Abstract
Background
The increasing incidence of thyroid cancer in the United States is well documented. In this study, we assessed the incidence patterns by histologic type according to demographic and tumor characteristics to further our understanding of these cancers.
Methods
We used the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program for cases diagnosed during 1992–2006 to investigate patterns for the four major histologic types of thyroid cancer by gender, race/ethnicity, and age as well as registry, tumor stage, and size.
Results
Among women, papillary thyroid cancer rates were highest among Asians (10.96 per 100,000 woman-years) and lowest among blacks (4.90 per 100,000 woman-years); follicular cancer rates did not vary substantially by race/ethnicity (p-values >0.05), medullary cancer rates were highest among Hispanics (0.21 per 100,000 woman-years) and whites (0.22 per 100,000 woman-years), and anaplastic rates were highest among Hispanics (0.17 per 100,000 woman-years). Among men, both papillary and follicular thyroid cancer rates were highest among whites (3.58 and 0.58 per 100,000 man-years, respectively), medullary cancer rates were highest among Hispanics (0.18 per 100,000 man-years), and anaplastic rates were highest among Asians (0.11 per 100,000 man-years). Racial/ethnic-specific rates did not vary notably across registries. In contrast to age-specific rates of papillary thyroid cancer that peaked in midlife (age 50), especially pronounced among women, rates for follicular, medullary, and anaplastic types continued to rise across virtually the entire age range, especially for anaplastic carcinomas. Female-to-male incidence rate ratios among whites decreased with age most steeply for the follicular type and least steeply for the medullary type; it was <1 until the very oldest ages for the anaplastic type.
Conclusion
We conclude that the similar age-specific patterns and lack of geographical variation across the SEER racial/ethnic groups indicate that detection effects cannot completely explain the observed thyroid cancer incidence patterns as variation in the amount or quality of healthcare provided has been shown to vary by SEER racial/ethnic groups, gender, and age. We find that the variations in age-specific patterns by gender and across histologic types are intriguing and recommend that future etiologic investigation focus on exogenous and endogenous exposures that are experienced similarly by racial/ethnic groups, more strongly among women, and distinctively by age.
Introduction
Arise in thyroid cancer incidence, especially of the papillary type, has been reported in several countries, including the United States, during the past several decades (1–6), and the factors responsible for the increase remain unknown. Thus far, the majority of the descriptive epidemiology of thyroid cancer has evaluated thyroid cancer overall or has been limited to the investigation of the papillary histologic type. A comparison of thyroid cancer types by gender across Surveillance, Epidemiology, and End Results (SEER) racial/ethnic groups, by age at diagnosis, and by tumor characteristics presents an opportunity to consider the role of detection and access to technology on incidence patterns. Consideration of the descriptive patterns of thyroid cancer could provide clues about the role of changing risk factors and detection methods in the dramatically increasing disease rates.
Five groups recently examined time trends in thyroid cancer incidence overall, and papillary cancer in particular, using SEER data (1,3,4,7,8). Enewold et al. examined thyroid cancer incidence by demographic and tumor characteristics and found that between 1992–1995 and 2003–2005, rates of papillary thyroid cancers increased nearly 100% among non-Hispanic white and black women but only 20%–50% among Hispanics, Asian/Pacific Islanders, and black men (4). Similarly, during a comparable period (1992–2004), Yu et al. found that the papillary carcinoma increases translated into an annual percent change of 5.6% among non-Hispanic whites, 4.3% among non-Hispanic blacks, 2.8% among Hispanic whites, and 1.5% among Asians (8). Additionally, both groups reported increases in large as well as small tumors among all SEER racial/ethnic groups studied, and Yu et al. further reported that local stage tumors increased 24% among blacks, compared to 14.4% among Hispanic whites, 14.3% among non-Hispanic whites, and 4.0% among Asians.
As race/ethnicity can be thought of as a proxy for healthcare access in the United States given that blacks, Hispanics, Asian/Pacific Islanders, and American Indian/Alaska Natives are more likely to be uninsured than non-Hispanic whites (9), these results seem to contradict the notion that thyroid cancer incidence is artificially inflated due to a detection effect (3) and suggest that more investigation into rates by sex-specific demographic and geographic factors is warranted. Further, as the overall increases in thyroid cancer in previous SEER studies were mainly due to rising papillary rates while the rates of other histologic types changed little (4), this also suggests that perhaps diagnosis is not as significant a factor as recently proposed by others as one would expect similar increases for follicular and medullary tumors. As such, further investigation of the incidence patterns for the follicular, medullary, and anaplastic subtypes by demographic and regional factors is of interest.
By considering the incidence patterns in light of different regional and demographic factors that might impact thyroid cancer diagnosis, we suggest that clues into the role of detection and opportunities for further etiologic research may be revealed. In this study, we were interested in evaluating the incidence patterns for the four major histologic types of thyroid cancer by gender, race/ethnicity, and age as well as registry, tumor stage, and size. Our investigation of all four thyroid cancer histologic types extends the recent analyses of trends for papillary thyroid carcinoma and explores factors that could further our understanding of recent incidence trends and disease etiology.
Methods
We used the National Cancer Institute's SEER 13 Registries Database, November 2008 Submission (10), to analyze male and female thyroid carcinoma incidence rates (IRs) by histologic type based on cases diagnosed during 1992 through 2006 among residents of the areas included in the 13 registries in Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, Utah, Los Angeles, San Jose–Monterey, Rural Georgia, and the Alaska Native Tumor Registry, covering ∼14% of the U.S. population.
Invasive thyroid cancer cases were coded using the International Classification of Diseases for Oncology, third edition (ICD-O-3) (11), and our analysis included the four major histological types: papillary carcinoma (ICD-O-3 codes 8050, 8260, 8340-8341, 8343-8344, and 8350), follicular carcinoma (ICD-O-3 codes 8290, 8330-8332, and 8335), medullary carcinoma (ICD-O-3 codes 8345-8346 and 8510), and anaplastic carcinoma (ICD-O-3 codes 8012, 8020-8021, and 8030-8032). All other ICD-O-3 codes were categorized as other or unknown.
In addition to analyzing thyroid cancer types by gender, we evaluated demographic and tumor characteristics, including age at diagnosis, race/ethnicity, SEER historic stage A, and tumor size. SEER racial/ethnic groups included non-Hispanic whites (hereafter referred to as whites), Hispanic whites (hereafter referred to as Hispanics), blacks, Asians/Pacific Islanders (hereafter referred to as Asians), and American Indians/Alaskan Natives combined. SEER historic stage A was used to classify thyroid cancers as localized (limited to the thyroid gland), regional (limited to surrounding tissues), and distant or systemic disease (11). Tumor size was the cancer's greatest diameter as recorded on surgical pathology reports, which we categorized as ≤1, >1, and ≤2 cm, >2 and ≤4 cm, and >4 cm based on the Extent of Disease-10 codes for 1992–2003 and the Collaborative Staging codes for 2004–2006 (10).
Data analysis
IRs were calculated using SEER*Stat 6.5.1 (12) and expressed per 100,000 person-years, man-years, or woman-years. Age-adjusted IRs were standardized to the 2000 U.S. population. Relative risks were expressed as IR ratios (IRRs), where a given characteristic was compared to a referent group rate assigned an IRR of 1.0 (13,14). Statistical significance of rates and IRR were assessed at the p < 0.05 alpha level; all hypothesis tests were two sided. Disease IRs were reported if there were at least 10 cases in a category, and IRRs were reported if both rates were based on at least 10 cases. Age-specific IRs and IRRs by racial/ethnic group for each histologic type were plotted on a log y and linear x scale, such that a slope of 10° equaled a change in rates of 1% per year (i.e., 40 years on the horizontal axis is the same length as one logarithmic cycle on the vertical axis) (15); single data points were not shown. The presentation of tumor stage and size in tables was restricted to whites due to small numbers of cases in other racial/ethnic groups, although our analyses considered all SEER racial/ethnic groups.
Results
A total of 43,644 thyroid cancer cases were diagnosed from 1992 through 2006, including 36,583 cases of papillary, 4560 cases of follicular, 976 cases of medullary, 556 cases of anaplastic, and 969 other or unspecified thyroid cancer. The overall incidence was 7.7 per 100,000 person-years, with a rate of 11.3 per 100,000 woman-years and 4.1 per 100,000 man-years.
Thyroid cancer by gender, type, and SEER racial/ethnic group
The proportion of thyroid cancer types was generally similar across sex and SEER racial/ethnic groups. Papillary carcinoma rates among women were 2.9–3.8 times those among men, and the IRRs for follicular carcinomas were somewhat lower (IRR = 1.9–3.6); all the female-to-male IRRs for both papillary and follicular carcinomas were significantly elevated for each racial/ethnic group (Table 1). Although all the IRRs for the medullary type were >1.0, it was significantly elevated only among whites (IRR = 1.3). In contrast, for the anaplastic type, the IRR was 1.0 among whites, but it was a significant 2.9 among Hispanics.
Table 1.
Thyroid Cancer Incidence in the Surveillance, Epidemiology, and End Results 13-Registry Database (1992–2006) by Gender, Type, and Racial/Ethnic Group
| |
Women |
Men |
Female/male |
95% CI |
Race/WNH women |
IRR |
IRR |
Race/WNH men |
IRR |
IRR |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 43,644) | n | Rate | SE | n | Rate | SE | IRR | LL | UL | IRR | LL | UL | IRR | LL | UL |
| Total | |||||||||||||||
| Whites | 21,681 | 12.06 | 0.08 | 7908 | 4.59 | 0.05 | 2.63 | 2.56 | 2.70 | 1.00 | 1.00 | ||||
| Hispanics | 4570 | 11.39 | 0.18 | 1075 | 3.26 | 0.11 | 3.50 | 3.25 | 3.78 | 0.94 | 0.91 | 0.98 | 0.71 | 0.66 | 0.76 |
| Asians | 4122 | 12.54 | 0.20 | 1064 | 3.93 | 0.12 | 3.19 | 2.98 | 3.43 | 1.04 | 1.01 | 1.08 | 0.86 | 0.80 | 0.91 |
| Blacks | 1951 | 6.44 | 0.15 | 537 | 2.37 | 0.11 | 2.72 | 2.46 | 3.01 | 0.53 | 0.51 | 0.56 | 0.52 | 0.47 | 0.57 |
| AI/ANs | 232 | 9.69 | 0.66 | 58 | 3.51 | 0.51 | 2.76 | 2.01 | 3.86 | 0.80 | 0.70 | 0.92 | 0.77 | 0.56 | 1.01 |
| Papillary | |||||||||||||||
| Whites | 18,523 | 10.39 | 0.08 | 6218 | 3.58 | 0.05 | 2.90 | 2.82 | 2.98 | 1.00 | 1.00 | ||||
| Hispanics | 3995 | 9.72 | 0.16 | 879 | 2.57 | 0.10 | 3.78 | 3.48 | 4.11 | 0.94 | 0.90 | 0.97 | 0.72 | 0.66 | 0.78 |
| Asians | 3619 | 10.96 | 0.18 | 882 | 3.20 | 0.11 | 3.43 | 3.18 | 3.70 | 1.05 | 1.02 | 1.09 | 0.89 | 0.83 | 0.96 |
| Blacks | 1504 | 4.90 | 0.13 | 366 | 1.56 | 0.09 | 3.14 | 2.78 | 3.55 | 0.47 | 0.45 | 0.50 | 0.44 | 0.39 | 0.49 |
| AI/ANs | 197 | 8.12 | 0.60 | 46 | 2.68 | 0.44 | 3.03 | 2.14 | 4.43 | 0.78 | 0.67 | 0.91 | 0.75 | 0.53 | 1.02 |
| Follicular | |||||||||||||||
| Whites | 2137 | 1.16 | 0.03 | 992 | 0.58 | 0.02 | 1.99 | 1.85 | 2.15 | 1.00 | 1.00 | ||||
| Hispanics | 382 | 1.04 | 0.06 | 94 | 0.29 | 0.03 | 3.55 | 2.75 | 4.64 | 0.90 | 0.80 | 1.01 | 0.50 | 0.39 | 0.64 |
| Asians | 345 | 1.07 | 0.06 | 110 | 0.42 | 0.04 | 2.53 | 2.03 | 3.19 | 0.92 | 0.82 | 1.03 | 0.72 | 0.59 | 0.89 |
| Blacks | 309 | 1.03 | 0.06 | 110 | 0.53 | 0.06 | 1.92 | 1.53 | 2.45 | 0.89 | 0.78 | 1.00 | 0.92 | 0.74 | 1.13 |
| AI/Ans | 22 | 1.02 | 0.23 | 9 | — | — | — | — | — | 0.88 | 0.53 | 1.37 | — | — | — |
| Medullary | |||||||||||||||
| Whites | 400 | 0.22 | 0.01 | 290 | 0.17 | 0.01 | 1.28 | 1.10 | 1.50 | 1.00 | 1.00 | ||||
| Hispanics | 75 | 0.21 | 0.03 | 56 | 0.18 | 0.03 | 1.19 | 0.80 | 1.80 | 0.98 | 0.75 | 1.27 | 1.06 | 0.74 | 1.47 |
| Asians | 46 | 0.14 | 0.02 | 27 | 0.10 | 0.02 | 1.47 | 0.89 | 2.47 | 0.65 | 0.47 | 0.89 | 0.57 | 0.37 | 0.85 |
| Blacks | 34 | 0.11 | 0.02 | 27 | 0.10 | 0.02 | 1.09 | 0.63 | 1.90 | 0.51 | 0.35 | 0.73 | 0.60 | 0.38 | 0.91 |
| AI/Ans | 4 | — | — | 0 | — | — | — | — | — | — | — | — | — | — | — |
| Anaplastic | |||||||||||||||
| Whites | 225 | 0.10 | 0.01 | 168 | 0.10 | 0.01 | 0.99 | 0.81 | 1.22 | 1.00 | 1.00 | ||||
| Hispanics | 41 | 0.17 | 0.03 | 10 | 0.06 | 0.02 | 2.92 | 1.45 | 6.77 | 1.62 | 1.12 | 2.27 | 0.55 | 0.25 | 1.03 |
| Asians | 39 | 0.14 | 0.02 | 23 | 0.11 | 0.02 | 1.32 | 0.77 | 2.34 | 1.36 | 0.94 | 1.91 | 1.02 | 0.62 | 1.58 |
| Blacks | 32 | 0.13 | 0.02 | 14 | 0.08 | 0.02 | 1.65 | 0.84 | 3.53 | 1.26 | 0.84 | 1.83 | 0.76 | 0.38 | 1.34 |
| AI/Ans | 1 | — | — | 2 | — | — | — | — | — | — | — | — | — | — | — |
Whites include non-Hispanic only, Hispanics include whites only, and AI/ANs include CHSDA counties only. There were 969 poorly specified/unknown type cancers that were not included in the analysis.
AI, American Indian; ANs, Alaska Natives; CI, confidence interval; n, number of cases; rate per 100,000 person-years, man-years, or woman-years (age-adjusted to the 2000 U.S. standard population); SE, standard error; IRR, incidence rate ratios based on unrounded rates; —, not calculated and/or not applicable; CHSDA, contract health service delivery areas; LL, low 95% limit; WNH, White non-Hispanic; UL, upper 95% limit.
Papillary carcinoma rates among both men and women were higher among whites than among Hispanics and American Indians/Alaskan Natives, and lowest among blacks; compared with whites, rates among Asians were significantly higher among women while lower among men. The follicular carcinoma rate among each female non-white group was about 90% that of whites; among men, racial differences were more pronounced, with rates significantly lower among Hispanics and Asians than among whites. Medullary carcinoma rates were virtually the same for whites and Hispanics of each gender, but they were significantly lower than among whites for each gender among both blacks and Asians. In contrast, the anaplastic carcinoma rate among each non-white female SEER racial/ethnic group was elevated compared to whites and significantly so among Hispanics; among men, the racial/ethnic differences were unremarkable.
Age-specific thyroid cancer incidence by type and racial/ethnic group
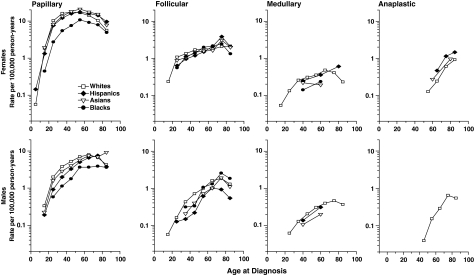
The age-specific incidence curves for men and women by type and racial/ethnic group are presented in Figure 1. For papillary carcinoma, the similarity in the incidence patterns among the SEER racial/ethnic groups within each gender is notable. The age-specific rates for whites, Asians, and Hispanics are quite similar, whereas the rates for black men and women are somewhat lower at all ages than for the other groups. For all SEER racial/ethnic groups, a clear difference between men and women is apparent for papillary carcinoma, with rates rising rapidly among women during reproductive years and then later in life falling to similar levels as men; rates among men rose less rapidly than among women until the older ages when they turned down among whites and Hispanics, plateaued among blacks, and continued to increase among Asians. For follicular carcinoma, the rates for the SEER racial/ethnic groups are more similar among women than men, and the consistently rising rates until the oldest age group among both genders are striking. The patterns for the medullary type among women and men were notably similar; in contrast, rates for the anaplastic type among both genders rose rapidly later in life.
FIG. 1.
Age-specific thyroid cancer incidence by type and racial/ethnic group, Surveillance, Epidemiology, and End Results (SEER)-13. All panels based on 10-year age groups except for the medullary type for Hispanics and the medullary and anaplastic types for Asians and blacks where the age groups were 0–49, 50–69, and 70+. All data points shown include 10 cases or more.
Female-to-male age-specific IRRs by type and racial/ethnic group
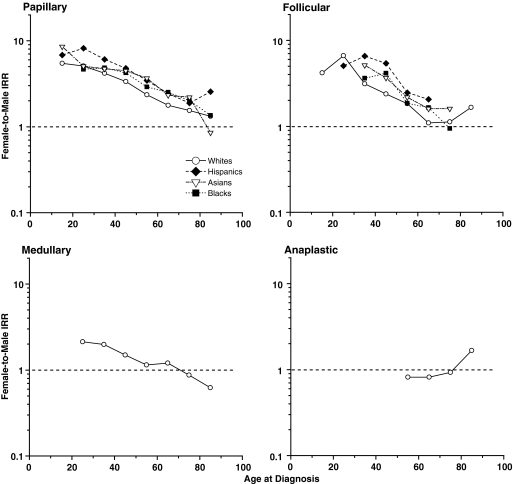
The age-specific female-to-male IRR patterns were quite consistent across the SEER racial/ethnic groups for the papillary and follicular types (Fig. 2). Female-to-male IRRs among whites decreased most steeply from more than six at ages 20–29 years to 1.1 at ages 70–79 years for the follicular type, less steeply from 5.1 to 1.5 for the papillary type, and least steeply from 2.2 to 0.9 for the medullary type; it was <1 until the very oldest ages for the anaplastic type. IRRs were generally higher among Hispanics and lower among whites for both the papillary and follicular types.
FIG. 2.
Female-to-male age-specific thyroid cancer incidence rate ratios (IRR) by type and racial/ethnic group, SEER-13. Data points shown for IRRs with female and male rates each based on 10 or more cases.
Thyroid cancer incidence by type and racial/ethnic group by SEER registry
Thyroid cancer incidence within type and racial/ethnic group varied barely twofold across registries (Table 2). Papillary carcinoma rates varied among whites from 3.9 in rural Georgia to 9.2 in New Mexico, among Hispanics from 4.9 in Detroit to 9.0 in Connecticut, among blacks from 2.6 in San Francisco to 4.2 in Hawaii, among Asians from 5.3 in Connecticut to 9.5 in Iowa, and among American Indians/Alaskan Natives from 5.0 in New Mexico to 7.8 in Utah. Follicular carcinomas rates ranged among whites from 0.6 in San Francisco to 1.4 in rural Georgia, among Hispanics from 0.6 in San Jose to 1.3 in Connecticut, among blacks from 0.5 in San Francisco to 1.6 in Iowa, and among Asians from 0.7 in Los Angeles to 1.0 in Atlanta. Medullary and anaplastic carcinoma rates among whites varied little geographically.
Table 2.
Thyroid Cancer Incidence in the Surveillance, Epidemiology, and End Results 13-Registry Database (1992–2006) by Type, Racial/Ethnic Group, and Registry for Women and Men Combined
| |
Papillary |
Follicular |
Medullary |
Anaplastic |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Registry | n | Rate | SE | n | Rate | SE | n | Rate | SE | n | Rate | SE |
| Whites | ||||||||||||
| San Francisco–Oakland SMSA | 1867 | 5.37 | 0.13 | 214 | 0.61 | 0.04 | 61 | 0.17 | 0.02 | 32 | 0.09 | 0.02 |
| Connecticut | 3295 | 7.70 | 0.14 | 378 | 0.85 | 0.04 | 101 | 0.23 | 0.02 | 69 | 0.14 | 0.02 |
| Detroit (Metropolitan) | 3028 | 6.89 | 0.13 | 428 | 0.97 | 0.05 | 101 | 0.23 | 0.02 | 59 | 0.13 | 0.02 |
| Hawaii | 279 | 5.96 | 0.36 | 49 | 1.05 | 0.15 | 9 | — | — | 4 | — | — |
| Iowa | 2684 | 6.51 | 0.13 | 439 | 1.02 | 0.05 | 73 | 0.17 | 0.02 | 36 | 0.07 | 0.01 |
| New Mexico | 1248 | 9.24 | 0.27 | 136 | 0.96 | 0.08 | 26 | 0.19 | 0.04 | 15 | 0.10 | 0.03 |
| Seattle (Puget Sound) | 3464 | 6.81 | 0.12 | 449 | 0.89 | 0.04 | 87 | 0.17 | 0.02 | 46 | 0.09 | 0.01 |
| Utah | 2132 | 8.51 | 0.19 | 214 | 0.89 | 0.06 | 41 | 0.17 | 0.03 | 22 | 0.10 | 0.02 |
| Atlanta (Metropolitan) | 1687 | 6.91 | 0.17 | 232 | 1.00 | 0.07 | 43 | 0.19 | 0.03 | 17 | 0.09 | 0.02 |
| San Jose–Monterey | 1156 | 6.22 | 0.18 | 141 | 0.76 | 0.06 | 32 | 0.17 | 0.03 | 18 | 0.09 | 0.02 |
| Los Angeles | 3860 | 7.27 | 0.12 | 435 | 0.80 | 0.04 | 113 | 0.21 | 0.02 | 74 | 0.12 | 0.01 |
| Rural Georgia | 41 | 3.92 | 0.63 | 14 | 1.41 | 0.39 | 3 | — | — | 1 | — | — |
| Hispanics | ||||||||||||
| San Francisco–Oakland SMSA | 465 | 5.63 | 0.28 | 51 | 0.72 | 0.11 | 11 | 0.16 | 0.05 | 5 | — | — |
| Connecticut | 277 | 8.98 | 0.60 | 31 | 1.27 | 0.25 | 8 | — | — | 2 | — | — |
| Detroit (Metropolitan) | 53 | 4.92 | 0.74 | 6 | — | — | 1 | — | — | 0 | — | — |
| Hawaii | 23 | 5.39 | 1.19 | 4 | — | — | 0 | — | — | 0 | — | — |
| Iowa | 36 | 5.20 | 1.05 | 1 | — | — | 0 | — | — | 0 | — | — |
| New Mexico | 720 | 7.79 | 0.30 | 66 | 0.75 | 0.10 | 12 | 0.13 | 0.04 | 13 | 0.17 | 0.05 |
| Seattle (Puget Sound) | 132 | 6.70 | 0.72 | 15 | 0.94 | 0.26 | 4 | — | — | 1 | — | — |
| Utah | 148 | 8.45 | 0.79 | 14 | 0.64 | 0.21 | 3 | — | — | 0 | — | — |
| Atlanta (Metropolitan) | 102 | 5.22 | 0.66 | 23 | 1.12 | 0.27 | 4 | — | — | 1 | — | — |
| San Jose–Monterey | 393 | 5.54 | 0.32 | 35 | 0.57 | 0.11 | 10 | 0.21 | 0.08 | 9 | — | — |
| Los Angeles | 2524 | 5.80 | 0.13 | 230 | 0.61 | 0.05 | 78 | 0.21 | 0.03 | 20 | 0.10 | 0.02 |
| Asians | ||||||||||||
| San Francisco–Oakland SMSA | 824 | 6.33 | 0.22 | 92 | 0.74 | 0.08 | 16 | 0.12 | 0.03 | 13 | 0.12 | 0.03 |
| Connecticut | 72 | 5.31 | 0.66 | 5 | — | — | 4 | — | — | 1 | — | — |
| Detroit (Metropolitan) | 81 | 5.83 | 0.71 | 6 | — | — | 2 | — | — | 2 | — | — |
| Hawaii | 1128 | 8.71 | 0.26 | 121 | 0.93 | 0.09 | 19 | 0.14 | 0.03 | 13 | 0.09 | 0.03 |
| Iowa | 39 | 9.53 | 1.81 | 6 | — | — | 1 | — | — | 1 | — | — |
| New Mexico | 25 | 8.53 | 1.83 | 1 | — | — | 0 | — | — | 0 | — | — |
| Seattle (Puget Sound) | 353 | 7.47 | 0.41 | 37 | 0.85 | 0.15 | 3 | — | — | 2 | — | — |
| Utah | 43 | 6.11 | 0.97 | 3 | — | — | 0 | — | — | 1 | — | — |
| Atlanta (Metropolitan) | 98 | 6.02 | 0.72 | 12 | 0.95 | 0.31 | 2 | — | — | 1 | — | — |
| San Jose–Monterey | 457 | 6.65 | 0.32 | 57 | 0.91 | 0.13 | 8 | — | — | 12 | 0.26 | 0.08 |
| Los Angeles | 1381 | 7.44 | 0.20 | 115 | 0.65 | 0.06 | 18 | 0.10 | 0.03 | 16 | 0.11 | 0.03 |
| Blacks | ||||||||||||
| San Francisco–Oakland SMSA | 158 | 2.62 | 0.21 | 28 | 0.48 | 0.09 | 5 | — | — | 7 | — | — |
| Connecticut | 156 | 3.65 | 0.30 | 29 | 0.72 | 0.14 | 2 | — | — | 3 | — | — |
| Detroit (Metropolitan) | 516 | 3.88 | 0.17 | 122 | 0.94 | 0.09 | 14 | 0.10 | 0.03 | 13 | 0.12 | 0.03 |
| Hawaii | 19 | 4.15 | 1.29 | 5 | — | — | 1 | — | — | 0 | — | — |
| Iowa | 26 | 3.50 | 0.72 | 10 | 1.59 | 0.53 | 0 | — | — | 1 | 0.17 | 0.17 |
| New Mexico | 14 | 2.74 | 0.76 | 6 | — | — | 2 | — | — | 0 | — | — |
| Seattle (Puget Sound) | 91 | 3.98 | 0.45 | 22 | 0.95 | 0.21 | 5 | — | — | 0 | — | — |
| Atlanta (Metropolitan) | 403 | 3.14 | 0.17 | 89 | 0.76 | 0.09 | 17 | 0.14 | 0.04 | 5 | — | — |
| San Jose–Monterey | 27 | 2.72 | 0.57 | 5 | — | — | 0 | — | — | 3 | — | — |
| Los Angeles | 430 | 3.18 | 0.16 | 96 | 0.75 | 0.08 | 14 | 0.10 | 0.03 | 13 | 0.12 | 0.03 |
| Rural Georgia | 26 | 3.72 | 0.73 | 7 | — | — | 1 | — | — | 0 | — | — |
| AIs/ANs | ||||||||||||
| New Mexico | 100 | 5.03 | 0.53 | 9 | — | — | 1 | — | — | 1 | — | — |
| Seattle (Puget Sound) | 53 | 7.33 | 1.19 | 7 | — | — | 3 | — | — | 0 | — | — |
| Utah | 14 | 7.75 | 2.18 | 2 | — | — | 0 | — | — | 1 | — | — |
| ANs | 71 | 5.43 | 0.67 | 10 | 0.89 | 0.32 | 0 | — | — | 1 | — | — |
Whites include non-Hispanic only, Hispanics include whites only, and AI/ANs include CHSDA counties only.
—, not applicable; SMSA, standard metropolitan statistical area.
Thyroid cancer incidence by gender, type, and tumor stage
Among whites, the incidence by type, gender, and tumor stage is presented in Table 3. For the papillary, follicular, and medullary types, the largest number of cases and the highest rates were for the localized stage compared to regional or distant stage tumors. In addition, the female-to-male IRR for papillary carcinomas decreased monotonically from 3.6 for localized stage to 2.2 for regional and 1.3 for distant stage disease. For follicular carcinomas, the IRRs declined from 2.4 to 1.6 and 1.3, and for medullary types from 1.8 to 1.1 and 0.8, respectively. In contrast, for the anaplastic type among both men and women, the rates for regional and distant stage were each higher than that for localized stage cancers; the female-to-male IRR was significantly elevated only for regional stage disease. Among Hispanics, blacks, Asians, and American Indians/Alaskan Natives, the female-to-male IRR for papillary thyroid cancer from localized to distant disease stages decreased monotonically as well (data not shown). The female-to-male papillary IRRs among Hispanics decreased from 5.0 for the localized stage to 3.3 for regional and 1.3 for distant stage disease, and among blacks declined from 3.6 to 3.0 and 1.3. Similar monotonic decreases in female-to-male IRRs by tumor stage were apparent for Asians and American Indians/Alaskan Natives.
Table 3.
Thyroid Cancer Incidence in the Surveillance, Epidemiology, and End Results 13-Registry Database (1992–2006) by Gender, Type, and Tumor Stage Among Whites
| |
Women |
Men |
Female/male |
95% CI |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Stage | n | Rate | SE | n | Rate | SE | IRR | LL | UL |
| Papillary | |||||||||
| Localized | 12,349 | 6.91 | 0.06 | 3316 | 1.90 | 0.03 | 3.63 | 3.50 | 3.78 |
| Regional | 5381 | 3.04 | 0.04 | 2421 | 1.40 | 0.03 | 2.18 | 2.08 | 2.29 |
| Distant | 493 | 0.27 | 0.01 | 363 | 0.22 | 0.01 | 1.26 | 1.09 | 1.44 |
| Unstaged | 300 | 0.16 | 0.01 | 118 | 0.07 | 0.01 | 2.34 | 1.88 | 2.92 |
| Follicular | |||||||||
| Localized | 1197 | 0.66 | 0.02 | 475 | 0.28 | 0.01 | 2.39 | 2.15 | 2.66 |
| Regional | 751 | 0.41 | 0.02 | 422 | 0.25 | 0.01 | 1.64 | 1.45 | 1.85 |
| Distant | 120 | 0.06 | 0.01 | 75 | 0.05 | 0.01 | 1.26 | 0.93 | 1.71 |
| Unstaged | 69 | 0.04 | 0.00 | 20 | 0.01 | 0.00 | 2.95 | 1.76 | 5.17 |
| Medullary | |||||||||
| Localized | 220 | 0.12 | 0.01 | 115 | 0.07 | 0.01 | 1.77 | 1.41 | 2.24 |
| Regional | 130 | 0.07 | 0.01 | 114 | 0.07 | 0.01 | 1.07 | 0.82 | 1.39 |
| Distant | 43 | 0.02 | 0.00 | 52 | 0.03 | 0.00 | 0.75 | 0.49 | 1.16 |
| Unstaged | 7 | — | — | — | — | — | — | — | — |
| Anaplastic | |||||||||
| Localized | 16 | 0.01 | 0.00 | 11 | 0.01 | 0.00 | 1.07 | 0.46 | 2.61 |
| Regional | 95 | 0.04 | 0.00 | 49 | 0.03 | 0.00 | 1.44 | 1.01 | 2.09 |
| Distant | 96 | 0.04 | 0.01 | 95 | 0.06 | 0.01 | 0.76 | 0.56 | 1.02 |
| Unstaged | 18 | 0.01 | 0.00 | 13 | 0.01 | 0.00 | 0.97 | 0.44 | 2.19 |
Whites include non-Hispanics only.
—, not calculated and/or not applicable.
Thyroid cancer incidence by gender, type, and tumor size
A clear decline in cases and rates according to tumor size was apparent only for papillary carcinomas among white women; among white men, similar numbers of cases were diagnosed at sizes <1.0, 1.0 to <2.0, and 2.0–4.0 cm (Table 4). In contrast, the highest follicular rates were for tumor sizes 2.0–4.0 cm among white women and 4+ cm among white men; for medullary type, the 2.0–4.0 cm size was the most frequent. There were very few anaplastic cancers diagnosed smaller than 2.0 cm, and most were at least 4.0 cm among both women and men. The female-to-male IRRs for whites with the papillary type decreased with tumor size from 3.7 to 1.3. The IRRs also generally declined with tumor size for the follicular and medullary types, and it was significantly <1.0 for the largest (4.0+ cm) size follicular carcinomas. In contrast, all the IRRs for anaplastic carcinomas were similar to 1.0. In general, among the non-white SEER racial/ethnic groups, larger female-to-male IRRs were observed for every tumor size for the papillary type when compared with whites (data not shown). For the follicular type among the non-white SEER racial/ethnic groups, the IRR was highest (4.5) for 1.0–2.0 cm tumors and was significantly <1 (0.8) for the largest tumor size (4.0+ cm). For the medullary and anaplastic types among the non-white SEER racial/ethnic groups, the IRR was also <1.0 cm for tumors 4.0+ cm.
Table 4.
Thyroid Cancer Incidence in the Surveillance, Epidemiology, and End Results 13-Registry Database (1992–2006) by Gender, Type, and Tumor Size Among Whites
| |
Women |
Men |
Female/male |
95% CI |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor size | n | Rate | SE | n | Rate | SE | IRR | LL | UL |
| Papillary | |||||||||
| <1 cm | 5551 | 3.09 | 0.04 | 1470 | 0.84 | 0.02 | 3.67 | 3.46 | 3.89 |
| 1 to <2.0 cm | 5199 | 2.95 | 0.04 | 1426 | 0.82 | 0.02 | 3.60 | 3.40 | 3.83 |
| 2.0 to <4.0 cm | 3849 | 2.18 | 0.04 | 1506 | 0.87 | 0.02 | 2.51 | 2.36 | 2.66 |
| 4.0+ cm | 907 | 0.50 | 0.02 | 651 | 0.38 | 0.02 | 1.32 | 1.19 | 1.46 |
| Unknown | 2931 | 1.63 | 0.03 | 1141 | 0.67 | 0.02 | 2.44 | 2.28 | 2.62 |
| Follicular | |||||||||
| <1 cm | 105 | 0.06 | 0.01 | 38 | 0.02 | 0.00 | 2.66 | 1.82 | 3.97 |
| 1 to <2.0 cm | 457 | 0.26 | 0.01 | 99 | 0.06 | 0.01 | 4.48 | 3.59 | 5.63 |
| 2.0 to <4.0 cm | 849 | 0.47 | 0.02 | 300 | 0.18 | 0.01 | 2.67 | 2.34 | 3.06 |
| 4.0+ cm | 375 | 0.19 | 0.01 | 397 | 0.24 | 0.01 | 0.83 | 0.72 | 0.96 |
| Unknown | 350 | 0.18 | 0.01 | 158 | 0.09 | 0.01 | 1.97 | 1.62 | 2.39 |
| Medullary | |||||||||
| <1 cm | 63 | 0.03 | 0.00 | 42 | 0.03 | 0.00 | 1.39 | 0.92 | 2.11 |
| 1 to <2.0 cm | 104 | 0.06 | 0.01 | 46 | 0.03 | 0.00 | 2.18 | 1.52 | 3.15 |
| 2.0 to <4.0 cm | 129 | 0.07 | 0.01 | 85 | 0.05 | 0.01 | 1.43 | 1.08 | 1.91 |
| 4.0+ cm | 50 | 0.03 | 0.00 | 60 | 0.04 | 0.01 | 0.74 | 0.50 | 1.10 |
| Unknown | 54 | 0.03 | 0.00 | 57 | 0.03 | 0.01 | 0.85 | 0.58 | 1.27 |
| Anaplastic | |||||||||
| <1 cm | 2 | — | — | 1 | — | — | — | — | — |
| 1 to <2.0 cm | 9 | — | — | 2 | — | — | — | — | — |
| 2.0 to <4.0 cm | 22 | 0.01 | 0.00 | 17 | 0.01 | 0.00 | 1.01 | 0.51 | 2.06 |
| 4.0+ cm | 93 | 0.04 | 0.00 | 80 | 0.05 | 0.01 | 0.83 | 0.61 | 1.14 |
| Unknown | 99 | 0.05 | 0.01 | 68 | 0.04 | 0.01 | 1.10 | 0.80 | 1.53 |
Whites include non-Hispanics only.
—, not calculated and/or not applicable.
Discussion
Papillary carcinoma was the most common type of thyroid cancer among both men and women and for all SEER racial/ethnic groups included in this study. Although papillary carcinoma rates among both men and women were higher among whites than Hispanics and American Indians/Alaskan Natives, and lowest among blacks, the age-specific patterns were similar. Specifically, for the papillary type, all four SEER racial/ethnic groups illustrated a similar pattern of gender disparities, with the burden among women peaking around 40 years of age. A similar pattern was seen for follicular and medullary types among women, whereas among men, rates were highest among whites but lowest among Hispanics. The incidence of all the histologic types of thyroid cancer varied by gender and by racial/ethnic group with two- to almost fourfold female-to-male IRRs for both papillary and follicular types and generally smaller IRRs for medullary and anaplastic types. Although a gender disparity was observed across all SEER racial/ethnic groups for the follicular type, the excess during reproductive years was not as pronounced as observed for the papillary type. A notable lack of gender disparity was noted for the medullary and anaplastic types with the incidence among both women and men generally increasing with age. The female-to-male IRRs subsequently decreased with age for all SEER racial/ethnic groups for both papillary and follicular thyroid cancer, and a similar pattern was observed for medullary thyroid cancer among whites, but the female-to-male IRR increased with age among whites for the anaplastic type.
Our findings build upon previous reports of racial/ethnic patterns in SEER (4,8). The results of our study are relevant as a principal concern in thyroid cancer research is whether the rising incidence over the past decades reflects a true increase in disease incidence or an artificial inflation in disease rates due to improvements and increased access to technologies such as ultrasonography. If detection plays a substantial role in diagnosis, we would expect to see racial differences in disease incidence and potentially different age-specific incidence patterns as those in lower socioeconomic groups would likely be diagnosed later in life once they are eligible for Medicare. We might also expect to see similar patterns across SEER racial/ethnic groups during reproductive years among women even if detection is an important factor, as women in lower socioeconomic groups would potentially qualify for reproductive health services (16,17). However, we observed similar racial/ethnic patterns across the lifespan. In addition, if the rising incidence was mostly due to diagnosis, we might also expect to see different patterns for SEER racial/ethnic groups by registry. However, our evaluation of thyroid cancer incidence by tumor registries did not reveal clear patterns of differential diagnosis.
When we looked at the IRRs within each type of thyroid cancer, comparing rates among SEER racial/ethnic groups to whites separately by gender, we found variation by histologic type. The similarity of follicular thyroid cancer rates among women of the other SEER racial/ethnic groups compared to white women was particularly notable. Thyroid cancer incidence may reflect a socioeconomic marker, as not all segments of the population may benefit equally from screening efforts, and these differences are often related to lack of access to healthcare. Given that health insurance status is associated with race/ethnicity, as blacks, Hispanics, Asian/Pacific Islanders, and American Indian/Alaska Natives are more likely to be uninsured than non-Hispanic whites (18), similarities in disease rates for a cancer that is thought to be increasingly diagnosed in asymptomatic persons are striking (including all types except anaplastic). Further support for this point is observed in the age-specific figure of female-to-male IRRs. The similarity in disease patterns (including gender disparities) across SEER racial/ethnic groups is notable and unexpected. Previous research by the U.S. Centers for Disease Control and Prevention has shown that Hispanics are twice as likely as non-Hispanic blacks and three times as likely as non-Hispanic whites to lack a regular healthcare provider (18). In short, these patterns argue against the notion that access to care accounts for the majority of incidence differences.
Further, if most or all of the rising incidence was due to improved disease detection, a more rapid increase in small early stage tumors than larger late stage would be expected. An increase across all histologic types would also be expected with the exception of the anaplastic type due to its symptomatic presentation and more aggressive progression. Recent reports (1,4) showed that the most rapid increases in thyroid cancer among both men and women were observed for larger size tumors (4.0+ cm) as well as microcarcinomas (<1 cm), and substantial increases in recent decades were observed for regional and distant stage tumors in addition to localized tumors (1). Our findings for papillary thyroid cancer among whites in a more recent study period showed that the highest rates for women were observed for the smallest size tumors but not men. For the other tumor types, we generally observed the lowest rates for the smallest tumors. Our results support the previous research showing the highest rates for localized papillary tumors, and we found that this is true for the follicular and medullary thyroid cancers as well.
It is unlikely that differences in clinical methods of tumor detection bias the rates of the papillary type compared to the follicular and medullary types. Patients presenting with thyroid cancer are typically asymptomatic with the exception of occasional neck swelling (19). Those with papillary tumors are not more likely to experience pain or discomfort than those with follicular or medullary thyroid cancer. In general, patients present for workup of nodular goiter, based on symptoms (such as neck mass, hoarseness, difficulty and or pain on swallowing) or most likely the tumor is incidentally found on routine imaging (20). Upon discovery of thyroid nodules by physical examination, a neck ultrasonography is typically obtained to assess characteristics of nodules. This is followed by fine-needle aspiration biopsies of suspicious nodules. There are no distinctive features by ultrasonography that can separate papillary from follicular or medullary thyroid cancer. Further, the ease of obtaining a biopsy is also similar for the different types (21).
However, once the biopsied specimen is sent to pathology for disease diagnosis, opportunities for biases in disease diagnoses may occur between specific types, which may be of concern in the interpretation of incidence patterns. There is great variability (up to 40%) between pathologists in how they read a fine-needle aspiration biopsy and subsequently determine the thyroid cancer tumor type (21). There are differences in the ease of tumor type identification by cell type; specifically, the detection of a papillary thyroid cancer is simple if papillary formations are present. However, papillary tumors are a mixture of thyroid follicles and neoplastic papillae. In the papillae of a thyroid tumor, cells are usually larger than normal, with large nuclei that appear crowded and overlapping and may contain hypodense chromatin, cytoplasmic pseudoinclusions, and/or nuclear grooves that are typically obvious to a pathologist. In contrast, follicular tumors can be more difficult to identify as follicular tumors are characterized with solid growth of small to medium-sized follicles, absence of colloid, and presence of vascular and/or capsular invasion, which can make it difficult to distinguish a follicular benign lesion from a follicular carcinoma. As such, it is possible that more papillary tumors are classified as malignant compared to follicular tumors, which are more likely to be classified as benign.
The female-to-male IRRs varied substantially by thyroid cancer histology, posing questions related to disparities by gender in diagnosis or incidence. It is known that women are more frequent users of healthcare services, particularly during childbearing years (22). However, as noted above, if the incidence patterns were due to diagnostic inflation, one might expect that a consistent pattern of similar increases in risk for women would be observed across all types of thyroid cancer with the possible exception the anaplastic type. When we looked at the age-specific incidence of the various types of thyroid cancer, we noted an early age-at-onset predominance for women for the papillary and follicular types, which is consistent with previous reports for papillary thyroid cancer (1). It was previously reported and we show here that IRs rose rapidly with increasing age among women, peaking at ages 40–49 years and then declined at ages 80+ years. In contrast, age-specific rates among men rose more slowly, peaking at ages 60–69 years and then declined at ages 80+ years (1). Such a hook pattern observed for women, particularly for the papillary type, is typically an indication of confounding by period and/or cohort (23). This phenomenon is observed when there is a progressive increase in cancer risk from one period and/or one generation to the next (23). This raises the question of why there would be differential period or cohort effects by histologic type. On the basis of prior reports (1), there is clearly a period and cohort effect affecting rates of papillary thyroid cancer; however, the different age-specific patterns by type indicate that such effects are impacting the types differently, suggesting different risk factors, different responses to exposure, or different opportunities for diagnosis.
A major strength of this study was the ability to evaluate thyroid cancer patterns using SEER's racially/ethnically diverse sample. Extensive efforts have been made since the program's inception to ensure that case ascertainment is as complete as possible and that data on all cancer patients are of the highest quality. However, our study was limited by the usual concerns related to analyses of registry data: nonreview of histopathologic diagnoses, potential incomplete data collection, and inconsistencies in tumor classification over time due to changing classification and staging systems. Despite these limitations, our results are consistent with other population-based studies that have largely used SEER data.
In sum, through the examination of the SEER database and its racially/ethnically diverse national sample, we were able to offer additional insights to what is currently known about the distribution of thyroid cancer in the U.S. population. We further suggest that there are etiologic clues and insights into the possible role of increased detection conferred by our analysis. Specifically, the similar age-specific patterns and lack of geographical variation across the SEER racial/ethnic groups indicate that a detection effect cannot completely explain the observed increases in thyroid cancer incidence. We conclude that the similar age-specific patterns and lack of geographical variation across the SEER racial/ethnic groups indicate that a detection effect cannot completely explain the observed increases in thyroid cancer incidence as variation in the amount or quality of healthcare provided is a function of SEER racial/ethnic groups, gender, and/or age. We suggest that the variations in age-specific patterns by gender and across histologic types are intriguing and recommend that future etiologic investigation focus on exogenous or endogenous exposures that are experienced similarly by REGs, more strongly among women, and distinctively by age.
Further investigation of the relationship between risk factors known to interfere with thyroid function that may be relevant in the development of thyroid cancer may be warranted.
Acknowledgment
This research was supported in part by the Intramural Research Program of the National Institutes of Health/National Cancer Institute.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Kilfoy BA. Devesa SS. Ward MH. Zhang Y. Rosenberg PS. Holford TR. Anderson WF. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009;18:1092–1100. doi: 10.1158/1055-9965.EPI-08-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilfoy BA. Zheng T. Holford TR. Han X. Ward MH. Sjodin A. Zhang Y. Bai Y. Zhu C. Guo GL. Rothman N. Zhang Y. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Enewold L. Zhu K. Ron E. Marrogi AJ. Stojadinovic A. Peoples GE. Devesa SS. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S. Semenciw R. Ugnat AM. Mao Y. Increasing thyroid cancer incidence in Canada, 1970–1996: time trends and age-period-cohort effects. Br J Cancer. 2001;85:1335–1339. doi: 10.1054/bjoc.2001.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds RM. Weir J. Stockton DL. Brewster DH. Sandeep TC. Strachan MW. Changing trends in incidence and mortality of thyroid cancer in Scotland. Clin Endocrinol. 2005;62:156–162. doi: 10.1111/j.1365-2265.2004.02187.x. [DOI] [PubMed] [Google Scholar]
- 7.Albores-Saavedra J. Henson DE. Glazer E. Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype—papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol. 2007;18:1–7. doi: 10.1007/s12022-007-0002-z. [DOI] [PubMed] [Google Scholar]
- 8.Yu G. Chun-Lun Li J. Branovan D. McCormick S. Schantz SP. Thyroid cancer incidence and survival in the National Cancer Institute surveillance, epidemiology, and end results race/ethnicity groups. Thyroid. 2010;20:465–473. doi: 10.1089/thy.2008.0281. [DOI] [PubMed] [Google Scholar]
- 9.Howell E. The impact of the Medicaid expansions for pregnant women: a synthesis of the evidence. Med Care Res Rev. 2001;58:3–30. doi: 10.1177/107755870105800101. [DOI] [PubMed] [Google Scholar]
- 10.Surveillance, Epidemiology, End Results (SEER) Program. (November 2008 submission.) Public-Use Database (1973–2006), National Cancer Institute DCCPS, Surveillance Research Program, Cancer Statistics Branch, Released April 2009, based on the November 2008 submission.
- 11.World Health Organization. International Classification of Diseases for Oncology. Third. WHO; Geneva: 2000. [Google Scholar]
- 12.Surveillance Research Program. National Cancer Institute SEER*Stat software. 2009. www.seer.cancer.gov/seerstat. www.seer.cancer.gov/seerstat version 6.4.4.
- 13.Fay MP. Approximate confidence intervals for rate ratios from directly standardized rates with sparse data. Commun Stat Theory Methods. 28:2141–2160. [Google Scholar]
- 14.Fay MP. Tiwari RC. Feuer EJ. Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006;62:847–854. doi: 10.1111/j.1541-0420.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 15.Devesa SS. Donaldson J. Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–304. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 16.Egerter S. Braveman P. Marchi K. Timing of insurance and use of prenatal care among low-income women. Am J Public Health. 2002;92:423–427. doi: 10.2105/ajph.92.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braveman P. Marchi K. Sarnoff R. Egerter S. Rittenhouse D. Promoting Access to Prenatal Care: Lessons from the California Experience. The Henry J. Kaiser Family Foundation; Washington, DC: 2003. [Google Scholar]
- 18.Pleis JR. Lethbridge-Cejku M. Summary Health Statistics for U.S Adults: National Health Interview Survey. National Center for Health Statistics Vital and Health Statistics Series 10; Atlanta, GA. 2007. 2006. [PubMed] [Google Scholar]
- 19.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules. Differentiated Thyroid Cancer; Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 20.Wartofsky L. The thyroid nodule: pathogenesis, evaluation, risk of malignancy. In: Wartosfsky L, editor. Thyroid Cancer: A Comprehensive Guide to Clinical Management. Humana Press; Totowa NJ: 2000. pp. 3–9. [Google Scholar]
- 21.Oertel Y. The thyroid nodule: fine needle aspiration. In: Wartosfsky L, editor. Thyroid Cancer: A Comprehensive Guide to Clinical Management. Humana Press; Totowa NJ: 2000. pp. 35–39. [Google Scholar]
- 22.Owens GM. Gender differences in health care expenditures, resource utilization, and quality of care. J Manag Care Pharm. 2008;14:2–6. doi: 10.18553/jmcp.2008.14.S6-A.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarone RE. Chu KC. Evaluation of birth cohort patterns in population disease rates. Am J Epidemiol. 1996;143:85–91. doi: 10.1093/oxfordjournals.aje.a008661. [DOI] [PubMed] [Google Scholar]