Abstract
We report the results of solid state nuclear magnetic (NMR) measurements on amyloid fibrils formed by the full-length prion protein PrP (residues 23-231, Syrian hamster sequence). Measurements of intermolecular 13C-13C dipole-dipole couplings in selectively carbonyl-labeled samples indicate that β-sheets in these fibrils have an in-register parallel structure, as previously observed in amyloid fibrils associated with Alzheimer’s disease and type 2 diabetes and in yeast prion fibrils. Two-dimensional 13C-13C and 15N-13C solid state NMR spectra of a uniformly 15N,13C-labeled sample indicate that a relatively small fraction of the full sequence, localized to the C-terminal end, forms the structurally ordered, immobilized core. Although unique site-specific assignments of the solid state NMR signals can not be obtained from these spectra, analysis with a Monte Carlo/simulated annealing algorithm suggests that the core is comprised primarily of residues in the 173-224 range. These results are consistent with earlier electron paramagnetic resonance studies of fibrils formed by residues 90-231 of the human PrP sequence, formed under somewhat different conditions, suggesting that an in-register parallel β-sheet structure formed by the C-terminal end may be a general feature of PrP fibrils prepared in vitro.
Keywords: amyloid, prion, cross-β, scrapie, dipolar recoupling, magic-angle spinning
PrP is a 210-residue prion protein whose aberrant aggregation leads to infections neurodegenerative diseases in higher animals (1). Although it has not been definitively established that the infectious or neurotoxic form of PrP is an amyloid fibril (2), the self-propagating nature of the infectious form (commonly called PrPSc, with “Sc” representing “scrapie”) can be readily understood if PrPSc has a molecular structure that at least resembles structures of amyloid fibrils (3). In particular, the existence of multiple, distinct, self-propagating strains or variants of PrPSc (4,5) is consistent with the observation of self-propagating polymorphs of amyloid fibrils, such as the β-amyloid fibrils associated with Alzheimer’s disease (6,7). Distinct strains of yeast prions have also been attributed to self-propagating amyloid polymorphism (8-11).
Amyloid fibrils are filamentous peptide or protein aggregates containing cross-β structures, i.e., ribbon-like β-sheets in which the β-strands run approximately perpendicular to the long axis and inter-strand hydrogen bonds run approximately parallel to the long axis. Full-length recombinant PrP and various PrP fragments are capable of forming amyloid fibrils in vitro (12-20), and recombinant PrP fibrils are cytotoxic (21). Fibrils formed by residues PrP127-147 and PrP106-126 have been shown by solid state NMR to contain in-register parallel β-sheets (18,20), while evidence for antiparallel β-sheets in PrP109-122 fibrils (16) and against in-register parallel β-sheets in PrP89-143 fibrils (14) has also been reported (with PrPn-m representing residues n to m of full-length PrP). Solid state NMR measurements on PrP23-144 fibrils indicate that the structured core involves only a relatively short segment, namely residues 112-141 (15,19), although the type of β-sheets within these fibrils has not yet been reported. Molecular structural studies of amyloid fibrils formed by the PrP prion folding domain PrP90-231 or PrP23-231 (the full-length protein after cleavage of an N-terminal signal peptide) have been limited to lower-resolution techniques, including atomic force microscopy (AFM) and electron microscopy (EM) (12,13,22,23), hydrogen/deuterium (H/D) exchange (24), proteolysis (13), electron paramagnetic resonance (EPR) (25), and Fourier-transform infrared spectroscopy (FTIR) (13,26). Recombinant full-length PrP fibrils are also polymorphic, with the predominant fibril morphology being dependent on growth conditions such as the intensity of agitation during incubation. Baskakov and coworkers have described two morphologies of fibrils formed by residues PrP23-231, called R and S fibrils, which differ in their appearance in AFM and EM images (17) as well as in conformational properties probed by FTIR, H/D exchange, proteolysis patterns, x-ray fiber diffraction, and dye-binding (27). In the PrP90-231 fibrils prepared by Surewicz and coworkers, the fibril core is comprised approximately of residues 160-220, which form in-register parallel β-sheets according to EPR measurements on spin-labeled samples (25).
In this paper, we report the results of solid state NMR measurements on recombinant PrP23-231 amyloid fibrils, specifically the R morphology of these fibrils (17,27). The principal conclusions are that these fibrils contain in-register parallel β-sheets and that the structurally ordered fibril core includes the C-terminal segment, approximately residues 175-225, which includes the second and third α-helices of monomeric PrP (28,29). Only a subset of residues within this segment contribute to solid state NMR signals, however, suggesting that structurally ordered sub-segments (presumably β-strands) coexist with disordered and dynamic sub-segments (presumably loops). The Discussion section below addresses how these results relate to previous studies of PrP fibrils and PrPSc.
Materials and Methods
Expression of uniformly and selectively labeled PrP23-231 and fibril formation
Uniformly 15N,13C-labeled recombinant Syrian hamster PrP23-231 (U-15N,13C-PrP; sequence in Figure 1) was expressed as described earlier (30), with the modification that, after transformation, cells were grown on 15N,13C-enriched (98% enrichment) Spectra-9 bacterial growth medium. PrP23-231 samples labeled selectively with 1-13C-isoleucine (13CO-I-PrP) and with 1-13C-phenylalanine (13CO-F-PrP) were expressed in BL21 Star E. coli as described by Baxa et al. (31) in synthetic medium with composition described by Cai et al. (32) with minor modification (10 mM MgSO4 was used instead of K2SO4). Spectra-9 medium, 1-13C-isoleucine, and 1-13C-phenylalanine were purchased from Cambridge Isotope Laboratories.
Figure 1.
(a) Experimental measurement of intermolecular 13C-13C dipole-dipole couplings using the PITHIRDS-CT solid state NMR technique, for 13CO-F-PrP and 13CO-I-PrP fibrils (squares and circles, respectively). Positions of labeled Phe and Ile residues are underlined in the PrP23-231 sequence at the bottom of the figure. Error bars represent the root-mean-squared noise in the NMR signals. Lines are guides to the eye. (b) Experimental PITHIRDS-CT data after correction for natural-abundance 13C signal contributions as explained in the text. (c) Simulated PITHIRDS-CT curves for linear chains of dipole-coupled 13C nuclei with the indicated spacings. T2 relaxation is not included. (d) Simulated PITHIRDS-CT curves for dipole-coupled 13C nuclei with a 4.8 Å spacing and with indicated values of the transverse relaxation time T2.
Lyophilized labeled protein was dissolved in 50 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer, pH 6.0, immediately before use. The protein concentration was determined by measuring the absorbance at 280 nm. Fibrils were formed at 37° C in the reaction mixture of 0.5 mg/ml labeled PrP23-231, 50 mM MES, pH 6.0, and 2 M guanidinium chloride, in a 15 ml or 50 ml centrifuge tube with a conical bottom (Sarstedt), under continuous 24 rpm rotation using a Clay Adams Nutator (model 1105) for several days. Formation of fibrils was monitored by a thioflavin T binding assay and confirmed by EM as previously described (33). The fibrils were dialyzed overnight into 10 mM Tris, pH 7.5, and centrifuged for 2 hr at 10,000 X g to obtain a slurry of concentrated fibrils.
EM and AFM images of isotopically labeled PrP23-231 fibrils are shown in Figure S1 of the Supporting Information. The morphologies of these fibrils match those of the R fibrils described previously (17).
Atomic force microscopy of PrP fibrils
Amyloid fibrils produced from uniformly or selectively labeled PrP23-231 were dialyzed for 4 hr against 10 mM sodium acetate buffer, pH 5.0, with one buffer change after 2 hr. 10 μl of each sample (PrP23-231 concentration ~10 μg/ml) were applied on freshly cleaved mica, incubated for 5 min at room temperature for fibril adsorption, gently washed by ultrapure water, and left to dry on the bench. AFM imaging was performed using a Pico LE system (Agilent Technologies). The AFM scanner was equipped with a PPP-NCH silicon cantilever PPP-NCH (Nanosensors) and operated in tapping mode at the resonant frequency of approximately 300 kHz. The images (512×512 pixels) were collected at a scan rate of one line per second.
Solid state NMR measurements
All NMR measurements were performed at room temperature. Measurements of intermolecular 13C-13C dipole-dipole couplings in Figure 1 were performed on the 13CO-F-PrP and 13CO-I-PrP fibrils samples with the PITHIRDS-CT technique (34) at 399.2 MHz 1H NMR frequency (100.4 MHz 13C NMR frequency) with magic-angle spinning (MAS) at 20.00 kHz, using a Varian InfinityPlus spectrometer and a Varian 3.2 mm MAS probe. Radio-frequency (rf) pulse sequence conditions were as previously described (6,34-36), with pulsed spin-locking to enhance signal-to-noise (37) and active MAS synchronization with tachometer filtering to permit a 76.8 ms 13C-13C recoupling period. Samples were lyophilized for these measurements and were approximately 2.0 mg for 13CO-F-PrP and 0.7 mg for 13CO-I-PrP, packed in thick-wall 3.2 mm MAS rotors with additional teflon spacers to contain the samples in the center of the probe’s rf coil. A total of 800 scans was acquired for each PITHIRDS-CT data point, with a 4.0 s recycle delay. Test measurements on U-15N,13C-PrP fibrils confirmed that lyophilization did not disrupt the PrP fibril structure (i.e., did not alter the 13C MAS NMR spectrum), consistent with previous observations for various other amyloid fibrils (38-40). PITHIRDS-CT data for 13CO-F-PrP and 13CO-I-PrP fibrils after rehydration by addition of 3 μl of H2O into the MAS rotors are shown in Figure S2 of the Supporting Information.
Two-dimensional (2D) NMR spectra of U-15N,13C-PrP fibrils were obtained at 747.6 MHz 1H NMR frequency (188.0 MHz 13C and 75.8 MHz 15N NMR frequencies) and 17.00 kHz MAS frequency, using a Varian Infinity spectrometer and a 1.8 mm MAS probe constructed in the group of Prof. Ago Samoson (Estonian Institute of Chemical Physics and Biophysics). The U-15N,13C-PrP fibril sample (approximately 5 mg of protein) was pelleted directly into the MAS rotor without lyophilization.
The 2D 13C-13C NMR spectrum in Figure 3 was acquired with a 2.82 ms mixing period, using finite-pulse radio-frequency-driven recoupling (fpRFDR) (41,42). 13C π pulses in the fpRFDR period were 18.00 μs at a carrier frequency of 69 ppm. Two-pulse phase-modulated (TPPM) decoupling (43) was applied in the t1 and t2 periods, with a 100 kHz 1H rf field, a 20.0 μs t1 increment, and 200 t1 points. A total of 2496 scans was acquired for each complex t1 point, with a 1.0 s recycle delay. 2D 15N-13C NMR spectra (NCACX and NCOCX) in Figure 4 were acquired with mixing periods consisting of a 4.0 ms frequency-selective 15N-13C cross-polarization period (44) followed by a 2.82 ms fpRFDR period and with 45.5 μs t1 increments and 150 t1 points. For the NCACX spectrum, the 13C carrier frequency was at 44 ppm and 13C π pulses were 20.0 μs in the fpRFDR period. For the NCOCX spectrum, the 13C carrier frequency was at 105 ppm and 13C π pulses were 10.0 μs in the fpRFDR period. A total of 3584 and 5120 scans were acquired for each complex t1 point in NCACX and NCOCX measurements, respectively, with a 1.0 s recycle delay.
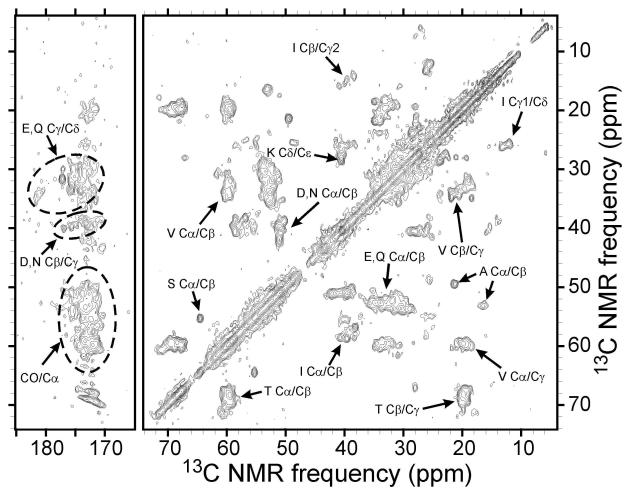
Figure 3.
2D 13C-13C solid state NMR spectrum of U-15N,13C-PrP fibrils. Partial residue-type assignments are shown for certain clusters of crosspeak signals.
Figure 4.
2D 15N-13C solid state NMR spectra of U-15N,13C-PrP fibrils, including the NCACX spectrum (a,b) and the NCOCX spectrum (c,d). Residue-type assignments for N/Cα crosspeaks that are used for automated site-specific assignments are shown in (a) and (c), with the following definitions for ambiguous residue-type assignments: X1=CDLNFY, X2=CDEFHKLMNQRWY, X3=EHKMQRW, X4=EHKMQRWA, X5=CEHKMQRW, Z1=IFYLV, Z2=IFYL, Z3=VT. Several N/Cβ crosspeaks are also indicated, for example by Tβ. Possible site-specific assignments of the same crosspeaks are shown in (b) and (d). These are the highest-scoring assignments, as explained in the text, but are not unique.
The 13C-detected 2D 1H-13C NMR spectrum in Figure 6 was obtained at 747.6 MHz 1H NMR frequency with conditions that select signals from highly mobile residues in hydrated U-15N,13C-PrP fibrils, i.e., conditions similar to those in solution NMR experiments. The MAS frequency was only 5.25 kHz. 1H decoupling in the t2 period was accomplished with a continuous train of 25 μs π pulses, using a 25-element phase pattern generated by the [0,0,120,60,120] iterative scheme of Tycko et al. (45) 1H evolution in the t1 period occurred without homonuclear decoupling and with heteronuclear decoupling by a single 13C π pulse at t1/2. 1H-13C polarization transfer was accomplished with a refocused INEPT sequence (46), determined empirically to produce maximal 13C NMR signals with a total mixing period of 3.6 ms. A total of 384 scans were acquired for each complex t1 point, with 110 t1 points and a 1.5 s recycle delay.
Figure 6.
2D 1H-13C NMR spectrum of U-15N,13C-PrP fibrils, obtained under conditions that select signals from highly mobile residues (see text). Assignments to sites on certain residue types are based on reported random-coil chemical shifts (62). Chemical shift scales are relative to 2,2-dimethyl-2-silapentane-5-sulfonate.
With the exception of Figure 6, 13C chemical shifts are relative to tetramethylsilane (TMS), based on an external 1-13C-alanine powder reference at 177.95 ppm. 15N chemical shifts are relative to liquid NH3, calculated using reported NMR frequency ratios (47).
Solid state NMR data analysis
Simulations of PITHIRDS-CT signals were performed with custom Fortran programs as previously described (34). Simulations without transverse (T2) spin relaxation in Figure 1c used a five-spin system, with polarization initially on the central spin to minimize end effects and approximate the behavior of an infinite chain of spins. Simulations of the effects of T2 relaxation in Figure 1d used a three-spin system and included continuous dephasing of coherences in the spin density matrix (using a direct-product basis), with dephasing rates equal to nqr/T2 where nqr is the number of nuclear spins that change state in the coherence between basis state |q> and basis state |r> and T2 is the transverse spin relaxation time.
2D NMR spectra of U-15N,13C-PrP fibrils were processed with NMRPipe (48) and analyzed and plotted with Sparky software (available at http://www.cgl.ucsf.edu/home/sparky/). As recently described (39), site-specific assignments were generated from tables of 15N/13Cα crosspeak chemical shifts, uncertainties, and degeneracies with a Monte Carlo/simulated annealing (MC/SA) algorithm in the program MCASSIGN1 (available upon request from robertty@mail.nih.gov). These tables are included in the Supporting Information (Tables S1 and S2). The crosspeak tables include residue-type assignments, which are allowed to be ambiguous whenever the data do not allow definite assignment of a 15N/13Cα crosspeak to one residue type. The MC/SA algorithm assigns crosspeak signals to specific sites, consistent with information in the crosspeak tables, so as to maximize the score S ≡ w1Ng - w2(Nb+Ne/4) - w3Nu. Ng and Nb are the numbers of “good connections” and “bad connections”, meaning pairs of sequentially assigned NCOCX and NCACX crosspeaks with consistent or inconsistent chemical shifts as described (39); Ne is the number of assignment “edges”, meaning dangling ends of sequentially assigned segments; Nu is the number of unassigned crosspeaks. In each MCASSIGN1 run, the parameters w1 and w2 were gradually incremented from 0 to 10 while assignment attempts are made. In this case, w3 was always 0, so that unassigned crosspeaks were not directly penalized (although good connections were rewarded and edges were penalized). Each run used 1.5 × 108 attempts and required roughly 250 s on an Acer TravelMate 6292 computer. 100 independent runs were executed, 98 of which ended with Nb = 0 (i.e., no inconsistencies in the final site-specific 15N and 13Cα chemical shifts). Final values of Nu were less than 5. Final scores ranged from 550 to 610.
The MC/SA algorithm is particularly well suited for data of the type reported below for U-15N,13C-PrP fibrils, in which residue-type assignments in NCACX and NCOCX spectra are highly ambiguous because of low resolution and signal-to-noise, and in which the number of crosspeaks is much less than the number of residues in the protein sequence. Manual sequential assignments are impossible in this case, because the information content of the data is insufficient to determine unique assignments. The MC/SA algorithm allows all assignments that are consistent with the available data to be identified. Examination of the complete set of possible assignments allows one to determine which residues have unique assignments, which residues definitely contribute to the solid state NMR spectra (and are therefore structurally ordered and relatively immobile), and which residues do not contribute to the solid state NMR spectra (and are therefore disordered and relatively dynamic). The MC/SA algorithm has been validated in experiments on uniformly 15N,13C-labeled HET-s218-289 fibrils (39).
Results
Intermolecular 13C-13C dipole-dipole couplings support a parallel β-sheet structure
As previously demonstrated, the organization of β-sheets within amyloid fibrils can be determined with measurements of intermolecular dipole-dipole couplings among 13C labels (6,7,31,35,36,40,49-57). An in-register parallel β-sheet structure leads to intermolecular 13C-13C distances of approximately 4.8 Å, the typical inter-strand spacing in a β-sheet. At this distance, 13C-13C dipole-dipole couplings are approximately 70 Hz, leading to decays of 13C NMR signals on the time scale of 30 ms when appropriate dipolar recoupling techniques are employed (34). Out-of-register or antiparallel β-sheets lead to longer 13C-13C distances and weaker couplings (proportional to the inverse cube of the distance) (38,58,59). Analogous experiments have also been performed with electron spins (60). When amyloid-forming polypeptides can be chemically synthesized, the 13C labels can be introduced at a single site in each polypeptide chain, so that the measured couplings have a unique structural assignment (6,7,38,40,49,51-55,61). In the case of larger proteins such as PrP, single-site labeling is generally not possible. However, studies of yeast prion fibrils have shown that similar information can be obtained if the protein is 13C-labeled biosynthetically at backbone carbonyl or sidechain methyl sites of residues that occur multiple times but are well separated in the amino acid sequence (31,35,36,56,57). In such cases, the solid state NMR data reflect the intermolecular couplings of all labeled residues simultaneously. If some, but not all, labeled residues participate in the in-register parallel β-sheet structure, then a combination of rapidly-decaying and slowly-decaying signals may be observed (57).
Figure 1a shows measurements of 13C-13C dipole-dipole couplings in lyophilized 13CO-F-PrP and 13CO-I-PrP fibrils obtained with the PITHIRDS-CT dipolar recoupling technique (34). These are measurements of the dependence of the total carbonyl 13C NMR signal on the effective recoupling period (i.e., the period of the PITHIRDS-CT pulse sequence during which 13C-13C couplings are active). Given the 1.1% natural abundance of 13C and the large number of unlabeled carbonyl sites, approximately 48% of the total signal from 13CO-F-PrP fibrils and approximately 41% of the total signal from 13CO-I-PrP fibrils arises from natural-abundance 13C sites, most of which are not coupled significantly to other 13C nuclei. Therefore, Figure 1b shows the same PITHIRDS-CT data after subtraction of the expected natural-abundance signals, which are assumed to decay linearly with increasing recoupling time to 70% of their initial value (as observed in earlier studies of yeast prion fibrils (57) and verified by PITHIRDS-CT measurements on PrP fibrils with a 1:4 dilution of 13CO-I-PrP in unlabeled PrP). The corrected data (and the raw data) show a decay on the 30 ms time scale, followed by a plateau at approximately 30% of the initial value for 13CO-F-PrP fibrils, or approximately 10% of the initial value for 13CO-I-PrP fibrils. Comparison with simulated PITHIRDS-CT data for an ideal linear chain of 13C nuclei, shown in Figure 1c, indicates 13C-13C distances of 5.0-5.5 Å. While the ideal simulated data for a 5.0 Å distance show oscillations at recoupling times beyond 30 ms that are not present in the experimental data, the simulations in Figure 1d show that these oscillations are damped by transverse spin relaxation when T2 < 200 ms. Actual T2 values for backbone 13CO sites under our experimental PITHIRDS-CT conditions are typically 50-100 ms. Thus, we conclude that the data in Figure 1a imply intermolecular 13C-13C distances of approximately 5.0 Å. Data for rehydrated samples, which agree with the data in Figure 1a but were acquired only to a maximum recoupling period of 38.4 ms, are shown in Figure S2 of the Supporting Information.
The most likely interpretation of these data is that the C-terminal half of PrP, which contains the three Phe and four Ile residues, forms in-register parallel β-sheets in PrP fibrils. The difference between the asymptotic values of 13CO-F-PrP and 13CO-I-PrP data suggests that one of the Phe residues (perhaps F141) may be outside the β-sheets. However, the asymptotic value of the corrected 13CO-F-PrP data is not much higher than corresponding values in the linear-chain simulations, and there is at least 10% uncertainty in the natural-abundance corrections. Relatively weak intramolecular dipole-dipole couplings between carbonyls of I182 and I184 and between carbonyls of I203 and I205 (6.5-7.0 Å distances in a β-strand) may also contribute to the lower asymptotic value of the 13CO-I-PrP data. Therefore, we conclude that at least two (and possibly all three) Phe residues and all four Ile residues participate in the parallel β-sheet structure.
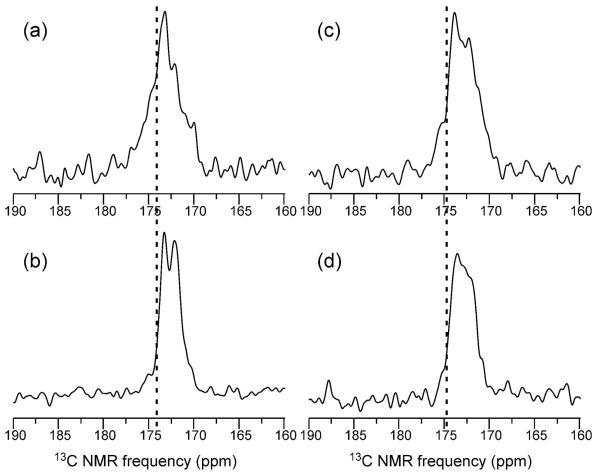
One-dimensional 13C NMR spectra of the 13CO-F-PrP and 13CO-I-PrP fibril samples are shown in Figure 2. Relatively broad carbonyl 13C NMR lines (4 ppm full width at half maximum, FWHM) are observed in the dry, lyophilized state (Figure 2. Rehydration in the MAS rotor reduces the total carbonyl signal intensities by factors of 0.46 and 0.71 for 13CO-F-PrP and 13CO-I-PrP fibrils, respectively, and also reduces the linewidths. These effects are due at least in part to elimination of natural-abundance signals from residues that are highly mobile in the hydrated state, i.e., residues that are outside of the β-sheet core. In the hydrated state, all carbonyl signals are shifted upfield relative to random-coil chemical shift values for Phe and Ile, consistent with β-strand conformations at the 13C-labeled sites. For 13CO-F-PrP, signals at 173.4 ppm and 172.1 ppm are resolved in the hydrated state, with an area ratio of approximately 5:8. These signals may arise from one and two labeled Phe sites, respectively.
Figure 2.
Solid state 13C NMR spectra of 13CO-F-PrP (a,b) and 13CO-I-PrP (c,d) fibrils in the dry, lyophilized state (a,c) and after rehydration by addition of 3 μl H2O. Spectra were obtained at 100.4 MHz 13C NMR frequency with 20.00 kHz MAS and 368 (a) or 4096 (b-d) scans. Vertical dashed lines indicate the random-coil chemical shift values for F or I carbonyl sites.
Identification of the core-forming segment in PrP23-231 fibrils
Solid state NMR measurements were performed on U-15N,13C-PrP fibrils in the hydrated state with the goals of identifying the segment or segments of the PrP23-231 sequence that form the rigid core structure and characterizing the secondary structure within the core. Figure 3 shows a 2D 13C-13C spectrum of these fibrils in which crosspeaks arise primarily from one-bond, intra-residue spin polarization transfers. Several features are noteworthy: (i) Strong CO/Cα signals from Gly residues are not observed, although the PrP23-231 sequence contains 41 Gly residues. Such signals would be expected around 170 ppm/45 ppm; (ii) One Ser Cα/Cβ crosspeak and two Ala Cα/Cβ crosspeaks are observed, although the sequence contains seven Ser residues and eight Ala residues; (iii) At least three sets of Ile crosspeaks are observed; (iv) Signals from approximately five Val residues are observed, out of the nine Val residues in the sequence; (v) Signals from eight or more Thr residues are observed, although they are not resolved from one another; (vi) Strong signals attributable to Pro residues are not observed. Taken together with the PrP23-231 sequence in Figure 1, these observations indicate that the core structure is composed primarily of residues in the 130-231 range.
In order to identify the segments that contribute to solid state NMR signals more precisely, we recorded the 2D 15N-13C spectra shown in Figure 4 and attempted to make sequential, site-specific resonance assignments. In favorable cases, assignments of signals from uniformly 15N,13C-labeled proteins can be made from such spectra by comparing NCACX crosspeaks (Figure 4a), which link backbone 15N chemical shifts with Cα and sidechain 13C chemical shifts within individual residues, with NCOCX crosspeaks (Figure 4c), which link the backbone 15N chemical shift of a given residue with 13C chemical shifts of the preceding residue in the amino acid sequence. If the crosspeaks are sufficiently well-resolved and if many crosspeaks can be assigned to residue types based on their characteristic 13C chemical shift ranges (62), then site-specific assignments can be made manually. Unfortunately, the 2D spectra in Figures 3 and 4 are only partially resolved (full-width-at-half-maximum linewidths of approximately 0.5-0.8 ppm and 1.0-1.3 ppm for 13C and 15N NMR signals, respectively) and only a small number of N/Cβ crosspeaks could be detected above the noise level, making residue-type assignments difficult. Therefore, manual sequential assignments could not be made uniquely. Instead, we used a computational approach to enumerate possible assignments that are consistent with the detected crosspeaks (39). In this approach, the program MCASSIGN1 is provided with input tables containing chemical shifts of N/Cα crosspeaks from NCACX and NCOCX spectra, uncertainties in the shifts, crosspeak degeneracies (in case more than one N/Cα pair contributes to a single crosspeak), and possible residue-type assignments. MCASSIGN1 then uses a MC/SA algorithm to find site-specific assignments that are consistent with these inputs. In principle, multiple independent runs allow all consistent assignments to be identified.
Crosspeak tables for the spectra in Figure 4 are given in the supporting information (Tables S1 and S2). Residue-type assignments in these tables (also shown in Figures 4a and 4c) were made primarily by comparing 13Cα chemical shifts in the NCACX and NCOCX spectra with crosspeak positions in the 2D 13C-13C spectrum. For many of the crosspeaks, residue-type assignments were highly ambiguous. One hundred MCASSIGN1 runs yielded 98 consistent assignments (not all different from one another). The highest-scoring assignment (score = 610; see Materials and Methods) is shown in Figures 4b and 4d.
The crosspeak tables allow only 36 residues to be assigned to NCACX crosspeaks and 35 residues to be assigned to NCOCX crosspeaks, limited by the number of definite crosspeaks in each 2D spectrum and their apparent degeneracies. The identities of residues in the PrP23-231 sequence that occurred in the final assignment varied significantly among the MCASSIGN1 runs. Figures 5a and 5b show the fractions of successful runs in which each residue was assigned to NCACX and NCOCX crosspeaks. These plots can be interpreted loosely as indications of the probability that a given residue contributes to the solid state NMR signals, i.e., participates in the rigid core of PrP23-231 fibrils. The core structure is comprised primarily of residues in the range from 173 to 224, but we can not rule out participation of certain segments in the range from 95 to 161 (including F141). For example, Figure 3 shows two Cα/Cβ crosspeaks from Ala residues, while Figure 5 suggests that these crosspeaks arise from A224 and A120.
Figure 5.
Summary of the results of automated site-specific assignments for U-15N,13C-PrP fibrils, using the spectra in Figures 3 and 4. Graphs show the fraction of MC/SA assignment runs in which each residue has assigned signals in the NCACX (a) and NCOCX (b) spectrum. In the 11 highest-scoring assignments, residues that never have assigned signals in either spectrum are struck through in the PrP sequence below the graphs; residues that always have assigned signals in at least one spectrum are underlined.
The 2D 13C-13C spectrum in Figure 3 shows a single Ser Cα/Cβ crosspeak (apparently S222) with β-strand-like chemical shifts (upfield relative to the random coil value for Cα, downfield for Cβ). All Ile Cα/Cβ crosspeak signals are also β-strand-like. Cα/Cβ crosspeak signals for Thr and Val are primarily at β-strand-like chemical shifts, but several Thr residues (out of 14 in PrP23-231) and at least one Val residue (out of nine) apparently contribute signals with non-β-strand shifts. (For these comparisons, random coil shifts reported by Wishart et al. are used (62), adjusted to our TMS reference by subtracting 1.7 ppm.)
Several residues have unique NCACX assignments (and are always assigned) in the 11 highest-scoring MCASSIGN1 runs, namely I182, T183, I184, I203, S222, Q223, and A224. For all of these residues, the 13Cα chemical shifts in the NCACX spectrum are upfield of random coil values, consistent with β-strand secondary structure. V189 and T192 are also always assigned (but not uniquely) in the 11 highest-scoring runs, with only β-strand-like 13Cα chemical shifts. No residues are consistently assigned with non-β-strand 13Cα chemical shifts. Thus, the MCASSIGN1 analysis indicates that residues 182-184 and 222-224 are most likely in β-strands. Current data do not permit us to draw conclusions regarding the length of these β-strands or the location of non-β-strand segments that may connect the β-strands.
MCASSIGN1 runs were also executed with the assumption that only residues 160-231 contribute to the solid state NMR spectra. Assignments with scores as high as 610 were obtained, but the assignments were not more unique than when the full PrP23-231 sequence was included.
Additional information about the identities of rigid and mobile residues comes from spectra obtained under conditions appropriate for solution NMR (rather than solid state NMR). Figure 6 shows a 2D 1H-13C spectrum obtained under such conditions. Signals that can be assigned to Ala, Gly, Lys, Met, and Thr residues are observed, with assignments based on random-coil shifts (62). All of these residues occur at multiple positions in the N-terminal half of the PrP23-231 sequence. However, the observation of relatively weak Gly signals, despite the fact that Gly accounts for 35% of residues from 23 to 131, indicates that the N-terminal half is not as mobile as a fully disordered polypeptide in solution (e.g., monomeric α-synuclein (63)) or as in monomeric PrP23-231, which exhibits sub-nanosecond orientational correlation times, minimal chemical shift dispersion, and negative 1H-15N nuclear Overhauser effects for residues 23-120 in solution NMR measurements (28,29). Steric restrictions or transient population of ordered structures may affect the time scale and amplitude of N-terminal motions, leading to relatively short T2 times and hence weak signals under the experimental conditions of Figure 6. Alternatively, the N-terminal half may contain a mixture of immobile and mobile segments in the fibrillar state. Recent evidence that N-terminal residues influence PrP23-231 fibril morphology may be relevant to our observation of restricted mobility for the N-terminal half (64).
Discussion
In monomeric PrP23-231, residues 144-156, 172-193, and 200-227 form α-helices (29). The data presented above indicate that a large part of these helical segments, most likely including most of the second and third helical segments, undergoes a major conformational change to form the amyloid fibril core in the recombinant PrP23-231 fibrils studied in this work. The β-sheets in these fibrils have the in-register parallel structure observed previously in several other amyloid fibrils (6,18,20,31,36,52,57,65,66). Although the precise location of β-strands can not be determined from our data, the analysis presented above indicates that the fibril core is most likely formed by residues in the 173-224 range, with residues 182-184 and 222-224 participating in β-strands.
These results from solid state NMR are consistent with previous studies where proteinase K (PK) digestion, immunofluorescence microscopy and site-specific conformational stability assays were employed to assess the structure of fibrils produced in vitro from full-length PrP (67-69). The PK resistant fragments of the amyloid fibrils were found to consist of residues 138/141-230, 152/153-230 and 162-230, where the fragment 162-230 was the most resistant to proteolytic treatment (13,70). Upon treatment with PK, the 152/153-230 and 162-230 fragments preserved a high β-sheet context, a fibrillar appearance, and high seeding activity in fibrillation assays (13), supporting the notion that the cross-β core is formed within the residues 162-230.
Our solid state NMR data indicate that less than 25% of the full-length PrP sequence acquires a β-strand conformation in the amyloid fibrils. This result is consistent with a relatively low level of amide hydrogen protection measured by H/D exchange FTIR spectroscopy (27). Despite the small fraction of residues that adopt β-sheet conformations, PrP fibrils show remarkably high conformational stability, with a GdnHCl denaturation midpoint above 4 M (67). This observation suggests that, in addition to hydrogen bonds, hydrophobic and electrostatic interactions contribute substantially to the conformational stability of PrP fibrils.
Our results are also consistent with the findings of Surewicz and coworkers, who obtained evidence from H/D exchange measurements that the second and third helical segments of human PrP90-231 form the core structure in amyloid fibrils prepared in vitro (24) and used EPR spectroscopy of spin-labeled to show that approximately residues 160-220 form an in-register parallel β-sheet structure (25). The agreement between our results and these earlier findings is remarkable in light of the facts that our PrP sequence is different, our fibril formation protocol is different, and our experimental measurements are different. An in-register parallel β-sheet core formed by the C-terminal portion may be a general feature of recombinant PrP fibrils formed in vitro.
Solid state NMR measurements on PrP23-144 fibrils indicate that the structured core consists of residues 112-141 (15,19). According to Figure 5, most of this segment is not included in the core of our PrP23-231 fibrils. For example, the AGAAAAGA segment (residues 113-120) does not contribute to the 2D 13C-13C spectrum in Figure 3, which shows only two Cα/Cβ crosspeaks from Ala residues and no strong CO/Cα crosspeaks from Gly residues. Previous biophysical measurements also indicate that this segment is solvent-expposed or unstructured in recombinant PrP23-231 fibrils (67-69).
The molecular structures of PrP fibrils formed in vitro may be significantly different from those of infectious PrPSc strains. For example, Wille et al. have shown that infectious PrP(27-30) rods produce different x-ray fiber diffraction patterns than recombinant fibrils(71), although both materials show the 4.8 Å meridional diffraction feature that is characteristic of a cross-β structure and both have the appearance of amyloid fibrils in EM images. The protease-resistant core of PrPSc also extends further towards the N-terminus than does the protease-resistant core of recombinant PrP fibrils (68,70,72). Therefore, our results do not necessarily apply to PrPSc.
While recombinant PrP fibrils and PrPSc may have different overall structures, they appear to share some common structural elements, as is evident from similarities in PK digestion patterns performed under partially denaturing conditions (68,73). Furthermore, transmissible prion diseases have been induced in wild type animals by inoculation with recombinant PrP fibril preparations produced in vitro (72,74), indicating that at least one component of these preparations structurally resembles PrPSc or is capable of converting to PrPSc (or catalyzing PrPSc formation) in the biological environment. We speculate that partial structural similarities between recombinant PrP fibrils and PrPSc may be sufficient to producing transmissible prion disease. Therefore, while recombinant PrP fibrils have limited infectivity, knowledge of their structures together with that of PrPSc is important for elucidating the molecular mechanisms responsible for the genesis and evolution of infectious prions.
An in-register parallel β-sheet structure formed by residues 173-224 is fundamentally different from the β-helical model for PrPSc proposed by Govaerts et al. (75), in which residues 89-174 form a left-handed β-helical structure and the second and third α-helices of monomeric PrP are retained. While the current solid state NMR results for PrP23-231 fibrils can not be used to judge whether the β-helical model of PrPSc is correct, previous studies argue that residues 90-140 are less likely to form the core structure in PrPSc. Antibodies specific to residues 90-110 were found to precipitate native PrPSc, arguing that this region is exposed to the solvent to an extent sufficient for immunoprecipitation (76). Furthermore, incubation of PrPSc with 2–3 M GdnHCl was shown to destabilize the central region (residues 90–140), which had acquired a PK-sensitive conformation, while the C-terminal part (residues 143–230) remained PK-resistant (77).
Supplementary Material
Abbreviations
- NMR
nuclear magnetic resonance
- EM
electron microscopy
- FTIR
Fourier-transform infrared
- H/D
hydrogen/deuterium
- EPR
electron paramagnetic resonance
- MES
2-(N-morpholino)ethanesulfonic acid
- MAS
magic-angle spinning
- 2D
two-dimensional
- rf
radio-frequency
- PITHIRDS-CT
constant-time π pulse recoupling
- fpRFDR
finite-pulse radio-frequency-driven recoupling
- TPPM
two-pulse phase-modulated
- NCACX
2D 15N-13Cα/13CX spectrum
- NCOCX
2D 15N-13CO/13CX spectrum
- INEPT
insensitive nuclei enhanced by polarization transfer
- MCA/SA
Monte Carlo/simulated annealing
- PK
proteinase K
- TMS
tetramethylsilane
Footnotes
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH, and by NIH grant NS045585 to I.V.B.
Supporting Information Available
Figures S1 and S2 showing EM and AFM images of isotopically labeled PrP23-231 fibrils and PITHIRDS-CT data for fibrils after rehydration; Tables S1 and S2 containing the input to MCASSIGN1 for automated assignment of NCACX and NCOCX corsspeaks. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Prusiner SB. Prions. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tycko R. Molecular structure of amyloid fibrils: Insights from solid state NMR. Q. Rev. Biophys. 2006;39:1–55. doi: 10.1017/S0033583506004173. [DOI] [PubMed] [Google Scholar]
- 4.Safar J, Wille H, Itrri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. Eight prion strains have PrPSc molecules with different conformations. Nat. Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 5.Bessen RA, Kocisko DA, Raymond GJ, Nandan S, Lansbury PT, Caughey B. Nongenetic propagation of strain-specific properties of scrapie prion protein. Nature. 1995;375:698–700. doi: 10.1038/375698a0. [DOI] [PubMed] [Google Scholar]
- 6.Paravastu AK, Leapman RD, Yau WM, Tycko R. Molecular structural basis for polymorphism in Alzheimer’s β-amyloid fibrils. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18349–18354. doi: 10.1073/pnas.0806270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petkova AT, Leapman RD, Guo ZH, Yau WM, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 8.Edskes HK, McCann LM, Hebert AM, Wickner RB. Prion variants and species barriers among Saccharomyces ure2 proteins. Genetics. 2009;181:1159–1167. doi: 10.1534/genetics.108.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toyama BH, Kelly MJS, Gross JD, Weissman JS. The structural basis of yeast prion strain variants. Nature. 2007;449:233–U8. doi: 10.1038/nature06108. [DOI] [PubMed] [Google Scholar]
- 10.King CY, Diaz-Avalos R. Protein-only transmission of three yeast prion strains. Nature. 2004;428:319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- 11.Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics. 1996;144:1375–1386. doi: 10.1093/genetics/144.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swietnicki W, Morillas M, Chen SG, Gambetti P, Surewicz WK. Aggregation and fibrillization of the recombinant human prion protein huPrP90-231. Biochemistry. 2000;39:424–431. doi: 10.1021/bi991967m. [DOI] [PubMed] [Google Scholar]
- 13.Bocharova OV, Breydo L, Parfenov AS, Salnikov VV, Baskakov IV. In vitro conversion of full-length mammalian prion protein produces amyloid form with physical properties of PrPSc. J. Mol. Biol. 2005;346:645–659. doi: 10.1016/j.jmb.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 14.Lim KH, Nguyen TN, Damo SM, Mazur T, Ball HL, Prusiner SB, Pines A, Wemmer DE. Solid state NMR structural studies of the fibril form of a mutant mouse prion peptide PrP89-143. Solid State Nucl. Magn. Reson. 2006;29:183–190. doi: 10.1016/j.ssnmr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Helmus JJ, Surewicz K, Nadaud PS, Surewicz WK, Jaroniec CP. Molecular conformation and dynamics of the Y145Stop variant of human prion protein. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6284–6289. doi: 10.1073/pnas.0711716105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SW, Mou Y, Lin SY, Chou FC, Tseng WH, Chen C, Lu CYD, Yu SSF, Chan JCC. Steric zipper of the amyloid fibrils formed by residues 109-122 of the Syrian hamster prion protein. J. Mol. Biol. 2008;378:1142–1154. doi: 10.1016/j.jmb.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Makarava N, Baskakov IV. The same primary structure of the prion protein yields two distinct self-propagating states. J. Biol. Chem. 2008;283:15988–15996. doi: 10.1074/jbc.M800562200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh P, Simonetti K, Sharpe S. Core structure of amyloid fibrils formed by residues 106-126 of the human prion protein. Structure. 2009;17:417–426. doi: 10.1016/j.str.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Helmus JJ, Surewicz K, Surewicz WK, Jaroniec CP. Conformational flexibility of Y145Stop human prion protein amyloid fibrils probed by solid state nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 2010;132:2393–2403. doi: 10.1021/ja909827v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin NS, Chao JCH, Cheng HM, Chou FC, Chang CF, Chen YR, Chang YJ, Huang SJ, Chan JCC. Molecular structure of amyloid fibrils formed by residues 127 to 147 of the human prion protein. Chem.-Eur. J. 2010;16:5492–5499. doi: 10.1002/chem.200903290. [DOI] [PubMed] [Google Scholar]
- 21.Novitskaya V, Bocharova OV, Bronstein I, Baskakov IV. Amyloid fibrils of mammalian prion protein are highly toxic to cultured cells and primary neurons. J. Biol. Chem. 2006;281:13828–13836. doi: 10.1074/jbc.M511174200. [DOI] [PubMed] [Google Scholar]
- 22.Kundu B, Maiti NR, Jones EM, Surewicz KA, Vanik DL, Surewicz WK. Nucleation-dependent conformational conversion of the Y145Stop variant of human prion protein: Structural clues for prion propagation. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12069–12074. doi: 10.1073/pnas.2033281100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Makarava N, Lee CI, Laksanalamai P, Robb FT, Baskakov IV. Conformational stability of PrP amyloid fibrils controls their smallest possible fragment size. J. Mol. Biol. 2008;376:1155–1167. doi: 10.1016/j.jmb.2007.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu XJ, Wintrode PL, Surewicz WK. β-Sheet core of human prion protein amyloid fibrils as determined by hydrogen/deuterium exchange. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1510–1515. doi: 10.1073/pnas.0608447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cobb NJ, Sonnichsen FD, McHaourab H, Surewicz WK. Molecular architecture of human prion protein amyloid: A parallel, in-register β-structure. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18946–18951. doi: 10.1073/pnas.0706522104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones EM, Surewicz K, Surewicz WK. Role of N-terminal familial mutations in prion protein fibrillization and prion amyloid propagation in vitro. J. Biol. Chem. 2006;281:8190–8196. doi: 10.1074/jbc.M513417200. [DOI] [PubMed] [Google Scholar]
- 27.Ostapchenko VG, Sawaya MR, Makarava N, Savtchenko R, Nilsson KPR, Eisenberg D, Baskakov IV. Two amyloid states of the prion protein display signficantly different folding patterns. J. Mol. Biol. 2010;400:908–921. doi: 10.1016/j.jmb.2010.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riek R, Hornemann S, Wider G, Glockshuber R, Wuthrich K. NMR characterization of the full-length recombinant murine prion protein, mPrP(23-231) FEBS Lett. 1997;413:282–288. doi: 10.1016/s0014-5793(97)00920-4. [DOI] [PubMed] [Google Scholar]
- 29.Donne DG, Viles JH, Groth D, Mehlhorn I, James TL, Cohen FE, Prusiner SB, Wright PE, Dyson HJ. Structure of the recombinant full-length hamster prion protein PrP29-231: The N terminus is highly flexible. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13452–13457. doi: 10.1073/pnas.94.25.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breydo L, Bocharova OV, Makarava N, Salnikov VV, Anderson M, Baskakov IV. Methionine oxidation interferes with conversion of the prion protein into the fibrillar proteinase K-resistant conformation. Biochemistry. 2005;44:15534–15543. doi: 10.1021/bi051369+. [DOI] [PubMed] [Google Scholar]
- 31.Baxa U, Wickner RB, Steven AC, Anderson DE, Marekov LN, Yau WM, Tycko R. Characterization of β-sheet structure in Ure2p1-89 yeast prion fibrils by solid state nuclear magnetic resonance. Biochemistry. 2007;46:13149–13162. doi: 10.1021/bi700826b. [DOI] [PubMed] [Google Scholar]
- 32.Cai ML, Huang Y, Sakaguchi K, Clore GM, Gronenborn AM, Craigie R. An efficient and cost-effective isotope labeling protocol for proteins expressed in Escherichia coli. J. Biomol. NMR. 1998;11:97–102. doi: 10.1023/a:1008222131470. [DOI] [PubMed] [Google Scholar]
- 33.Baskakov IV, Bocharova OV. In vitro conversion of mammalian prion protein into amyloid fibrils displays unusual features. Biochemistry. 2005;44:2339–2348. doi: 10.1021/bi048322t. [DOI] [PubMed] [Google Scholar]
- 34.Tycko R. Symmetry-based constant-time homonuclear dipolar recoupling in solid state NMR. J. Chem. Phys. 2007;126:064506. doi: 10.1063/1.2437194. [DOI] [PubMed] [Google Scholar]
- 35.Shewmaker F, Ross ED, Tycko R, Wickner RB. Amyloids of shuffled prion domains that form prions have a parallel in-register β-sheet structure. Biochemistry. 2008;47:4000–4007. doi: 10.1021/bi7024589. [DOI] [PubMed] [Google Scholar]
- 36.Wickner RB, Dyda F, Tycko R. Amyloid of Rnq1p, the basis of the [PIN+] prion, has a parallel in-register β-sheet structure. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2403–2408. doi: 10.1073/pnas.0712032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petkova AT, Tycko R. Sensitivity enhancement in structural measurements by solid state NMR through pulsed spin locking. J. Magn. Reson. 2002;155:293–299. doi: 10.1006/jmre.2002.2519. [DOI] [PubMed] [Google Scholar]
- 38.Petkova AT, Buntkowsky G, Dyda F, Leapman RD, Yau WM, Tycko R. Solid state NMR reveals a pH-dependent antiparallel β-sheet registry in fibrils formed by a β-amyloid peptide. J. Mol. Biol. 2004;335:247–260. doi: 10.1016/j.jmb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 39.Tycko R, Hu K-N. A Monte Carlo/simulated annealing algorithm for sequential resonance assignment in solid state NMR of uniformly labeled proteins with magic-angle spinning. J. Magn. Reson. 2010;205:304–314. doi: 10.1016/j.jmr.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paravastu AK, Petkova AT, Tycko R. Polymorphic fibril formation by residues 10-40 of the Alzheimer’s β-amyloid peptide. Biophys. J. 2006;90:4618–4629. doi: 10.1529/biophysj.105.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett AE, Rienstra CM, Griffiths JM, Zhen WG, Lansbury PT, Griffin RG. Homonuclear radio frequency-driven recoupling in rotating solids. J. Chem. Phys. 1998;108:9463–9479. [Google Scholar]
- 42.Ishii Y. 13C-13C dipolar recoupling under very fast magic angle spinning in solid state nuclear magnetic resonance: Applications to distance measurements, spectral assignments, and high-throughput secondary-structure determination. J. Chem. Phys. 2001;114:8473–8483. [Google Scholar]
- 43.Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 1995;103:6951–6958. [Google Scholar]
- 44.Baldus M, Petkova AT, Herzfeld J, Griffin RG. Cross polarization in the tilted frame: Assignment and spectral simplification in heteronuclear spin systems. Mol. Phys. 1998;95:1197–1207. [Google Scholar]
- 45.Tycko R, Pines A, Guckenheimer J. Fixed-point theory of iterative excitation schemes in NMR. J. Chem. Phys. 1985;83:2775–2802. [Google Scholar]
- 46.Morris GA, Freeman R. Enhancement of nuclear magnetic-resonance signals by polarization transfer. J. Am. Chem. Soc. 1979;101:760–762. [Google Scholar]
- 47.Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD. 1H, 13C, and 15N chemical shift referencing in biomolecular NMR. J. Biomol. NMR. 1995;6:135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]
- 48.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRpipe: a multidimensional spectral processing system based on Unix pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 49.Benzinger TLS, Gregory DM, Burkoth TS, Miller-Auer H, Lynn DG, Botto RE, Meredith SC. Propagating structure of Alzheimer’s β-amyloid(10-35) is parallel β-sheet with residues in exact register. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13407–13412. doi: 10.1073/pnas.95.23.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gregory DM, Benzinger TLS, Burkoth TS, Miller-Auer H, Lynn DG, Meredith SC, Botto RE. Dipolar recoupling NMR of biomolecular self-assemblies: Determining inter- and intrastrand distances in fibrilized Alzheimer’s β-amyloid peptide. Solid State Nucl. Magn. Reson. 1998;13:149–166. doi: 10.1016/s0926-2040(98)00086-1. [DOI] [PubMed] [Google Scholar]
- 51.Antzutkin ON, Balbach JJ, Leapman RD, Rizzo NW, Reed J, Tycko R. Multiple quantum solid state NMR indicates a parallel, not antiparallel, organization of β-sheets in Alzheimer’s β-amyloid fibrils. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13045–13050. doi: 10.1073/pnas.230315097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antzutkin ON, Leapman RD, Balbach JJ, Tycko R. Supramolecular structural constraints on Alzheimer’s β-amyloid fibrils from electron microscopy and solid state nuclear magnetic resonance. Biochemistry. 2002;41:15436–15450. doi: 10.1021/bi0204185. [DOI] [PubMed] [Google Scholar]
- 53.Balbach JJ, Petkova AT, Oyler NA, Antzutkin ON, Gordon DJ, Meredith SC, Tycko R. Supramolecular structure in full-length Alzheimer’s β-amyloid fibrils: Evidence for a parallel β-sheet organization from solid state nuclear magnetic resonance. Biophys. J. 2002;83:1205–1216. doi: 10.1016/S0006-3495(02)75244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan JCC, Oyler NA, Yau WM, Tycko R. Parallel β-sheets and polar zippers in amyloid fibrils formed by residues 10-39 of the yeast prion protein Ure2p. Biochemistry. 2005;44:10669–10680. doi: 10.1021/bi050724t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luca S, Yau WM, Leapman R, Tycko R. Peptide conformation and supramolecular organization in amylin fibrils: Constraints from solid state NMR. Biochemistry. 2007;46:13505–13522. doi: 10.1021/bi701427q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shewmaker F, Kryndushkin D, Chen B, Tycko R, Wickner RB. Two prion variants of Sup35p have in-register parallel β-sheet structures, independent of hydration. Biochemistry. 2009;48:5074–5082. doi: 10.1021/bi900345q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shewmaker F, Wickner RB, Tycko R. Amyloid of the prion domain of Sup35p has an in-register parallel β-sheet structure. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19754–19759. doi: 10.1073/pnas.0609638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tycko R, Sciarretta KL, Orgel J, Meredith SC. Evidence for novel β-sheet structures in Iowa mutant β-amyloid fibrils. Biochemistry. 2009;48:6072–6084. doi: 10.1021/bi9002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shewmaker F, McGlinchey RP, Thurber KR, McPhie P, Dyda F, Tycko R, Wickner RB. The functional curli amyloid is not based on in-register parallel β-sheet structure. J. Biol. Chem. 2009;284:25065–25076. doi: 10.1074/jbc.M109.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Margittai M, Langen R. Fibrils with parallel in-register structure constitute a major class of amyloid fibrils: Molecular insights from electron paramagnetic resonance spectroscopy. Q. Rev. Biophys. 2008;41:265–297. doi: 10.1017/S0033583508004733. [DOI] [PubMed] [Google Scholar]
- 61.Balbach JJ, Ishii Y, Antzutkin ON, Leapman RD, Rizzo NW, Dyda F, Reed J, Tycko R. Amyloid fibril formation by Aβ16-22, a seven-residue fragment of the Alzheimer’s β-amyloid peptide, and structural characterization by solid state NMR. Biochemistry. 2000;39:13748–13759. doi: 10.1021/bi0011330. [DOI] [PubMed] [Google Scholar]
- 62.Wishart DS, Bigam CG, Holm A, Hodges RS, Sykes BD. 1H, 13C, and 15N random coil NMR chemical shifts of the common amino acids: 1. Investigations of nearest-neighbor effects. J. Biomol. NMR. 1995;5:67–81. doi: 10.1007/BF00227471. [DOI] [PubMed] [Google Scholar]
- 63.Wu KP, Kim S, Fela DA, Baum J. Characterization of conformational and dynamic properties of natively unfolded human and mouse α-synuclein ensembles by NMR: Implication for aggregation. J. Mol. Biol. 2008;378:1104–1115. doi: 10.1016/j.jmb.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ostapchenko VG, Makarava N, Savtchenko R, Baskakov IV. The polybasic N-terminal region of the prion protein controls the physical properties of both the cellular and fibrillar forms of PrP. J. Mol. Biol. 2008;383:1210–1224. doi: 10.1016/j.jmb.2008.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ladner CL, Chen M, Smith DP, Platt GW, Radford SE, Langen R. Stacked sets of parallel, in-register β-strands of β2-microglobulin in amyloid fibrils revealed by site-directed spin labeling and chemical labeling. J. Biol. Chem. 285:17137–17147. doi: 10.1074/jbc.M110.117234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen M, Margittai M, Chen J, Langen R. Investigation of α-synuclein fibril structure by site-directed spin labeling. J. Biol. Chem. 2007;282:24970–24979. doi: 10.1074/jbc.M700368200. [DOI] [PubMed] [Google Scholar]
- 67.Sun Y, Breydo L, Makarava N, Yang QY, Bocharova OV, Baskakov IV. Site-specific conformational studies of prion protein (PrP) amyloid fibrils revealed two cooperative folding domains within amyloid structure. J. Biol. Chem. 2007;282:9090–9097. doi: 10.1074/jbc.M608623200. [DOI] [PubMed] [Google Scholar]
- 68.Bocharova OV, Breydo L, Salnikov VV, Gill AC, Baskakov IV. Synthetic prions generated in vitro are similar to a newly identified subpopulation of PrPSc from sporadic Creutzfeldt-Jakob disease. Protein Sci. 2005;14:1222–1232. doi: 10.1110/ps.041186605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Novitskaya V, Makarava N, Bellon A, Bocharova OV, Bronstein IB, Williamson RA, Baskakov IV. Probing the conformation of the prion protein within a single amyloid fibril using a novel immunoconformational assay. J. Biol. Chem. 2006;281:15536–15545. doi: 10.1074/jbc.M601349200. [DOI] [PubMed] [Google Scholar]
- 70.Bocharova OV, Makarava N, Breydo L, Anderson M, Salnikov VV, Baskakov IV. Annealing prion protein amyloid fibrils at high temperature results in extension of a proteinase K-resistant core. J. Biol. Chem. 2006;281:2373–2379. doi: 10.1074/jbc.M510840200. [DOI] [PubMed] [Google Scholar]
- 71.Wille H, Bian W, McDonald M, Kendall A, Colby DW, Bloch L, Ollesch J, Borovinskiy AL, Cohen FE, Prusiner SB, Stubbs G. Natural and synthetic prion structure from x-ray fiber diffraction. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16990–16995. doi: 10.1073/pnas.0909006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Makarava N, Kovacs GG, Bocharova O, Savtchenko R, Alexeeva I, Budka H, Rohwer RG, Baskakov IV. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol. 2010;119:177–187. doi: 10.1007/s00401-009-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sajnani G, Pastrana MA, Dynin I, Onisko B, Requena JR. Scrapie prion protein structural constraints obtained by limited proteolysis and mass spectrometry. J. Mol. Biol. 2008;382:88–98. doi: 10.1016/j.jmb.2008.06.070. [DOI] [PubMed] [Google Scholar]
- 74.Wang F, Wang X, Yuan C-G, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Govaerts C, Wille H, Prusiner SB, Cohen FE. Evidence for assembly of prions with left-handed β-helices into trimers. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8342–8347. doi: 10.1073/pnas.0402254101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khalili-Shirazi A, Summers L, Linehan J, Mallinson G, Anstee D, Hawke S, Jackson GS, Collinge J. PrP glycoforms are associated in a strain-specific ratio in native PrPSc. J. Gen. Virol. 2005;86:2635–2644. doi: 10.1099/vir.0.80375-0. [DOI] [PubMed] [Google Scholar]
- 77.Kocisko DA, Lansbury PT, Caughey B. Partial unfolding and refolding of scrapie-associated prion protein: Evidence for a critical 16-kda C-terminal domain. Biochemistry. 1996;35:13434–13442. doi: 10.1021/bi9610562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.