Summary
BACKGROUND
The sheep is routinely used in experimental cardiac electrophysiology and surgery.
OBJECTIVE
We aimed at (1) ascertaining the topography and architecture of the ovine epicardial neural plexus (ENP), (2) determining the relationships of the ENP with the vagal and sympathetic cardiac nerves and ganglia, and (3) evaluating gross anatomical differences and similarities among ENPs in humans, sheep and other species.
METHODS
The ovine ENP, extrinsic sympathetic and vagal nerves were revealed histochemically for acetylcholinesterase on whole heart and/or thorax-dissected preparations from 23 newborn lambs with subsequent examination by a stereomicroscope.
RESULTS
The intrinsic cardiac nerves extend from the venous part of the ovine heart hilum (HH) along the roots of the cranial (superior) caval and left azygos veins to both atria and ventricles via five epicardial routes; i.e. the dorsal right atrial (DRA), middle (MD), left dorsal (LD), right ventral (VR) and ventral left atrial (VLA) nerve subplexuses. Intrinsic nerves proceeding from the arterial part of the HH along the roots of the aorta and pulmonary trunk extend exclusively into the ventricles as the right and left coronary subplexuses. The DRA, RV, and MD subplexuses receive the main extrinsic neural input from the right cervicothoracic and the right thoracic sympathetic T2, T3 ganglia, as well as from the right vagal nerve. The LD is supplied by sizeable extrinsic nerves from the left thoracic T4-T6 sympathetic ganglia and the left vagal nerve. Sheep hearts contained on average 769±52 epicardial ganglia. Cumulative areas of epicardial ganglia on the root of the cranial vena cava and on the wall of the coronary sinus were the largest of all regions (p<0.05).
CONCLUSION
Despite substantial interindividual variability in the morphology of the ovine ENP, the right-sided epicardial neural subplexuses supplying the sinuatrial and atrioventricular nodes are mostly concentrated at a fat pad between the right pulmonary veins and the cranial vena cava. This is in sharp contrast with a solely left lateral neural input to the human atrioventricular node which extends mainly from the LD and MD subplexuses. The abundance of epicardial ganglia distributed widely along the ovine ventricular nerves over respectable distances below the coronary groove implies a distinctive neural control of the ventricles in human and sheep hearts.
Keywords: Heart innervation, Autonomic ganglia, Extrinsic and intrinsic cardiac neural plexus, Anatomy, Sheep
Introduction
The intrinsic cardiac nervous system (ICNS) is a crucial regulator of heart rate, atrial and ventricular refractoriness, contractility and coronary blood flow.1-3 The ICNS also modulates intrathoracic and central cardiovascular–cardiac reflexes and coordinates parasympathetic and sympathetic efferent postganglionic neuronal input to the heart.4,5 Morphologically, the ICNS forms a neural ganglionated plexus that may be subdivided into epicardial, myocardial and endocardial subplexuses.6 Several studies indicate that the ICNS is a complex of distinct subplexuses, and that the cardiac ganglia are mainly distributed at certain atrial regions around the sinuatrial node, the roots of the venae cavae and pulmonary veins (PVs), and near the atrioventricular node.7-9 Postganglionic neurons distributed within discrete fat pads on the heart surface mediate certain aspects of cardiac function.10,11 For example, ganglion cells in a fat pad adjacent to the epicardium of the right PVs selectively mediate negative chronotropic effects,12,13 whereas ganglion cells in a fat pad at the junction between the inferior right atrium and the inferior the vena cava mediate AV conduction slowing.14 Furthermore, a fat pad found ventrally at the cranial margin of the left ventricle near its junction with the right ventricle contains ganglionic cells that selectively mediate negative inotropic vagal effects on the left ventricle without influencing the chronotropic or dromotropic cardiac functions.15
The sheep heart is used extensively in cardiovascular research because of its many similarities with the human,16 including its electrophysiologic properties.17-22 Yet, to our knowledge, the topography and architecture of the ovine cardiac neural plexus have been reported by only one published article to date.23 Therefore, we aimed at (1) ascertaining both the topography and architecture of the ovine epicardial neural plexus (ENP); (2) determining the relationships of ENP with the vagal and sympathetic cardiac nerves and ganglia; and (3) evaluating gross anatomical differences and similarities of ENP in sheep, humans and other species with the objective of determining the suitability of the sheep heart as a model in experimental cardiac electrophysiology and surgery.
Materials and Methods
Twenty-three black-faced newborn lambs (3±0.5 kg) of either sex, were used in accordance with local and state guidelines for the care and use of experimental animals. Preparations, staining procedures using acetylcholinesterase (AChE) and tyrosine hydroxylase (TH), microscopic examination, and quantitative and statistical analyses are described in detail in the Online Supplement (OLS).
To evaluate quantitatively the distribution of epicardial ganglia in homologous regions of different specimens, the surface of the ovine heart was subdivided as described previously,24 and in Fig. 2.
Fig 2.
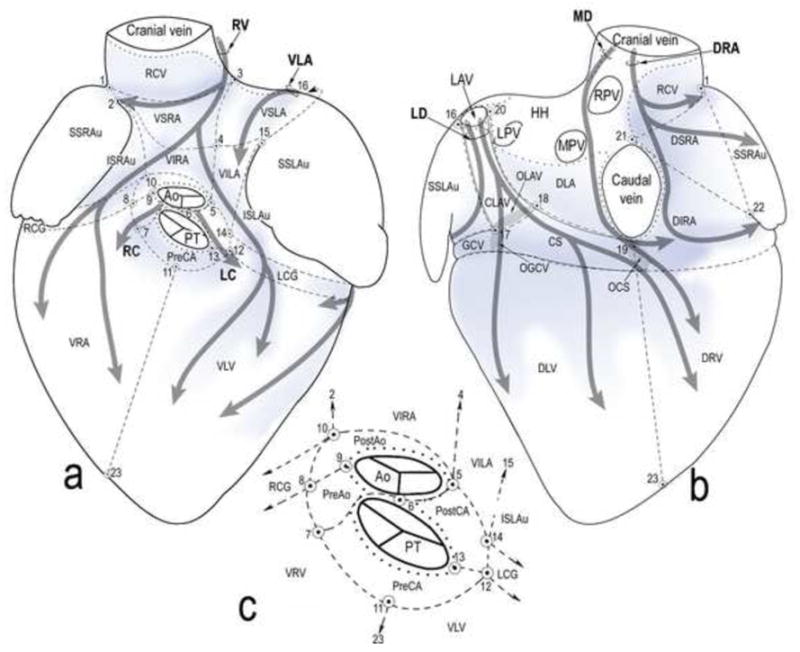
Schematic ventral (a) and dorsal (b) views of the pressure-inflated lamb heart. The heart surface is subdivided into regions for quantitative analysis of ganglia and representation of the course and innervation regions of the seven epicardial subplexuses. Inset (c), cardiac zone around the roots of the aorta and the pulmonary trunk. Dotted lines, limits of the HH; thick grey semitransparent arrows, course of neural subplexuses; blue shadowed areas, main areas with the highest density of subplexal ganglia. Ao – aorta; PT – pulmonary trunk; OCS – opening of coronary sinus; OGCV – opening of great cardiac vein; OLAV – opening of left azygos vein; veins: GCV – great cardiac; LPV – left pulmonary; MPV – middle pulmonary; RPV – right pulmonary; LAV – left azygos; subplexuses: DRA – dorsal right atrial; LC – left coronary; LD – left dorsal; MD – middle dorsal; RC – right coronary; VLA – ventral LA; RV – right ventral; regions: CLAV – cardiac portion of the LAV; CS – coronary sinus; DIRA – dorsal inferior right atrial; DLA – dorsal LA; DLV – dorsal left ventricular; DRV – dorsal right ventricular; DSRA – dorsal superior right atrial; HH – heart hilum; ISLAu – inferior surface of left auricle; ISRAu - inferior surface of right auricle; LCG – left side of coronary groove; PostAo – post-aortic; PreAo – pre-aortic; PostCA – region laying behind the conus arteriosus; PreCA – region laying in front of the conus arteriosus; RCG – right side of coronary groove; RCV – root of the cranial caval vein; SSLAu – superior surface of left auricle; SSRAu - superior surface of right auricle; VILA – ventral inferior LA; VIRA – ventral inferior right atrial; VLV – ventral left ventricular; VRV – ventral right ventricular; VSLA – ventral superior LA; VSRA – ventral superior right atrial; points (1-23): (1) approximate mid-point of terminal groove; (2) cranial end of free margin of right auricle; (3) cranial dorsal bend of ventral interatrial groove; (4) middle of ventral interatrial groove; (5) base of ventral interatrial groove; (6) mid-point of the aorta and pulmonary trunk on the level of HH; (7) ventral edge of the bed of the root of the right coronary artery; (8) branching site of the first branches from the right coronary artery to the conus arteriosus; (9) right lateral edge of aorta; (10) dorsal edge of the bed right coronary artery root; (11) basal midst of conus arteriosus; (12) cranial end of ventral interventricular groove or ventral edge of the left coronary artery root; (13) left lateral edge of pulmonary trunk; (14) dorsal edge of the bed of the left coronary artery root; (15) ventral end of the free margin of left auricle; (16) lateral edge of left azygos vein; (17) point of juncture of left azygos vein and great cardiac vein; (18) point of juncture of left azygos vein and coronary sinus; (19) lower edge of caudal caval vein; (20) medial edge of left azygos vein; (21) upper edge of caudal caval vein; (22) caudal end of free margin of right auricle; (23) notch of heart apex.
Results
TH immunoreactive nerve fibers within AChE positive intrinsic cardiac nerves
Immunohistochemistry for TH, performed either before or after histochemical staining for AChE (see OLS for technical details), demonstrates the concurrent distribution of AChE with the TH positive nerve fibers (Fig. 1). Although the intensity of AChE staining was not quantified, it was obvious that intraneural staining for AChE occurred even in nerves that involved almost exclusively the TH immunoreactive nerve fibers (Fig. 1). While TH positive nerve fibers were distributed in most ovine intrinsic cardiac nerves, they were predominant in thick epicardial nerves. All epicardial ganglia examined lacked TH immunoreactive neurons but were well stained for AChE (Fig. 1).
Fig 1.
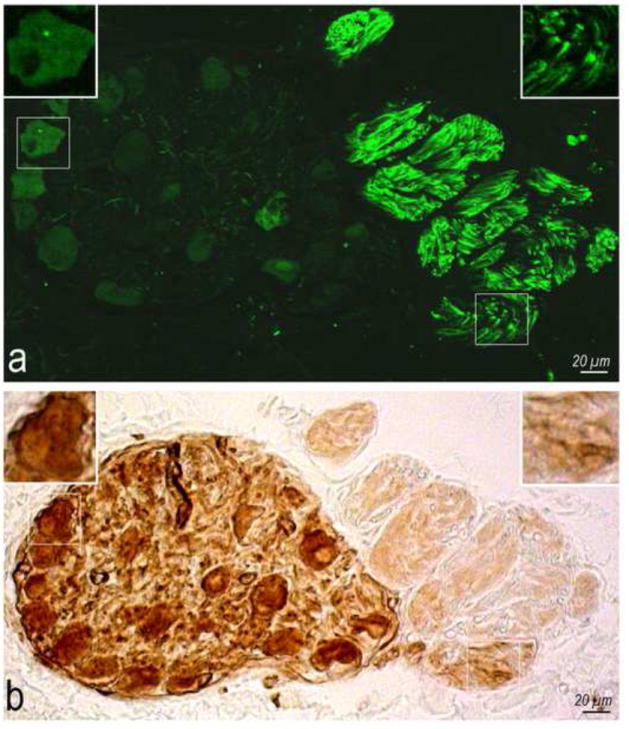
Coexistence of TH positive nerve fibers (a) with AChE (b). Boxed areas on both images are enlarged as top insets to show the adrenergic nerve fibers (a) surrounded by brown diffuse precipitates ensued by AChE histochemical reaction (b).
Structure of the Ovine ENP
Extrinsic cardiac nerves access the ovine heart helium (HH) mainly at three locations: (1) medial side of cranial (superior) vena cava; (2) around the left azygos vein; and (3) between the aorta and pulmonary trunk (Fig. 2). Nerves that access the heart at the first two locations extend epicardially to both atria and ventricles, while nerves from the third location proceed almost directly onto the ventricles (Fig. 2). Epicardially, intrinsic nerves were mostly ganglionated and clustered into seven subplexuses (Figs. 2-4). Individual subplexal epicardial ganglia were distributed within specific regions, but interconnected among themselves via thin nerves (Figs. 2-4). On the dorsal walls of both ventricles, many epicardial nerves were also abundantly ganglionated (Figs. 2b; 3c; Table 1 OLS). The largest proportion (59%) of all epicardial ganglia was identified on the dorsal heart side. The coronary sinus (CS) walls, root of the cranial (superior) caval vein (RCV), dorsal inferior right atrial (DIRA) and ventral superior right atrial (VSRA) regions were particularly ganglionated (Figs. 3 and 4; see also Table 1 OLS). Many ventricular ganglia were distributed along epicardial nerves whereas the atrial ganglia were most frequent at intersections of epicardial nerves (Fig. 5).
Fig 4.
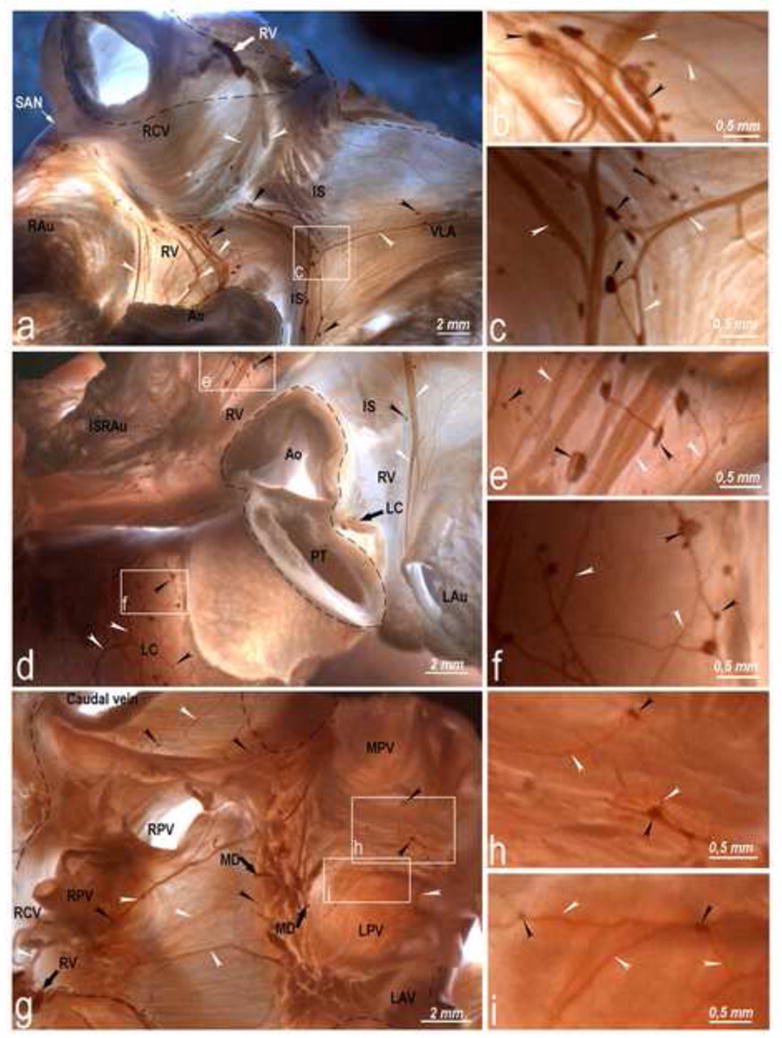
Right ventral, ventral LA (a, b, c, d, e), and left coronary (d, f) epicardial subplexuses and intrinsic ganglionated nerves in the ovine HH (g, h, i). Boxed areas in a, d and g are enlarged as b, c, e, f, h and i panels to show detail. Black arrowheads, epicardial ganglia; white arrowheads, subplexal nerves; white and black solid arrows, preganglionated nerves entering epicardium through the HH; dashed line, limits of the HH. Abbreviations: IS – ventral groove of the interatrial septum; RAu – right auricle; other abbreviations as in Figs 2 and 3.
Fig 3.
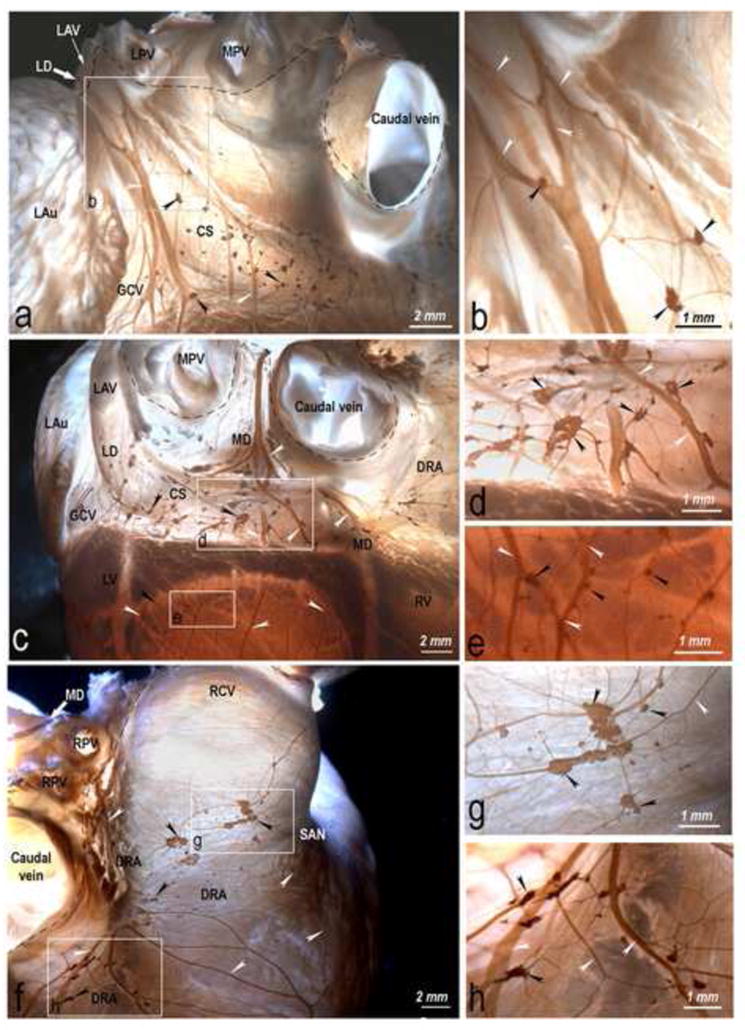
Topography and structure of the dorsal subplexuses of the lamb heart, i.e. left (a, b) and middle (c, d, e) dorsal subplexuses and dorsal right atrial (f, g, h) subplexus, stained for acetylcholinesterase. Boxed areas in a, c and f are enlarged on the left as b, d, e, g and h to illustrate details of plexus. Black arrowheads, epicardial ganglia: white arrowheads, subplexal nerves: white solid arrows, preganglionated nerves entering epicardium through the HH, and the dashed line shows the limits of the HH (broken lines). Note in panel c numerous ventricular ganglia scattered on the dorsal side of the left ventricle. Abbreviations: LAu – left auricle; LV – left ventricle; RV – right ventricle; SAN – sinuatrial nodal zone; other abbreviations as in Fig 2.
Fig 5.
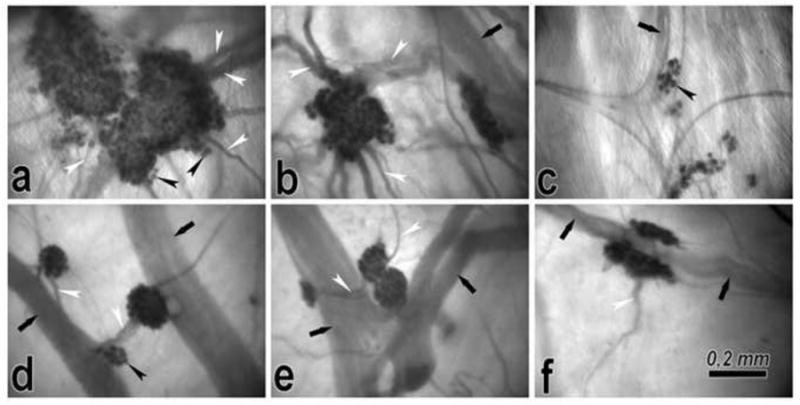
Morphologic pattern of atrial (a-c) and ventricular (d-f) epicardial ganglia. Some regions of ganglia are out of sharp focus because of their deep location in the epicardium. Black arrows, longitudinally oriented epicardial nerves; black arrowheads, ganglionic cells; white arrowheads, interganglionic nerves. Atrial ganglia are irregular in shape due to their extensions at the sites where nerves connect to a ganglion; ventricular ganglia are oval or spindle-shaped and situated along the epicardial nerves.
Although the largest clusters of epicardial ganglia were scattered in atrial regions near the HH, quantitative analysis revealed their uneven distribution. The largest ganglia in terms of both mean ganglion area and cumulative ganglion area were distributed in the CS, RCV and DIRA regions (Table 1). The cumulative ganglion areas of the RCV and CS regions differed statistically from other regions (Table 1).
Table 1.
Mean and cumulative epicardial ganglion areas in atrial regions indicated in Fig. 2. Abbreviations are the same as in the legend for Fig. 2.
| Regions | Number of measured ganglia | Mean ganglion area (mm2) | Cumulative ganglion area |
Range of cumulative ganglion area | |
|---|---|---|---|---|---|
| Area (mm2) | Approximate neuronal number* | ||||
| Ganglia related to the coronary sinus and atrioventricular node region | |||||
| † CS | 72 ± 6 | 0,05 ± 0,007 | † 3,5 ± 0,52 | 4600 | 2,31 – 5,5 |
| ‡ DIRA | 62 ± 9 | 0,025 ± 0,005 | ‡1,42 ± 0,36 | 1900 | 0,57 – 2,94 |
| Ganglia related to the sinuatrial node region | |||||
| † RCV | 80 ± 13 | 0,036 ± 0,01 | † 2,32 ± 0,65 | 3000 | 0,27 – 4,64 |
| SSRAu | 14 ± 4 | 0,013 ± 0,002 | 0,18 ± 0,05 | 200 | 0,11 – 0,28 |
| Ganglia distributed more distal to the sinuatrial node region | |||||
| DSRA | 17 ± 4 | 0,03 ± 0,01 | 0,43 ± 0,1 | 600 | 0,07 – 0,93 |
| VSRA | 24 ± 2 | 0,04 ± 0,009 | 0,96 ± 0,19 | 1250 | 0,36 – 1,58 |
| Ganglia related to the cardiac portion of the left azygos vein | |||||
| CLAV | 23 ± 2 | 0,03 ± 0,006 | 0,7 ± 0,15 | 900 | 0,38 – 1,49 |
| Ventral atrial ganglia | |||||
| VSLA | 26 ± 3 | 0,027 ± 0,005 | 0,7 ± 0,17 | 900 | 0,25 – 1,45 |
| VILA | 24 ± 4 | 0,027 ± 0,007 | 0,74 ± 0,21 | 950 | 0,08 – 1,79 |
| VIRA | 12 ± 2 | 0,019 ± 0,003 | 0,2 ± 0,04 | 250 | 0,01 – 0,36 |
| ISRAu | 13 ± 3 | 0,014 ± 0,003 | 0,23 ± 0,07 | 300 | 0,06 – 0,56 |
| Ganglia within the heart hilum | |||||
| HH | 10 ± 3 | 0,007 ± 0,001 | 0,07 ± 0,006 | 100 | 0,06 – 0,09 |
| In all regions | 358 ± 18 | 0,028 ± 0,003 | 13,05 ± 0,1 | 17 000 | 4,47 – 21,52 |
Approximations were made basing on earlier findings by Pauza et al.32
The cumulative ganglion areas of the RCV and CS regions differed statistically compared with the rest regions at p<0,05.
The cumulative ganglion area of the DIRA differed statistically (p<0,05) from the DSRA, VIRA, ISRAu, SSRAu and CS regions.
The ovine epicardial ganglia varied in shape and size (Fig. 5). Atrial epicardial ganglia (0.029 ± 0.002 mm2) were significantly larger than ventricular epicardial ganglia (0.013 ± 0.001 mm2; p<0.05).
Dorsal Right Atrial (DRA) Subplexus
Extracardiac nerves proceeding toward the DRA subplexus access the epicardium between the roots of the cranial caval vein and right PV (Fig. 2). The ganglionated field of this subplexus is located between the cranial and caudal venae cavae and involves up to 27% of all epicardial ganglia (Figs. 2 and 3f; see Table 2 OLS). However, the largest proportion of epicardial ganglia in this subplexus are concentrated on the RCV and DIRA regions (Tables 1 and 1 OLS). The DRA subplexal postganglionated nerves spread widely on the right atrial dorsal and lateral walls, being particularly abundant near the sinuatrial node and the superior surface of the right auricle (Figs. 2, 3f and Table 2 OLS).
Middle Dorsal (MD) Subplexus
The access of the MD subplexal nerves into the epicardium is complex because these nerves spread broadly ahead of the ganglionated field between the right, middle and left PVs, as well as between the right PV and caudal caval vein (Figs. 2, 3c; Table 2 OLS). The MD subplexal ganglia were less than 3% of all counted ganglia (Fig. 3c; Table 2 OLS). Epicardial nerves of the MD subplexus passed through the coronary groove and spread onto the dorsal side of both ventricles (Figs. 2, 3c). Noteworthy, the ventricular nerves of this subplexus were accompanied by many epicardial ganglia (Fig. 3c, e).
Left Dorsal (LD) Subplexus
Thick nerves accessing the LD subplexus concentrate next to the left azygos vein (Figs. 2, 3a). The dorsal LD subplexal nerves proceed toward epicardial ganglia scattered on the walls of the left azygos vein (CLAV), coronary sinus (CS), dorsal left (DLV) and right (DRV) ventricular regions (Figs. 2, 3a and c; Table 2 OLS). The ventral portion of the LD subplexal nerves extend toward epicardial ganglia distributed on the ventral left ventricular (VLV) region (Fig. 4; Table 2 OLS). Overall, the LD subplexal ganglia constituted about 39% of all counted epicardial ganglia (Table 2 OLS). The vast majority of the postganglionated LD subplexal nerves passaged the coronary groove and extended onto the dorsal side of both ventricles (Figs. 2, 3a, c). Like the MD subplexal nerves, the ventricular nerves of the LD subplexus were accompanied by numerous epicardial ganglia as well (Figs. 2, 3c; Tables 1 and 1 OLS).
Right Ventral (RV) Subplexus
The RV subplexal nerves access the epicardium at the medial surface of RCV and at the anterior interatrial groove (Fig. 2, 4a; Table 2 OLS). Although the ganglionated field of this subplexus involves only up to 17% of all epicardial ganglia, its distribution includes the ventral side of the RCV, VSRA and VILA regions, the ISRAu and the ventral side of both ventricles adjacent to the anterior coronary groove (Figs. 2, 4a-f; Table 2 OLS). The postganglionated RV subplexal nerves spread widely onto the ventral and lateral sides of the right atrium, including the sinuatrial node and inferior surface of the right auricle, and both ventricles, (Figs. 2; 4a-f; Table 2 OLS).
Ventral Left Atrial (VLA) Subplexus
The VLA subplexal nerves enter the epicardium at the root of left azygos vein and distribute only on the VSLA and VILA regions (Fig. 2; Table 2 OLS). The VLA subplexal ganglia comprised up to 3.8% of all epicardial ganglia and were mostly scattered in the VSLA region (Table 2 OLS). The comparatively sparse VLA subplexal postganglionated nerves spread to the VSLA and VILA regions where they overlapped in part with the ventral right atrial subplexal nerves (Fig. 4a; Table 2 OLS).
Left (LC) and Right (RC) Coronary Subplexuses
Nerves of the coronary subplexuses penetrate into the heart through the arterial part of the HH between the roots of the ascending aorta and pulmonary trunk (Figs. 2, 4d; Table 2 OLS). The vast majority of coronary subplexal ganglia concentrate near the pulmonary trunk, but related sparse ganglia may be distributed on the anterior coronary groove and ventral side of both ventricles (Fig. 2; Table 2 OLS). Both coronary subplexuses included up to 10% of all counted epicardial ganglia (Table 2 OLS). The distribution of postganglionated nerves from both coronary subplexuses is restricted to the ventral side of both ventricles, where they overlap in part with the numerous RV subplexal nerves (Fig. 2; Table 2 OLS).
Nerves and Ganglia in the Heart Hilum (HH)
Nerves and ganglia distributed at the limits of the HH are not covered by epicardium but lay in a fatty connective tissue on the cardiac base beneath the pulmonary arteries at bifurcation of the pulmonary trunk (Fig. 4g). The extrinsic nerves access the ganglia on the cardiac base from three sites of the HH; namely, the right cranial caval vein, the left azygos vein, and between left and middle pulmonary veins (PVs). These nerves extend along the cranial caval vein and join ganglia scattered between the RCV and right PVs, as well as on the root of the caudal caval vein. Nerves coursing from the left azygos vein and the left and middle PVs reach the ganglia located in the HH between the left, middle and right pulmonary vein roots. Generally, the ovine HH included less than 2% of all identified cardiac ganglia. Slim and short nerves originating from ganglia of the HH extended towards the extra-pulmonary segments of the left, middle and right pulmonary, right cranial and caudal caval veins.
Individual Variability of the Ovine ENP
The distribution of epicardial nerves and the number of ganglia in separate subplexuses, as well as in homologous cardiac regions varied substantially from heart to heart (Fig. 6; Tables 1 and 1 OLS). Presumably, the number of subplexal nerves is related to their branching pattern; i.e. if extrinsic cardiac nerves ramify above the HH (Fig. 6a), their number within that structure is larger and they are thinner than those that branch below the HH, which appear thicker (Fig. 6b). On the other hand, the anastomoses of subplexal nerves within the same cardiac region were evidently idiosyncratic. The number and regional density of subplexal ganglia varied greatly from heart to heart (Table 1 OLS). For instance, in the dorsal DRV, VRV, and DSRA regions, the ganglion number differed in different lambs by up to 50 fold (Table 1 OLS).
Fig 6.
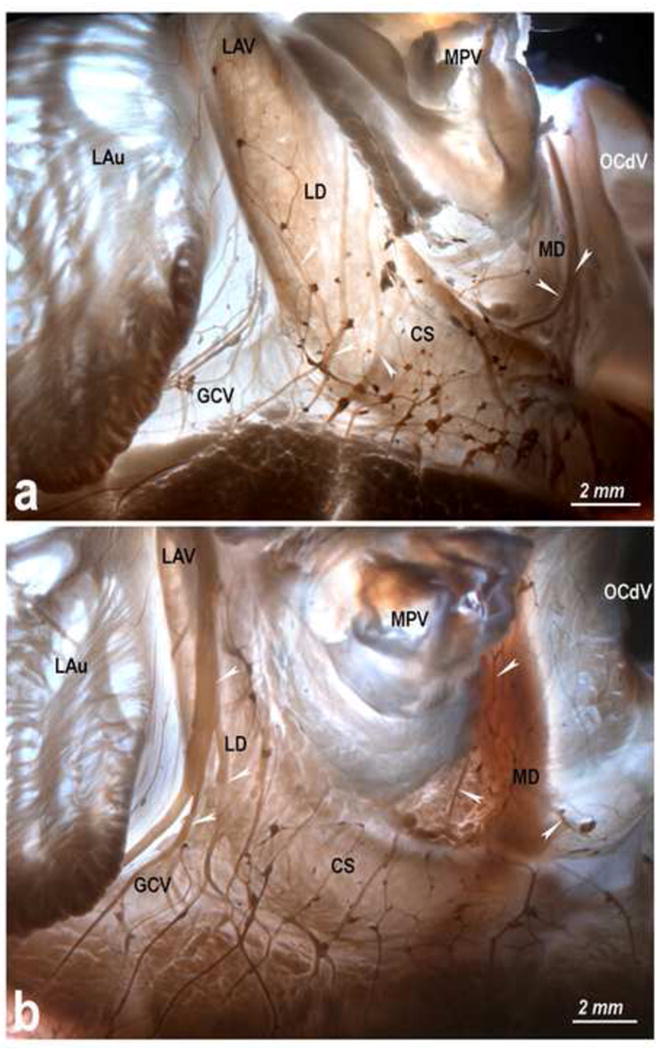
Variability in morphology of the left and the middle dorsal subplexuses in hearts from newborn lambs. Black arrowheads, left dorsal subplexal nerves; white arrowheads, middle dorsal subplexal nerves. Note the distinct branching patterns of epicardial nerves coursing along the left azygos vein. Abbreviations: OCdV – orifice of the caudal vein; others – as in Figs 2-4.
Relationships between Extracardiac Autonomic Nerves and Epicardial Ganglionated Nerve Plexus
The two-sided cervicothoracic and thoracic (T2-T6) sympathetic ganglia and the two-sided vagal nerves were macroscopically evident neural sources, from which extrinsic cardiac nerves extended toward and reached the ovine HH (Fig. 7; Table 3 OLS). The DRA, RV and MD epicardial neural subplexuses were supplied by nerves originated from the right cervicothoracic (RCT) and T2-T4 sympathetic ganglia, as well as from the right vagal nerve (RVN), between which anastomoses developed (Fig. 7a; Table 3 OLS).
Fig 7.
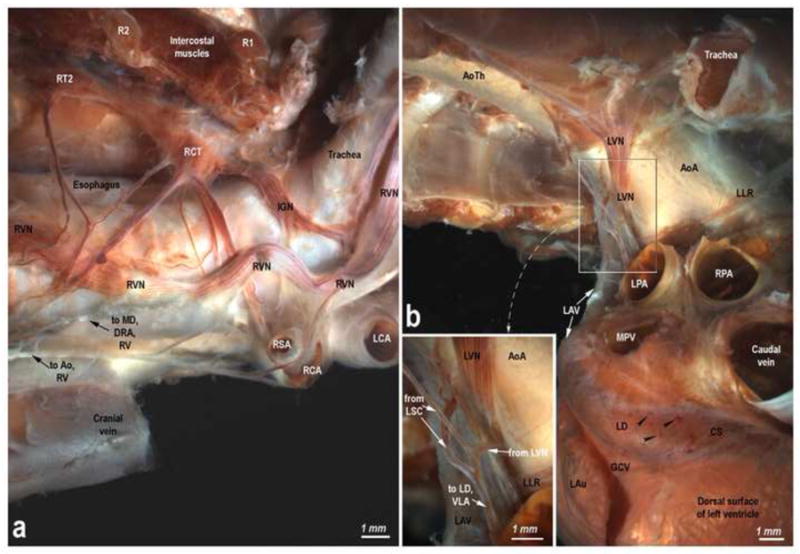
Lamb thorax-dissected preparations showing the origin and course of the right (a) and left (b) extrinsic cardiac nerves. Abbreviations: Ao – aorta; AoA – aortic arch; AoTh – thoracic aorta; IGN – interganglionated nerve; LCA – left common carotid artery; LLR – left laryngeal recurrent nerve; LPA – left pulmonary artery; LSC – left sympathetic chain; LV – left vagus; R – rib; RCA – right common carotid artery; RCT – right cervicothoracic ganglion; RPA – right pulmonary artery; RSA – right subclavian artery; RT – right thoracic ganglion; RV – right vagus; other abbreviations as for Figs 2-6.
Comparatively thick mediastinal nerves derived from the left thoracic (T4-T6) sympathetic and partly from the left cervicothoracic ganglia reached the HH at the left azygos vein, on which they formed the left dorsal neural subplexus that stretched out toward the posterior and lateral walls of the left ventricle (Fig. 7b; Table 3 OLS). At the access site of the left azygos vein into the ovine HH, branches from the above mentioned sympathetic ganglia prolonged toward the anterior LA wall where the ventral LA neural subplexus was distributed. The finer branches of the left vagal nerves passed into the left dorsal and ventral LA neural subplexuses exactly at the same sites of the HH as the extracardiac nerves from the above indicated left sympathetic ganglia (Fig. 7b).
In the arterial part of the ovine HH, the access of the extrinsic cardiac nerves that extended along the anterior coronary groove and on the ventral sides of both ventricles as the right and left coronary subplexuses was narrowly concentrated between ascending aorta and pulmonary trunk. These nerves could be tracked up to the two-sided vagal nerves, cervicothoracic and right T4-T5 sympathetic ganglia (Table 3 OLS).
Discussion
This is the first detailed investigation of the gross anatomy of the complete ovine epicardial neural ganglionated plexus, revealed histochemically in the whole, unsectioned heart using AChE staining. Simultaneous histochemistry for AChE and immunohistochemistry for TH confirmed earlier findings25,26 demonstrating that the histochemical stain of Karnovsky and Roots, which detects AChE, allows visualization of both parasympathetic and sympathetic structures. However, since postganglionic sympathetic nerve fibers have less AChE activity and/or content than cholinergic fibers,26 presumably the lighter histochemical staining of some epicardial nerves demonstrated on our images indicate predominance of adrenergic fibers. Importantly, immunohistochemistry for general neuronal marker (PGP 9.5) suggests that the architecture of the intrinsic cardiac nerve plexus is entirely similar to that revealed by AChE histochemistry.27
Similar to human, canine, and porcine hearts,7,9,24 each subplexus in the ovine ENP threads in a particular site of the HH, occupies a specific location on the epicardium, extends to a restricted cardiac zone, and includes its preganglionated nerves, ganglionated field, and postganglionated nerves. In the sheep, the RA, including the sinuatrial nodal region, is supplied by the ganglionated nerves from the RV and DRA subplexuses, the walls of the LA from the VLA and RV, LD and MD subplexuses, and the ventricles by nerves from the LD and MD, VLA and both coronary subplexuses. Some postganglionated nerves from LD, MD, RV and DRA subplexuses extend towards the interatrial septum and presumably supply the atrioventricular nodal region. Moreover, we have shown that from their access into the ovine HH the extrinsic right vago-sympathetic cardiac nerves descend mainly to the DRA, the RV and MD epicardial neural ganglionated subplexuses, whereas the left vago-sympathetic nerve branches extend typically to the LD and VLA subplexuses (Table 3 OLS). Most likely, both coronary epicardial nerve subplexuses are equally supplied by the left and right vago-sympathetic nerve branches. Altogether, our findings are in part consistent with earlier observations in humans.28,29 We demonstrate the asymmetry in neural sources of sympathetic nerves because the right sympathetic cardiac nerves as a rule derive from the RCT – RT5, while the left ones – from the LT4 – LT6 sympathetic ganglia. Notably, we did not identify main extrinsic cardiac nerves in the lamb that would originate from the LCT and both upper cervical sympathetic ganglia, which normally occurs in humans.28,29 Both in lamb and humans, right and left vagal extracardiac branches derive from the proximal laryngeal recurrent nerve.28,29
Although in general the morphology of the ovine ENP coincides with the human, canine and porcine plexuses, there are evident topographical and quantitative interspecies differences. In Table 2 OLS, the lamb RV and DRA nerve subplexuses involve about 44% of all epicardial ganglia, which is very similar to the human heart. In contrast, in the dog the right atrial subplexuses include about 70% of all epicardial ganglia. Similarly, the distribution of the RV subplexal postganglionated nerves is more expansive in sheep than human and canine hearts. The RV subplexal postganglionated nerves of the porcine heart are most similar to the sheep heart in that numerous nerves extend onto the ventral side of both ventricles.9
The relative ganglion number of the LA subplexuses (i.e. LD, MD and VLA) is very similar in the sheep and human hearts. They contain, respectively, about 46% and 52% of the intrinsic cardiac ganglia, whereas the dog contains only about 30%.7,24 The left atrial part of the ovine and porcine RV subplexus is more developed and ganglionated (up to 4% of all epicardial ganglia) than the more tenuous VLA subplexus in man and dog. A clear interspecies difference is that, in the ovine ventricles, the number of ventricular ganglia distributed on the dorsal side, on which we identified abundant epicardial ganglia, is larger than in dog, pig and human.7-9,24,33 (Table 1 OLS). Since the ventricular neural ganglia selectively mediated the negative inotropic effects of vagal stimulation on the left ventricle without influencing the negative chronotropic or dromotropic actions,15,30 our findings imply that neurons from profuse ventricular ganglia specifically influence the neural control of the ovine ventricles.
In dog and human, abundant thick nerves access both coronary subplexuses around the roots of the ascending aorta and pulmonary trunk.7,24 In the lamb, we identified an obviously lesser neural supply to the left and right coronary subplexuses. Interestingly, in the pig there was no neural input between the ascending aorta and the root of the pulmonary trunk as the porcine coronary blood vessels are mainly supplied by epicardial nerves extending from the right ventral subplexus.9
The ovine and human intrinsic cardiac plexuses are very similar with respect to the density of neural structures within the neural plexus of the HH on the heart base, where very rare ganglia were identified.24 Similar to the lamb, the hearts of rats, guinea pigs, rabbits, dogs and pigs, involve abundant intrinsic neurons clustered on the heart base within the limits of their comparatively wide HH.7,24,31
The morphology of the ovine epicardial nerve subplexuses varies from heart to heart. The number of epicardial ganglia and interganglionic nerves in every subplexus varies greatly from one specimen to another; presumably the number of the preganglionated subplexal nerves is related to the branching pattern of these nerves.
Based on our earlier findings showing a direct correlation between the area of an epicardial ganglion and the number of its neurons,32 we calculate that on average the ovine heart contains 17,000 intrinsic neurons, 6,500 of which are associated with the AV-nodal region, 5,000 with the sinuatrial-nodal region, and 1000 are topographically related to the sinus of the left azygos vein. Noteworthy, the number of intrinsic neurons identified in the pig9 was smaller than the ovine heart, but the number of intrinsic neurons in the human and canine hearts is almost sixfold larger.7,24
Our data lead us to conclude that, despite some obvious morphologic differences, the topography of the ovine epicardial neural subplexuses corresponds to the human subplexuses, which supports the use of the ovine heart as a model for experimental cardiac electrophysiology. Hopefully, the detailed neuroanatomy demonstrated here will facilitate further immunohistochemical and functional experiments using the ovine ICNS.
Supplementary Material
Acknowledgments
We thank Mr. Zilvinas Augustinavicius, Association of Lithuanian sheep-breeders, for providing material for this study. Supported by grant from the Science Foundation of Kaunas University of Medicine (PAR 18) and by NHLBI Grants P01-HL039707, P01-HL087226 and R01-HL080159 (JJ).
Footnotes
No Conflicts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gorman MW, Tune JD, Richmond KN, Feigl EO. Quantitative analysis of feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol. 2000;89:1903–1911. doi: 10.1152/jappl.2000.89.5.1903. [DOI] [PubMed] [Google Scholar]
- 2.Tsuboi M, Furukawa Y, Nakajima K, Kurogouchi F, Chiba S. Inotropic, chronotropic, and dromotropic effects mediated via parasympathetic ganglia in the dog heart. Am J Physiol Heart Circ Physiol. 2000;279:H1201–1207. doi: 10.1152/ajpheart.2000.279.3.H1201. [DOI] [PubMed] [Google Scholar]
- 3.Cifelli C, Rose RA, Zhang H, et al. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res. 2008;103:527–535. doi: 10.1161/CIRCRESAHA.108.180984. [DOI] [PubMed] [Google Scholar]
- 4.Levy MN, Warner MR. Parasympathetic effects on cardiac function. In: Armour JA, Ardell JL, editors. Neurocardiology. New York: Oxford University Press; 1994. pp. 53–76. [Google Scholar]
- 5.Armour JA, Kember GC. Cardiac sensory neurons. In: Armour JA, Ardell JL, editors. Basic and clinical neurocardiology. New York: Oxford University Press; 2004. pp. 79–117. [Google Scholar]
- 6.Marron K, Wharton J, Sheppard MN, et al. Distribution, morphology, and neurochemistry of endocardial and epicardial nerve terminal arborizations in the human heart. Circulation. 1995;92:2343–2351. doi: 10.1161/01.cir.92.8.2343. [DOI] [PubMed] [Google Scholar]
- 7.Pauza DH, Skripka V, Pauziene N. Morphology of the intrinsic cardiac nervous system in the dog: a whole-mount study employing histochemical staining with acetylcholinesterase. Cells Tissues Organs. 2002;172:297–320. doi: 10.1159/000067198. [DOI] [PubMed] [Google Scholar]
- 8.Arora RC, Waldmann M, Hopkins DA, Armour JA. Porcine intrinsic cardiac ganglia. Anat Rec A Discov Mol Cell Evol Biol. 2003;271:249–258. doi: 10.1002/ar.a.10030. [DOI] [PubMed] [Google Scholar]
- 9.Batulevicius D, Skripka V, Pauziene N, Pauza DH. Topography of the porcine epicardial nerve plexus as revealed by histochemistry for acetylcholinesterase. Auton Neurosci. 2008;138:64–75. doi: 10.1016/j.autneu.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol. 1986;251:H764–773. doi: 10.1152/ajpheart.1986.251.4.H764. [DOI] [PubMed] [Google Scholar]
- 11.Chiou CW, Eble JN, Zipes DP. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. The third fat pad. Circulation. 1997;95:2573–2584. doi: 10.1161/01.cir.95.11.2573. [DOI] [PubMed] [Google Scholar]
- 12.Fee JD, Randall WC, Wurster RD, Ardell JL. Selective ganglionic blockade of vagal inputs to sinoatrial and/or atrioventricular regions. J Pharmacol Exp Ther. 1987;242:1006–1012. [PubMed] [Google Scholar]
- 13.Kamada S, Kawamura Y, Iida Y, Sato N, Hasebe N. An experimental study on the site dependency and mechanism of vagal denervation following radiofrequency catheter ablation for supraventricular arrhythmias including atrial fibrillation. Int Heart J. 2008;49:493–506. doi: 10.1536/ihj.49.493. [DOI] [PubMed] [Google Scholar]
- 14.Schauerte P, Mischke K, Plisiene J, et al. Catheter stimulation of cardiac parasympathetic nerves in humans: a novel approach to the cardiac autonomic nervous system. Circulation. 2001;104:2430–2435. doi: 10.1161/hc4501.099307. [DOI] [PubMed] [Google Scholar]
- 15.Gatti PJ, Johnson JA, McKenzie J, Lauenstein JM, Gray A, Massari VJ. 1997. Vagal control of left ventricular contractility is selectively mediated by a cranioventricular intracardiac ganglion in the cat. J Auton Nerv Syst. 1997;66:138–144. doi: 10.1016/s0165-1838(97)00071-4. [DOI] [PubMed] [Google Scholar]
- 16.Markovitz LJ, Savage EB, Ratcliffe MB, et al. Large animal model of left ventricular aneurysm. Ann Thorac Surg. 1989;48:838–845. doi: 10.1016/0003-4975(89)90682-6. [DOI] [PubMed] [Google Scholar]
- 17.Jardine DL, Charles CJ, Melton IC, et al. Continual recordings of cardiac sympathetic nerve activity in conscious sheep. Am J Physiol Heart Circ Physiol. 2002;282:H93–99. doi: 10.1152/ajpheart.2002.282.1.H93. [DOI] [PubMed] [Google Scholar]
- 18.Bugge E, Nicholson IA, Thomas SP. Comparison of bipolar and unipolar radiofrequency ablation in an in vivo experimental model. Eur J Cardiothorac Surg. 2005;28:76–80. doi: 10.1016/j.ejcts.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Addis A, Vanosi G, Manasse E, Mainetti M, Monaco A, Addis F. An experimental sheep model used to develop an ablation procedure for chronic atrial fibrillation. Surg Endosc. 2007;21:1626–1630. doi: 10.1007/s00464-007-9213-0. [DOI] [PubMed] [Google Scholar]
- 20.Anne W, Willems R, Holemans P, et al. Self-terminating AF depends on electrical remodeling while persistent AF depends on additional structural changes in a rapid atrially paced sheep model. J Mol Cell Cardiol. 2007;43:148–158. doi: 10.1016/j.yjmcc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Doll N, Aupperle H, Borger M, Czesla M, Mohr FW. [Efficacy and safety of various energy sources and application techniques for the surgical treatment of atrial fibrillation] Herzschrittmacherther Elektrophysiol. 2007;18:83–91. doi: 10.1007/s00399-007-0559-8. [DOI] [PubMed] [Google Scholar]
- 22.Pruvot E, Jousset F, Ruchat P, et al. Propagation velocity kinetics and repolarization alternans in a free-behaving sheep model of pacing-induced atrial fibrillation. Europace. 2007;9(Suppl 6):vi, 83–88. doi: 10.1093/europace/eum211. [DOI] [PubMed] [Google Scholar]
- 23.McKibben JS, Getty R. A comparative study of the cardiac innervation in domestic animals: sheep. Acta Anat (Basel) 1969;74:228–242. doi: 10.1159/000143379. [DOI] [PubMed] [Google Scholar]
- 24.Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259:353–382. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 25.Karnovsky MJ, Roots L. A “Direct-Coloring” Thiocholine Method for Cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- 26.Koelle GB, Massoulie J, Eugene D, Melone MA, Boulla G. Distributions of molecular forms of acetylcholinesterase and butyrylcholinesterase in nervous tissue of the cat. Proc Natl Acad Sci USA. 1987;84:7749–7752. doi: 10.1073/pnas.84.21.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crick SJ, Sheppard MN, Ho SY, Anderson RH. Localisation and quantitation of autonomic innervation in the porcine heart I: conduction system. J Anat. 1999;195:341–357. doi: 10.1046/j.1469-7580.1999.19530341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janes RD, Brandys JC, Hopkins DA, Johnstone DE, Murphy DA, Armour JA. Anatomy of human extrinsic cardiac nerves and ganglia. Am J Cardiol. 1986;57:299–309. doi: 10.1016/0002-9149(86)90908-2. [DOI] [PubMed] [Google Scholar]
- 29.Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol (Berl) 2005;209:425–438. doi: 10.1007/s00429-005-0462-1. [DOI] [PubMed] [Google Scholar]
- 30.Dickerson LW, Rodak DJ, Fleming TJ, et al. Parasympathetic neurons in the cranial medial ventricular fat pad on the dog heart selectively decrease ventricular contractility. J Auton Nerv Syst. 1998;70:129–141. doi: 10.1016/s0165-1838(98)00048-4. [DOI] [PubMed] [Google Scholar]
- 31.Pauza DH, Pauziene N, Tamasauskas KA, Stropus R. Hilum of the heart. Anat Rec. 1997;248:322–324. doi: 10.1002/(SICI)1097-0185(199707)248:3<322::AID-AR3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 32.Pauza DH, Pauziene N, Pakeltyte G, Stropus R. Comparative quantitative study of the intrinsic cardiac ganglia and neurons in the rat, guinea pig, dog and human as revealed by histochemical staining for acetylcholinesterase. Ann Anat. 2002;184:125–136. doi: 10.1016/S0940-9602(02)80005-X. [DOI] [PubMed] [Google Scholar]
- 33.Saburkina I, Pauza DH. Location and variability of epicardiac ganglia in human fetuses. Anat Embryol (Berl) 2006;211:585–594. doi: 10.1007/s00429-006-0110-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


