Abstract
Hypoparathyroidism is a hormone deficiency syndrome that leads to low blood calcium levels and for which current replacement therapy is inadequate. Gene transfer to salivary glands leads to safe and abundant secretion of therapeutic protein into either saliva or the bloodstream. We previously reported the successful transduction of rat submandibular glands with an adenoviral vector encoding human parathyroid hormone (Ad.hPTH), but unfortunately most of the hPTH was secreted into saliva. Because submandibular and parotid glands are morphologically and functionally different, we hypothesized that hPTH sorting might be different in parotid glands. After 2 days, the pattern of hPTH secretion from transduced parotid glands of intact rats was reversed from that of transduced submandibular glands, that is, most transgenic hPTH was detected in serum (5 × 1010 viral particles per gland; the saliva-to-serum ratio of total hPTH secreted was 0.04). Vector copies were localized to the targeted parotid glands, with none detected in liver or spleen. Ad.hPTH next was administered to parotid glands of parathyroidectomized rats. Two days after delivery no hPTH was detectable in saliva, but high levels were found in serum, leading to normalization of serum calcium and a significant increase in the urinary phosphorus-to-creatinine ratio. This study demonstrates for the first time differential sorting of transgenic hPTH between submandibular and parotid glands, suggesting that hPTH may be a valuable model protein for understanding the molecular basis of transgenic secretory protein sorting in these exocrine glands. We also show the clinical potential of salivary gland hPTH gene therapy for patients with hypoparathyroidism.
Physiologically sufficient levels of calcium in the blood are important for a range of vital functions and hypocalcemia can lead to seizures, tetany, or heart failure. Parathyroid hormone (PTH) is central to maintaining adequate blood calcium concentration. In this study, Adriaansen et al. demonstrate that delivery of an adenoviral vector encoding human PTH to the parotid gland of hypocalcemic rats leads to a normalization of serum calcium levels.
Introduction
Physiologically sufficient levels of calcium in the blood are crucially important for a range of vital functions; that is, hypocalcemia can lead to seizures, tetany, altered mental state, and heart failure (Shoback, 2008). Parathyroid hormone (PTH) is central to maintaining adequate blood calcium concentrations and is normally secreted by the parathyroid glands in response to low blood calcium levels (Poole and Reeve, 2005). PTH increases blood calcium levels by mobilizing calcium from bone (Potts, 2005; Blair and Zaidi, 2006) and, in the kidney, increases calcium reabsorption from the distal nephrons and stimulates renal formation of active 1,25-dihydroxyvitamin D for efficient intestinal calcium absorption (Zierold et al., 2001). Hypocalcemia can be the result of a PTH deficiency, vitamin D deficiency, or hypomagnesemia (Cooper and Gittoes, 2008). Whereas the latter two are relatively easy to restore, the treatment of hypoparathyroidism poses more challenges.
Hypoparathyroidism can be congenital, autoimmune, or acquired (mostly through surgery), but in all cases low levels of PTH and hypocalcemia occur and, without the calcium-retaining action of PTH at the kidney, hypercalciuria follows. Although supplying patients with recombinant PTH seems logical, this approach is hampered by its short half-life in vivo, the resultant need for multiple injections per day, particularly when PTH (1–34) is used, and the consequent expense. Despite the potential physiological superiority of recombinant PTH injections (e.g., Winer et al., 1996, 1998, 2003, 2008), conventional therapy, which usually involves supplementation with calcium and active forms of vitamin D3, continues as the mainstay of treatment. However, this approach can lead to hypercalciuria and nephrocalcinosis, nephrolithiasis, impaired renal function, and, not infrequently, renal failure (e.g., Tal and Powers, 1996; Chou et al., 2009; Medarov, 2009). Hypoparathyroidism is thus a serious disorder and one of the few remaining hormone deficiencies for which no satisfactory hormone replacement therapy is approved. Taken together, there is a clear need for a different, more efficient PTH delivery method to treat these patients.
We have proposed using the efficient protein-producing capacity of the salivary glands for gene therapeutics (e.g., Baum et al., 2004; Voutetakis et al., 2004a,b). Salivary glands are easily accessible by retroductal instillation and nonessential for life in case of a serious adverse reaction to the gene transfer vector. This approach can yield high circulating transgenic protein levels (typically nanograms per milliliter; Perez et al., 2010) without the need for systemic vector administration. Salivary gland gene transfer shows considerable potential for treating systemic single-protein deficiencies, provided the transgenic proteins will sort toward the bloodstream. Salivary glands are polarized epithelia with the ability to secrete proteins into both saliva as well as the bloodstream (Baum et al., 1999; Isenman et al., 1999).
We have reported a successful proof-of-principle study in which rodent submandibular glands were transduced with a serotype 5 adenoviral (Ad) vector encoding full-length human PTH (1–84) (Adriaansen et al., 2008). In rat submandibular glands, unfortunately, almost all hPTH was sorted in an exocrine direction. This resulted in high hPTH concentrations in saliva, which would then be degraded in the stomach and thus be of no therapeutic use. In the same report, we also described the opposite sorting behavior of hPTH in the mouse submandibular gland, with hPTH being secreted primarily into the bloodstream.
Differences in sorting of soluble secretory proteins between different cell types are not uncommon (Perez et al., 2010). Although submandibular and parotid glands share important characteristics, at a cellular and physiological level there are differences. For example, the main secretion of submandibular glands is mucinous, whereas the parotid secretion is serous and watery. Such differences suggested that interactions in the formation and organization of secretory granules in the two gland types could be different. With this in mind we postulated that the sorting of hPTH in rat parotid glands would be different from that in submandibular glands. In the present study, we describe for the first time the predominant sorting of transgenic hPTH from transduced rat parotid glands toward the bloodstream and show clearly that the parotid gland-produced transgenic hPTH was physiologically active in a rat model of hypoparathyroidism.
Materials and Methods
Animals
Male Wistar rats (n = 8 per treatment group), both untreated controls and parathyroidectomized (PTX) animals, were obtained at 7 weeks of age from Charles River Laboratories (Wilmington, MA), where the parathyroidectomies were performed. All animals retained intact thyroid gland function. They were acclimatized for 1 week before the start of any experiments, and standard water and food were provided ad libitum. The Animal Care and Use Committee of the National Institute of Dental and Craniofacial Research (National Institutes of Health [NIH], Bethesda, MD), and the National Institutes of Health Biosafety Committee, approved all animal experiments.
Construction of recombinant adenovirus
The hPTH carrier plasmid (GeneCopoeia, Germantown, MD) was enzymatically digested and pre-pro-hPTH1–84 cDNA was directionally cloned into the adenoviral expression shuttle plasmid pACCMV-pLpA after a cytomegalovirus (CMV) promoter and before a simian virus 40 polyadenylation signal, as previously described (Adriaansen et al., 2008). The sequence of the resultant plasmid, pACCMV-hPTH, was confirmed by automated sequencing and hPTH expression was tested in vitro with 293 cells. After that, an E1-deleted recombinant adenoviral vector was generated as previously described (He et al., 1998a). In brief, pACCMV-hPTH and pJM17, providing most of the adenoviral genome, were cotransfected into C7 cells (Amalfitano and Chamberlain, 1997), using calcium phosphate precipitation. The created Ad.hPTH was amplified in 293 cells and purified by two rounds of CsCl gradient centrifugation as previously reported (Delporte et al., 1996; Adriaansen et al., 2008). The vector preparation was tested with the SMIE (He et al., 1998b) and A5 (Brown et al., 1989) cell lines for hPTH production.
Measurement of vector copies
After Ad.hPTH administration, we measured the presence of vector particles (VP) in glands, liver, and spleen by real-time quantitative polymerase chain reaction (qPCR) against a standard curve. We chose these tissues, versus performing an extensive vector biodistribution study, for two reasons: (1) previous experiments by us have shown that after administration of an Ad5 vector encoding human α1-antitrypsin (hα1AT), transgenic hα1AT was found predominantly in the targeted parotid gland (97%; Kagami et al., 1996); and (2) in case of an inadvertent intravascular administration, many experiments by others, with several different animal species, have shown that intravascular delivery of an Ad5 vector results overwhelmingly in liver transduction, with many fewer copies of vector or transgenic protein found in the spleen, among other tissues (e.g., see Jaffe et al., 1992; Herz and Gerard, 1993; Kay et al., 1994; Wood et al., 1999). SYBR green PCR master mix (Applied Biosystems, Foster City, CA) was used in an ABI Prism 7700 sequence detector (Applied Biosystems). The conditions were as follows: 95°C for 2 min, 95°C for 8 min, 95°C for 15 sec and 60°C for 1 min, for 40 cycles. The primers were designed to amplify part of the CMV promoter region: Fwd, 5′-CAT-CTA-CGT-ATT-AGT-CAT-CGC-TAT-TAC-3′; Rev, 5′-TGG-AAA-TCC-CCG-TGA-GTC-A-3′. qPCR assays were performed twice, with all samples and standards in triplicate. DNA extracted from excised glands, liver, and spleen was analyzed the same way (see below).
Transduction of salivary glands in vivo
Rats were anesthetized by intramuscular injection of a mixture of ketamine (60 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (6 mg/kg; Phoenix Scientific, St. Joseph, MO). Five hundred microliters of whole blood was collected from the retro-orbital plexus, serum-separated, and stored at −80°C. Thereafter, left and right submandibular glands (Kagami et al., 1996; Wang et al., 2006a; Zheng et al., 2008) or parotid glands (Kagami et al., 1996, 1998) were cannulated with modified PE-10 polyethylene tubing (BD Diagnostic Systems, Sparks, MD), as previously reported. Atropine was delivered intramuscularly (0.5 mg/kg; Sigma-Aldrich, St. Louis, MO) 10 min before vector infusion to stop salivary flow. The vector doses were suspended in 200 μl of saline and delivered to both ducts by retrograde infusion at a dose of 5 × 109, 5 × 1010, or 1 × 1011 VP per gland. After infusion, syringes were left in place for 15 min to prevent backflow of fluid. Retroductal saline administration was used as control (Adriaansen et al., 2008). In addition, some animals received an intravenous injection of 2 × 1011 VP of Ad.hPTH in 100 μl of saline.
Forty-eight hours after vector administration, the treated animals were reanesthetized with ketamine–xylazine and given a subcutaneous injection of pilocarpine (0.5 mg/kg in saline) to stimulate salivary flow. The 48-hr time point was selected for this proof-of-concept study because, on the basis of previous experiments, it represents a time when transgenic protein production and secretion into serum is at near-maximal values (Kagami et al., 1996, 1998). Saliva was collected as described (Wang et al., 2006a) and snap-frozen immediately. Animals were killed in a CO2 chamber and, after death was ensured by bilateral thoracotomy, blood was collected as described previously, and serum was separated and stored at −80°C. Submandibular or parotid glands were excised and cleaned, and each gland was cut in half longitudinally. One part was snap-frozen and the other part was embedded in O.C.T. medium and frozen in liquid nitrogen (see below). Spleen and sections of the liver were removed and snap-frozen. Urine was collected from the bladder and snap-frozen as well.
Analytical determinations
Total DNA was extracted from glands, liver, and spleen, using a DNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The quality and quantity of the obtained DNA were measured spectrophotometrically and samples were diluted with Tris–EDTA (TE) buffer to 100 ng/μl. One microliter of each sample, in triplicate, was then used in the real-time qPCR assay as described previously to determine the amount of vector copies present.
We used a two-site enzyme-linked immunosorbent assay (ELISA) (MD Biosciences, St. Paul, MN), with a sensitivity of 1.72 pg/ml, to measure intact hPTH, in accordance with the manufacturer's directions. A separate set of control samples was spiked with hPTH protein to assess for the possibility of assay interference in saliva or serum. Each sample was measured twice and in duplicate. The serum-to-saliva distribution ratio for hPTH in rats was calculated generally as described previously (Hoque et al., 2001; Adriaansen et al., 2008). Specifically, the concentration of hPTH measured by ELISA in saliva or serum samples was multiplied by the specific volume of saliva collected from each animal or an assumed serum volume of 4 ml, respectively, to give the total amount of hPTH found in each compartment during the collection time period. Other potential compartments of hPTH accumulation, for example, extracellular fluid, were not considered for this calculation. To generate the serum-to-saliva distribution ratio the calculated total amount of hPTH found in the saliva pool was divided by the calculated total amount of hPTH found in the serum pool.
All clinical chemistry assays were performed in the NIH Clinical Center's Department of Laboratory Medicine and employed standard procedures.
Immunofluorescence detection of hPTH
Salivary glands, collected 48 hr after transduction, were embedded in O.C.T. compound (Electron Microscopy Sciences, Hatfield, PA) and frozen in liquid nitrogen. Sections (15 μm) were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) in phosphate-buffered saline (PBS), pH 7.4, for 10 min at room temperature. Free aldehyde was blocked with a solution of 100 mM glycine in PBS. Sections were mounted in ProLong Gold antifade 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, OR) to stain nuclei. After incubation for 30 min with a blocking solution containing 10% goat serum, 0.02% azide, and 0.4% saponin in PBS, slides were incubated with mouse anti-hPTH clone 5E745 (diluted 1:10; Santa Cruz Biotechnology, Santa Cruz, CA) in 10% fetal bovine serum in PBS overnight at 4°C. As an internal control, an irrelevant mouse immunoglobulin G was used as a primary antibody. After extensive washing in PBS, slides were incubated for 1 hr with a secondary antibody, goat anti-mouse Alexa Fluor 488 (diluted 1:200; Molecular Probes). Images were collected with a Leica confocal microscope (Leica Microsystems, Wetzlar, Germany), MetaMorph software (Molecular Devices, Sunnyvale, CA), and ImageJ software (National Institutes of Health).
Statistics
The significance of differences in means between groups was determined by the Kruskal–Wallis test, followed by a Mann–Whitney U rank-sum test. p < 0.05 was considered statistically significant. All analyses were done with SPSS version 15.0 (SPSS, Chicago, IL). Data are expressed as means ± SEM.
Results
Vector biodistribution
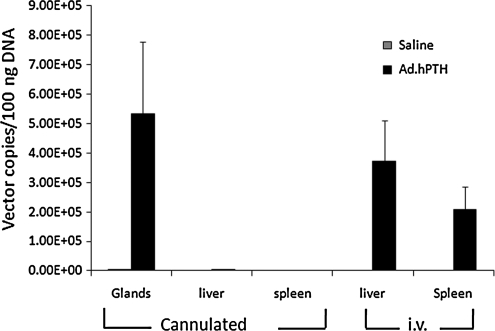
Two days after vector infusion into salivary glands of intact rats, or intravenous injection, the salivary glands, liver, and spleen were excised and total DNA was extracted. Subsequently, a qPCR assay was performed to evaluate the amount of vector copies in these tissues (Fig. 1). The Ad.hPTH vector was present in high amounts in the parotid glands of treated rats. Apparently, no vector escaped into the circulation from these glands, as essentially no vector was found in the DNA samples from liver and spleen. In contrast, for animals receiving an intravenous injection of Ad.hPTH, high vector copy numbers were present in their livers and, to a lesser extent, spleens. No vector copies were detected in any of the control, saline-treated animals.
FIG. 1.
Detection of vector particles. Intact animals (n = 8 per group) were infused with 1 × 1011 VP of Ad.hPTH in each parotid gland or injected intravenously with 2 × 1011 VP. Two days later, parotid glands, liver, and spleen were removed and qPCR was performed on extracted total DNA. No vector copies were detected in liver and spleen of the cannulated and vector-treated animals, but a high amount of Ad.hPTH was found in the targeted parotid glands. In rats injected intravenously with the vector, high copy numbers were found in liver and spleen. No copies were detected in control (saline-administered) animals. Values are expressed as means ± SEM.
Human PTH production after Ad.hPTH delivery to salivary glands
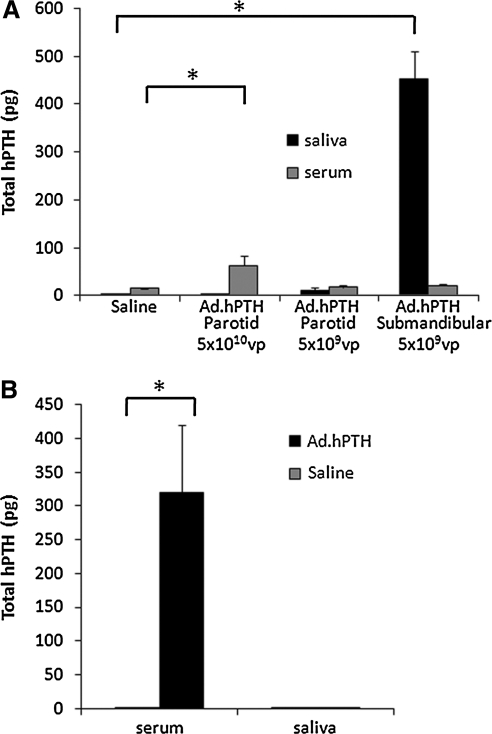
In intact rats, Ad.hPTH was infused into the salivary glands: 5 × 109 or 5 × 1010 VP per gland (parotid) or 5 × 109 VP per gland (submandibular). Two days later, when adenovirus-mediated transgene expression in rat parotid glands is near maximal (Kagami et al., 1998), blood and saliva were collected and assayed for hPTH (Fig. 2A; total hPTH secreted into serum or saliva). As we reported previously, hPTH secreted from rat submandibular glands overwhelmingly sorted toward the saliva (total secreted hPTH saliva-to-serum ratio, 70; p < 0.01 compared with control). However, when 5 × 1010 VP of Ad.hPTH was administered to the parotid glands, the sorting of hPTH was reversed and was now almost exclusively secreted into the bloodstream (ratio of saliva to serum, 0.04; p < 0.01 compared with control). An average serum hPTH concentration of 9.1 ± 2.8 pg/ml was achieved after parotid gland delivery of 5 × 1010 VP. To assess the therapeutic potential of parotid gland hPTH gene delivery, PTX rats were used as a model for human hypoparathyroidism (Fig. 2B, total hPTH secreted). In these experiments, a higher dose (1 × 1011 VP per gland) of Ad.hPTH was used in order to increase the hPTH level in the blood, because hPTH is not highly biologically active in rats (Usatii et al., 2007). An even more pronounced sorting of hPTH into the serum was found with this higher dose (total secreted hPTH saliva-to-serum ratio, 0.004; p < 0.01 compared with control) 2 days after vector delivery and the average hPTH concentration achieved in serum was clinically relevant, 43 ± 11 pg/ml.
FIG. 2.
Production of transgenic hPTH from transduced salivary glands. Two days after Ad.hPTH vector infusion, blood and saliva were collected and assayed for hPTH by ELISA. Total hPTH secreted, that is, detected in serum and saliva (calculated as described in Materials and Methods), is displayed as means ± SEM. In intact animals (A, n = 6 per group), vector infusion (5 × 109 VP per gland) into both submandibular glands led to abundant detection of hPTH in the saliva and low levels in serum (saliva-to-serum ratio, 70). However, at this same dose, little transduction of parotid glands occurred. When both parotid glands were administered the higher dose (5 × 1010 VP per gland) used in this experiment, there was significant secretion of hPTH into the serum, with little hPTH found in saliva (ratio, 0.04). At a dose of 1011 VP per gland in PTX animals (B, n = 8 per group), hPTH secretion was directed specifically toward the bloodstream (ratio, 0.004). No hPTH was detected in serum or saliva of control (saline-administered) animals. *p < 0.01.
Biological effects of Ad.hPTH parotid gland gene transfer
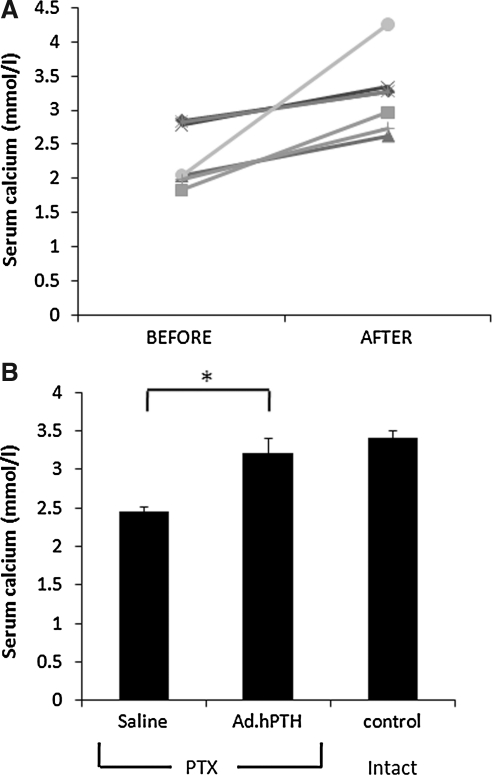
Before and 2 days after hPTH gene transfer to parotid glands of PTX animals, blood was collected and calcium concentrations were determined. We observed an increase in serum calcium levels in all individual animals after vector treatment (Fig. 3A), and the average increase in serum calcium was significantly greater (32%) when compared with saline-treated controls (Fig. 3B; p < 0.01).
FIG. 3.
Effects of hPTH gene transfer on serum calcium in parathyroidectomized (PTX) rats. Blood was collected before and 2 days after Ad.hPTH-mediated gene transfer to both parotid glands of PTX animals (1011 VP per gland) and serum was assayed for calcium. In (A), values for individual rats (n = 7) are shown before vector treatment and joined by a line to values in the same animal after vector treatment. All individual rats had higher serum calcium levels after vector treatment. This resulted in a significant (*p < 0.01) average increase of 32% in serum calcium levels in treated animals compared with saline-treated rats (n = 8), reaching levels similar to those of intact control animals (n = 5; B).
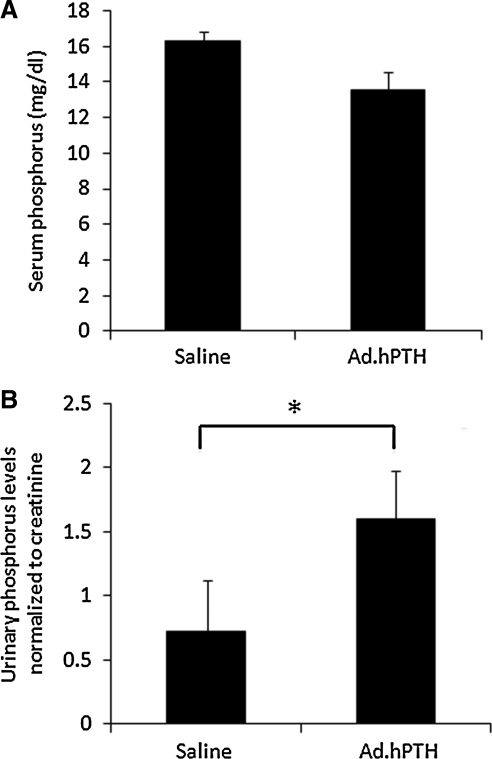
PTH is known to have a robust effect on the kidneys, stimulating the excretion of phosphorus and resulting in lower blood phosphorus levels and higher concentrations of phosphorus in the urine. Ad.hPTH gene transfer resulted in a trend (p = 0.07) toward lower serum phosphorus levels (Fig. 4A). To assess the phosphaturic effect of the transgenic hPTH, the urine phosphorus-to-creatinine ratio was determined. The results are shown in Fig. 4B and demonstrate a physiologically significant (p = 0.028), >2-fold increase in the urinary phosphorus-to-creatinine ratio in treated animals compared with saline-administered control rats.
FIG. 4.
Phosphorus changes after Ad.hPTH administration to PTX rats. Ad.hPTH (1011 VP per gland) was infused into both parotid glands of PTX rats (n = 8 per group) and blood was collected 2 days after vector infusion. Urine was collected from the bladder after sacrifice. There was a trend (p = 0.07) toward lower phosphorus concentrations in the serum of rats after treatment with Ad.hPTH (A). The urinary phosphorus-to-creatinine ratio was significantly elevated (*p = 0.028; 124% increase) in Ad.hPTH-treated rats compared with saline-treated controls (B).
Immunofluorescence detection of transgenic hPTH in transduced salivary glands
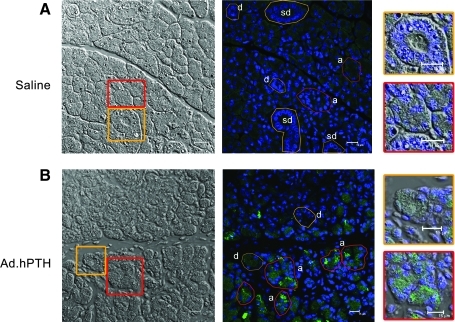
Two days after the transduction of salivary glands, the glands were removed and examined for the expression of hPTH by immunostaining and confocal microscopy. Human PTH was visible in all parotid glands to which Ad.hPTH was administered. In all glands, the immunostaining was visible throughout, but predominantly in acinar rather than ductal cells (Fig. 5B). Intracellularly, hPTH seemed evenly distributed, in a punctate pattern without any defined polarity. No immunofluorescence signal was found in saline-treated animals (Fig. 5A). The irrelevant immunoglobulin G control also did not exhibit any immunostaining.
FIG. 5.
Immunofluorescence detection of transgenic hPTH in parotid glands. Parotid glands were removed 2 days after transduction, embedded in O.C.T. compound, and frozen in liquid nitrogen; 15-μm sections were fixed in 4% paraformaldehyde. Sections were immunostained for hPTH (green) and nuclei were stained with DAPI (blue). (A) Saline-treated glands. (B) Ad.hPTH-treated glands. In each panel a phase-contrast image is shown on the left for visualization of gland morphology. Next, an immunostained image is shown. In the phase-contrast images are two boxes, one red (outlining acinar cells) and one orange (outlining duct cells), that highlight a region of tissue that is shown at higher magnification on the right of each panel. For the immunostained images, different cell types are indicated as either a (acinar), d (intralobular duct), or sd (striated duct), and encircled regions of interest in either red (acinar) or orange (duct) show corresponding cells of interest. Transduced parotid glands show distinct hPTH staining, in a punctate appearance, throughout the gland (B). No polarity in hPTH expression was observed intracellularly and there were overwhelmingly more hPTH-positive acinar cells, compared with ductal cells. No significant signal was found in saline-infused animals (A). Representative pictures are shown.
Discussion
PTH is a key hormone regulating systemic calcium levels. Consequently, hypoparathyroidism of whatever etiology, with its attendant reduction in PTH production and secretion, is a serious condition leading to low blood calcium levels. Therapeutically, it would seem that the most logical approach for such patients would be to supply them with recombinant PTH. However, because of the short half-life, need for multiple daily injections, and expense of recombinant PTH, this approach to therapy is not in wide use.
For these reasons, we thought that hypoparathyroidism would be a good candidate disease for possible gene therapy. However, systemic hPTH gene transfer to critical-for-life organs or tissues not normally intended for protein production and secretion may present drawbacks for clinical applications (Zhou et al., 2005; Wen et al., 2006; Chou et al., 2009). Safety, certainly, is crucially important for any successful application of gene therapy (e.g., Marshall, 2000; Hohmann, 2009), as are considerations of the levels of transgenic protein that can be achieved. Salivary glands have some attractive features as target tissues for clinical gene transfer. They are noncritical for life and have remarkable protein-secretory capabilities (Baum et al., 2004). Furthermore, direct delivery of vectors to human parotid glands appears generally safe (Zheng et al., 2010).
We have previously shown that transgenic hPTH could be successfully produced in the submandibular glands of rats (Adriaansen et al., 2008). The transgenic hPTH, however, was secreted primarily into saliva. Salivary glands are polarized epithelia with the ability to secrete transgenic proteins at the apical pole (predominantly via a regulated secretory pathway) as well as the basolateral pole (primarily constitutive or constitutive-like pathways) of the cells (Baum et al., 1999; Isenman et al., 1999; Voutetakis et al., 2008). A molecular understanding of this sorting process is still elusive, but it most likely involves the interaction of structural signals on the protein with the cellular sorting machinery. Exocrine secretory proteins are directed to large dense-core granules, which will mature into secretory granules to be secreted into the forming saliva on extracellular stimulation (Castle and Castle, 1998). Secretory proteins that are not directed toward or retained within dense-core granules can be secreted constitutively. In this step, the maturing secretory granule may act as a final distribution center (Gorr et al., 2005). It is unclear how much of this cellular protein-sorting machinery is shared between submandibular and parotid glands. These glands are morphologically different, and the secretion produced by parotid glands is serous in nature, whereas that produced by submandibular glands is more mucinous. In this study, we initially hypothesized that the sorting of transgenic hPTH in rat parotid glands may be different compared with that observed in submandibular glands. Our results strongly support that hypothesis.
We found that 2 days after infusion of Ad.hPTH into the parotid glands a significant amount of vector was detected in the targeted tissue. In contrast, no vector copies were found in the liver and spleen of these animals, providing strong evidence that the vector did not spread beyond the glands and that all produced transgenic hPTH originated from the transduced glands. Whereas infusion of Ad.hPTH into the submandibular glands of intact animals resulted, as previously reported (Adriaansen et al., 2008), in the predominant secretion of hPTH into saliva, that is, exocrine secretion, transduction of the parotid glands led to the reverse pattern of hPTH sorting. Although the lowest dose of vector (5 × 109 VP per gland) tested was insufficient to detect hPTH production, at a dose of 5 × 1010 VP per gland significant levels of hPTH were found in the serum of all treated rats. Consistent with this, hPTH did not display any polarity in parotid glands as detected by immunofluorescence and confocal microscopy. Interestingly, a dose of 5 × 109 VP per gland led to a marked production of hPTH by the submandibular glands compared with the parotid glands. The reason for this dramatic difference in vector dose responsiveness between the two glands is likely due to several factors including differences in secretory pathways, size (Wells and Peronace, 1967; Johannsson and Ryberg, 1991), protein synthesis (Kuijper-Lenstra et al., 1975; Proctor et al., 1993), and adenoviral receptor distribution (Delporte et al., 1997). Nonetheless, the present findings on the pattern of transgenic hPTH secretion from rat parotid glands suggest the potential for a clinical application of parotid gland gene transfer in patients with hypoparathyroidism.
To test this, we studied the biological effects of hPTH parotid gene transfer in rats that had their parathyroid glands surgically removed. To achieve a more therapeutically relevant concentration of hPTH in the serum for these experiments with PTX rats we increased the vector dose administered to 1 × 1011 VP per gland. We found substantial hPTH secretion into the serum 2 days after vector infusion into the parotid glands of PTX rats. Despite the lower biological activity of hPTH in rats (Usatii et al., 2007), we found impressive biological effects of the parotid gland-produced hPTH transgene product. Although the serum calcium levels in PTX rats suggested some residual endogenous PTH activity, in each individual Ad.hPTH-treated animal we observed higher serum calcium levels after vector infusion, reaching on average levels similar to those seen in control animals. In the present study, we did not directly determine the source of the elevated serum calcium, but it is probably the result of a combination of the well-established direct effect of PTH in stimulating renal calcium reabsorption (decreased urinary calcium excretion), and increased absorption of dietary calcium from the gastrointestinal tract (Shoback, 2008). In future, long-term, therapeutic studies it will be important to measure both urinary calcium levels and the serum levels of active 1,25(OH)2 vitamin D, which would stimulate dietary calcium absorption from the gastrointestinal tract. An additional important physiological effect of PTH is the stimulation of phosphaturia. Consistent with this, we found a significantly increased phosphorus-to-creatinine ratio in the urine of treated rats. This resulted in generally lower average serum phosphorus levels in the treated PTX rats. Taken together, our data clearly show that the transgenic hPTH produced in the parotid glands of PTX rats is biologically active and support the notion of potential clinical utility in patients with hypoparathyroidism.
For the latter suggestion to reach fruition, however, in addition to detailed toxicological evaluations, considerably more study is required. This would include demonstration of long-term, stable, parotid hPTH gene transfer to PTX animals with, for example, an adeno-associated viral vector (Voutetakis et al., 2004a, 2007; Hai et al., 2009) that would allow for extended transgenic hPTH production, tight regulation of hPTH expression levels (e.g., Wang et al., 2006b) and scaling to a large animal model (e.g., Shan et al., 2005; Hai et al., 2009). In particular, the large animal studies should be helpful in predicting results in humans (e.g., Casal and Haskins, 2006). Initial clinical studies of parotid gland gene transfer, using methods similar to those employed herein, indicate that the procedure is safe and well tolerated by patients (Zheng et al., 2010).
However, a major biological consideration is that it is not known how human parotid glands will sort hPTH. Indeed, the exact sorting mechanisms involved in secretory protein sorting by salivary glands are still unknown (Voutetakis et al., 2008). The present study has demonstrated that there is a difference in how transgenic hPTH is routed intracellularly between rat submandibular and parotid glands, and ultimately secreted. Using hPTH as a model protein to study the sorting pathways operative in these two exocrine glands should lead to a better understanding of how transgenic proteins are handled in salivary epithelial cells and, presumably, result in the identification of the key intracellular molecules involved (Baum, 2009; Sramkova et al., 2009). The latter, along with large animal studies, likely would permit a reasonable assessment of the potential to find similar endocrine sorting of transgenic hPTH in human parotid glands as seen herein with the rat parotid gland.
Acknowledgments
The Division of Intramural Research of the National Institute of Dental and Craniofacial Research supported this research. The authors thank Dr. John Fox for valuable comments on an earlier version of this manuscript.
Author Disclosure Statement
The authors declare no conflict of interests.
References
- Adriaansen J. Perez P. Goldsmith C.M. Zheng C. Baum B.J. Differential sorting of human parathyroid hormone after transduction of mouse and rat salivary glands. Hum. Gene Ther. 2008;19:1021–1028. doi: 10.1089/hum.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalfitano A. Chamberlain J.S. Isolation and characterization of packaging cell lines that coexpress the adenovirus E1, DNA polymerase, and preterminal proteins: Implications for gene therapy. Gene Ther. 1997;4:258–263. doi: 10.1038/sj.gt.3300378. [DOI] [PubMed] [Google Scholar]
- Baum B.J. In vivo veritas: The power of in situ manipulation of cells in a living animal. Am. J. Physiol. Cell Physiol. 2009;297:C1333–C1335. doi: 10.1152/ajpcell.00437.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B.J. Berkman M.E. Marmary Y. Goldsmith C.M. Baccaglini L. Wang S.L. Wellner R.B. Hoque A.T.M.S. Atkinson J.C. Yamagishi H. Kagami H. Parlow A.F. Chao J.L. Polarized secretion of transgene products from salivary glands in vivo. Hum. Gene Ther. 1999;10:2789–2797. doi: 10.1089/10430349950016528. [DOI] [PubMed] [Google Scholar]
- Baum B.J. Voutetakis A. Wang J.H. Salivary glands: Novel target sites for gene therapeutics. Trends Mol. Med. 2004;10:585–590. doi: 10.1016/j.molmed.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Blair H.C. Zaidi M. Osteoclastic differentiation and function regulated by old and new pathways. Rev. Endocr. Metab. Disord. 2006;7:23–32. doi: 10.1007/s11154-006-9010-4. [DOI] [PubMed] [Google Scholar]
- Brown A.M. Rusnock E.J. Sciubba J.J. Baum B.J. Establishment and characterization of an epithelial cell line from the rat submandibular gland. J. Oral Pathol. Med. 1989;18:206–213. doi: 10.1111/j.1600-0714.1989.tb00764.x. [DOI] [PubMed] [Google Scholar]
- Casal M. Haskins M. Large animal models and gene therapy. Eur. J. Hum. Genet. 2006;14:266–272. doi: 10.1038/sj.ejhg.5201535. [DOI] [PubMed] [Google Scholar]
- Castle D. Castle A. Intracellular transport and secretion of salivary proteins. Crit. Rev. Oral Biol. Med. 1998;9:4–22. doi: 10.1177/10454411980090010301. [DOI] [PubMed] [Google Scholar]
- Chou F.F. Huang S.C. Hung P.H. Chang S.F. Liaw J. Oral gene therapy for hypoparathyroidism: A rat model. Hum. Gene Ther. 2009;20:1344–1350. doi: 10.1089/hum.2009.015. [DOI] [PubMed] [Google Scholar]
- Cooper M.S. Gittoes N.J.L. Diagnosis and management of hypocalcaemia. Br. Med. J. 2008;336:1298–1302. doi: 10.1136/bmj.39582.589433.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delporte C. O'Connell B.C. He X.J. Ambudkar I.S. Agre P. Baum B.J. Adenovirus-mediated expression of aquaporin-5 in epithelial cells. J. Biol. Chem. 1996;271:22070–22075. doi: 10.1074/jbc.271.36.22070. [DOI] [PubMed] [Google Scholar]
- Delporte C. Redman R.S. Baum B.J. Relationship between the cellular distribution of the αv, β3/5 integrins and adenoviral infection in salivary glands. Lab. Invest. 1997;77:167–173. [PubMed] [Google Scholar]
- Gorr S.U. Venkatesh S.G. Darling D.S. Parotid secretory granules: Crossroads of secretory pathways and protein storage. J. Dent. Res. 2005;84:500–509. doi: 10.1177/154405910508400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai B. Yan X. Voutetakis A. Zheng C. Cotrim A.P. Shan Z. Ding G. Zhang C. Xu J. Goldsmith C.M. Afione S. Chiorini J.A. Baum B.J. Wang S. Long-term transduction of miniature pig parotid glands using serotype 2 adenoassociated viral vectors. J. Gene Med. 2009;11:506–514. doi: 10.1002/jgm.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X. Goldsmith C.M. Marmary Y. Wellner R.B. Parlow A.F. Nieman L.K. Baum B.J. Systemic action of human growth hormone following adenovirus-mediated gene transfer to rat submandibular glands. Gene Ther. 1998a;5:537–541. doi: 10.1038/sj.gt.3300622. [DOI] [PubMed] [Google Scholar]
- He X. Kuijpers G.A.J. Goping G. Kulakusky J.A. Zheng C.Y. Delporte C. Tse C.M. Redman R.S. Donowitz M. Pollard H.B. Baum B.J. A polarized salivary cell monolayer useful for studying transepithelial fluid movement in vitro. Pflugers Arch. 1998b;435:375–381. doi: 10.1007/s004240050526. [DOI] [PubMed] [Google Scholar]
- Herz J. Gerard R.D. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann E.L. Gene therapy: Still a work in progress. N. Engl. J. Med. 2009;361:193–195. doi: 10.1056/NEJMe0902716. [DOI] [PubMed] [Google Scholar]
- Hoque A.T.M.S. Baccaglini L. Baum B.J. Hydroxychloroquine enhances the endocrine secretion of adenovirus-directed growth hormone from rat submandibular glands in vivo. Hum. Gene Ther. 2001;12:1333–1341. doi: 10.1089/104303401750270986. [DOI] [PubMed] [Google Scholar]
- Isenman L. Liebow C. Rothman S. The endocrine secretion of mammalian digestive enzymes by exocrine glands. Am. J. Physiol. 1999;276:E223–E232. doi: 10.1152/ajpendo.1999.276.2.E223. [DOI] [PubMed] [Google Scholar]
- Jaffe H.A. Danel C. Longenecker G. Metzger M. Setoguchi Y. Rosenfeld M.A. Gant T.W. Thorgeirsson S.S. Stratford-Perricaudet L.D. Perricaudet M. Pavirani A. Lecocq J.-P. Crystal R.G. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat. Genet. 1992;1:372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- Johannsson I. Ryberg M. The effects of moderate protein deficiency on β-adrenoceptor density in rat parotid and submandibular salivary glands. Arch. Oral Biol. 1991;36:591–594. doi: 10.1016/0003-9969(91)90109-8. [DOI] [PubMed] [Google Scholar]
- Kagami H. O'Connell B.C. Baum B.J. Evidence for the systemic delivery of a transgene product from salivary glands. Hum. Gene Ther. 1996;7:2177–2184. doi: 10.1089/hum.1996.7.17-2177. [DOI] [PubMed] [Google Scholar]
- Kagami H. Atkinson J.C. Michalek S.M. Handelman B. Yu S. Baum B.J. O'Connell B. Repetitive adenovirus administration to the parotid gland: Role of immunological barriers and induction of oral tolerance. Hum. Gene Ther. 1998;9:305–313. doi: 10.1089/hum.1998.9.3-305. [DOI] [PubMed] [Google Scholar]
- Kay M.A. Landen C.N. Rothenberg S.R. Taylor L.A. Leland F. Wiehle S. Fang B. Bellinger D. Finegold M. Thompson A.R. Read M. Brinkhous K.M. Woo S.L.C. In vivo hepatic gene therapy: Complete albeit transient correction of factor IX deficiency in hemophilia dogs. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2353–2357. doi: 10.1073/pnas.91.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper-Lenstra A.H. Kramer M.F. Van Venroij W.J. Rate of protein synthesis in rat salivary gland cells after pilocarpine or feeding. II. Protein synthesis in vivo in cells of the submandibular and parotid gland after secretion. Cell Tissue Res. 1975;164:447–456. doi: 10.1007/BF00219936. [DOI] [PubMed] [Google Scholar]
- Marshall E. Clinical research: FDA halts all gene therapy trials at Penn. Science. 2000;287:565. [PubMed] [Google Scholar]
- Medarov B.I. Milk-alkali syndrome. Mayo Clin. Proc. 2009;84:261–267. doi: 10.4065/84.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez P. Rowzee A.M. Zheng C. Adriaansen J. Baum B.J. Salivary epithelial cells: An unassuming target for gene therapeutics. Int. J. Biochem. Cell Biol. 2010;42:773–777. doi: 10.1016/j.biocel.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K.E.S. Reeve J. Parathyroid hormone: A bone anabolic and catabolic agent. Curr. Opin. Pharmacol. 2005;5:612–617. doi: 10.1016/j.coph.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Potts J.T. Parathyroid hormone: Past and present. J. Endocrinol. 2005;187:311–325. doi: 10.1677/joe.1.06057. [DOI] [PubMed] [Google Scholar]
- Proctor G.B. Shori D.K. Preedy V.R. Protein synthesis in the major salivary glands of the rat and the effects of re-feeding and acute ethanol injection. Arch. Oral Biol. 1993;38:971–978. doi: 10.1016/0003-9969(93)90110-8. [DOI] [PubMed] [Google Scholar]
- Shan Z. Lim J. Zheng C. Liu X. Fan Z. Zhang C. Goldsmith C.M. Wellner R.B. Baum B.J. Wang S. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol. Ther. 2005;11:444–451. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Shoback D. Hypoparathyroidism. N. Engl. J. Med. 2008;359:391–403. doi: 10.1056/NEJMcp0803050. [DOI] [PubMed] [Google Scholar]
- Sramkova M. Masedunskas A. Parente L. Molinolo A. Weigert R. Expression of plasmid DNA in the salivary gland epithelium: Novel approaches to study dynamic cellular processes in live animals. Am. J. Physiol. Cell Physiol. 2009;297:C1347–C1357. doi: 10.1152/ajpcell.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal A. Powers K. Milk-alkali syndrome induced by 1,25(OH)2D in a patient with hypoparathyroidism. J. Natl. Med. Assoc. 1996;88:313–314. [PMC free article] [PubMed] [Google Scholar]
- Usatii M. Rousseau L. Demers C. Petit J.L. Brossard J.H. Gascon-Barre M. Lavigne J.R. Zahradnik R.J. Nemeth E.F. D'amour P. Parathyroid hormone fragments inhibit active hormone and hypocalcemia-induced 1,25(OH) 2D synthesis. Kidney Int. 2007;72:1330–1335. doi: 10.1038/sj.ki.5002532. [DOI] [PubMed] [Google Scholar]
- Voutetakis A. Kok M.R. Zheng C.Y. Bossis I. Wang J.H. Cotrim A.P. Marracino N. Goldsmith C.M. Chiorini J.A. Loh Y.P. Nieman L.K. Baum B.J. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc. Natl. Acad. Sci. U.S.A. 2004a;101:3053–3058. doi: 10.1073/pnas.0400136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutetakis A. Wang J. Baum B.J. Utilizing endocrine secretory pathways in salivary glands for systemic gene therapeutics. J. Cell. Physiol. 2004b;199:1–7. doi: 10.1002/jcp.10429. [DOI] [PubMed] [Google Scholar]
- Voutetakis A. Zheng C. Mineshiba F. Cotrim A.P. Goldsmith C.M. Schmidt M. Afione S. Roescher N. Metzger M. Eckhaus M.A. Chiorini J.A. Dunbar C.E. Donahue R.E. Baum B.J. Adeno-associated virus serotype 2-mediated gene transfer to the parotid glands of nonhuman primates. Hum. Gene Ther. 2007;18:142–150. doi: 10.1089/hum.2006.154. [DOI] [PubMed] [Google Scholar]
- Voutetakis A. Zheng C. Metzger M. Cotrim A.P. Donahue R.E. Dunbar C.E. Baum B.J. Sorting of transgenic secretory proteins in rhesus macaque parotid glands after adenovirus-mediated gene transfer. Hum. Gene Ther. 2008;19:1401–1405. doi: 10.1089/hum.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Voutetakis A. Mineshiba F. Illei G.G. Dang H.W. Yeh C.K. Baum B.J. Effect of serotype 5 adenoviral and serotype 2 adeno-associated viral vector-mediated gene transfer to salivary glands on the composition of saliva. Hum. Gene Ther. 2006a;17:455–463. doi: 10.1089/hum.2006.17.455. [DOI] [PubMed] [Google Scholar]
- Wang J. Voutetakis A. Papa M. Rivera V.M. Clackson T. Lodde B.M. Mineshiba F. Baum B.J. Rapamycin control of transgene expression from a single AAV vector in mouse salivary glands. Gene Ther. 2006b;13:187–190. doi: 10.1038/sj.gt.3302647. [DOI] [PubMed] [Google Scholar]
- Wells H. Peronace A.A. Functional hypertrophy and atrophy of the salivary glands of rats. Am. J. Physiol. 1967;212:247–251. doi: 10.1152/ajplegacy.1967.212.2.247. [DOI] [PubMed] [Google Scholar]
- Wen J.M. Zhong Y.H. Sun Y. Huang Z. Zhang L.W. Wu Q.L. Zeng D.C. pcDPG parathyroid hormone gene therapy of hypoparathyroidism: An experimental study. Zhonghua Yi Xue Za Zhi. 2006;86:260–265. [PubMed] [Google Scholar]
- Winer K.K. Yanovski J.A. Cutler G.B. Synthetic human parathyroid hormone 1–34 vs calcitriol and calcium in the treatment of hypoparathyroidism: Results of a short-term randomized crossover trial. JAMA. 1996;276:631–636. [PubMed] [Google Scholar]
- Winer K.K. Yanovski J.A. Sarani B. Cutler G.B. A randomized, crossover trial of once-daily versus twice-daily parathyroid hormone 1–34 in treatment of hypoparathyroidism. J. Clin. Endocrinol. Metab. 1998;83:3480–3486. doi: 10.1210/jcem.83.10.5185. [DOI] [PubMed] [Google Scholar]
- Winer K.K. Ko C.W. Reynolds J.C. Dowdy K. Keil M. Peterson D. Gerber L.H. McGarvey C. Cutler G.B., Jr. Long-term treatment of hypoparathyroidism: A randomized controlled study comparing parathyroid hormone(1–34) versus calcitriol and calcium. J. Clin. Endocrinol. Metab. 2003;88:4214–4220. doi: 10.1210/jc.2002-021736. [DOI] [PubMed] [Google Scholar]
- Winer K.K. Sinaii N. Peterson D. Sainz B. Cutler G.B., Jr. Effects of once versus twice-daily parathyroid hormone 1–34 therapy in children with hypoparathyroidism. J. Clin. Endocrinol. Metab. 2008;93:3389–3395. doi: 10.1210/jc.2007-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. Perrotte P. Onishi E. Harper M.E. Dinney C. Pagliaro L. Wilson D.R. Biodistribution of an adenoviral vector carrying the luciferase reporter gene following intravesical or intravenous administration to a mouse. Cancer Gene Ther. 1999;6:367–372. doi: 10.1038/sj.cgt.7700090. [DOI] [PubMed] [Google Scholar]
- Zheng C. Vitolo J.M. Zhang W. Mineshiba F. Chiorini J.A. Baum B.J. Extended transgene expression from a nonintegrating adenoviral vector containing retroviral elements. Mol. Ther. 2008;16:1089–1097. doi: 10.1038/mt.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C. Nikolov N.P. Alevizos I. Cotrim A.P. Liu S. McCullagh L. Chiorini J.A. Illei G.G. Baum B.J. Transient detection of E1-containing adenovirus in saliva after the delivery of a first-generation adenoviral vector to human parotid gland. J. Gene Med. 2010;12:3–10. doi: 10.1002/jgm.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. Lu B.J. Xu P. Song C.F. Optimising gene therapy of hypoparathyroidism with hematopoietic stem cells. Chinese Med. J. 2005;118:204–209. [PubMed] [Google Scholar]
- Zierold C. Mings J.A. Deluca H.F. Parathyroid hormone regulates 25-hydroxyvitamin D3-24-hydroxylase mRNA by altering its stability. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13572–13576. doi: 10.1073/pnas.241516798. [DOI] [PMC free article] [PubMed] [Google Scholar]