolic regulators of the cell, it is not surprising that they play an intricate role in diabetes and hyperglycemia, ailments triggered by metabolic excess. More surprisingly perhaps is the intimate role that mitochondrial dynamics appears to play in the progression of this disease state, through insult to β-cells, and observed pathologies in other tissue systems. Observations in multiple cell and tissue types correlate the fragmentation of mitochondria with hyperglycemia and/or hyperlipidemia but the nature of this, causative or resulting, remains an open question.
Mitochondrial fission itself, which we have described as a result of hyperglycemic insult, can be detrimental to the cell through the production of ROS molecules. ROS damage to mitochondria has a compounding effect on ROS production and damage to cellular contents, and, as such, damaged mitochondria are degraded through autophagy, also termed “mitophagy.” This completes what can be viewed as the mitochondrial life cycle, in which mitochondrial dynamics represent a crossroads for maintenance required to adjust to perturbations in the cellular environment (Fig. 8). Studies of morphological disintegration of mitochondria in apoptosis have also brought Bcl-2 family proteins to the mitochondrial dynamics. Through this, mitochondrial dynamics controls the proper maintenance of tissues and organ systems, which, when misregulated, progresses to pathologies associated with disease.
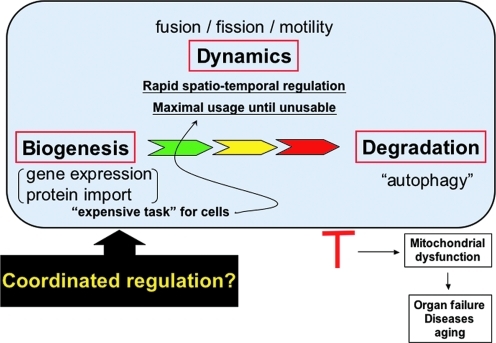
FIG. 8.
Life cycle of mitochondria. Mitochondrial biogenesis requires new syntheses and transport of proteins and lipids. Old and dysfunctional mitochondria are eliminated by the autophagic process. Between their birth and death, mitochondria carry out essential cellular processes, which requires proper maintenance of mitochondrial activity. Through a spatio-temporal regulation of shape and location (mitochondrial dynamics), mitochondria maximize their functional efficiency and life span. Therefore, mitochondrial homeostasis, including biogenesis, dynamics, and autophagic removal, needs to be regulated in a coordinated manner, and dysregulation of any of these processes leads to mitochondrial dysfunction and disease conditions. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Mitochondrial dynamics which started with a simple microscopic observation has come a long way to find an important place in the human pathophysiology. The field of mitochondrial dynamics is still young and many questions remain to be answered. As discussed, mounting evidence suggests that proper control of mitochondrial morphology has an immense impact on the diabetic pathophysiology. Elucidating the functional interactions between the machineries participating in mitochondrial dynamics, and differential signaling pathways operating in normal and pathologic conditions will assist in delineating the pathophysiological role of mitochondrial dynamics in diabetes and lead to novel therapeutic interventions in combating this disease.
Abbreviations Used
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- CaMKIα
calmodulin-dependent kinase Iα
- DLP1
dynamin-like protein 1
- DN
dominant negative
- DRG
dorsal root ganglia
- Drp1
dynamin-related protein 1
- ETC
electron transport chain
- Fis1
fission protein 1
- GDAP1
ganglioside-induced differentiation associated protein 1
- GTP
guanosine triphosphate
- HR
heptad repeat
- MARCH-V
membrane-associated RING-CH-V
- Mff
mitochondrial fission factor
- Mfn
mitofusin
- MIB
Mfn-binding protein
- MIDD
maternally inherited diabetes and deafness
- MitoPLD
mitochondrial phospholipase D
- MOMP
mitochondrial outer membrane permeabilization
- MPT
mitochondrial permeability transition
- MTP18
mitochondrial protein 18 kDa
- N
nucleus
- OPA1
optic atrophy 1
- OXPHOS
oxidative phosphorylation
- PKA
cyclic AMP-dependent protein kinase
- ROS
reactive oxygen species
- SLP-2
stomatin-like protein 2
- SNO
S-nitrosylation
- TPR
tetratricopeptide repeat
Acknowledgment
This work was supported by National Institutes of Health grants DK 078618 and DK 061991.
References
- 1.Alexander C. Votruba M. Pesch UE. Thiselton DL. Mayer S. Moore A. Rodriguez M. Kellner U. Leo-Kottler B. Auburger G, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 2.Anello M. Lupi R. Spampinato D. Piro S. Masini M. Boggi U. Del Prato S. Rabuazzo AM. Purrello F. Marchetti P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48:282–289. doi: 10.1007/s00125-004-1627-9. [DOI] [PubMed] [Google Scholar]
- 3.Arnoult D. Rismanchi N. Grodet A. Roberts RG. Seeburg DP. Estaquier J. Sheng M. Blackstone C. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol. 2005;15:2112–2118. doi: 10.1016/j.cub.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Autret A. Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell. 2009;36:355–363. doi: 10.1016/j.molcel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Bach D. Naon D. Pich S. Soriano FX. Vega N. Rieusset J. Laville M. Guillet C. Boirie Y. Wallberg-Henriksson H, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–2693. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 6.Bach D. Pich S. Soriano FX. Vega N. Baumgartner B. Oriola J. Daugaard JR. Lloberas J. Camps M. Zierath JR, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 7.Barsoum MJ. Yuan H. Gerencser AA. Liot G. Kushnareva Y. Graber S. Kovacs I. Lee WD. Waggoner J. Cui J, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxter RV. Ben Othmane K. Rochelle JM. Stajich JE. Hulette C. Dew-Knight S. Hentati F. Ben Hamida M. Bel S. Stenger JE, et al. Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot-Marie-Tooth disease type 4A/8q21. Nat Genet. 2002;30:21–22. doi: 10.1038/ng796. [DOI] [PubMed] [Google Scholar]
- 9.Benard G. Bellance N. James D. Parrone P. Fernandez H. Letellier T. Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 10.Benard G. Rossignol R. Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid Redox Signal. 2008;10:1313–1342. doi: 10.1089/ars.2007.2000. [DOI] [PubMed] [Google Scholar]
- 11.Bereiter-Hahn J. Behavior of mitochondria in the living cell. Int Rev Cytol. 1990;122:1–63. doi: 10.1016/s0074-7696(08)61205-x. [DOI] [PubMed] [Google Scholar]
- 12.Bereiter-Hahn J. Voth M. Dynamics of mitochondria in living cells: shape changes, dislocation, fusion, and fission of mitochondria. Microsc Res Tech. 1994;27:198–219. doi: 10.1002/jemt.1070270303. [DOI] [PubMed] [Google Scholar]
- 13.Berggren PO. Larsson O. Ca2+ and pancreatic B-cell function. Biochem Soc Trans. 1994;22:12–18. doi: 10.1042/bst0220012. [DOI] [PubMed] [Google Scholar]
- 14.Berman SB. Chen YB. Qi B. McCaffery JM. Rucker EB., 3rd Goebbels S. Nave KA. Arnold BA. Jonas EA. Pineda FJ, et al. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J Cell Biol. 2009;184:707–719. doi: 10.1083/jcb.200809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bindokas VP. Kuznetsov A. Sreenan S. Polonsky KS. Roe MW. Philipson LH. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem. 2003;278:9796–9801. doi: 10.1074/jbc.M206913200. [DOI] [PubMed] [Google Scholar]
- 16.Bonnard C. Durand A. Peyrol S. Chanseaume E. Chauvin MA. Morio B. Vidal H. Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brady NR. Elmore SP. van Beek JJ. Krab K. Courtoy PJ. Hue L. Westerhoff HV. Coordinated behavior of mitochondria in both space and time: a reactive oxygen species-activated wave of mitochondrial depolarization. Biophys J. 2004;87:2022–2034. doi: 10.1529/biophysj.103.035097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braschi E. Zunino R. McBride HM. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep. 2009;10:748–754. doi: 10.1038/embor.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brehm A. Krssak M. Schmid AI. Nowotny P. Waldhausl W. Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes. 2006;55:136–140. [PubMed] [Google Scholar]
- 20.Brookes PS. Yoon Y. Robotham JL. Anders MW. Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 21.Brooks C. Wei Q. Cho SG. Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks C. Wei Q. Feng L. Dong G. Tao Y. Mei L. Xie ZJ. Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci U S A. 2007;104:11649–11654. doi: 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 24.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 25.Butler AE. Janson J. Bonner-Weir S. Ritzel R. Rizza RA. Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 26.Cai L. Li W. Wang G. Guo L. Jiang Y. Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome c-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- 27.Cave AC. Brewer AC. Narayanapanicker A. Ray R. Grieve DJ. Walker S. Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 28.Chang CR. Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 29.Chen H. Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;14:R283–R289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 30.Chen H. Chan DC. Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H. Chomyn A. Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 32.Chen H. Detmer SA. Ewald AJ. Griffin EE. Fraser SE. Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho DH. Nakamura T. Fang J. Cieplak P. Godzik A. Gu Z. Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi SY. Huang P. Jenkins GM. Chan DC. Schiller J. Frohman MA. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol. 2006;8:1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- 35.Cipolat S. Martins de Brito O. Dal Zilio B. Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cipolat S. Rudka T. Hartmann D. Costa V. Serneels L. Craessaerts K. Metzger K. Frezza C. Annaert W. D'Adamio L, et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Cribbs JT. Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuesta A. Pedrola L. Sevilla T. Garcia-Planells J. Chumillas MJ. Mayordomo F. LeGuern E. Marin I. Vilchez JJ. Palau F. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat Genet. 2002;30:22–25. doi: 10.1038/ng798. [DOI] [PubMed] [Google Scholar]
- 39.Da Cruz S. Parone PA. Gonzalo P. Bienvenut WV. Tondera D. Jourdain A. Quadroni M. Martinou JC. SLP-2 interacts with prohibitins in the mitochondrial inner membrane and contributes to their stability. Biochim Biophys Acta. 2008;1783:904–911. doi: 10.1016/j.bbamcr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Danial NN. Walensky LD. Zhang CY. Choi CS. Fisher JK. Molina AJ. Datta SR. Pitter KL. Bird GH. Wikstrom JD, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies VJ. Hollins AJ. Piechota MJ. Yip W. Davies JR. White KE. Nicols PP. Boulton ME. Votruba M. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum Mol Genet. 2007;16:1307–1318. doi: 10.1093/hmg/ddm079. [DOI] [PubMed] [Google Scholar]
- 42.de Brito OM. Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 43.Delettre C. Griffoin JM. Kaplan J. Dollfus H. Lorenz B. Faivre L. Lenaers G. Belenguer P. Hamel CP. Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet. 2001;109:584–591. doi: 10.1007/s00439-001-0633-y. [DOI] [PubMed] [Google Scholar]
- 44.Delettre C. Lenaers G. Griffoin JM. Gigarel N. Lorenzo C. Belenguer P. Pelloquin L. Grosgeorge J. Turc-Carel C. Perret E, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 45.Delivani P. Adrain C. Taylor RC. Duriez PJ. Martin SJ. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell. 2006;21:761–773. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 46.Deng S. Vatamaniuk M. Huang X. Doliba N. Lian MM. Frank A. Velidedeoglu E. Desai NM. Koeberlein B. Wolf B, et al. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes. 2004;53:624–632. doi: 10.2337/diabetes.53.3.624. [DOI] [PubMed] [Google Scholar]
- 47.Detaille D. Guigas B. Chauvin C. Batandier C. Fontaine E. Wiernsperger N. Leverve X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 48.Ding H. Aljofan M. Triggle CR. Oxidative stress and increased eNOS and NADPH oxidase expression in mouse microvessel endothelial cells. J Cell Physiol. 2007;212:682–689. doi: 10.1002/jcp.21063. [DOI] [PubMed] [Google Scholar]
- 49.Dlaskova A. Spacek T. Santorova J. Plecita-Hlavata L. Berkova Z. Saudek F. Lessard M. Bewersdorf J. Jezek P. 4Pi microscopy reveals an impaired three-dimensional mitochondrial network of pancreatic islet beta-cells, an experimental model of type-2 diabetes. Biochim Biophys Acta. 2010;1797:1327–1341. doi: 10.1016/j.bbabio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Dohm JA. Lee SJ. Hardwick JM. Hill RB. Gittis AG. Cytosolic domain of the human mitochondrial fission protein Fis1 adopts a TPR fold. Proteins. 2004;54:153–156. doi: 10.1002/prot.10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donath MY. Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47:581–589. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- 52.Duvezin-Caubet S. Jagasia R. Wagener J. Hofmann S. Trifunovic A. Hansson A. Chomyn A. Bauer MF. Attardi G. Larsson NG, et al. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem. 2006;281:37972–37979. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- 53.Duvezin-Caubet S. Koppen M. Wagener J. Zick M. Israel L. Bernacchia A. Jagasia R. Rugarli EI. Imhof A. Neupert W, et al. OPA1 processing reconstituted in yeast depends on the subunit composition of the m-AAA protease in mitochondria. Mol Biol Cell. 2007;18:3582–3590. doi: 10.1091/mbc.E07-02-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehses S. Raschke I. Mancuso G. Bernacchia A. Geimer S. Tondera D. Martinou JC. Westermann B. Rugarli EI. Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187:1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Estaquier J. Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 2007;14:1086–1094. doi: 10.1038/sj.cdd.4402107. [DOI] [PubMed] [Google Scholar]
- 56.Eura Y. Ishihara N. Oka T. Mihara K. Identification of a novel protein that regulates mitochondrial fusion by modulating mitofusin (Mfn) protein function. J Cell Sci. 2006;119:4913–4925. doi: 10.1242/jcs.03253. [DOI] [PubMed] [Google Scholar]
- 57.Figueroa-Romero C. Iniguez-Lluhi JA. Stadler J. Chang CR. Arnoult D. Keller PJ. Hong Y. Blackstone C. Feldman EL. SUMOylation of the mitochondrial fission protein Drp1 occurs at multiple nonconsensus sites within the B domain and is linked to its activity cycle. FASEB J. 2009;23:3917–3927. doi: 10.1096/fj.09-136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frank S. Gaume B. Bergmann-Leitner ES. Leitner WW. Robert EG. Catez F. Smith CL. Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 59.Frezza C. Cipolat S. Martins de Brito O. Micaroni M. Beznoussenko GV. Rudka T. Bartoli D. Polishuck RS. Danial NN. De Strooper B, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 60.Gandre-Babbe S. van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao CL. Zhu C. Zhao YP. Chen XH. Ji CB. Zhang CM. Zhu JG. Xia ZK. Tong ML. Guo XR. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol Cell Endocrinol. 2010;320:25–33. doi: 10.1016/j.mce.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 62.Goldman S. Zhang Y. Jin S. Autophagy and adipogenesis: implications in obesity and type II diabetes. Autophagy. 2010;6:179–181. doi: 10.4161/auto.6.1.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Green K. Brand MD. Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 2004;53:S110–S118. doi: 10.2337/diabetes.53.2007.s110. [DOI] [PubMed] [Google Scholar]
- 64.Griparic L. Kanazawa T. van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griparic L. van der Wel NN. Orozco IJ. Peters PJ. van der Bliek AM. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J Biol Chem. 2004;279:18792–18798. doi: 10.1074/jbc.M400920200. [DOI] [PubMed] [Google Scholar]
- 66.Hackenbrock CR. Ultrastructural bases for metabolically linked mechanical activity in mitochondria: I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol. 1966;30:269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hajek P. Chomyn A. Attardi G. Identification of a novel mitochondrial complex containing mitofusin 2 and stomatin-like protein 2. J Biol Chem. 2007;282:5670–5681. doi: 10.1074/jbc.M608168200. [DOI] [PubMed] [Google Scholar]
- 68.Han D. Yang B. Olson LK. Greenstein A. Baek SH. Claycombe KJ. Goudreau JL. Yu SW. Kim EK. Activation of autophagy through modulation of 5′-AMP-activated protein kinase protects pancreatic beta-cells from high glucose. Biochem J. 2010;425:541–551. doi: 10.1042/BJ20090429. [DOI] [PubMed] [Google Scholar]
- 69.Han XJ. Lu YF. Li SA. Kaitsuka T. Sato Y. Tomizawa K. Nairn AC. Takei K. Matsui H. Matsushita M. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harder Z. Zunino R. McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004;14:340–345. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 71.Head B. Griparic L. Amiri M. Gandre-Babbe S. van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187:959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 73.Hoeks J. Hesselink MK. Russell AP. Mensink M. Saris WH. Mensink RP. Schrauwen P. Peroxisome proliferator-activated receptor-gamma coactivator-1 and insulin resistance: acute effect of fatty acids. Diabetologia. 2006;49:2419–2426. doi: 10.1007/s00125-006-0369-2. [DOI] [PubMed] [Google Scholar]
- 74.Ishihara N. Fujita Y. Oka T. Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishihara N. Jofuku A. Eura Y. Mihara K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem Biophys Res Commun. 2003;301:891–898. doi: 10.1016/s0006-291x(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 76.Ishihara N. Nomura M. Jofuku A. Kato H. Suzuki SO. Masuda K. Otera H. Nakanishi Y. Nonaka I. Goto Y, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 77.Jahani-Asl A. Cheung EC. Neuspiel M. MacLaurin JG. Fortin A. Park DS. McBride HM. Slack RS. Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. J Biol Chem. 2007;282:23788–23798. doi: 10.1074/jbc.M703812200. [DOI] [PubMed] [Google Scholar]
- 78.James DI. Parone PA. Mattenberger Y. Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 79.Jendrach M. Mai S. Pohl S. Voth M. Bereiter-Hahn J. Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA after transient oxidative stress. Mitochondrion. 2008;8:293–304. doi: 10.1016/j.mito.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 80.Kahn SE. Montgomery B. Howell W. Ligueros-Saylan M. Hsu CH. Devineni D. McLeod JF. Horowitz A. Foley JE. Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001;86:5824–5829. doi: 10.1210/jcem.86.12.8105. [DOI] [PubMed] [Google Scholar]
- 81.Kamimoto T. Nagai Y. Onogi H. Muro Y. Wakabayashi T. Hagiwara M. Dymple, a novel dynamin-like high molecular weight GTPase lacking a proline-rich carboxyl-terminal domain in mammalian cells. J Biol Chem. 1998;273:1044–1051. doi: 10.1074/jbc.273.2.1044. [DOI] [PubMed] [Google Scholar]
- 82.Karbowski M. Jeong SY. Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. J Cell Biol. 2004;166:1027–1039. doi: 10.1083/jcb.200407046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karbowski M. Neutzner A. Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007;178:71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karbowski M. Norris KL. Cleland MM. Jeong SY. Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 85.Kelley DE. He J. Menshikova EV. Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 86.Kelley DE. McKolanis TM. Hegazi RA. Kuller LH. Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285:E906–E916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 87.Kim JY. Hickner RC. Cortright RL. Dohm GL. Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–E1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 88.Knott AB. Bossy-Wetzel E. Impairing the mitochondrial fission and fusion balance: a new mechanism of neurodegeneration. Ann N Y Acad Sci. 2008;1147:283–292. doi: 10.1196/annals.1427.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Knott AB. Perkins G. Schwarzenbacher R. Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kong D. Xu L. Yu Y. Zhu W. Andrews D. Yoon Y. Kuo TH. Regulation of Ca2+-induced permeability transition by Bcl-2 is antagonized by Drp1 and hFis1. Mol Cell Biochem. 2005;272:187–199. doi: 10.1007/s11010-005-7323-3. [DOI] [PubMed] [Google Scholar]
- 91.Koshiba T. Detmer SA. Kaiser JT. Chen H. McCaffery JM. Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- 92.Lagouge M. Argmann C. Gerhart-Hines Z. Meziane H. Lerin C. Daussin F. Messadeq N. Milne J. Lambert P. Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 93.Larson-Meyer DE. Heilbronn LK. Redman LM. Newcomer BR. Frisard MI. Anton S. Smith SR. Alfonso A. Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leach JK. Van Tuyle G. Lin PS. Schmidt-Ullrich R. Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61:3894–3901. [PubMed] [Google Scholar]
- 95.Leahy JL. Natural history of beta-cell dysfunction in NIDDM. Diabetes Care. 1990;13:992–1010. doi: 10.2337/diacare.13.9.992. [DOI] [PubMed] [Google Scholar]
- 96.Lee YJ. Jeong SY. Karbowski M. Smith CL. Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Legros F. Lombes A. Frachon P. Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13:4343–4354. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leinninger GM. Backus C. Sastry AM. Yi YB. Wang CW. Feldman EL. Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol Dis. 2006;23:11–22. doi: 10.1016/j.nbd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 99.Lemasters JJ. Nieminen AL. Qian T. Trost LC. Elmore SP. Nishimura Y. Crowe RA. Cascio WE. Bradham CA. Brenner DA, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 100.Li H. Chen Y. Jones AF. Sanger RH. Collis LP. Flannery R. McNay EC. Yu T. Schwarzenbacher R. Bossy B, et al. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:2169–2174. doi: 10.1073/pnas.0711647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liot G. Bossy B. Lubitz S. Kushnareva Y. Sejbuk N. Bossy-Wetzel E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009;16:899–909. doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lowell BB. Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 103.Lu B. Mitochondrial dynamics and neurodegeneration. Curr Neurol Neurosci Rep. 2009;9:212–219. doi: 10.1007/s11910-009-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maassen JA. ’T Hart LM. Van Essen E. Heine RJ. Nijpels G. Jahangir Tafrechi RS. Raap AK. Janssen GM. Lemkes HH. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 2004;53(Suppl 1):S103–S109. doi: 10.2337/diabetes.53.2007.s103. [DOI] [PubMed] [Google Scholar]
- 105.Maechler P. Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001;414:807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- 106.Maechler P. Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402:685–689. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- 107.Maechler P. Wollheim CB. Mitochondrial signals in glucose-stimulated insulin secretion in the beta cell. J Physiol. 2000;529:49–56. doi: 10.1111/j.1469-7793.2000.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maechler P. Wollheim CB. Role of mitochondria in metabolism-secretion coupling of insulin release in the pancreatic beta-cell. Biofactors. 1998;8:255–262. doi: 10.1002/biof.5520080313. [DOI] [PubMed] [Google Scholar]
- 109.Mannella CA. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta. 2006;1763:542–548. doi: 10.1016/j.bbamcr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 110.Marchetti P. Masini M. Autophagy and the pancreatic beta-cell in human type 2 diabetes. Autophagy. 2009;5:1055–1056. doi: 10.4161/auto.5.7.9511. [DOI] [PubMed] [Google Scholar]
- 111.Masini M. Bugliani M. Lupi R. del Guerra S. Boggi U. Filipponi F. Marselli L. Masiello P. Marchetti P. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52:1083–1086. doi: 10.1007/s00125-009-1347-2. [DOI] [PubMed] [Google Scholar]
- 112.Mattenberger Y. James DI. Martinou JC. Fusion of mitochondria in mammalian cells is dependent on the mitochondrial inner membrane potential and independent of microtubules or actin. FEBS Lett. 2003;538:53–59. doi: 10.1016/s0014-5793(03)00124-8. [DOI] [PubMed] [Google Scholar]
- 113.Meijer AJ. Codogno P. Autophagy: regulation and role in disease. Crit Rev Clin Lab Sci. 2009;46:210–240. doi: 10.1080/10408360903044068. [DOI] [PubMed] [Google Scholar]
- 114.Men X. Wang H. Li M. Cai H. Xu S. Zhang W. Xu Y. Ye L. Yang W. Wollheim CB, et al. Dynamin-related protein 1 mediates high glucose induced pancreatic beta cell apoptosis. Int J Biochem Cell Biol. 2009;41:879–890. doi: 10.1016/j.biocel.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 115.Molina AJ. Wikstrom JD. Stiles L. Las G. Mohamed H. Elorza A. Walzer G. Twig G. Katz S. Corkey BE, et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mootha VK. Lindgren CM. Eriksson KF. Subramanian A. Sihag S. Lehar J. Puigserver P. Carlsson E. Ridderstrale M. Laurila E, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 117.Nakamura N. Kimura Y. Tokuda M. Honda S. Hirose S. MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep. 2006;7:1019–1022. doi: 10.1038/sj.embor.7400790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nelis E. Erdem S. Van Den Bergh PY. Belpaire-Dethiou MC. Ceuterick C. Van Gerwen V. Cuesta A. Pedrola L. Palau F. Gabreels-Festen AA, et al. Mutations in GDAP1: autosomal recessive CMT with demyelination and axonopathy. Neurology. 2002;59:1865–1872. doi: 10.1212/01.wnl.0000036272.36047.54. [DOI] [PubMed] [Google Scholar]
- 119.Neuspiel M. Zunino R. Gangaraju S. Rippstein P. McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem. 2005;280:25060–25070. doi: 10.1074/jbc.M501599200. [DOI] [PubMed] [Google Scholar]
- 120.Niemann A. Ruegg M. La Padula V. Schenone A. Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J Cell Biol. 2005;170:1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nishikawa T. Edelstein D. Du XL. Yamagishi S. Matsumura T. Kaneda Y. Yorek MA. Beebe D. Oates PJ. Hammes HP, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 122.Noske AB. Costin AJ. Morgan GP. Marsh BJ. Expedited approaches to whole cell electron tomography and organelle mark-up in situ in high-pressure frozen pancreatic islets. J Struct Biol. 2008;161:298–313. doi: 10.1016/j.jsb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nunnari J. Marshall WF. Straight A. Murray A. Sedat JW. Walter P. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol Biol Cell. 1997;8:1233–1242. doi: 10.1091/mbc.8.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Olichon A. Baricault L. Gas N. Guillou E. Valette A. Belenguer P. Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 125.Ostergard T. Andersen JL. Nyholm B. Lund S. Nair KS. Saltin B. Schmitz O. Impact of exercise training on insulin sensitivity, physical fitness, and muscle oxidative capacity in first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;290:E998–E1005. doi: 10.1152/ajpendo.00012.2005. [DOI] [PubMed] [Google Scholar]
- 126.Paltauf-Doburzynska J. Malli R. Graier WF. Hyperglycemic conditions affect shape and Ca2+ homeostasis of mitochondria in endothelial cells. J Cardiovasc Pharmacol. 2004;44:423–436. doi: 10.1097/01.fjc.0000139449.64337.1b. [DOI] [PubMed] [Google Scholar]
- 127.Park KS. Wiederkehr A. Kirkpatrick C. Mattenberger Y. Martinou JC. Marchetti P. Demaurex N. Wollheim CB. Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J Biol Chem. 2008;283:33347–33356. doi: 10.1074/jbc.M806251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Parone PA. Da Cruz S. Tondera D. Mattenberger Y. James DI. Maechler P. Barja F. Martinou JC. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE. 2008;3:e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Parone PA. James DI. Da Cruz S. Mattenberger Y. Donze O. Barja F. Martinou JC. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Patti ME. Butte AJ. Crunkhorn S. Cusi K. Berria R. Kashyap S. Miyazaki Y. Kohane I. Costello M. Saccone R, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pattingre S. Tassa A. Qu X. Garuti R. Liang XH. Mizushima N. Packer M. Schneider MD. Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 132.Pich S. Bach D. Briones P. Liesa M. Camps M. Testar X. Palacin M. Zorzano A. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet. 2005;14:1405–1415. doi: 10.1093/hmg/ddi149. [DOI] [PubMed] [Google Scholar]
- 133.Pitts KR. Yoon Y. Krueger EW. McNiven MA. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell. 1999;10:4403–4417. doi: 10.1091/mbc.10.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Plecita-Hlavata L. Lessard M. Santorova J. Bewersdorf J. Jezek P. Mitochondrial oxidative phosphorylation and energetic status are reflected by morphology of mitochondrial network in INS-1E and HEP-G2 cells viewed by 4Pi microscopy. Biochim Biophys Acta. 2008;1777:834–846. doi: 10.1016/j.bbabio.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 135.Pospisilik JA. Knauf C. Joza N. Benit P. Orthofer M. Cani PD. Ebersberger I. Nakashima T. Sarao R. Neely G, et al. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell. 2007;131:476–491. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 136.Quagliaro L. Piconi L. Assaloni R. Martinelli L. Motz E. Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 137.Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 138.Ricci J-E. Munoz-Pinedo C. Fitzgerald P. Bailly-Maitre B. Perkins GA. Yadava N. Scheffler IE. Ellisman MH. Green DR. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 139.Richardson DK. Kashyap S. Bajaj M. Cusi K. Mandarino SJ. Finlayson J. DeFronzo RA. Jenkinson CP. Mandarino LJ. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem. 2005;280:10290–10297. doi: 10.1074/jbc.M408985200. [DOI] [PubMed] [Google Scholar]
- 140.Rimbert V. Boirie Y. Bedu M. Hocquette JF. Ritz P. Morio B. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB J. 2004;18:737–739. doi: 10.1096/fj.03-1104fje. [DOI] [PubMed] [Google Scholar]
- 141.Robertson RP. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr Opin Pharmacol. 2006;6:615–619. doi: 10.1016/j.coph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 142.Rojo M. Legros F. Chateau D. Lombes A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci. 2002;115:1663–1674. doi: 10.1242/jcs.115.8.1663. [DOI] [PubMed] [Google Scholar]
- 143.Rossignol R. Gilkerson R. Aggeler R. Yamagata K. Remington SJ. Capaldi RA. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res. 2004;64:985–993. doi: 10.1158/0008-5472.can-03-1101. [DOI] [PubMed] [Google Scholar]
- 144.Russell JW. Golovoy D. Vincent AM. Mahendru P. Olzmann JA. Mentzer A. Feldman EL. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 145.Sakuraba H. Mizukami H. Yagihashi N. Wada R. Hanyu C. Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 146.Santel A. Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 147.Sato A. Nakada K. Hayashi J. Mitochondrial dynamics and aging: mitochondrial interaction preventing individuals from expression of respiratory deficiency caused by mutant mtDNA. Biochim Biophys Acta. 2006;1763:473–481. doi: 10.1016/j.bbamcr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 148.Schrauwen-Hinderling VB. Mensink M. Hesselink MK. Sels JP. Kooi ME. Schrauwen P. The insulin-sensitizing effect of rosiglitazone in type 2 diabetes mellitus patients does not require improved in vivo muscle mitochondrial function. J Clin Endocrinol Metab. 2008;93:2917–2921. doi: 10.1210/jc.2008-0267. [DOI] [PubMed] [Google Scholar]
- 149.Scorrano L. Oakes SA. Opferman JT. Cheng EH. Sorcinelli MD. Pozzan T. Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 150.Serasinghe MN. Yoon Y. The mitochondrial outer membrane protein hFis1 regulates mitochondrial morphology and fission through self-interaction. Exp Cell Res. 2008;314:3494–3507. doi: 10.1016/j.yexcr.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sesaki H. Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J Cell Biol. 1999;147:699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sheridan C. Delivani P. Cullen SP. Martin SJ. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome c release. Mol Cell. 2008;31:570–585. doi: 10.1016/j.molcel.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 153.Shin HW. Shinotsuka C. Torii S. Murakami K. Nakayama K. Identification and subcellular localization of a novel mammalian dynamin-related protein homologous to yeast Vps1p and Dnm1p. J Biochem. 1997;122:525–530. doi: 10.1093/oxfordjournals.jbchem.a021784. [DOI] [PubMed] [Google Scholar]
- 154.Silva JP. Kohler M. Graff C. Oldfors A. Magnuson MA. Berggren PO. Larsson NG. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet. 2000;26:336–340. doi: 10.1038/81649. [DOI] [PubMed] [Google Scholar]
- 155.Simoneau JA. Veerkamp JH. Turcotte LP. Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999;13:2051–2060. doi: 10.1096/fasebj.13.14.2051. [DOI] [PubMed] [Google Scholar]
- 156.Smirnova E. Griparic L. Shurland DL. van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Song Z. Chen H. Fiket M. Alexander C. Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Song Z. Ghochani M. McCaffery JM. Frey TG. Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20:3525–3532. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sparks LM. Xie H. Koza RA. Mynatt R. Hulver MW. Bray GA. Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 160.Stojanovski D. Koutsopoulos OS. Okamoto K. Ryan MT. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci. 2004;117:1201–1210. doi: 10.1242/jcs.01058. [DOI] [PubMed] [Google Scholar]
- 161.Su B. Wang X. Zheng L. Perry G. Smith MA. Zhu X. Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim Biophys Acta. 2010;1802:135–142. doi: 10.1016/j.bbadis.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sugioka R. Shimizu S. Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–52734. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- 163.Suzuki M. Jeong SY. Karbowski M. Youle RJ. Tjandra N. The solution structure of human mitochondria fission protein Fis1 reveals a novel TPR-like helix bundle. J Mol Biol. 2003;334:445–458. doi: 10.1016/j.jmb.2003.09.064. [DOI] [PubMed] [Google Scholar]
- 164.Szabadkai G. Simoni AM. Chami M. Wieckowski MR. Youle RJ. Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 165.Taguchi N. Ishihara N. Jofuku A. Oka T. Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 166.Toledo FG. Menshikova EV. Azuma K. Radikova Z. Kelley CA. Ritov VB. Kelley DE. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57:987–994. doi: 10.2337/db07-1429. [DOI] [PubMed] [Google Scholar]
- 167.Toledo FG. Menshikova EV. Ritov VB. Azuma K. Radikova Z. DeLany J. Kelley DE. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142–2147. doi: 10.2337/db07-0141. [DOI] [PubMed] [Google Scholar]
- 168.Tondera D. Czauderna F. Paulick K. Schwarzer R. Kaufmann J. Santel A. The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J Cell Sci. 2005;118:3049–3059. doi: 10.1242/jcs.02415. [DOI] [PubMed] [Google Scholar]
- 169.Tondera D. Grandemange S. Jourdain A. Karbowski M. Mattenberger Y. Herzig S. Da Cruz S. Clerc P. Raschke I. Merkwirth C, et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28:1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tondera D. Santel A. Schwarzer R. Dames S. Giese K. Klippel A. Kaufmann J. Knockdown of MTP18, a novel phosphatidylinositol 3-kinase-dependent protein, affects mitochondrial morphology and induces apoptosis. J Biol Chem. 2004;279:31544–31555. doi: 10.1074/jbc.M404704200. [DOI] [PubMed] [Google Scholar]
- 171.Twig G. Elorza A. Molina AJ. Mohamed H. Wikstrom JD. Walzer G. Stiles L. Haigh SE. Katz S. Las G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Twig G. Hyde B. Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vanhorebeek I. De Vos R. Mesotten D. Wouters PJ. De Wolf-Peeters C. Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365:53–59. doi: 10.1016/S0140-6736(04)17665-4. [DOI] [PubMed] [Google Scholar]
- 174.Van Laar VS. Berman SB. Mitochondrial dynamics in Parkinson's disease. Exp Neurol. 2009;218:247–256. doi: 10.1016/j.expneurol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang X. Su B. Zheng L. Perry G. Smith MA. Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J Neurochem. 2009;109(Suppl 1):153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 177.Wasiak S. Zunino R. McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Waterham HR. Koster J. van Roermund CW. Mooyer PA. Wanders RJ. Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 179.Westermann B. Merging mitochondria matters: cellular role and molecular machinery of mitochondrial fusion. EMBO Rep. 2002;3:527–531. doi: 10.1093/embo-reports/kvf113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wredenberg A. Freyer C. Sandstrom ME. Katz A. Wibom R. Westerblad H. Larsson NG. Respiratory chain dysfunction in skeletal muscle does not cause insulin resistance. Biochem Biophys Res Commun. 2006;350:202–207. doi: 10.1016/j.bbrc.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 181.Yonashiro R. Ishido S. Kyo S. Fukuda T. Goto E. Matsuki Y. Ohmura-Hoshino M. Sada K. Hotta H. Yamamura H, et al. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J. 2006;25:3618–3626. doi: 10.1038/sj.emboj.7601249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yoon KH. Ko SH. Cho JH. Lee JM. Ahn YB. Song KH. Yoo SJ. Kang MI. Cha BY. Lee KW, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 183.Yoon Y. Regulation of mitochondrial dynamics: another process modulated by Ca2+ signal? Sci STKE. 2005;2005:pe18. doi: 10.1126/stke.2802005pe18. [DOI] [PubMed] [Google Scholar]
- 184.Yoon Y. Sharpening the scissors: mitochondrial fission with aid. Cell Biochem Biophys. 2004;41:193–205. doi: 10.1385/CBB:41:2:193. [DOI] [PubMed] [Google Scholar]
- 185.Yoon Y. Krueger EW. Oswald BJ. McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yoon Y. Pitts KR. Dahan S. McNiven MA. A novel dynamin-like protein associates with cytoplasmic vesicles and tubules of the endoplasmic reticulum in mammalian cells. J Cell Biol. 1998;140:779–793. doi: 10.1083/jcb.140.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yoon Y. Pitts KR. McNiven MA. Mammalian dynamin-like protein DLP1 tubulates membranes. Mol Biol Cell. 2001;12:2894–2905. doi: 10.1091/mbc.12.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yu T. Fox RJ. Burwell LS. Yoon Y. Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1. J Cell Sci. 2005;118:4141–4151. doi: 10.1242/jcs.02537. [DOI] [PubMed] [Google Scholar]
- 189.Yu T. Robotham JL. Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yu T. Sheu SS. Robotham JL. Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zinkel S. Gross A. Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 2006;13:1351–1359. doi: 10.1038/sj.cdd.4401987. [DOI] [PubMed] [Google Scholar]
- 192.Zorov DB. Filburn CR. Klotz LO. Zweier JL. Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zuchner S. Mersiyanova IV. Muglia M. Bissar-Tadmouri N. Rochelle J. Dadali EL. Zappia M. Nelis E. Patitucci A. Senderek J, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 194.Zunino R. Schauss A. Rippstein P. Andrade-Navarro M. McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120:1178–1188. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]