Abstract
INTRODUCTION
This is a 7-year retrospective review summarising the North of England Bone and Soft Tissue Tumour Service's experience of managing 13 cases of groin sarcoma requiring soft tissue flap reconstruction. This study was performed to try to identify where national referral guidelines in sarcoma management had been followed and reasons for any delays. The study also includes outcome data relating to these patients.
PATIENTS AND METHODS
A retrospective, case-note review was undertaken using the local sarcoma database to identify approriate patients.
RESULTS
In nine patients, national referral guidelines were not followed. This resulted in a mean delay of presentation to the multidisciplinary team of 4.4 months. Ten patients had unplanned excision or exploration of tumours before referral. There were no lower limb amputations. All patients with narrow margins or high grade tumours were referred for radiotherapy. Four patients died; three as a result of distant metastases and one as a result of local recurrence.
CONCLUSIONS
Despite delays in referral, treatment by wide excision and plastic surgical reconstruction allowed for local control of these tumours with functional limb salvage. Implementation of National Institute for Health and Clinical Excellence (NICE) guidelines and local strategies could improve the expedient management of these patients.
Keywords: Sarcoma, Groin, Reconstruction, Diagnosis, Referral
Groin sarcoma management is a challenge which should ideally be met by a dedicated multidisciplinary team in order to achieve the best results for patients.
Achieving complete resection margins in recurrent or persistent groin sarcoma is difficult. These tumours may be bulky, they develop in constrained anatomical areas where specific structures such as the femoral vessels, the anterior abdominal wall, the anterior urethra and the contralateral testis may be involved and can add to the complexity of surgery.1 In addition, there is often surgical scarring from previous attempts to resect the tumour and from the effects of radiotherapy.2,3
Amputation is occasionally necessary to resect these tumours completely;4 more recently, limb salvage surgery has been considered preferable because it is associated with lower morbidity. Also, when limb salvage is combined with radiotherapy, equivalent survival rates can be achieved.5
The reconstruction following extirpation of recurrent groin sarcoma usually requires restoration of abdominal wall integrity and a fasciocutaneous or musculocutaneous flap for durable and reliable soft tissue cover, thus enabling wide macroscopic margins to be taken.
Groin sarcoma surgery is acknowledged to be an influence on functional outcome, possibly more than other anatomical sites, which is likely to be due to the reduction in hip flexion postoperatively.6 This can have an effect on the patient's mobility. Successful reconstruction is, therefore, an important factor in the early rehabilitation of the patient.
Patients and Methods
All patients requiring plastic surgery intervention for groin reconstruction following sarcoma resection were included in this single centre, retrospective review. Details were retrieved from the Northern Regional Sarcoma Service Database; 13 patients were identified in the period 1 January 2001 to 1 January 2008. The patient demographics, referral patterns, histological types, resection margins and the type of the reconstructions performed were analysed. Information was also gathered regarding adjuvant therapy, recurrences and survival.
Results
Demographics
Thirteen patients met the entry criteria. Ten patients were male and the mean age at the time of surgery was 59 years (range, 18–80 years). The majority of patients presented initially to their general practitioner with a groin or inguinal mass (7 patients), four with a scrotal swelling and in two no details were available.
Five patients were referred with recurrent disease, seven were referred after biopsy or incomplete excision and one patient had treatment of primary disease entirely within the regional centre. All 12 patients referred from other specialities (general surgery and urology) underwent some type of biopsy before referral; 10 of these were incomplete at the time of referral.
There was a delay of between 1–12 months (mean, 4.4 months) after biopsy, before referral to the regional centre.
All patients underwent pre-operative staging investigations within the multidisciplinary team and none were shown to have distant metastasis at the time of surgery.
The mean follow up period recorded for these patients was 30 months (range, 4–81 months).
Operative management
Surgical excision of groin sarcomas is most often performed via an abdomino-inguinal incision, incorporating the biopsy scar, tumour and any involved structures, such as the abdominal wall, spermatic cord and testis. There were no resections of femoral vessels or femoral nerve in this series.
Flap coverage was required to allow for radical excision of the tumour with the recommended 3–5 cm macroscopic margin and achieve wound closure without tension. Flaps were chosen based on the defect size and position, medical condition of the patient and the preference of the surgeon. Pedicled flaps were used exclusively. The most frequently used option was a vertically orientated rectus abdominis musculocuta-neous (VRAM) flap (Figs 1–3). The VRAM flap was used in 10 patients, gracilis flaps were used in two patients and a fascio-cutaneous transposition flap in one patient.
Figure 1.
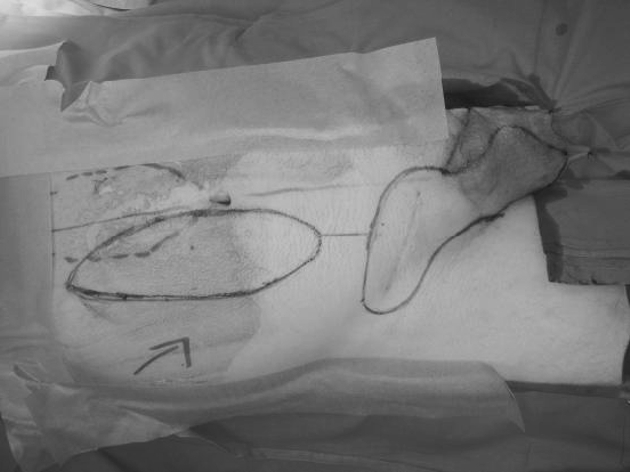
Pre-operative appearance of patient with right groin sarcoma recurrence. Intended excision margins are marked in inguino-scrotal region, incorporating previous scar. Markings for VRAM flap incision present on the abdomen.
Figure 3.
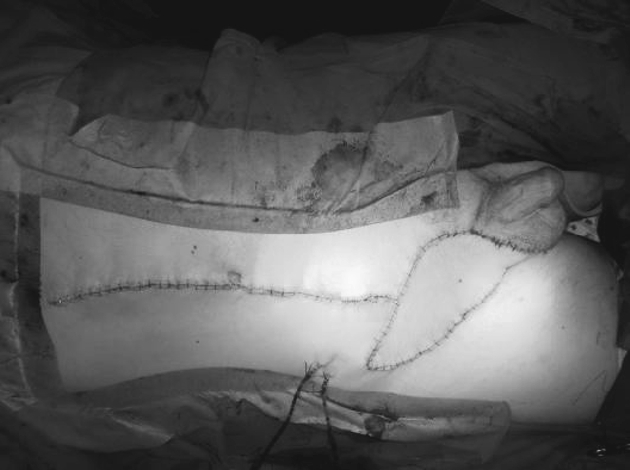
Completed VRAM flap groin reconstruction. Flap inset and donor site closed over suction drains.
Figure 2.

Sarcoma excised including ipsilateral testis and anterior abdominal wall at deep margin. VRAM flap is raised. Prolene mesh beneath flap is used to reconstruct the anterior abdominal wall defect.
Abdominal wall repair was required in six cases and was performed using polypropylene mesh or acellular porcine dermis sheet.
There were seven complications in six patients, these were; seroma (three), wound breakdown (two), haematoma (one) and lymphoedema (one).
Histopathology
The histopathology of all tumours is summarised in Table 1. Complete resection was achieved in seven patients, narrow or marginal resection was reported in four patients and two patients had an incomplete excision. The involved margins were posterior in a dermatofibromasarcoma protuberans and deep adjacent to the femoral vein in a malignant fibrous histiocytoma. These patients underwent further procedures to achieve complete margins.
Table 1.
Histopathology of groin sarcomas
| Histological subtype | High or low grade | Size | Superficial or deep |
|---|---|---|---|
| Dedifferentiated liposarcoma | High grade | ∼60 mm | Deep – surrounding cord structures and testis |
| Epithelioid sarcoma, proximal type | High grade | 50 × 35 mm | Deep – recurrence found in cord and testis |
| Epithelioid sarcoma of proximal type | High grade | 85 × 65 × 60 mm | Deep – fungating through scrotum |
| Dedifferentiated liposarcoma | High grade | 130 × up to 109 × up to 60 mm | Deep |
| Leiomyosarcoma | Trojani grade 2 | 170 × 130 × up to 100 mm | Deep |
| Leiomyosarcoma | Trojani grade 2 | 35 mm | Deep |
| De-differentiated sarcoma of para-testicular tissues, of uncertain type | Trojani grade 3 | 110 × 80 × 65 mm | Deep |
| Dermatofibroma sarcoma protuberans | Not designated | 40 mm | Superficial |
| Synovial sarcoma | High | 35 mm | Superficial |
| Dedifferentiated liposarcoma | High | 65 mm | Deep |
| Sclerosing liposarcoma | Low | ∼75 mm | Deep |
| Dedifferentiated liposarcoma | High | 120 × 90 × 60 mm | Deep – spermatic cord and testis |
| Chondrosarcoma | Some areas Trojani grade 3 | 45 × 42 × 38 mm | Deep – pelvic |
All patients with narrow margins were referred for radiotherapy as were the majority of patients with high-grade tumours. Six patients received postoperative radiotherapy. A further three patients were offered radiotherapy but refused treatment.
Clinical outcome
There were four mortalities in the patients in this review of which only one had local recurrence. The patient with local recurrence was too frail for further treatment and succumbed to his disease. The three other mortalities were as a direct result of metastatic sarcoma. The mean time to presentation with metastasis was 11 months (range, 6–19 months).
Discussion
Thirteen is a small cohort for such a review, but this is a specific group who have all undergone groin sarcoma excision and local flap reconstruction. Some of the largest published series of groin sarcomas include details of only 20 and 12 patients, respectively, who have required similar reconstructive procedures.1,7 This number does not allow a statistical analysis to be performed but does highlight some of the management difficulties encountered when dealing with this condition.
Diagnosis
It is recognised that the diagnosis of groin sarcoma is complicated by the fact that sarcoma is probably the least likely explanation for a lump in the groin or inguinal region. Five patients in this series were initially diagnosed with a ‘hernia’ and underwent herniorrhaphy before the correct diagnosis was realised. Sarcomas may be misdiagnosed,8,9 in this way, or may co-exist with hernias10 in the form of a cord liposarcoma. Less than 0.1% of hernia operations are considered to be complicated by this phenomenon.11 Other reports suggest that unexpected tumours are found in only 0.00098% of specimens from hernia operations.12.
Pre-operative clinical findings of fixed or rubbery masses at the deep inguinal ring might prompt further investigation, as this seems to be a common feature in the cases accidentally explored in this series.
If an incidental tumour is discovered, the correct management is to biopsy the lesion (either incisional or excisional biopsy), complete the intended procedure and refer promptly to the regional centre for further advice.1 Nine of the patients in this review were not managed according to these guidelines.
Complications
Complication rates for extensive surgical procedures in the groin are substantial. Large series of lower limb sarcoma surgery report wound complication rates of 27–32 %;2,3 however, these are studies of a very heterogeneous patient mix. An overall complication rate of 68% has been quoted,7 in a cohort more analogous to the patients in this review and compares favourably with our results. Seroma is a frequent postoperative problem for this anatomical region, even routine inguinal lymph node dissection is followed by a high seroma rate of 5–16%.14,15 The 23% seroma rate in our series would, therefore, seem to be acceptable.
Referral
The National Institute for Health and Clinical Excellence (NICE) has set out guidelines for the management of patients with soft tissue sarcoma, with particular reference to correct organisation of services and referral processes.13 These represent a wide-ranging and robust means of ensuring patients are referred expediently to the sarcoma treatment centre and are applicable to primary and secondary health care settings.
Outcome
This is a group of patients with inevitably adverse prognostic features; recurrent (five) or persistent (seven) disease, deeply situated (eleven) and of high grade (eleven), presenting for radical resection and complex reconstruction. It is arguable that patients with such extensive tumours of the groin as these are essentially undergoing palliative procedures. The primary objectives are to remove the tumour before it erodes into the femoral vessels or develops into a fungating mass, and to maintain good quality of life with limb salvage and early rehabilitation.7 By these criteria, success was achieved: limb salvage was 100%, all patients were mobilised with satisfactory soft tissue reconstruction and there were no peri-operative deaths. However, the high late mortality rates reported here and by others, are sobering.1,7
Conclusions
Despite considerable surgical challenges in management, it could be argued that the greatest challenge of all remains the persistent delays associated with referral of these patients to the regional centre. The mean time to referral after biopsy (4.4 months) seems to be unaccountable time wasted. These patients were not designated to a UK Government cancer target ‘pathway’ for the reason that most were not identified as having sarcoma until after the incidental biopsy was performed or that these targets simply had not been introduced.
The implementation of cancer targets and NICE referral guidelines should improve the expediency of service for patients with groin sarcoma. However, patients may still remain undiagnosed for some time due to the nature of the condition. Attempting to increase levels of awareness in the primary health care setting may be valuable to promote correct referral procedures.
It remains to be seen if referral times are reduced in the future as a result of these strategies and if this has an influence on the outcome for this group of patients.
References
- 1.Brooks AD, Bowne WB, Delgado R, Leung DH, Woodruff J, et al. Soft tissue sarcoma of the grodiagnosis, management and prognosis. J Am Coll Surg. 2001;193:130–6. doi: 10.1016/s1072-7515(01)00982-6. [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP, Ballo MT, Zagars GK, Mirza AN, Lin PP, et al. Complications of combined modality treatment of primary lower extremity soft tissue sarcomas. Cancer. 2006;107:2455–61. doi: 10.1002/cncr.22298. [DOI] [PubMed] [Google Scholar]
- 3.Tseng JF, Ballo MT, Langstein HN, Wayne JD, Cormier JN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13:1209–15. doi: 10.1245/s10434-006-9028-6. [DOI] [PubMed] [Google Scholar]
- 4.Shiu MH, Castro EB, Hajdu SI, Fortner JG. Surgical treatment of 297 soft-tissue sarcoma of the lower extremity. Ann Surg. 1975;182:597–602. doi: 10.1097/00000658-197511000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, et al. The treatment of soft-tissue sarcoma of the extremities: prospective randomised evaluation of (1) limb sparing surgery plus radiation therapy combined with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–15. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerrand CH, Wunder JS, Kandel RA, O'Sullivan B, Catton CN, et al. The influence of anatomical location on functional outcome in lower extremity soft-tissue sarcoma. Ann Surg Oncol. 2004;11:476–82. doi: 10.1245/ASO.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Reece GP, Gillis TA, Pollock RE. Lower extremity salvage after radical resection of malignant tumours in the groin and lower abdominal wall. J Am Coll Surg. 1997;185:260–7. doi: 10.1016/s1072-7515(97)00044-6. [DOI] [PubMed] [Google Scholar]
- 8.Bell RS, O'Sullivan B, Mahoney JL, Nguyen C, Langer F, Catton C. The inguinal sarcoma: a review of five cases. Can J Surg. 1990;33:309–12. [PubMed] [Google Scholar]
- 9.Geelhoed GW, Millar RC, Ketcham AS. Hernia presentation of cancer in the groin. Surgery. 1974;75:436–41. [PubMed] [Google Scholar]
- 10.Fieber SS, Wolstenholme JT. Primary tumors in inguinal hernial sacs. Arch Surg. 1955;71:254–6. doi: 10.1001/archsurg.1955.01270140102018. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery E, Buras R. Incidental liposarcomas identified during hernia repair operations. J Surg Oncol. 1999;71:50–3. doi: 10.1002/(sici)1096-9098(199905)71:1<50::aid-jso10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Kassan MA, Munoz E, Laughlin A, Margolis IB, Wise L. Value of routine pathology in herniorrhaphy performed upon adults. Surg Gynecol Obstet. 1986;163:518–22. [PubMed] [Google Scholar]
- 13.National Institute for Health and Clinical Excellence. Improving Outcomes for People with Sarcoma: Guidance Recommendations. London: NICE; 2006. [Google Scholar]
- 14.Karakousis CP, Driscoll DL. Groin dissection in malignant melanoma. Br J Surg. 1994;81:1771–4. doi: 10.1002/bjs.1800811221. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DE, Lo RK. Complications of groin dissection in penile cancer: experience with 101 lymphoadenectomies. Urology. 1984;24:312–4. doi: 10.1016/0090-4295(84)90198-5. [DOI] [PubMed] [Google Scholar]


