Abstract
Human exposures to organophosphate insecticides are ubiquitous. Although regarded as neurotoxicants, increasing evidence points toward lasting metabolic disruption from early-life organophosphate exposures. We gave neonatal rats chlorpyrifos, diazinon or parathion in doses devoid of any acute signs of toxicity, straddling the threshold for barely-detectable cholinesterase inhibition. Organophosphate exposure during a critical developmental window altered the trajectory of hepatic adenylyl cyclase/cyclic AMP signaling, culminating in hyperresponsiveness to gluconeogenic stimuli. Consequently, the animals developed metabolic dysfunction resembling prediabetes. When the organophosphate-exposed animals consumed a high fat diet in adulthood, metabolic defects were exacerbated and animals gained excess weight compared to unexposed rats on the same diet. At the same time, the high fat diet ameliorated many of the central synaptic defects caused by organophosphate exposure, pointing to nonpharmacologic therapeutic interventions to offset neurodevelopmental abnormalities, as well as toward fostering dietary choices favoring high fat intake. These studies show how common insecticides may contribute to the increased worldwide incidence of obesity and diabetes.
Keywords: Adenylyl cyclase signaling, Chlorpyrifos, Cyclic AMP, Diabetes, Diazinon, Dietary Fat, Liver, Neurodevelopmental disorders, Obesity, Organophosphate pesticides, Parathion
INTRODUCTION
Obesity and consequent type II diabetes are rising at epidemic rates in the US and many other countries around the world, burdening individual lives and the community with substantial morbidity and mortality, as well as attendant financial costs. Two out of three US adults are now classified as overweight [1], contributing to a parallel increase in the incidence of type II diabetes [2, 3]. The potential role of early-life chemical exposures in obesity and diabetes has garnered far less interest in comparison to factors such as diet and lifestyle, genetics, race and ethnicity, and socioeconomic status [4–6] but there is increasing evidence that such exposures may have a significant impact. As just one example, it is clear that maternal smoking during pregnancy produces metabolic reprogramming that leads to subsequent risk of obesity in the offspring [7–12]. Recent attention has turned to the organophosphate insecticides, which represent 50% of all the insecticide use worldwide [13] and to which virtually all children are exposed [14]. There are epidemiologic links between insecticide exposure and diabetes [15, 16] and the same subpopulations that have the highest rates of obesity — inner city, low socioeconomic status, agricultural populations — are also those that have greater exposure to organophosphates and other insecticides [17–23]. Superimposed on these relationships, children are exposed more than adults because of their higher food and water consumption per kg body weight, and higher surface-to-volume ratio [24–28].
Organophosphates produce their insecticidal activity, as well as their systemic toxicity in off-target species, by inhibiting cholinesterase, the enzyme that breaks down the neurotransmitter, acetylcholine. Most of these agents are actually the phosphorothioate precursors of the active organophosphate compounds and need to be converted metabolically to their oxon metabolites, the agents that actually possess potent anticholinesterase activity. We will focus first on chlorpyrifos, one of the most extensively used, and widely studied of the organophosphates. Chlorpyrifos is converted by cytochrome P450 to chlorpyrifos oxon; when the resultant cholinesterase inhibition exceeds about 70%, signs of intoxication emerge as “cholinergic syndrome,” marked by salivation, lachrymation, urination, defecation, gastrointestinal distress and emesis. Since nearly every person shows organophosphate residues in their bodies [13], it is obvious that most exposures are below that threshold and thus go unnoticed and undetected. It was thus of pivotal importance that, beginning in the mid-1990s, we were able to show that these agents acted potently as developmental neurotoxicants at exposures not only below the threshold for signs of exposure, but even in the absence of significant inhibition of cholinesterase [29, 30]. In exploring the mechanisms underlying the noncholinesterase actions of the organophosphates, we noted that these agents target cell signaling cascades that control neural cell replication and differentiation, leading to cell damage and loss in the immature brain, mis-wiring of neuronal circuits, and corresponding behavioral deficits that continue to emerge later in adolescence and adulthood [29, 30]. Of these, the pathway synthesizing cyclic AMP, controlled by adenylyl cyclase is among the most prominent sites for disruption by organophosphates [31–40]. The critical finding was that organophosphate-induced interference with this signaling cascade during critical developmental periods permanently reprograms the future expression and function of the signaling proteins themselves. This means that cellular responses to the multiple neurotransmitters, hormones, cytokines and trophic signals that operate through cyclic AMP are permanently altered.
It is thus important to note that adenylyl cyclase and cyclic AMP play equally important roles outside the central nervous system, controlling metabolic, cardiovascular and hormonal functions. Indeed, animal studies confirm that organophosphate administration in adults can lead to enhanced weight gain [41] and diabetes-like changes in hepatic energy metabolism [42]. These relationships may be even more important when exposure occurs during development, as fetal and neonatal rats exposed to chlorpyrifos show excess weight gain and leptin dysregulation in adulthood [43]. We therefore set out to explore the likelihood that early-life organophosphate exposure could lead to metabolic dysfunction later in life, and this review will summarize the results of our work in this area over the last several years.
Early-Life Chlorpyrifos Exposure Leads to Prediabetes
In our studies of the developmental neurotoxicity of chlorpyrifos, we developed exposure paradigms to simulate nonsymptomatic exposures just at the threshold for barely-detectable inhibition of cholinesterase and noted that the most sensitive stage for eliciting neurodevelopmental abnormalities centered around the immediate perinatal period [29, 30]. When we examined the long-term impact on cell signaling in peripheral tissues, we found a similar relationship [32]. In the liver, this was characterized by upregulation of adenylyl cyclase itself, the enzyme that synthesizes cyclic AMP and that serves as the point of convergence for the various receptors that work through this second messenger pathway. Consequently, all inputs operating through cyclic AMP showed “heterologous sensitization,” namely a parallel increase in their effects. The enhancement included the responses to β-adrenergic receptors and glucagon receptors, the main inputs that control hepatic gluconeogenesis and lipolysis. We therefore expected to find hyperglycemia and hyperlipidemia in the chlorpyrifos-exposed animals. Whereas we did find hyperlipidemia, we were surprised to find normal serum glucose levels in either the fed or fasting states [44]. More detailed metabolic profiling revealed that the animals were maintaining their circulating glucose within normal limits only by hypersecreting insulin; thus, when we fasted the animals to reduce the impact of hepatic gluconeogenesis, insulin levels returned to normal. Our metabolic profiling also revealed deficits in glucose utilization and in several other important aspects of both glucose and lipid metabolism, with an overall pattern essentially resembling prediabetes. These initial results showed that an apparently “safe” exposure to a common environmental toxicant, even when normal growth parameters were maintained, leads to metabolic dysfunction culminating in prediabetes, and provided a mechanistic link between cell signaling targets and the subsequent emergence of hyperinsulinemia and hyperlipidemia.
Diazinon, Parathion and the Role of Dietary Fat Intake
Once we abandon the idea that organophosphates work solely through their ability to inhibit cholinesterase, we can no longer assume that they will all act alike, since the various agents in this class may diverge in their actions mediated by other mechanisms. We recently conducted comparative studies of the effects of diazinon and parathion to elicit long-term changes in hepatic adenylyl cyclase, paralleling our work on chlorpyrifos [37–40, 45]. We designed the dose regimens to produce a toxicodynamic match to the effects of chlorpyrifos, straddling the threshold for barely-detectable cholinesterase inhibition as the benchmark for dosing-equivalents. Diazinon, like chlorpyrifos, produced a lasting upregulation of adenylyl cyclase and a corresponding heterologous sensitization to the critical gluconeogenic inputs mediated through β-adrenergic receptors and glucagon receptors. However, parathion differed in that it produced the upregulation effect in adolescence, but the effect waned in adulthood. Nevertheless, as discussed below, parathion still produced prediabetes. It is thus likely that the lasting metabolic dysfunction wrought by early-life organophosphate exposure reflects a change in the trajectory of metabolic regulation initiated by earlier signaling changes, and that the effects at the level of cell signaling do not need to be maintained in order for their consequences to be expressed. We confirmed this by studying the programming of adenylyl cyclase expression with in vitro models, identifying a critical period in cell differentiation during which organophosphate exposure, whether from chlorpyrifos, diazinon or parathion, would lead to permanent changes in the regulation of cell signaling, without requiring a continued exposure to the organophosphates [39].
Our studies with diazinon and parathion gave us a greater depth of understanding of how the metabolic abnormalities caused by early-life organophosphate exposure interact with other factors that are relevant to human obesity and diabetes. Given recent concerns about human dietary choices, we focused on the role of saturated fat. In these studies, we exposed the animals to diazinon or parathion immediately after birth, using the same nonsymptomatic, low-level exposures as in our previous work; then, in adulthood, we switched half the animals to a diet where the 58% of the calories came from fat, and with a high proportion of saturated fat [45–48]. Animals exposed to either of the organophosphates showed much greater weight gain on the high-fat diet than did unexposed animals given the same dietary manipulation, again in association with prediabetic metabolic profiles; this did not reflect differences in food consumption, indicating a difference in fat metabolism between the exposed and unexposed groups. When we examined metabolic profiles, adipokines and indices of adipose inflammation, it was evident that the high-fat diet exacerbated the metabolic defects in the organophosphate-exposed animals. Our results thus provide some of the first evidence that early-life exposures to common chemical contaminants can enhance the effects of adverse dietary choices. However, we also identified a more worrisome interaction, pointing to a specific relationship between the effects of organophosphates on the response to a high fat diet and their effects on neural function, the subject of the next section.
Does Early-Life Organophosphate Exposure Contribute to Poor Dietary Choice?
In our studies of the interactions between neonatal organophosphate exposure and consumption of a high-fat diet in adulthood, we also made observations on the impact in a variety of brain regions. To our surprise, some of the effects of neonatal parathion exposure were ameliorated or reversed by the high-fat diet, including effects on lipid peroxidation involved associated with synaptic activity [46]. Accordingly, we explored whether the dietary manipulation could offset the adverse effects of the organophosphate on synaptic function, focusing on acetylcholine systems, one of the most prominent targets for disruption by organophosphates [49]. By itself, neonatal parathion exposure evoked widespread abnormalities in markers of synaptic activity, encompassing effects in brain regions possessing acetylcholine projections as well as the corresponding cell bodies. The high fat diet eliminated nearly all the ACh marker abnormalities.
The point is thus inescapable: a high-fat diet reverses some of the neurodevelopmental effects of neonatal parathion exposure. Does this mean that a nonpharmacologic intervention could be of practical use in offsetting neurobehavioral damage caused by these agents? Certainly, approaches using a high-fat, “ketogenic” diet have met with some success in pilot studies of drug-resistant childhood epilepsies [50–53], attention deficit hyperactivity disorder and autism [51, 54, 55]. Similarly, a few animal studies have examined the effects of a high-fat diet on neural function, and provide some evidence for a generalized decrease in excitability and reduced motor activity [56, 57], including amelioration of behavioral anomalies of genetic origin [58]. The ability of dietary manipulations to evoke widespread changes in neurotransmitter function is likely due to changes in the composition of membrane lipids in which receptors and cell signaling molecules are embedded [59, 60], and therefore can span multiple brain regions and neurotransmitter systems. Indeed, diet-induced changes in neural membrane lipids are known to alter neurotransmitter uptake and release, and function of neurotransmitter receptors and their signaling pathways [61–63]. Our findings thus provide a proof-of-principle that such strategies may be similarly applicable to offset the effects of developmental neurotoxicants. Obviously, there are other liabilities imposed by consumption of a high-fat diet that render its use problematic but it would be worthwhile to determine if there are specific dietary components that provide the major effect.
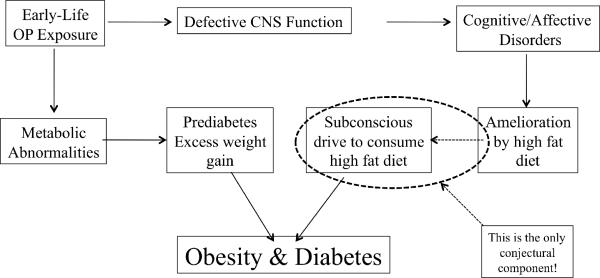
Notwithstanding this potentially beneficial effect, there is a more sinister aspect to the ability of high dietary fat to offset the synaptic defects caused by early-life organophosphate exposure. It is clear that neurodevelopmental disorders can influence apparent “life-style” choices, most notably in the increased incidence of drug abuse or cigarette smoking [64–68]. A recent study showed that abstinence in adolescent smokers whose mothers smoked during pregnancy leads to cognitive impairment, whereas abstinence in those who were born to nonsmokers showed cognitive improvement upon abstinence [69]. In other words, where there was preexisting neurodevelopmental damage from prenatal tobacco exposure, adolescents were able to use smoking to offset cognitive impairment; this likely contributes to the higher likelihood of the children born to smoking women becoming smokers themselves [67, 68]. By the same token, our studies point to the possibility that exposure to developmental neurotoxicants could contribute to a subsequent subconscious preference for a high-fat diet as a way of ameliorating the effects, thus providing an indirect but potentially potent driving force for consuming an unhealthy diet (Figure 1). If this turns out to be true, then our findings point to a potentially important factor contributing to dietary choices that ultimately promote obesity and diabetes, expanding the public health implications of the “silent pandemic” caused by developmental neurotoxicant exposure [70].
Figure 1.
How early-life exposure to organophosphate pesticides could contribute to later dietary choice, obesity and diabetes. Abbreviations: OP = organophosphate, CNS = central nervous system.
Acknowledgments/disclaimers
Research was supported by NIH ES10356. The sponsor had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. In some places, the author has paraphrased or quoted directly from his own previously-published primary research papers and review articles; in those instances, citations are provided but quotation marks have been omitted for clarity of presentation. The author has provided expert witness testimony in the past three years for: The Calwell Practice (Charleston WV), Frost Brown Todd (Charleston WV), Weltchek Mallahan & Weltchek (Lutherville MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Frommer Lawrence Haug (Washington DC), Carter Law (Peoria IL), Corneille Law (Madison WI), Angelos Law (Baltimore MD), Kopff, Nardelli & Dopf (New York NY), Gutglass Erickson Bonville & Larson (Madison WI), The Killino Firm (Philadelphia PA) and Alexander Hawes (San Jose, CA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- [2].Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia. 1999;42:1395–403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- [3].Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- [4].Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2004;89:2522–5. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- [5].Wang Y, Beydoun MA. The obesity epidemic in the United States -- gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- [6].Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Al Mamun A, Lawlor DA, Alati R, O'Callaghan MJ, Williams GM, Najman JM. Does maternal smoking during pregnancy have a direct effect on future offspring obesity? Evidence from a prospective birth cohort study. Am J Epidemiol. 2006;164:317–25. doi: 10.1093/aje/kwj209. [DOI] [PubMed] [Google Scholar]
- [8].Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. Intl J Epidemiol. 2006;35:121–30. doi: 10.1093/ije/dyi218. [DOI] [PubMed] [Google Scholar]
- [9].Gao YJ, Holloway AC, Zeng ZH, Lim GE, Petrik JJ, Foster WG, et al. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obesity Res. 2005;13:687–92. doi: 10.1038/oby.2005.77. [DOI] [PubMed] [Google Scholar]
- [10].Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. Int J Epidemiol. 2002;31:413–9. [PubMed] [Google Scholar]
- [11].Toschke AM, Ehlin AG, von Kries R, Ekbom A, Montgomery SM. Maternal smoking during pregnancy and appetite control in offspring. J Perinatal Med. 2003;31:251–6. doi: 10.1515/JPM.2003.034. [DOI] [PubMed] [Google Scholar]
- [12].von Kries R, Toschke AM, Koletzko B, Slikker W., Jr Maternal smoking during pregnancy and childhood obesity. Am J Epidemiol. 2002;156:954–61. doi: 10.1093/aje/kwf128. [DOI] [PubMed] [Google Scholar]
- [13].Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–98. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- [14].Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, et al. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J Exposure Anal Environ Epidemiol. 2005;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- [15].Morgan DP, Lin LI, Saikaly HH. Morbidity and mortality in workers occupationally exposed to pesticides. Arch Environ Contam Toxicol. 1980;9:349–82. doi: 10.1007/BF01057414. [DOI] [PubMed] [Google Scholar]
- [16].Saldana TM, Basso O, Hoppin JA, Baird DD, Knott C, Blair A, et al. Pesticide exposure and self-reported gestational diabetes mellitus in the Agricultural Health Study. Diabetes Care. 2007;30:529–34. doi: 10.2337/dc06-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Curl CL, Fenske RA, Kissel JC, Shirai JH, Moate TF, Griffith W, et al. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ Health Perspect. 2002;110:A787–A92. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fenske RA, Kissel JC, Lu C, Kalman DA, Simcox NJ, Allen EH, et al. Biological based pesticide dose estimates for children in an agricultural community. Environ Health Perspect. 2000;108:515–20. doi: 10.1289/ehp.00108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Petchuay C, Visuthismajarn P, Vitayavirasak B, Hore P, Robson MG. Biological monitoring of organophosphate pesticides in preschool children in an agricultural community in Thailand. Intl J Occup Environ Health. 2006;12:134–41. doi: 10.1179/oeh.2006.12.2.134. [DOI] [PubMed] [Google Scholar]
- [20].Rothlein J, Rohlman D, Lasarev M, Phillips J, Muniz J, McCauley L. Organophosphate pesticide exposure and neurobehavioral performance in agricultural and nonagricultural Hispanic workers. Environ Health Perspect. 2006;114:691–6. doi: 10.1289/ehp.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, et al. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26:573–87. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- [22].Whyatt RM, Camann D, Perera FP, Rauh VA, Tang D, Kinney PL, et al. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol Appl Pharmacol. 2005;206:246–54. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]
- [23].Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Whyatt RM, Garfinkel R, Hoepner LA, Holmes D, Borjas M, Williams MK, et al. Within-and between-home variability in indoor-air insecticide levels during pregnancy among an inner-city cohort from New York City. Environ Health Perspect. 2007;115:383–9. doi: 10.1289/ehp.9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Garry VF. Pesticides and children. Toxicol Appl Pharmacol. 2004;198:152–63. doi: 10.1016/j.taap.2003.11.027. [DOI] [PubMed] [Google Scholar]
- [26].Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, et al. Pesticides and inner-city children: exposures, risks, and prevention. Environ Health Perspect. 1999;107(suppl 3):431–7. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].National Research Council . Pesticides in the diets of infants and children. National Academy Press; Washington D.C.: 1993. [PubMed] [Google Scholar]
- [28].Physicians for Social Responsibility . Pesticides and Children. Physicians for Social Responsibility; Washington DC: 1995. [Google Scholar]
- [29].Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–51. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- [30].Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- [31].Meyer A, Seidler FJ, Aldridge JE, Tate CA, Cousins MM, Slotkin TA. Critical periods for chlorpyrifos-induced developmental neurotoxicity: alterations in adenylyl cyclase signaling in adult rat brain regions after gestational or neonatal exposure. Environ Health Perspect. 2004;112:295–301. doi: 10.1289/ehp.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Meyer A, Seidler FJ, Slotkin TA. Developmental effects of chlorpyrifos extend beyond neurotoxicity: critical periods for immediate and delayed-onset effects on cardiac and hepatic cell signaling. Environ Health Perspect. 2004;112:170–8. doi: 10.1289/ehp.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Meyer A, Seidler FJ, Cousins MM, Slotkin TA. Developmental neurotoxicity elicited by gestational exposure to chlorpyrifos: when is adenylyl cyclase a target? Environ Health Perspect. 2003;111:1871–6. doi: 10.1289/ehp.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang HS, Liu J, Pope CN. Age-related effects of chlorpyrifos on muscarinic receptor-mediated signaling in rat cortex. Arch Toxicol. 2002;75:676–84. doi: 10.1007/s00204-001-0309-3. [DOI] [PubMed] [Google Scholar]
- [35].Olivier K, Liu J, Pope C. Inhibition of forskolin-stimulated cAMP formation in vitro by paraoxon and chlorpyrifos oxon in cortical slices from neonatal, juvenile, and adult rats. J Biochem Mol Toxicol. 2001;15:263–9. doi: 10.1002/jbt.10002. [DOI] [PubMed] [Google Scholar]
- [36].Ward TR, Mundy WR. Organophosphorus compounds preferentially affect second messenger systems coupled to M2/M4 receptors in rat frontal cortex. Brain Res Bull. 1996;39:49–55. doi: 10.1016/0361-9230(95)02044-6. [DOI] [PubMed] [Google Scholar]
- [37].Adigun AA, Ryde IT, Seidler FJ, Slotkin TA. Organophosphate exposure during a critical developmental stage reprograms adenylyl cyclase signaling in PC12 cells. Brain Res. 2010;1329:36–44. doi: 10.1016/j.brainres.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Adigun AA, Seidler FJ, Slotkin TA. Disparate developmental neurotoxicants converge on the cyclic AMP signaling cascade, revealed by transcriptional profiles in vitro and in vivo. Brain Res. 2010;1316:1–16. doi: 10.1016/j.brainres.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Adigun AA, Wrench N, Seidler FJ, Slotkin TA. Neonatal organophosphorus pesticide exposure alters the developmental trajectory of cell signaling cascades controlling metabolism: differential effects of diazinon and parathion. Environ Health Perspect. 2010;118:210–5. doi: 10.1289/ehp.0901237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Adigun AA, Wrench N, Seidler FJ, Slotkin TA. Neonatal dexamethasone treatment leads to alterations in cell signaling cascades controlling hepatic and cardiac function in adulthood. Neurotoxicol Teratol. 2010;32:193–9. doi: 10.1016/j.ntt.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Meggs WJ, Brewer KL. Weight gain associated with chronic exposure to chlorpyrifos in rats. J Med Toxicol. 2007;3:89–93. doi: 10.1007/BF03160916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Abdollahi M, Donyavi M, Pournourmohammadi S, Saadat M. Hyperglycemia associated with increased hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase in rats following subchronic exposure to malathion. Comp Biochem Physiol Toxicol Pharmacol. 2004;137:343–7. doi: 10.1016/j.cca.2004.03.009. [DOI] [PubMed] [Google Scholar]
- [43].Lassiter TL, Brimijoin S. Rats gain excess weight after developmental exposure to the organophosphorothionate pesticide, chlorpyrifos. Neurotoxicol Teratol. 2008;30:125–30. doi: 10.1016/j.ntt.2007.10.004. [DOI] [PubMed] [Google Scholar]
- [44].Slotkin TA, Brown KK, Seidler FJ. Developmental exposure of rats to chlorpyrifos elicits sex-selective hyperlipidemia and hyperinsulinemia in adulthood. Environ Health Perspect. 2005;113:1291–4. doi: 10.1289/ehp.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Adigun AA, Wrench N, Levin ED, Seidler FJ, Slotkin TA. Neonatal parathion exposure and interactions with a high-fat diet in adulthood: adenylyl cyclase-mediated cell signaling in heart, liver and cerebellum. Brain Res Bull. 2010;81:605–12. doi: 10.1016/j.brainresbull.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lassiter TL, Ryde IT, Levin ED, Seidler FJ, Slotkin TA. Neonatal exposure to parathion alters lipid metabolism in adulthood: interactions with dietary fat intake and implications for neurodevelopmental deficits. Brain Res Bull. 2010;81:85–91. doi: 10.1016/j.brainresbull.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lassiter TL, Ryde IT, MacKillop EA, Brown KK, Levin ED, Seidler FJ, et al. Exposure of neonatal rats to parathion elicits sex-selective reprogramming of metabolism and alters the response to a high-fat diet in adulthood. Environ Health Perspect. 2008;116:1456–62. doi: 10.1289/ehp.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res Bull. 2008;75:166–72. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Slotkin TA, Lassiter TL, Ryde IT, Wrench N, Levin ED, Seidler FJ. Consumption of a high-fat diet in adulthood ameliorates the effects of neonatal parathion exposure on acetylcholine systems in rat brain regions. Environ Health Perspect. 2009;117:916–22. doi: 10.1289/ehp.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48:43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- [51].Connolly MB, Hendson G, Steinbok P. Tuberous sclerosis complex: a review of the management of epilepsy with emphasis on surgical aspects. Childs Nervous System. 2006;22:896–908. doi: 10.1007/s00381-006-0130-7. [DOI] [PubMed] [Google Scholar]
- [52].Hallbook T, Lundgren J, Rosen I. Ketogenic diet improves sleep quality in children with therapy-resistant epilepsy. Epilepsia. 2007;48:59–65. doi: 10.1111/j.1528-1167.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- [53].Hartman AL, Vining EPG. Clinical aspects of the ketogenic diet. Epilepsia. 2007;48:31–42. doi: 10.1111/j.1528-1167.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- [54].Evangeliou A, Vlachonikolis I, Mihailidou H, Spilioti M, Skarpalezou A, Makaronas N, et al. Application of a ketogenic diet in children with autistic behavior: pilot study. J Child Neurol. 2003;18:113–8. doi: 10.1177/08830738030180020501. [DOI] [PubMed] [Google Scholar]
- [55].Pulsifer MB, Gordon JM, Brandt J, Vining EP, Freeman JM. Effects of ketogenic diet on development and behavior: preliminary report of a prospective study. Dev Med Child Neurol. 2001;43:301–6. doi: 10.1017/s0012162201000573. [DOI] [PubMed] [Google Scholar]
- [56].Murphy P, Burnham WM. The ketogenic diet causes a reversible decrease in activity level in Long-Evans rats. Exp Neurol. 2006;201:84–9. doi: 10.1016/j.expneurol.2006.03.024. [DOI] [PubMed] [Google Scholar]
- [57].Murphy P, Likhodii SS, Hatamian M, McIntyre Burnham W. Effect of the ketogenic diet on the activity level of Wistar rats. Pediatr Res. 2005;57:353–7. doi: 10.1203/01.PDR.0000150804.18038.79. [DOI] [PubMed] [Google Scholar]
- [58].Teegarden SL, Nestler EJ, Bale TL. DeltaFosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol Psychiat. 2008;64:941–50. doi: 10.1016/j.biopsych.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gudbjarnason S, Benediktsdottir VE. Regulation of β-adrenoceptor properties and the lipid milieu in heart muscle membranes during stress. Mol Cell Biochem. 1996;164:137–43. doi: 10.1007/978-1-4613-1289-5_16. [DOI] [PubMed] [Google Scholar]
- [60].Ponsard B, Durot I, Delerive P, Oudot F, Cordelet C, Grynberg A, et al. Cross-influence of membrane polyunsaturated fatty acids and hypoxia-reoxygenation on α- and β-adrenergic function of rat cardiomyocytes. Lipids. 1999;34:457–66. doi: 10.1007/s11745-999-0385-5. [DOI] [PubMed] [Google Scholar]
- [61].Clandinin MT, Foot M, Robson L. Plasma membrane: can its structure and function be modulated by dietary fat? Comp Biochem Physiol B. 1983;76:335–9. doi: 10.1016/0305-0491(83)90079-2. [DOI] [PubMed] [Google Scholar]
- [62].Geiser F. Influence of polyunsaturated and saturated dietary lipids on adipose tissue, brain and mitochondrial membrane fatty acid composition of a mammalian hibernator. Biochim Biophys Acta. 1990;1046:159–66. doi: 10.1016/0005-2760(90)90183-x. [DOI] [PubMed] [Google Scholar]
- [63].Kelly JF, Joseph JA, Denisova NA, Erat S, Mason RP, Roth GS. Dissociation of striatal GTPase and dopamine release responses to muscarinic cholinergic agonists in F344 rats: influence of age and dietary manipulation. J Neurochem. 1995;64:2755–64. doi: 10.1046/j.1471-4159.1995.64062755.x. [DOI] [PubMed] [Google Scholar]
- [64].Deas D, Brown ES, Deas D, Brown ES. Adolescent substance abuse and psychiatric comorbidities. J Clin Psychiat. 2006;67:e02. doi: 10.4088/jcp.0706e02. [DOI] [PubMed] [Google Scholar]
- [65].Pliszka SR, Pliszka SR. Psychiatric comorbidities in children with attention deficit hyperactivity disorder: implications for management. Paediatr Drugs. 2003;5:741–50. doi: 10.2165/00148581-200305110-00003. [DOI] [PubMed] [Google Scholar]
- [66].Wilens TE, Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship, subtypes at risk, and treatment issues. Psychiat Clinics N Am. 2004;27:283–301. doi: 10.1016/S0193-953X(03)00113-8. [DOI] [PubMed] [Google Scholar]
- [67].Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;84:1407–13. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiat. 1999;38:892–9. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- [69].Jacobsen LK, Slotkin TA, Mencl WE, Frost SJ, Pugh KR. Gender specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007;32:2453–64. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- [70].Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]