Abstract
INTRODUCTION
Retrograde transpopliteal angioplasty (PA) is a potentially useful alternative technique for endovascular treatment of infra-inguinal arterial disease when antegrade transfemoral puncture (FA) is technically not possible or appropriate. This study aimed to investigate the outcomes of PA compared with FA during a 5-year period.
PATIENTS AND METHODS
A retrospective study was performed to assess 88 PA and 275 FA performed between January 2003 and January 2008. Assessments of patients, indication for procedure, disease site, stenosis severity, procedure outcomes and time to further intervention were recorded.
RESULTS
FA was used to treat more patients with critical ischaemia (42.2% vs 30.7%; P = 0.014)). PA was used to treat more proximal superficial femoral lesions (P < 0.001) and occlusive lesions (P = 0.001). Overall, 84.1% of PA and 82.5 % of FA were technically successful. There was no difference in local puncture site complication rates. Significantly more FA resulted in distal thrombus/embolism (8.4% vs 3.4%; P = 0.044). Further intervention was required in 27.3% of PA and 36.0% of FA. The time interval to re-intervention was not different between the groups.
CONCLUSIONS
PA is safe with comparable success rates and long-term outcomes to an FA. PA is a useful alternative approach or treating occlusive, proximal disease.
Keywords: Angioplasty, Access site, Popliteal artery, Long-term patency, Complications
Endovascular treatment of peripheral arterial disease is commonly performed using an antegrade, transfemoral approach via the common femoral artery (FA).1 An alternative technique is to use a retrograde, transpopliteal approach obtaining access via the ipsilateral popliteal artery (PA). First described in 1988,2 indications for PA include: (i) an absent femoral pulse secondary to an iliac or common femoral occlusion; (ii) severe calcification; (iii) combined iliac and femoral lesions; (iv) occlusion or a high take-off of the origin of the superficial femoral artery; and (v) severe obesity. PA can also be used to avoid scar tissue and when femoral angioplasty has failed.3–6 However, concerns have been expressed regarding PA puncture site complications.4,7 and there are no published data comparing long-term outcomes between the two approaches. The aim of this study was to compare the immediate and long-term results of PA with FA.
Patients and Methods
Data were collected retrospectively on all patients who underwent PA and or FA between January 2003 and January 2008 in our institution. Angioplasties were performed by three interventional radiologists. Intervention was planned at weekly multidisciplinary meetings. Clinical indications included symptomatic peripheral arterial disease with severe claudication, ischaemic rest pain, ischaemic ulcera-tion, or ischaemic tissue loss/gangrene (Rutherford stages 3–6).8 The arterial approach used to perform angioplasty was determined on a case-by-case basis according to clinical findings and the results of arterial imaging. Indications for PA are documented in Table 1.
Table 1.
Indications used for performing retrograde trans-popliteal angioplasty
| Difficult femoral artery access |
| • Impalpable femoral pulse |
| • Heavily calcified femoral artery |
| • Graft prosthesis |
| • High femoral bifurcation |
| • Groin scar tissue |
| • Obesity |
| Arterial lesion anatomy |
| • Flush SFA occlusion |
| • Large collaterals arising from occlusion point |
| • Ipsilateral combined lesions, e.g. femoral with either iliac or CFA lesions |
| Failed femoral approach |
Technique
All patients had intravenous digital subtraction angiography (IVDSA) or magnetic resonance angiography (MRA) prior to procedure. Intentional subintimal angioplasty was performed for occlusions, and in multiple diffuse stenoses.9
Procedures were performed under local anaesthetic (5 ml of 1% lignocaine) with or without intravenous sedation (Diazemuls 2–10 mg). PA patients were placed prone and an ultrasound scan was used to identify the popliteal artery and its relation to the popliteal vein. The popliteal artery was punctured just above the knee joint line using a Doppler ultrasound ‘Smart’ needle (Cardiovascular Dynamics, Irvine, CA, USA). A standard 0.035 inch 1.5 mm J guide-wire (Cook, Bjaeverkov, Denmark) and 5Fr Van Andel catheter (Cook) were then introduced retrogradely into the popliteal artery. FA patients were placed supine and the common femoral artery was punctured below the inguinal ligament using fluoroscopic guidance. A standard J guide-wire and either a 5Fr or 6Fr Van Andel catheter were then introduced antegradely. Both procedures were similar following guide-wire introduction.
Angiographic images were obtained and 3000 units of heparin given intra-arterially. The first attempt to cross the lesion(s) either transluminally or subintimally was made with the guide-wire/catheter combination. If unsuccessful, the guide wire was exchanged for an angled hydrophilic guide-wire (Terumo guide-wire and 5-F Cobra 2 glide catheter; Terumo Corp. Tokyo Japan). After crossing the lesion and confirming the luminal position, dilatation was undertaken using an appropriately sized balloon catheter (4–7 × 40 mm Smash balloon catheters; Boston Scientific Ltd, Natick, USA). A post-procedure angiogram was then performed and balloon dilation repeated for any residual stenosis greater than 30%. Catheters and guide-wires were removed and haemostasis achieved by hand. If there was distal embolisation, a 24∧18 h heparin infusion was administered and suction embolectomy was attempted at time of procedure. Technical success was defined as restored patency with no stenosis greater than 30% as per reporting standards.10 A poor result was defined as improvement in patency but with stenosis greater than 30% whilst failure was defined as no change in patency. All patients were prescribed a daily dose of 75 mg aspirin to be taken indefinitely post-procedure.
Follow-up
Patients were routinely reviewed 1 day and 6 weeks post-procedure. Follow-up patency was assessed on a symptomatic basis only. If symptoms persisted or returned, patients were re-imaged with IVDSA or MRA. Long-term outcomes were measured using length of time to the end of primary patency. The end of primary patency being defined as when any procedure that might preserve or extend patency or performed as described by the standards of Ahn et al.10 Length of time to further intervention (radiological or surgical) and deaths were recorded. Patency rates stated are primary patency rates. The study was approved by the local ethics committee.
Statistical analysis
Statistical analyses were performed using Pearson's chi-squared test and Mann-Whitney U-test for unpaired two group non-parametric discrete and continuous data, respectively. The Kaplan–Meier method was used to create probability curves and analyse length of time to further vascular intervention. Probability curves were compared using a log-rank (Mantel–Cox) test. Multivariate analysis was performed to assess the significance of independent factors upon time to further intervention by calculating hazard ratios and 95% confidence intervals in a Cox proportional hazards model. Statistical significance was set at P < 0.05. All data were analysed using SPSS v.11.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 326 patients were treated: 88 PA were performed on 83 patients; 275 FA were performed on 243 patients. Of total angioplasties performed, 24.2% were PA. Table 2 summarises patient demographics and clinical indications for intervention. There was no significant difference in demographics between the groups. Five-year mortality was not significantly different between PA and FA patients (19.3% and 21.1%, respectively). Arterial disease risk factor demographics were typical of any series of patients with peripheral arterial disease. Significantly more FA were performed for critical ischaemia (P = 0.020). There was no significant difference in procedural success rates when comparing severe claudicants with those with critical ischaemia.
Table 2.
Patient demographics and clinical indication for angioplasty in popliteal and femoral approach angioplasty groups?
| Popliteal angioplasty | Femoral angioplasty | P-value | |
|---|---|---|---|
| Number of procedures | 88 | 275 | |
| Number of patients | 83 | 243 | |
| Age median (mean, SD) | 70.5(70.6, 9.4) | 72.0(71.9, 10.8) | 0.13* |
| Sex(M:F) | 47:41 | 154:121 | 0.76** |
| Race | |||
| (Caucasian: AfroCarri bean: Asian) | 81:3:4 | 258:6:11 | 0.57* |
| Clinical indication | |||
| (Cluadicationcritical ischaemia) | 61:27 | 159:116 | 0.02* |
| Critical ischaemia | |||
| (Rest pain:ulceration:tissue necrosis/gangrene) | 5:17:5 | 35:62:19 |
Mann–Whitney U-test
Pearson's chi-squared test.
Table 3 demonstrates the distribution of atherosclerotic lesions and the incidence of occlusive or stenotic lesions. The proximal SFA was more frequently treated in the PA group (21.6% [PA] vs 8.4% [FA];P = 0.001). Occlusive lesions were more frequently treated using PA (51.1% [PA] vs 33.5% [FA]; P = 0.004). In both groups there was the potential at angioplasty to treat multiple lesions; however, results detailed include only the significant lesions treated. Occlusion lengths ranged from 1–16 cm (median, 4.5 cm) with no significant difference between the two groups.
Table 3.
Site distribution of atherosclerotic arterial lesions and lesion type (occlusive or stenotic) in popliteal and femoral approach angioplasty groups
| Popliteal angioplasty | Femoral angioplasty | P-value* | |
|---|---|---|---|
| Lesion site | |||
| Proximal SFA | 19(21.6%) | 23 (8.4%) | 0.001 |
| Distal SFA | 46 (52.3%) | 133 (48.4%) | 0.606 |
| Multiple SFA | 18 (20.5%) | 73 (26.5%) | 0.314 |
| CIA | 1 (1.1%) | 2 (0.7%) | 0.712 |
| EIA | 1 (1.1%) | 1 (0.4%) | 0.980 |
| Graft | 3 (3.4%) | 26 (9.7%) | 0.111 |
| Tibio/peroneal | 0 (0%) | 11 (4.1%) | 0.121 |
| Lesion type | |||
| Occlusion :stenosis | 45:43 | 92:183 | 0.004 |
Pearson's chi-squared test.
Proximal SFA = proximal one-third of SFA.
Distal SFA = distal one-third of SFA.
PA was successful in 74 cases (84.1%), seven (8%) had poor results and seven (8 %) failed. FA was successful in 227 cases (82.5%), 18 (6.5%) had poor results and25 (9.1%) failed. The most common reasons for technical failure in both PA and FA were inability to cross the arterial lesion or an inability to break back into the native lumen due to a calcified plaque.
Complications following PA angioplasty included puncture site haematoma in two cases and distal embolisation in three cases (treated with heparinisation plus suction embolectomy in one case). Following FA there were six cases of puncture site haematoma and 24 cases of distal embolisation/thrombus which required heparinisation. Suction embolectomy was performed in 14 cases. Significantly more FA patients suffered postoperative distal embolisation/thrombus (P = 0.044).
Further surgical or radiological intervention was required in 25 (28.4%) PA and 97 (35.3%) FA. Thirteen PA cases required surgical intervention (8 bypass procedures, 5 distal amputations) and 12 had repeat angioplasty (2 PA). Forty-four FA cases required surgical intervention (17 bypass procedures, 5 endarterectomies and 22 distal amputations) and 53 underwent repeat angioplasty (3 PA).
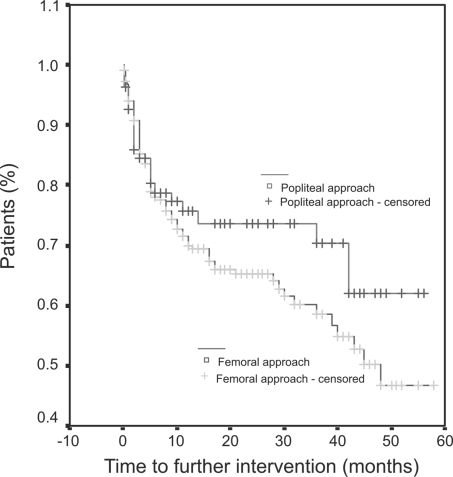
The median length of time to further intervention post PA was 12 months (mean, 19.46 ± 17.61 months) and post FA was 12 months (mean, 16.75 ± 15.11 months). There was no significant difference in time to further intervention between the two groups (Fig. 1). Patients with critical ischaemia had a significantly shorter time interval to further intervention than those treated for severe claudication (P = 0.003). Patients with occlusive lesions had a significantly shorter time to further intervention compared to patients with stenotic lesions (P = 0.007). Lesion site and patient demographics did not significantly affect time to further intervention. Multivariate analysis demonstrated clinical indication (critical ischaemia) and lesion type (occlusive) significantly influenced time to further intervention (P = 0.005 andP = 0.008, respectively; Table 4).
Figure 1.
Results of survival analysis (Kaplan–Meier method) assessing time to further vascular intervention (long-term patency) according to angioplasty approach.
Table 4.
Multivariate analysis of to assess independent factors influence upon time to further vascular intervention
| Regression coefficient | SE | Hazard ratio | P-value* | |
|---|---|---|---|---|
| Angioplasty approach (popliteal/femoral) | 0.90 | 0.254 | 1.27 (0.77, 2.10) | 0.342 |
| Clinical indication (claudication/critical ischaemia) | 7.00 | 0.206 | 1.72 (1.15, 2.58) | 0.008 |
| Lesion site | 1.18 | 0.066 | 1.07 (0.94, 1.22) | 0.174 |
| Lesion type (occlusive/stenosis) | 7.86 | 0.205 | 0.56 (0.38, 0.84) | 0.005 |
Values in parentheses are 95% confidence intervals.
Cox proportional hazards model.
Discussion
PA treatment of iliofemoral lesions has been demonstrated to be a useful alternative to FA,11 increasing the number of femoral artery occlusions considered technically feasible for angioplasty by about one-fifth.12 However, PA remains a relatively infrequently used technique, possibly as a result of anecdotal concerns regarding puncture site complications.13
To date, outcomes have only been published from consecutive PA series or case reports with no direct comparisons to FA and long-term PA outcomes not specifically assessed.1–6,11,13 PA is used in a significant proportion (24.2%) of our total angioplas-ties and this study presents our experience of the technique. In an attempt to minimise experimental bias within this retrospective study, all PA and FA performed within the research period were analysed. Although selection criteria were used to select PA cases, we believe the wide range of lesions treated between both groups ensured that an appropriate comparison could be made.
This study demonstrates that PA can be performed safely with a high rate of technical success.3,13 It is noted that a relatively small number of infragenicular interventions were performed within this study. However, both the FA success rates and FA long-term patency rates (in terms of symptomatic control and requirement for further vascular intervention) are within accepted published ranges.14,15 This, therefore, enables a valid comparison of PA to FA to be made with similar rates of technical success and matched long-term outcomes identified. In this study, primary patency was assessed by clinical need for further intervention, which is ultimately the most important factor in determining the success of any intervention. It is accepted that objective, radiological measurements of patency would have provided further information to compare the techniques but was not possible due to the retrospective nature of the study.
It has been previously demonstrated that long-term patency rates are significantly reduced after treating critical ischaemic cases as opposed claudicants16 plus that long-term patency rates are significantly worse following treatment of occlusive lesions compared to stenotic ones.17 Both these findings were demonstrated in our current research; indeed, multivariate analysis identified only critical ischaemia and occlusive lesions to have a significant impact upon long-term outcomes. The arterial approach used for angioplasty had no influence and it can be concluded that PA does not impact upon clinical long-term patency.
Concerns have been highlighted with regards to the incidence of local complications following PA. The formation of arteriovenous fistulas has had a reported incidence as high as 14%;4 puncture site arterial dissection or thrombosis and per-oneal nerve palsy secondary to haematoma have also been described.7 In this study, the popliteal artery was punctured using the Doppler ultrasound ‘Smart’ needle. This technique has been demonstrated to be safe and requires less effort, preparation and skill to perform compared to other visualisation techniques.3 Only two local haematomas and no fistulas found in this study helps confirm the safety of this technique.
Similar to other institutions, instances of distal embolisa-tion/thrombus rates were less common following PA.18 This might be explained by the retrograde fashion of inserting the catheter and guide wire, making it potentially harder for emboli to travel distally. It is important to note that the treatment of distal embolisation/thrombus following PA is more complex as it is not always possible to perform aspiration embolectomy or thrombolysis from the same puncture site.1,13
There are now also alternative endovascular techniques as opposed to solely angioplasty including the use of bare-metal or drug-eluting stents or stent grafts for arterial lesions.16 Given the changing treatment options and variability in indications for treatment strategies, only angioplasty results were reviewed within this study. However, PA has been used to deploy arterial stents successfully and the choice of device used should not influence puncture site.19
Our institution has previously documented PA to be of particular benefit in crossing flush SFA origin occlusions.1 In this study, a higher proportion of lesions treated by PA were within the proximal SFA although distal SFA lesions were still more frequently treated. This potentially reflects the fact that proximal lesions arising at the origin of the SFA are considered more suitable for surgical intervention8 but also demonstrates the broadening use of PA as increased experience with the technique is obtained. Bypass grafting remains the gold standard of treatment for most infra-inguinal lesions20 and endarterectomy the preferred management for occlusive disease involving the CFA and femoral bifurcation.21 However, given the matched immediate and long-term outcomes demonstrated when comparing PA to FA, it is believed a prospective randomised study including clinical matching and objective patency assessment could now be performed.
Conclusions
PA is safe with comparable success rates and long-term outcomes to FA. There no increase in local complication rates following PA when a Doppler ultrasound ‘Smart’ needle is used to identify the popliteal vasculature. PA is a useful alternative technique for endovascular therapy if there is difficulty accessing the femoral artery. The approach can be considered when treating occlusive, proximal disease when surgical intervention is not the primary treatment.
References
- 1.Saha S, Gibson M, Magee TR, Galland RB, Torrie EP. Early results of retrograde transpopliteal angioplasty of iliofemoral lesions. Cardiovasc Intervent Radiol. 2001;24:378–82. doi: 10.1007/s00270-001-0043-5. [DOI] [PubMed] [Google Scholar]
- 2.Tonnesen KH, Sager P, Karle A, Henriksen L, Jorgensen B. Percutaneous transluminal angioplasty of the superficial femoral artery by retrograde catheterization via the popliteal artery. Cardiovasc Intervent Radiol. 1988;11:127–31. doi: 10.1007/BF02577101. [DOI] [PubMed] [Google Scholar]
- 3.Kluge A, Rauber K, Breithecker A, Rau WS, Bachmann G. Puncture of the popliteal artery using a Doppler-equipped (SMART) needle in transpopliteal interventions. Eur Radiol. 2003;13:1972–8. doi: 10.1007/s00330-002-1749-8. [DOI] [PubMed] [Google Scholar]
- 4.McCullough KM. Retrograde transpopliteal salvage of the failed antegrade transfemoral angioplasty. Aust Radiol. 1993;37:329–31. doi: 10.1111/j.1440-1673.1993.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 5.Zaitoun R, Iyer SS, Lewin RF, Dorros G. Percutaneous popliteal approach for angioplasty of superficial femoral artery occlusions. Cathet Cardiovasc Diagn. 1990;21:154–8. doi: 10.1002/ccd.1810210306. [DOI] [PubMed] [Google Scholar]
- 6.Henry M, Amicabile C, Amor M, Beron R, Henry I, Mentre B. [Peripheral arterial angioplasty: value of the popliteal approach. Apropos of 30 cases] Arch Mal Coeur Vaiss. 1993;86:463–9. [PubMed] [Google Scholar]
- 7.Yilmaz S, Altinbas H, Senol U, Sindel T, Mete A, Luleci E. Common peroneal nerve palsy after retrograde popliteal artery puncture. Eur J Vasc Endovasc Surg. 2002;23:467–9. doi: 10.1053/ejvs.2002.1629. [DOI] [PubMed] [Google Scholar]
- 8.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31:S1–296. [PubMed] [Google Scholar]
- 9.Bolia A, Miles KA, Brennan J, Bell PR. Percutaneous transluminal angioplasty of occlusions of the femoral and popliteal arteries by subintimal dissection. Cardiovasc Intervent Radiol. 1990;13:357–63. doi: 10.1007/BF02578675. [DOI] [PubMed] [Google Scholar]
- 10.Ahn SS, Rutherford RB, Becker GJ, Comerota AJ, Johnston KW, et al. Reporting standards for lower extremity arterial endovascular procedures. Society for Vascular Surgery/International Society for Cardiovascular Surgery. J Vasc Surg. 1993;17:1103–7. doi: 10.1067/mva.1993.45889. [DOI] [PubMed] [Google Scholar]
- 11.Villas PA, Cohen G, Goyal A, Putnam III SG, Ball D. The merits of percutaneous transluminal angioplasty of a superficial femoral artery stenosis via a retrograde popliteal artery approach. J Vasc Intervent Radiol. 1999;10:325–8. doi: 10.1016/s1051-0443(99)70038-2. [DOI] [PubMed] [Google Scholar]
- 12.Matsi PJ, Manninen HI, Soder HK, Mustonen P, Kouri J. Percutaneous translu minal angioplasty in femoral artery occlusions: primary and long-term results in 107 claudicant patients using femoral and popliteal catheterization techniques. Clin Radiol. 1995;50:237–44. doi: 10.1016/s0009-9260(05)83478-6. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz S, Sindel T, Luleci E. Ultrasound-guided retrograde popliteal artery catheterization: experience in 174 consecutive patients. J Endovasc Ther. 2005;12:714–22. doi: 10.1583/05-1576MR.1. [DOI] [PubMed] [Google Scholar]
- 14.Fowkes F, Leng GC. Bypass surgery for chronic lower limb ischaemia. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD002000.pub2. (2): CD002000. [DOI] [PubMed] [Google Scholar]
- 15.Fowkes FG, Gillespie IN. Angioplasty (versus non surgical management) for intermittent claudication. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000017. (2): CD000017. [DOI] [PubMed] [Google Scholar]
- 16.Meier GH. Current literature for evidence-based infrainguinal endovascular treatment. Semin Vasc Surg. 2008;21:210–6. doi: 10.1053/j.semvascsurg.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Balmer H, Mahler F, Do DD, Triller J, Baumgartner I. Balloon angioplasty in chronic critical limb ischemia: factors affecting clinical and angiographic out come. J Endovasc Ther. 2002;9:403–10. doi: 10.1177/152660280200900403. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz S, Sindel T, Luleci E. Re: the merits of percutaneous transluminal angioplasty of a superficial femoral artery stenosis via a retrograde popliteal artery approach. J Vasc Intervent Radiol. 2001;12:1457–8. doi: 10.1016/s1051-0443(07)61711-4. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz S, Sindel T, Ceken K, Alimoglu E, Luleci E. Subintimal recanalization of long superficial femoral artery occlusions through the retrograde popliteal approach. Cardiovasc Intervent Radiol. 2001;24:154–60. doi: 10.1007/s002700001751. [DOI] [PubMed] [Google Scholar]
- 20.Lenti M, Cieri E, De RP, Pozzilli P, Coscarella C, et al. Endovascular treatment of long lesions of the superficial femoral artery: results from a multi-center registry of a spiral, covered polytetrafluoroethylene stent. J Vasc Surg. 2007;45:32–9. doi: 10.1016/j.jvs.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 21.Al-Khoury G, Marone L, Chaer R, Rhee R, Cho J, et al. Isolated femoral endarterectomy: impact of SFA TASC classification on recurrence of symptoms and need for additional intervention. J Vasc Surg. 2009;50:784–9. doi: 10.1016/j.jvs.2009.05.053. [DOI] [PubMed] [Google Scholar]