Abstract
Autophagy, a cellular “self-eating” process in eukaryotic cells, exists in both a basal and an activated state that is induced in response to starvation. Basal and induced autophagy are associated with the packaging of cellular components, including damaged and/or redundant organelles into double-membrane vesicles called autophagosomes, followed by autophagosome fusion with lysosomes, wherein their contents are degraded and recycled. Recent results highlight a novel role for autophagy that does not involve lysosomal degradation of autophagosomal contents but instead involves their redirection towards the extracellular delivery of an unconventionally secreted protein. Here, we discuss these findings, evaluate the strength of evidence, consider their implications for the field of protein trafficking and suggest the next steps required to probe this interesting pathway.
What is unconventional protein secretion?
Most proteins secreted extracellularly have signal peptides that engage the conventional secretory pathway to direct them from the ER, via the Golgi apparatus, to the plasma membrane using vesicular carriers (classical secretory mode). However, a growing list of unconventionally-secreted proteins has been reported over the past two decades that neither have a signal sequence nor transit via the ER-Golgi route [1,2] (Table 1). The cellular mechanisms employed by such unconventionally-secreted proteins have not been extensively elucidated, although several models have been proposed and, in fact, different unconventionally secreted proteins may use alternative mechanisms to mediate their transit across the plasma membrane [3].
Table 1.
Some unconventionally secreted proteins that have important implications in health and disease
| Protein | Proposed secretion mechanism | References |
|---|---|---|
| FGF1 | Non-vesicular | [57] |
| FGF2 | Non-vesicular | [58] |
| Interleukin 1β | Vesicular | [59] |
| Galectins 1 and 3 | Vesicular / Non-vesicular | [58,60] |
| HMGB1 | Vesicular | [61] |
| AcbA | Vesicular | [25] |
| Thioredoxin | Non-vesicular | [62] |
| Engrailed Homeoproteins | Vesicular | [63] |
| HIV Tat | Non-vesicular | [64] |
| Tubby | Unclear | [65] |
| Yeast MATa | Non-vesicular | [4] |
Models for unconventional protein secretion
In the broadest sense, unconventional secretion refers to a collection of mechanisms that deviate from the normal classical pathways in transporting proteins to the cell surface or into the extracellular medium. However, for the purposes of this review, we use this term more restrictively to consider the transport of soluble proteins from the cytosol to the cell surface or extracellular medium without direct involvement of traditional secretory signal sequences or the ER and the Golgi apparatus. As discussed in a recent review [2], the modes of unconventional protein secretion of this type may be classified broadly into non-vesicular and vesicular categories. Examples of non-vesicular secretion include the yeast mating factor MATa, which is transported directly from the cytosol to the extracellular space via a specific plasma-membrane ABC transporter, encoded by the STE6 gene [4]. Another well studied example is FGF2, which binds to lipids, such as phosphatidylinositol 4,5 bisphosphate (PI(4,5)P2) in the inner leaflet of the plasma membrane and is then transported, after phosphorylation, in a folded configuration across the membrane, but in a manner that is dependent on the proteoglycan, heparan sulfate, on the extracellular side (reviewed in detail [2]).
The unconventionally secreted protein, IL-1β exemplifies several vesicular models that have been proposed [5], however, the precise mechanism remains elusive because to date no proteins directly required for IL-1β secretion have been defined. The suggested mechanisms include the lysosome-dependent pathway, microvesicle shedding and exosome release. In the lysosome-dependent pathway, pro-IL-1β is translocated into secretory lysosomes together with caspase-1. The mature form of IL-1β is produced within the lysosome by caspase-1 cleavage, after which the lysosomes fuse with the plasma membrane and the contents are released into the extracellular space. In the microvesicle shedding model, caspase-1 activates IL-1β in the cytoplasm and it is exported along with the mature cytokine into the extracellular space via vesicles budding from the plasma membrane. Neither of these secretion mechanisms were suggested as major pathways in studies of the secretion of IL-1β in primary bone marrow-derived macrophages, in which the P2X7 receptor (P2X7R) has been activated by extracellular ATP [6]. This leaves open the third pathway, exosome release, in which cytoplasmic caspase-1 and IL-1β would be packaged within endosomes and released as exosomes upon endosome fusion with the plasma membrane. Although this study suggested that the prevailing mechanism might be exosome release, it is possible that multiple pathways exist for IL1-β secretion [7,8].
Recently, we and others uncovered a new vesicular mechanism which suggests the capture of a particular cargo, an acyl-CoA binding protein known as Acb1 in yeasts (or AcbA in Dictyostelium discoideum and ACBP in mammals), into autophagosomes, which fuse with multivesicular bodies (MVBs) to form amphisomes, followed by the fusion of amphisomes with the plasma membrane [9,10]. We discuss below the evidence for this new mechanism involving autophagy, suggest additional experiments needed to strengthen it and then explore whether this mechanism could play a broader role in the unconventional secretion of other proteins.
The process of autophagy
To understand how autophagy is involved in unconventional secretion it is necessary to consider briefly how this process occurs. Studies in yeast have made significant contributions to our present understanding of the autophagy pathway. The discovery of approximately 34 autophagy-related genes (ATG) has shed light on how autophagy is orchestrated [11]. Although basal autophagy exists in many cells, it is generally triggered as a response to nutritional starvation and is suppressed under nutrient-rich conditions primarily by the Target of rapamycin (Tor) [12] and cAMP-dependent Protein Kinase A (PKA) [13] signaling pathways in yeast. Two modes of autophagy have been identified, microautophagy and macroautophagy. The former involves invaginations of the yeast vacuole (lysosomes, in higher eukaryotes) membrane to sequester cytosolic constituents for vacuolar degradation. Macroautophagy (herein referred to as autophagy) has been investigated in greater detail and involves the formation of a double-membrane structure, the crescent-shaped isolation membrane, which eventually expands to form a double-membrane vesicle called the autophagosome that engulfs a portion of cytosol. The outer membrane of each autophagosome then fuses with the vacuolar membrane, delivering the single-membrane-enclosed “autophagic body” into the vacuolar lumen for degradation. In the case of mammalian cells, autophagosomes fuse with endosomes and/or MVBs to form amphisomes, which then fuse with the lysosomes to generate autolysosomes [14]. Alternatively, autophagosomes can also fuse directly with lysosomes to form autolysosomes. Further breakdown of autophagic bodies by vacuolar degradative enzymes, such as hydrolases, results in the generation of basic nutrient pools of amino acids, nucleotides, sugars and lipids for further use by the cell (degradation of bulk cytosol in the vacuole/lysosome, Figure 1) [15,16].
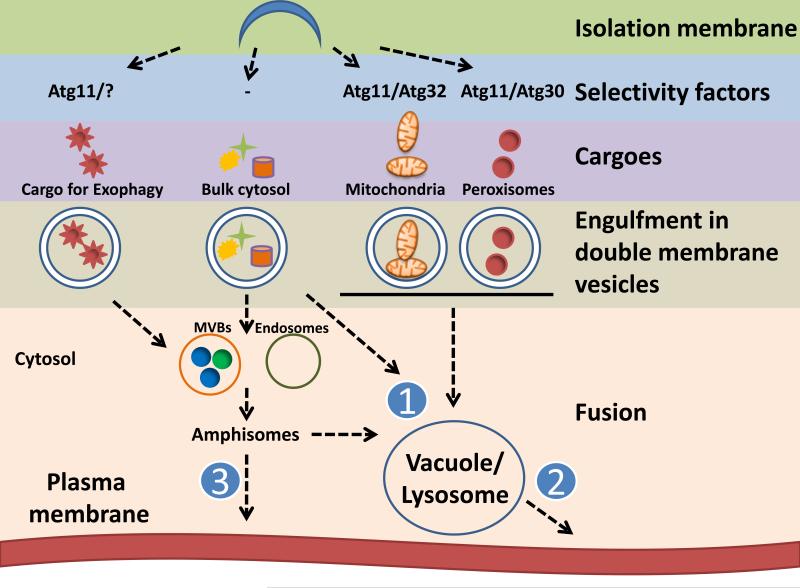
Figure 1. Autophagic delivery of cargoes.
Constitutive autophagy or its induction leads to formation and expansion, from the PAS, of an isolation membrane that begins to engulf non-specific (bulk cytosol) or specific cargo (e.g. Acb1 and organelles). Selectivity factors such as Atg30 (Atg11, not shown) and Atg32 direct the capture of cargoes, such as peroxisomes and mitochondria, respectively. While bulk autophagy may not require such factors, determinants of packaging of Acb1 (that might interact with Atg11) are unknown. The fates of these diverse cargoes within autophagosome-like vesicles could be either (1) degradation through vacuolar fusion (bulk and selective autophagy) either directly with autophagosomes or with amphisomes, or via direct fusion of autophagosomes with the plasma membrane. Alternatively, (2) degraded proteins could be used for antigen presentation, or (3) autophagosome-derived vesicles with cargoes, such as Acb1, could generate amphisomes that fuse with the plasma membrane, causing cargo release to the extracellular medium.
Autophagy-related pathways
Although autophagy degrades cytoplasmic constituents non-selectively, related pathways target selective cargoes for lysosomal turnover. These pathways employ the core autophagic machinery required for the formation of autophagosomes, but specificity is generally achieved by the interaction of soluble “receptor” proteins with the cargo on the one hand and with the autophagic machinery on the other. Additionally in yeast, Atg11 is necessary to assemble the phagophore assembly site (PAS, where the isolation membrane originates) for the engulfment of selective cargoes [17]. The absence of these receptor proteins or Atg11 typically does not perturb general autophagy, but the degradation of selective cargoes is severely affected [18]. Thus, in the case of the cytosol to vacuole transport (Cvt) pathway, cargoes such as prApe1, alpha-mannosidase (Ams1) and misfolded plasma membrane H(+)-ATPase (Pma1) are transported via autophagosome-like vesicular intermediates (Cvt vesicles) to the vacuole and require the receptor protein, Atg19 [19]. Similarly, the specific degradation of mitochondria (mitophagy) requires the mitochondrial protein Atg32 [20,21], while the phosphoprotein Atg30 plays a key role in targeting peroxisomes for selective autophagic degradation (pexophagy) in the yeast Pichia pastoris (Figure 1) [22]. Importantly, the Cvt, pexophagy and mitophagy pathways all need Atg11 [20,21,23,24]. The mechanistic steps involved in autophagy-related pathways are summarized in Box 1.
Classical autophagy has been characterized primarily as a degradative pathway required for survival during starvation and for innate immunity. However, as described below, a growing body of evidence suggests that all autophagy pathways do not necessarily lead towards complete degradation of macromolecules and their recycling, but also have other important cellular functions, ranging from antigen presentation to unconventional protein secretion (Figure 1).
Role of autophagy in unconventional protein secretion
Acyl-CoA binding protein is a highly conserved protein of approximately 10 kDa that lacks a signal peptide and is known to be secreted in an unconventional manner [25]. Yeast Acb1 serves as an excellent model to characterize one of multiple unconventional secretory pathways suggested to use vesicular carriers [9,10]. Acb1 secretion in yeast is regulated; it is released as a single pulse within approximately 4 hours either after nitrogen starvation or in response to the induction of autophagy by rapamycin. Recently, rapamycin-induced rapid release of the related mammalian protein, ACBP, has also been demonstrated in primary astrocytes [26]. Genetic evidence using gene deletions showed that the secretion of Acb1 is dependent on the Golgi reassembly and stacking protein (GRASP) and on several core autophagy machinery proteins such as Atg1, Atg6 and Atg8 that are necessary for autophagosome formation [10]. Based on these data, it was suggested that Acb1 is captured into autophagosomes as a necessary step in its unconventional secretion. Supporting this suggestion is a recent study in Dictyostelium showing that AcbA is in a vesicular fraction, although the nature of this vesicle was undefined [27]. Acb1 secretion also requires peroxisomally-derived medium chain fatty-acyl-CoA (MCFA-CoA) either to bind to Acb1 or for some step of Acb1 vesicle formation [10].
Emerging evidence for different fates of autophagosomes and Acb1 vesicles
If yeast Acb1 is indeed captured into autophagosomes, how do the contents of these autophagosomes end up being secreted outside the cells, and could such a mechanism operate also in mammalian cells? We address this question first in yeast and then deal with the possibility that it might occur also in mammalian cells.
Genetic analyses elucidating the fate of Acb1 vesicles in yeast revealed that the proteins involved in autophagosome-vacuole fusion (Vam7 and Ypt7), which would correspond to the normal fate of autophagosomes during autophagy, are unnecessary for Acb1 secretion. However, proteins required for autophagosome closure (Tlg2), for fusion of vesicles (perhaps including autophagosomes) with endosomes (Ypt6), and for the formation of MVBs (Vps4 and Vps23) are necessary for Acb1 secretion [9]. This requirement of endosomes and/or MVBs was interpreted to mean that Acb1 vesicles fuse with endosomes and/or MVBs to generate amphisomes (as in mammalian cells). An alternative mechanism by which such amphisomes might be generated would be by the capture of Acb1 into endosomes by a microautophagic process. Irrespective of how exactly these putative amphisomes are generated, they are proposed to subsequently fuse with the plasma membrane, as deduced from the genetic observation that Acb1 secretion in yeast requires the t-SNARE, Sso1, and a phospholipase D (Spo14) required to load subunits of the Sso1 SNARE complex onto the plasma membrane [9,10]. As a consequence of the fusion event suggested by the model, it is exosomes containing Acb1 that would be secreted, and it is presumed that they would rupture extracellularly to release Acb1, as has been reported in low pH environments [28]. Alternatively, the Acb1 vesicles would have to rupture within amphisomes prior to the fusion with the plasma membrane. This pathway for unconventional secretion of Acb1 has been dubbed ‘exophagy’ (Figure1) [29].
Further tests of the exophagy model for Acb1 secretion
The proposed model for exophagy in yeast is based primarily on genetic evidence showing the requirement of particular proteins for this process (see Table 2). These genetic data have been interpreted in terms of a hypothetical model for which additional evidence will be needed. First, the requirement of Atg proteins for autophagosome formation (e.g. Atg1, Atg5, Atg7, Atg8, etc), and for selective autophagy (Atg11) has been interpreted to mean that Acb1 is selectively captured into autophagosomes. Proof of this requires evidence that Acb1 is transiently in double-membrane Acb1 vesicles in cells. However, this has been technically difficult to prove biochemically because of several factors. First, only about 5% or less of the intracellular pool of Acb1/AcbA/ACBP is secreted in yeast, astrocytes and Dictyostelium, respectively, making it difficult to distinguish true signal from noise associated with a membranous fraction [9,10,26,30]. However, the low efficiency of secretion of Acb1 should not be equated with physiological irrelevance because Acb1 secretion, even at this level, is clearly required to stimulate sporulation in both yeast and Dictyostelium [10,31]. Second, the co-existence of a large cytosolic pool of Acb1 and a small potentially vesicle-associated pool would render it difficult to clearly define an autophagosomal pool by immunoelectron microscopy, although this might be revealed in mutants that accumulate Acb1 containing vesicles. This has not been attempted. Finally, at least in P. pastoris, the detection of Acb1 awaits the availability of antibodies to this protein to follow it biochemically.
Table 2.
| Protein | Protein function |
|---|---|
| Grh1 | Unconventional secretion |
| Bug1 | Interacts with Grh1 |
| Sec18 | Intracellular membrane fusion |
| Atg1 | Autophagosome formation |
| Atg5 | Autophagosome formation |
| Atg6 | Autophagosome formation |
| Atg7 | Autophagosome formation |
| Atg8 | Autophagosome formation |
| Atg9 | Autophagosome formation |
| Atg11 | Autophagosome formation with selective cargoes |
| Atg12 | Autophagosome formation |
| Ypt6 | Endosomal sorting |
| Tlg2 | Autophagosome completion |
| Vps23 | MVB sorting |
| Vps4 | MVB sorting |
| Sso1 | Exocytic vesicle fusion with plasma membrane |
| Spo14 | Exocytic vesicle fusion with plasma membrane |
| Pex3 | Peroxisome membrane biogenesis |
| Pex8 | Peroxisomal matrix protein import |
| Pex5 | Peroxisomal matrix protein import, PTS1 receptor |
| Pex11 | Medium chain fatty acid transport from cytosol to peroxisome matrix |
| Faa2 | Medium chain fatty Acyl-CoA synthesis in peroxisome matrix |
The requirement for these proteins in the unconventional secretion of Acb1 in yeast was determined primarily using mutants either deleted for the corresponding genes, or expressing temperature-sensitive versions of these proteins. The functions of these proteins are based on their involvement in cellular processes based on previously published studies.
A key tenet of the exophagy model, however, is indeed that Acb1 is in intracellular vesicles, and that such vesicles would accumulate in mutants (such as GRASP-deficient cells) in which Acb1 vesicle (in amphisomes) fusion with the plasma membrane is blocked. While this has not been demonstrated as yet in yeast, it has been documented in Dictyostelium cells lacking GRASP, and furthermore these vesicles have been shown to accumulate just next to the plasma membrane where they would be expected to reside if the final fusion step with the plasma membrane is blocked [27]. However, these vesicles would need to be characterized further in Dictyostelium for the colocalization of Acb1 with MVB or endosomal markers to show that they are amphisomes. To date, amphisomes have not been described in yeast.
Exosomes have been characterized in yeast but not under conditions of Acb1 release [32] but this is clearly feasible. Furthermore, the site and mechanism of Acb1 release from Acb1 vesicles remains unclear. The various possibilities include: a) rupture of Acb1 vesicles in amphisomes prior to fusion with the plasma membrane, or b) release of Acb1 from exosomes. Although exosomes from mammalian cells appear to be stable and have been purified and characterized from various cell types, it is possible that other signals such as low extracellular pH could contribute to the rupture and release of their contents [28], but these possibilities need further investigation.
Interestingly, while many of the proposed steps in the exophagy pathway of yeast have not been biochemically or morphologically documented in this organism, these processes including autophagosome fusion with endosomes and/or MVBs to generate amphisomes and fusion of amphisomes with the plasma membrane, has been documented in mammalian cells, but they have not been linked to a pathway involving secretion of unconventional proteins. We document below the evidence for these early and late steps of the exophagy pathway and suggest that this might be an evolutionarily-conserved process that could also exist in yeast.
Autophagosome-endosome fusion in mammalian cells
Is there support in other systems for an intersection between the autophagy and endosomal pathways? Indeed, there is ample evidence that in mammalian cells the formation of amphisomes depends on endosome-autophagosome fusion [33-37]. Furthermore, specific protein requirements for the formation of amphisomes by fusion of autophagosomes and MVBs have also been studied, for example in the human leukemic cell line, K562 [38]. Rab11, located on the MVBs, is necessary, along with the v-SNARE proteins Vamp3/cellubrevin for the membrane fusion event that generates these amphisomes [39]. Another v-SNARE, Vamp7, and N-ethyl maleimide-sensitive factor (NSF), are required for amphisome/lysosome fusion, as well as for MVB fusion with the plasma membrane [39].
The fusion of autophagosomes with early and late endosomes allows autophagosomes to mature into degradative autophagic vacuoles or amphisomes [40]. The fusion between autophagosomes and both early and late endosomes has been reconstituted in vitro [37]. The SNARE, Vti1, is required for the fusion of autophagosomes with the late endosomes and the small GTPase, Rab7, is necessary for the fusion of degradative autophagic vacuoles with lysosomes [33].
Amphisome-plasma membrane fusion in mammalian cells
In mammalian cells, autophagosomes as well as amphisomes, can under certain circumstances bypass fusion with lysosomes, and fuse instead with the plasma membrane. This would release single-membrane enclosed exosomes to the exterior of cells. A clue regarding what activates this alternative route might be differentiation programs or stress conditions, such as nitrogen starvation. For example, in the human K562 cells mentioned above, the activation of a differentiation program causes subcellular organelles to be captured into autophagosomes, which would normally be delivered to lysosomes via amphisomes. However, these cells also exhibit fusion of their MVBs with the plasma membrane, so it seems plausible that some amphisomes derived from MVBs might fuse with the plasma membrane to release exosomes. Direct evidence of such amphisome/plasma membrane fusion might be obtained by in vitro studies or genetic studies using knockdown of candidate proteins involved in this process. During Acb1 secretion in yeast, nitrogen starvation, or the induction of autophagy by rapamycin, triggers Acb1 capture into autophagosomes, which are proposed to transition via amphisomes to the plasma membrane for secretion.
It will be interesting to test whether some common mechanism, such as elevated intracellular calcium, diverts the fate of autophagosomes towards amphisomes and perhaps extracellular secretion. Support for this idea comes from the fact that induction of autophagy, by either starvation or rapamycin, stimulates autophagosome fusion with MVBs even in K562 cells [38]. Interestingly, autophagy induction causes an elevation of calcium in autophagic compartments, and calcium chelators inhibit autophagosome-MVB fusion [38].
Thus, since there is evidence for the individual steps of the proposed exophagy pathway in mammalian cells, it should be fairly easy to ask, using inhibitors of autophagosome formation, whether any of the mammalian proteins secreted in an unconventional manner might be dependent on autophagy. If so, mammalian homologs of the yeast candidate proteins might be knocked down to see if they play a broader role in other unconventional secretion pathways.
Evolutionary advantages of unconventional secretion
One might ask why cells need multiple modes of secretion and what evolutionary pressures preserve these mechanisms. We argue that the following possibilities could offer an answer to this issue.
Avoidance of detrimental cellular environments or modifications
Release of proteins in the form of vesicles protects the contents against extracellular degradation by agents such as proteases. Such exosomes, released extracellularly after fusion with plasma membrane, serve as intercellular communication vehicles [41,42]. Additionally, some unconventionally secreted proteins, when routed via the secretory pathway acquire modifications that block their normal function [43].
Bypassing conditions where conventional secretion is either blocked or inefficient
Toxins or drugs that paralyze the conventional pathway could be lethal if all cellular proteins followed the conventional secretory route. However, studies have shown that several proteins are secreted unconventionally even upon treatment with Brefeldin A, a fungal antibiotic that inhibits ER to Golgi transport [44-46]. Additionally, during starvation, cells are blocked in conventional secretion [47,48], but may need to use unconventional mechanisms for cell-cell communication using proteins and peptides secreted by other mechanisms. Thus cell survival and/or communication during such situations is perhaps still possible due to an alternate secretion pathway.
Co-factor access and transport of folded proteins
Certain unconventionally secreted proteins employing either vesicular or non-vesicular modes require binding to specific lipids for efficient secretion (e.g. FGF2, Tubby, Acb1, Tat). In the case of FGF2 and HIV-Tat protein, binding to PI(4,5)P2 recruits them to the plasma membrane - a first step in membrane translocation for their efficient unconventional secretion [49,50]. FGF2 is then secreted directly across the plasma membrane in a folded conformation after binding to PI(4,5)P2 associated with the inner leaflet of plasma membrane [51]. MCFA-CoA derived from peroxisomes is required for the unconventional secretion of Acb1 [10] and acyl-CoA binding is a prerequisite for the Dictyostelium AcbA to be incorporated into vesicles [27]. Unconventional secretion pathways such as the non-vesicular mode used by FGF2, Tubby and Tat or the vesicular mechanism proposed for Acb1 may provide access to such cofactors more readily.
Concluding remarks
The induction of unconventional secretion of Acb1 by starvation or conditions that induce autophagy, and the novel role of autophagy proteins in unconventional secretion, suggest that there is an evolutionarily conserved mechanistic link between the two processes. The inhibition of normal conventional secretion during stressed conditions, such as starvation, might activate unconventional secretion pathways as the principal mode of intercellular communication using secreted proteins. Intriguingly, only a small fraction of the unconventionally secreted proteins, such as Acb1 and Engrailed, are released extracellularly. Mechanisms that govern this remain completely unanswered. These studies also raise a number of important questions for future research (Box 2). A greater understanding of the mechanism of unconventional secretion will help address the important question of whether unconventional secretion preceded the evolution of conventional secretion pathways or whether the two processes co-evolved.
Box 1 – Molecular mechanism of autophagy and autophagy-related pathways (based on studies in yeast).
Cargo recognition - Selective autophagy involves receptor proteins that engage selective cargoes, such as proteins or organelles, to target them for lysosomal degradation. The receptors engage the autophagic machinery through interactions with both the cargo and components of the autophagy machinery. For example, Atg19 is the yeast receptor for the Cvt pathway, interacting with its cargo, prApe1 and with the autophagy proteins, Atg11 and Atg8 [24]. Atg30 is the peroxisome receptor that binds Pex3 and Pex14 on the peroxisome membrane and also interacts with the autophagy proteins Atg11 and Atg17 [22].
Regulation of induction - Initiation of autophagy involves relief from the suppressive effects of the Tor [12] and PKA [13] pathways, which negatively regulate autophagy. Upon induction of starvation, the characteristic events seen are the dephosphorylation of the phosphoproteins Atg1 and Atg13, resulting in their interaction. These proteins further associate with other autophagy proteins like Atg17, Atg20, Atg24, Atg29 and Atg31 forming the Atg1 kinase complex [52].
Vesicle nucleation, expansion and completion - A complex of 4 proteins – Vps15, Vps34 [the catalytic subunit of phosphatidylinositol (PI) 3 kinase], Atg14 and Atg6 – acts at the PAS, where Vps34 generates PI3 phosphate (PI3P) to nucleate assembly and expansion of the isolation membrane. This process is facilitated by the recruitment of PI3P-binding proteins to this site to enable membrane expansion [53].
The expansion of the isolation membrane involves two ubiquitin-like proteins, Atg8 and Atg12. Both of these proteins undergo modifications [16]. Thus Atg8 is coupled to phosphatidylethanolamine (PE), while Atg12 is conjugated to Atg5, using E1-, E2- and E3-like enzymes analogous to those used for classical ubiquitin-conjugation reactions. Atg5 binds to Atg16 leading to the formation of a multimeric complex. The formation of autophagosomes or pexophagosomes relies on this step [16].
Retrieval - Many protein components required for autophagosome formation (e.g. Atg9, Atg12-Atg5, Atg16, etc) are retrieved from the autophagosomal membrane as vesicle completion occurs [54,55]. Atg8-PE conjugated to the outer autophagosome membrane is removed by the proteolytic activity of Atg4, whereas Atg8-PE associated with the inner autophagosome membrane is degraded in the vacuole.
Vesicle docking and fusion, vesicle breakdown and nutrient recycling – Autophagosomes fuse with the vacuole membrane to deliver autophagic bodies into the vacuole. Vacuolar hydrolases break down the autophagic bodies and recycle the components (reviewed in [56]).
Box 2 - Unanswered Questions.
What are the other proteins involved in the unconventional secretion of Acb1 in yeast and other organisms?
What biochemical roles do the proteins/genes involved in the unconventional secretion of Acb1 in yeast play and can the vesicular intermediates in this process be shown biochemically and/or morphologically?
How exactly are autophagosomes containing unconventionally-secreted proteins diverted to the plasma membrane, while autophagosomes containing autophagic cargo are still targeted for fusion with vacuoles/lysosomes?
What is the mechanism by which unconventionally secreted proteins are released either within amphisomes or extracellularly?
Is autophagy involved broadly in the unconventional secretion of other proteins?
Are there human diseases in which exophagy is compromised?
Acknowledgement
This work was supported by an NIH grant GM 069373 to SS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nickel W, Seedorf M. Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu. Rev. Cell Dev. Biol. 2008;24:287–308. doi: 10.1146/annurev.cellbio.24.110707.175320. [DOI] [PubMed] [Google Scholar]
- 2.Nickel W. Pathways of unconventional protein secretion. Curr. Opin. Biotechnol. 2010 doi: 10.1016/j.copbio.2010.06.004. doi:10.1016/j.copbio.2010.1006.1004. [DOI] [PubMed] [Google Scholar]
- 3.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 4.McGrath JP, Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989;340:400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- 5.Becker CE, et al. Rab39a binds caspase-1 and is required for caspase-1-dependent interleukin-1beta secretion. J. Biol. Chem. 2009;284:34531–34537. doi: 10.1074/jbc.M109.046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu Y, et al. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 7.Thomas LM, Salter RD. Activation of macrophages by P2X7-induced microvesicles from myeloid cells is mediated by phospholipids and is partially dependent on TLR4. J. Immunol. 2010;185:3740–3749. doi: 10.4049/jimmunol.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelegrin P, et al. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J. Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 9.Duran JM, et al. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 2010;188:527–536. doi: 10.1083/jcb.200911154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manjithaya R, et al. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J. Cell. Biol. 2010;188:537–546. doi: 10.1083/jcb.200911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todde V, et al. Autophagy: principles and significance in health and disease. Biochim. Biophys. Acta. 2009;1792:3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 13.Budovskaya YV, et al. The Ras/cAMP-dependent protein kinase signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae. J. Biol. Chem. 2004;279:20663–20671. doi: 10.1074/jbc.M400272200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fader CM, Colombo MI. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 2009;16:70–78. doi: 10.1038/cdd.2008.168. [DOI] [PubMed] [Google Scholar]
- 15.Nakatogawa H, et al. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 16.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 17.Geng J, Klionsky DJ. Quantitative regulation of vesicle formation in yeast nonspecific autophagy. Autophagy. 2008;4:955–957. doi: 10.4161/auto.6791. [DOI] [PubMed] [Google Scholar]
- 18.Yu L, et al. The selectivity of autophagy and its role in cell death and survival. Autophagy. 2008;4:567–573. doi: 10.4161/auto.5902. [DOI] [PubMed] [Google Scholar]
- 19.Lynch-Day MA, Klionsky DJ. The Cvt pathway as a model for selective autophagy. FEBS Lett. 2010;584:1359–1366. doi: 10.1016/j.febslet.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto K, et al. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev. Cell. 2009;17:87–97. doi: 10.1016/j.devcel.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Kanki T, et al. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev. Cell. 2009;17:98–109. doi: 10.1016/j.devcel.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farre JC, et al. PpAtg30 tags peroxisomes for turnover by selective autophagy. Dev. Cell. 2008;14:365–376. doi: 10.1016/j.devcel.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nazarko TY, et al. Peroxisome size provides insights into the function of autophagy-related proteins. Mol. Biol. Cell. 2009;20:3828–3839. doi: 10.1091/mbc.E09-03-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yorimitsu T, Klionsky DJ. Atg11 links cargo to the vesicle-forming machinery in the cytoplasm to vacuole targeting pathway. Mol. Biol. Cell. 2005;16:1593–1605. doi: 10.1091/mbc.E04-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinseth MA, et al. The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell. 2007;130:524–534. doi: 10.1016/j.cell.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Loomis WF, et al. Pregnenolone sulfate and cortisol induce secretion of acyl coa binding protein and its conversion into endozepines from astrocytes. J. Biol. Chem. 2010;285:21359–21365. doi: 10.1074/jbc.M110.105858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabral M, et al. Unconventional secretion of AcbA in Dictyostelium discoideum through a vesicular intermediate. Eukaryot. Cell. 2010;9:1009–1017. doi: 10.1128/EC.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taraboletti G, et al. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006;8:96–103. doi: 10.1593/neo.05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrahamsen H, Stenmark H. Protein secretion: unconventional exit by exophagy. Curr. Biol. 2010;20:R415–418. doi: 10.1016/j.cub.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Anjard C, Loomis WF. Peptide signaling during terminal differentiation of Dictyostelium. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7607–7611. doi: 10.1073/pnas.0501820102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anjard C, et al. Steroids initiate a signaling cascade that triggers rapid sporulation in Dictyostelium. Development. 2009;136:803–812. doi: 10.1242/dev.032607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues ML, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell. 2008;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jager S, et al. Role for Rab7 in maturation of late autophagic vacuoles. J. Cell Sci. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 34.Razi M, et al. Early endosomes and endosomal coatomer are required for autophagy. J. Cell Biol. 2009;185:305–321. doi: 10.1083/jcb.200810098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon PB, Seglen PO. Prelysosomal convergence of autophagic and endocytic pathways. Biochem. Biophys. Res. Commun. 1988;151:40–47. doi: 10.1016/0006-291x(88)90556-6. [DOI] [PubMed] [Google Scholar]
- 36.Liou W, et al. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J. Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morvan J, et al. In vitro reconstitution of fusion between immature autophagosomes and endosomes. Autophagy. 2009;5:676–689. doi: 10.4161/auto.5.5.8378. [DOI] [PubMed] [Google Scholar]
- 38.Fader CM, et al. Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in K562 cells. Traffic. 2008;9:230–250. doi: 10.1111/j.1600-0854.2007.00677.x. [DOI] [PubMed] [Google Scholar]
- 39.Fader CM, et al. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim. Biophys. Acta. 2009;1793:1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Berg TO, et al. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J. Biol. Chem. 1998;273:21883–21892. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- 41.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Thery C, et al. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 43.Wegehingel S, et al. Rerouting of fibroblast growth factor 2 to the classical secretory pathway results in post-translational modifications that block binding to heparan sulfate proteoglycans. FEBS Lett. 2008;582:2387–2392. doi: 10.1016/j.febslet.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 44.Lecellier CH, et al. Intra- and intercellular trafficking of the foamy virus auxiliary bet protein. J. Virol. 2002;76:3388–3394. doi: 10.1128/JVI.76.7.3388-3394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toyokawa K, et al. Cellular localization and function of the antiviral protein, ovine Mx1 (oMx1): I. Ovine Mx1 is secreted by endometrial epithelial cells via an ‘unconventional’ secretory pathway. Am. J. Reprod. Immunol. 2007;57:13–22. doi: 10.1111/j.1600-0897.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J, et al. Insulin-degrading enzyme is exported via an unconventional protein secretion pathway. Mol. Neurodegener. 2009;4:4. doi: 10.1186/1750-1326-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geng J, et al. Post-Golgi Sec proteins are required for autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell. 2010;21:2257–2269. doi: 10.1091/mbc.E09-11-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shorer H, et al. Modulation of N-ethylmaleimide-sensitive factor activity upon amino acid deprivation. J. Biol. Chem. 2005;280:16219–16226. doi: 10.1074/jbc.M500554200. [DOI] [PubMed] [Google Scholar]
- 49.Rayne F, et al. Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J. 2010;29:1348–1362. doi: 10.1038/emboj.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Temmerman K, et al. A direct role for phosphatidylinositol-4,5-bisphosphate in unconventional secretion of fibroblast growth factor 2. Traffic. 2008;9:1204–1217. doi: 10.1111/j.1600-0854.2008.00749.x. [DOI] [PubMed] [Google Scholar]
- 51.Torrado LC, et al. An intrinsic quality-control mechanism ensures unconventional secretion of fibroblast growth factor 2 in a folded conformation. J. Cell Sci. 2009;122:3322–3329. doi: 10.1242/jcs.049791. [DOI] [PubMed] [Google Scholar]
- 52.Cheong H, et al. The Atg1 kinase complex is involved in the regulation of protein recruitment to initiate sequestering vesicle formation for nonspecific autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell. 2008;19:668–681. doi: 10.1091/mbc.E07-08-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kihara A, et al. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Legakis JE, et al. A cycling protein complex required for selective autophagy. Autophagy. 2007;3:422–432. doi: 10.4161/auto.4129. [DOI] [PubMed] [Google Scholar]
- 55.Reggiori F, et al. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev. Cell. 2004;6:79–90. doi: 10.1016/s1534-5807(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 56.Farre JC, et al. Turnover of organelles by autophagy in yeast. Curr. Opin. Cell Biol. 2009;21:522–530. doi: 10.1016/j.ceb.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prudovsky I, et al. The intracellular translocation of the components of the fibroblast growth factor 1 release complex precedes their assembly prior to export. J. Cell Biol. 2002;158:201–208. doi: 10.1083/jcb.200203084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schafer T, et al. Unconventional secretion of fibroblast growth factor 2 is mediated by direct translocation across the plasma membrane of mammalian cells. J. Biol. Chem. 2004;279:6244–6251. doi: 10.1074/jbc.M310500200. [DOI] [PubMed] [Google Scholar]
- 59.Rubartelli A, et al. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooper DN, Barondes SH. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J. Cell Biol. 1990;110:1681–1691. doi: 10.1083/jcb.110.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gardella S, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rubartelli A, et al. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J. Biol. Chem. 1992;267:24161–24164. [PubMed] [Google Scholar]
- 63.Joliot A, et al. Identification of a signal sequence necessary for the unconventional secretion of Engrailed homeoprotein. Curr. Biol. 1998;8:856–863. doi: 10.1016/s0960-9822(07)00346-6. [DOI] [PubMed] [Google Scholar]
- 64.Chang HC, et al. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Caberoy NB, Li W. Unconventional secretion of tubby and tubby-like protein 1. FEBS. Lett. 2009;583:3057–3062. doi: 10.1016/j.febslet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]