Abstract
Dystonia is an involuntary movement disorder characterized by repetitive patterned or sustained muscle contractions causing twisting or abnormal postures. Several lines of evidence suggest that abnormalities of dopaminergic pathways contribute to the pathophysiology of dystonia. In particular dysfunction of D2-like receptors that mediate function of the indirect pathway in the basal ganglia may play a key role. We have demonstrated with positron emission tomography (PET) that patients with primary focal cranial or hand dystonia have reduced putamenal specific binding of [18F]spiperone a non-selective D2-like radioligand with nearly equal affinity for serotonergic 5-HT(2A) sites. We then repeated the study with [18F]N-methyl-benperidol (NMB), a more selective D2-like receptor radioligand with minimal affinity for 5-HT(2A). Surprisingly, there was no decrease in NMB binding in the putamen of subjects with dystonia. Our findings excluded reductions of putamenal uptake greater than 20% with 95% confidence intervals. Following analysis of the in vitro selectivity of NMB and spiperone demonstrated that NMB was highly selective for D2 receptors relative to D3 receptors (200-fold difference in affinity), whereas spiperone has similar affinity for all three of the D2-like receptor subtypes. These findings coupled with other literature suggest that a defect in D3, rather than D2, receptor expression may be associated with primary focal dystonia.
Keywords: dystonia, dopamine, NMB, D2-like dopamine receptors, D3 dopamine receptor, PET
Introduction
Dystonia is an involuntary movement disorder characterized by repetitive patterned or sustained muscle contractions causing twisting or abnormal postures.1 Several lines of evidence suggest that abnormalities of dopaminergic pathways contribute to the pathophysiology of dystonia. Dystonia can be the initial manifestation in some patients with Parkinson disease (PD) which respond to L-dopa.2,3 Patients with PD can develop dystonia as a side effect of L-dopa treatment.4 Hereditary forms of dystonia due to abnormal synthesis of dopamine respond to treatment with L-dopa.5 Further, a mutation in the gene coding for D5 receptors might be a susceptibility factor for cervical dystonia.6 Some people with familial dystonia have reduced [18F]dopa uptake in putamen.7 Finally, dopamine release may be impaired in a mouse model of DYT1dystonia8 and is also diminished in a non-human primate model of transient dystonia.9
In particular, dysfunction of D2-like receptors that mediate function of the indirect pathway in the basal ganglia may play a key role.10,11 Exposure to neuroleptics that block D2-like receptors can cause acute dystonia and long term treatment with these drugs can produce tardive dystonia.12–14 Non-manifesting carriers of DYT1 have decreased D2-like binding.11 Further, non-human primates treated with intra-carotid MPTP developed transient dystonia that corresponded to a decrease in D2-like striatal receptors.15
We demonstrated that patients with primary focal hand or cranial dystonia have a reduction of the putamenal specific binding of [18F]spiperone, a non-selective D2-like radioligand with nearly equal affinity to serotonergic 5-HT(2A) receptors.10,16 In contrast, [18F]N-methyl-benperidol (NMB) is a more specific D2-like receptor ligand with minimal affinity for 5-HT(2A) or D1-like receptors.17
A goal of this study was to determine whether patients with primary focal hand or cranial dystonia have reduced striatal D2-like dopamine receptor binding as measured with the more selective positron emission tomography (PET) radioligand [18F]NMB. This would exclude the possibility that a defect in 5-HT(2A) receptors accounted for the previous findings with [18F]spiperone. Given the unanticipated finding that PET studies with [18F]NMB demonstrated no difference in receptor binding within dystonic patients and controls, we proceeded to investigate the subtype selectivity of NMB and spiperone for dopaminergic receptors.
Methods
Subjects
Twenty four subjects with primary cranial or arm dystonia were included (age: 52±11; 16 women, 8 men). No subject had evidence of a secondary cause of dystonia as assessed by history or physical examination. No one had a history of exposure to dopamine antagonistic or dopaminergic medication (except for a few weeks trial of levodopa at least one month prior to study), reserpine, tetrabenazine. Treatment with botulinum toxin was not excluded. The eleven subjects with a history of botulinum toxin exposure had received the injection within 3 months preceding the study. Twenty-two individuals without any neurologic or psychiatric history served as normal controls (age: 49± 14; 14 women, 8 men). Six subjects in the dystonic and six in the control group reported a history of smoking. All subjects underwent Mini-Mental Status examination and Hamilton Depression Inventory.18,19
These studies were approved by the Washington University Human Research Protection Office and the Radioactive Drug Research Committee. Each participant provided written informed consent.
Radiopharmaceutical preparation
[18F]NMB was synthesized from [18F]fluoride using a three step procedure.20 The radiopharmaceutical had a radiochemical purity exceeding 95% and a specific activity ≥1000 Ci/mmol. The in vivo kinetics and radiation dosimetry of [18F]NMB are published elsewhere.17,21
MRI procedure
All subjects had 3-D MPRAGE MRI scans of the brain on a Siemens Magnetom Vision 1.5 T scanner. These scans were used for identification of volumes of interest.
PET procedure
Most PET studies were performed using a Siemens/CTI ECAT 953B scanner; five (normals) were performed using a Siemens/CTI ECAT EXACT HR. All studies were acquired in 3D mode and reconstructed using measured attenuation and model-based scatter correction followed by Gaussian filtering to a common resolution of 16 mm FWHM in all three dimensions.22,23 We have compared high-count FDG images using a Hoffman 3D phantom on both scanners which were similarly processed and observed no differences.
Subjects were placed within the scanner and attenuation scans were obtained as described elsewhere.24 A maximum of 8 mCi of [18F]NMB was injected over 20 seconds followed by 10 second saline flush. Emission scans were started with the injection and continued for 2 hours. The initial 4 frames were 2 minutes with the remaining frames each lasting 5 minutes.
Data analysis
A blinded observer outlined volumes of interest (VOIs) for the left and right caudate, putamen and cerebellum by following the anatomical outlines on the T1-weighted MRI. All dynamic PET frames were aligned to correct for movement during the scans for each individual. A composite PET was co-registered to Talairach25 atlas with the help of the corresponding MPRAGE.26,27 We used a graphical analysis method with the cerebellum as the reference region to calculate the binding potential (BP). BP is an index of specific binding, as validated for this particular radioligand.28 The BP for each voxel was calculated and the average BP for each VOI was computed.29 The left and right regional values were averaged for each participant.
Statistical analysis
The mean age of the dystonic subjects was not significantly different from the controls (p > 0.5). Nevertheless, since age may be a factor in D2-like uptake in striatum, we corrected the data for age, as done by others.11 Linear regression analysis was used to correlate age with striatal BP values across the normals. We divided the observed BP value in each dystonic subject by the age-predicted normal value. A two tailed unpaired t-test was applied to compare the BP between normal controls and individuals with focal dystonia.11
In Vitro Radioligand binding studies
A filtration binding assay was used to characterize the binding properties of spiperone and NMB at human D2, D3 and D4 dopamine receptors. Competition curves were performed using [125I]-IABN30 and human D2, D3 or D4 dopamine receptors stably expressed in HEK 293 cells. The binding buffer contained 50 mM Tris–HCl, 10 mM EDTA, and 150 mM NaCl at pH 7.4. Nonspecific binding was defined using 4 µM (+)-butaclamol. For competition experiments the radioligand concentration was approximately equal to the Kd value and the concentration of the competitive inhibitor ranged over 5 orders of magnitude. Binding was terminated by the addition of cold wash buffer (10 mM Tris-HCl/150 mM NaCl, pH 7.4) and filtration over a glass-fiber filter (Schleicher and Schuell No. 32). Filters were washed with 10 ml of cold buffer and the radioactivity was quantitated. A Packard Cobra gamma counter was used for 125I-labeled radioligands (efficiency = 75%). The protein concentration was determined using a BCA reagent (Pierce) with bovine serum albumin as the protein standard.
Data from competitive inhibition experiments was modeled using nonlinear regression analysis31 to determine the concentration of inhibitor that inhibits 50% of the specific binding of the radioligand (IC50 value). Competition curves were modeled for a single site using the following equation:
where B is the amount of ligand bound to tissue, Bo is the amount of ligand bound in the absence of competitive inhibitor, L is the concentration of the competitive inhibitor, Bns is the nonspecific binding of the radioligand (defined using a high concentration of a structurally dissimilar competitive inhibitor) and IC50 is the concentration of competitive inhibitor that inhibits 50% of the total specific binding. Data from competition dose response curves were analyzed using Table Curve program (Jandel). IC50 values were converted to equilibrium dissociation constants (Ki values) using the Cheng and Prusoff correction.32
Results
PET Imaging studies
Normals and patients with dystonia were not significantly different in age. All participants scored less than 10 on Hamilton Depression Inventory and greater than 26 on the Mini-Mental Status examination. Additional details are presented in Table 1. The mean BP’s for caudate and for putamen were not significantly different comparing the dystonia group and the normals regardless of age correction (Table 2). The 95% confidence intervals for the difference between the means made it highly unlikely that striatal NMB specific binding was decreased more than 20%.
Table 1.
Characteristics of participants with focal hand or cranial dystonia
| Subjects with dystonia (total) | 24 |
| Mean age (years) | 52±11 |
| Cranial dystonia | 12 |
| Hand dystonia | 12 |
| Blepharospasm alone | 8 |
| Pure writing cramp | 4 |
| Right handedness | 20 |
| Median duration of dystonia (years) | 5 |
| Ever treated with botulinum toxin | 11 |
Table 2.
Mean NMB binding potential in patients with dystonia and normal subjects
| Normals | Dystonics | ||
|---|---|---|---|
| caudate | Age correction | 2.42 ± 0.22 | 2.47 ± 0.28 |
| No age correction |
2.35 ± 0.33 | 2.47 ± 0.35 | |
| putamen | Age correction | 3.12 ±0.26 | 3.18 ± 0.32 |
| No age correction |
3.14 ±0.35 | 3.18 ± 0.42 |
A two tailed unpaired t-test was applied to compare the binding potential between normal controls and individuals with focal dystonia. No significant difference (confidence interval 95%) was detected in the PET measured NMB binding potential with or without age correction. The BP values are mean± standard deviation.
In vitro receptor affinity studies
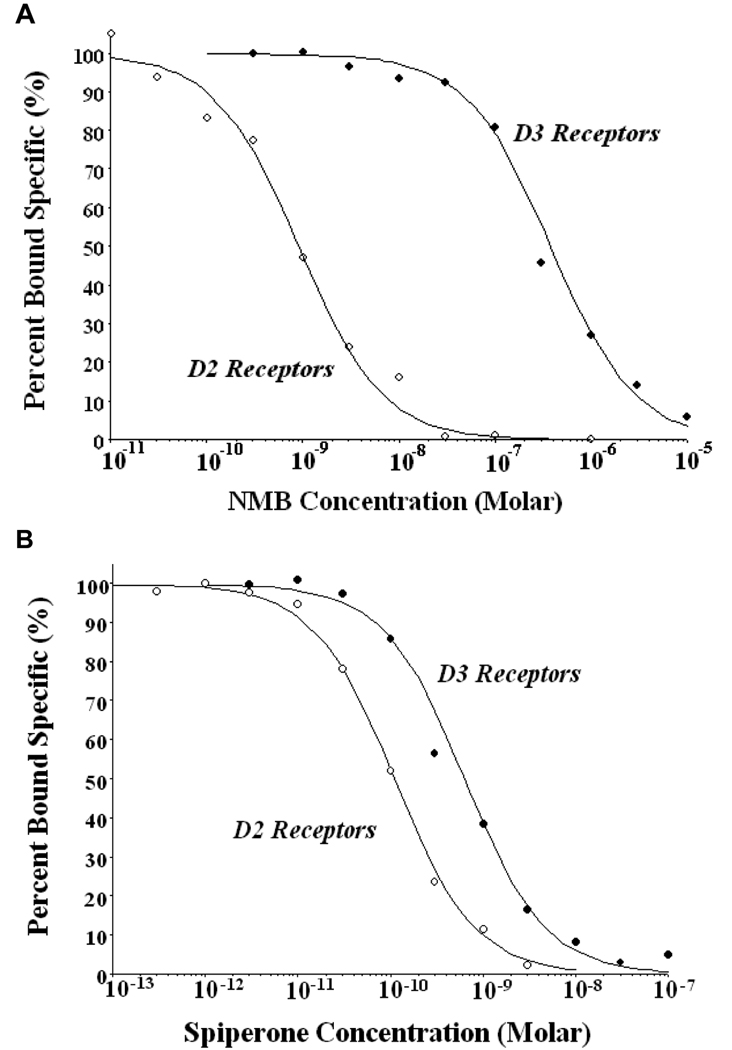
In vitro radioligand binding studies were performed to compare the affinity of NMB and spiperone for D2, D3 and D4 receptors which comprise the D2-like family of dopamine receptors. Figure 1 shows representative competition curves for the binding of these compounds at human D2 and D3 receptors and Table 3 lists the affinity of these ligands for binding to D2, D3 and D4 receptors. Spiperone was found to exhibit approximately 5-fold greater selectivity for D2 compared to D3 receptors and 7-fold greater selectivity for D2 compared to D4 receptor subtype. In contrast, NMB was found to be almost 200-fold selective for D2 relative to D3 receptors but only 8-fold selective compared to D4 dopamine receptor subtype.
Figure 1. Competitive radioligand binding of NMB and spiperone to human D2 and D3 dopamine receptor subtypes in vitro.
Competitive radioligand binding studies were performed to compare the binding affinity and selectivity of spiperone and NMB for human D2 (○) and D3 (●) dopamine receptors. Competition experiments were performed using 125I-IABN and human dopamine receptors expressed in stably transfected HEK-293 cells. Representative competition curves obtained using NMB (A) and spiperone (B) are shown.
Table 3.
Comparison of the affinity of spiperone and NMB for human D2-Like dopamine receptors
| Ki values (nMolar) | ||
|---|---|---|
| Receptors | spiperone | NMB |
| D2 receptors | 0.06 ± 0.01 (18) | 0.58 ± 0.21 (3) |
| D3 receptors | 0.33 ± 0.02 (21) | 114 ± 27 (4) |
| D4 receptors | 0.45 ± 0.01 (4) | 4.9 ± 0.26 (4) |
The affinity (Ki value) for the binding to human D2 and D3 dopamine receptors was obtained using competitive radioligand binding experiments with 125I-IABN. The Ki values are the mean ± the S.E.M. and the number in the parentheses is the number of independent experiments (n). Dopamine receptors were expressed in stably transfected HEK 293 cells.
Discussion
Previous studies report decreased striatal D2-like radioligand uptake in various forms of primary dystonia. These include hand or cranial dystonias, cervical dystonia, DYT1, DYT 6 and DYT11 dystonias regardless of clinical manifestation.11,33–36 In the present study, we found no significant differences between caudate or putamenal [18F]NMB binding in patients with adult-onset primary focal arm or cranial dystonias compared to normals. This conclusion applies to the findings whether or not corrected for age. Several explanations may account for this apparent discrepancy. The simplest issue is whether the current study had sufficient power to detect the magnitude of differences found in other studies. This study was designed to have 90% power to detect a 20% difference in BP between healthy control subjects and those with cranial or arm dystonia (95% confidence intervals). For this reason, it is unlikely that insufficient power is what led to a failure to detect a difference in uptake in the healthy and diseased.
A more plausible explanation for the discrepancy between our study and previous studies is the improved selectivity of the radioligand [18F]NMB. Earlier imaging protocols used either [18F]spiperone10, [11C]raclopride11 or [123I]-iodobenzamide.36 All of these radioligands have lower receptor specificity compared to NMB. The least specific of these ligands is [18F]spiperone which binds to both D2-like and 5-HT(2A) receptors.16 Although [11C]raclopride has much lower 5-HT(2A) affinity than spiperone, it binds with nearly equal affinity to D2 and D3 receptors.37 IBZM is known to have only 12% of raclopride’s affinity to D2-like receptors.37 The specificity analysis in this paper demonstrates that NMB has 200-fold higher affinity for D2 compared to D3 receptors. The D2 specificity of NMB is thus superior to raclopride, which has only an 11-fold greater affinity for binding to D2 receptors38, as well as spiperone, which has only about a 5-fold higher affinity for D2 receptor binding. The high selectivity of NMB for D2 versus D3 and D4 receptors may shed light on the discrepancy between the results of the current study and previous investigations that employed less specific radioligands for PET imaging, including our own study involving [18F]spiperone.
One explanation for the discrepancy between the spiperone and the NMB study could be spiperone’s high affinity for 5HT(2A). However, such interpretation would dismiss the evidence of dopaminergic involvement in various primary and secondary dystonias.2–4,11,33,35,36,39 Further, raclopride studies although mainly performed in genetic forms showed a reduced uptake making serotonergic binding a less likely explanation for the observed discrepancies.33
An alternative explanation for the findings is that there are differences in the levels of endogenous striatal dopamine between dystonics and normals. Due to its relatively low receptor binding affinity, raclopride may be displaced or blocked by endogenous dopamine, whereas NMB with its higher D2 affinity is far less susceptible to this effect.17 However, this explanation is less likely, since studies suggest that striatal dopamine is reduced rather than increased in primary dystonia40, and in a non-human primate model of dystonia.9 Future studies of patients with focal dystonia designed to measure endogenous striatal dopamine, such as PET measurement of amphetamine induced [11C]raclopride displacement, would offer more conclusive information regarding the role of endogenous dopamine on D2-like receptor binding.
The more likely explanation for the divergent findings using the various D2-like selective radiopharmaceuticals is that primary dystonia is associated not with reduced striatal D2 receptors, but instead with a decrease in D3 or D4 binding sites. The literature supporting the presence of low D4 receptors in striatum is limited41, while the presence of D3 receptors in the striatum at a concentration below D2 receptors is supported by a number of studies.42,43 In this interpretation, if only D3 binding were decreased, the highly D2 selective radioligand NMB would not be expected to demonstrate reduced striatal binding, since it has relatively low affinity for the affected D3 receptor sites. Evaluation of this hypothesis must await the development of D3 and D4-selective radiopharmaceutical for PET imaging.
Possible role of D3 receptors in the pathophysiology of dystonia
The classic basal ganglia model describes cortical projections to putamen, which in turn project to the internal segment of the pallidum (GPi) via two major pathways: the direct pathway and the indirect pathway via globus pallidus pars externa (GPe) and subthalamic nucleus. GPi output neurons target thalamus which then projects to the cortical areas.44–46 Some proposed models of dystonia suggest that dysfunction of indirect pathways leads to a loss of inhibition of unwanted muscle activity surrounding a selected movement. This is consistent with clinical observations that a selected movement may begin nearly normally while excessive muscle activity quickly impairs the intended function.47
D1-like and D2-like receptors are mostly segregated to different medium spiny neurons (MSN) with D2-like receptors that predominantly localize to and inhibit the striatopallidal neurons of the indirect pathway that project to GPe, whereas D1-like receptors localize to and facilitate the neurons of the direct pathway that project from striatum to GPi.46,48,49 Hence, any abnormality in D2-like receptors could lead to dysfunction of the indirect pathway and contribute to the pathophysiology of dystonia. Indeed, D2-like receptors act presynaptically to reduce striatal GABA release. It has been shown in a DYT1 mouse model that there is disinhibition of striatal GABAergic synaptic activity in cells containing D2, GABA and enkephalin receptors projecting to GPe, suggestive of D2 receptor dysfunction in the indirect pathway.50 Interestingly, the agonist was a D2-like ligand. However, the findings were attributed to D2 receptors assuming there were no D3 receptors present in the striatum.
Alternatively, abnormal plasticity with reduced cortical inhibition has been regarded a key factor in the pathophysiology of dystonia.51–53 There is mounting evidence suggesting that striatal synaptic plasticity plays a pivotal role in procedural and sensorimotor learning,54–56 which could explain abnormal plasticity at basal ganglia, in addition to, cortical levels in dystonia.57 Several observations may provide insights into how dopamine plays a central role in mediating such synaptic plasticity. Striatal MSNs receive excitatory glutamatergic cortical and thalamic input and nigral dopaminergic afferents.48 Dopamine receptors are strategically positioned on the neck of the dendritic spines of MSNs close to the glutamatergic cortical synapses at the spine heads.58 In addition all striatal interneurons express dopamine receptors and can be modulated by dopamine.59–61 In fact numerous studies suggest that corticostriatal long term potentiation and depression, which are forms of plasticity, are regulated and likely induced by dopamine.62,63
While the main focus has been on D2 receptor, D3 receptors have not received much attention given the low level of their expression in the striatum. Interestingly, an L-type calcium channel agonist provoked dystonia in mice via affecting the D3 and possibly D1-like receptors, while D2 receptor did not seem to play a role.64 Indeed, D3 receptor could be a major contributor to dopaminergic synaptic modulations: A small subpopulation of striatal MSN contain both D1 and D2-like receptors65–66 and D3 receptor may interact with other receptors at pre-synaptic sites contributing to auto-regulation.67–68 In addition, recent studies indicate that D1 and D3 receptors can form heterodimers capable of enhancing D1 receptor mediated activity.69,70 Finally, D3 receptor affects the extracellular dopamine level most likely via regulation of DAT expression.71 These features could allow D3 receptor to contribute to striatal neuroplasticity despite its relative low density compared to D2 and D1 receptors. Hence, a disturbance in D3 receptors might have far reaching effects on interactions of the various components of the basal ganglia dopaminergic system. Of course, evaluation of a possible D3 or even D4 abnormality in dystonia requires in vivo measurements with a D3 or D4-specific radioligands. Most importantly such studies need to be conducted in various forms of dystonia to prove commonality.
Acknowledgment
We thank Lori McGee-Minnich,BSN for expert assistance with clinical care of participants, John Hood, Jr. and Lennis Lich for technical assistance, Kimberly Kania for assisting with the image preparation and the Cyclotron Facility of Washington University for production of radioisotopes. This study was funded by NIH grants NS031001, R 21 NS050658-01A1, NS041509, NS050425, NS058714 UL1 RR024992 (Clinical Science Translational Award at Washington University), P30NS05710 (Neuroscience Blueprint Grant at Washington University), the Murphy Fund, American Parkinson Disease Association (APDA) Center for Advanced PD Research at Washington University; Greater St. Louis Chapter of the APDA; and the Barnes-Jewish Hospital Foundation (the Elliot H. Stein Family Fund and the Jack Buck Fund for PD Research).
Footnotes
Financial disclosure/conflict: M. Karimi has received a one year fellowship from Allergan.
Author roles:
M. Karimi: analyzed data, wrote the manuscript
S. Moerlein: radiopharm synthesis and production, reviewed manuscript
R. Luedtke and M.Taylor: in vitro specificity analysis, reviewed manuscript
T .Videen: helped with data analyses , reviewed manuscript
R. Mach: coordinated in vitro analyses and helped with interpretation, reviewed manuscript
J. Perlmutter: designed the study, recruited subjects, implemented the study, reviewed and edited manuscript
Full financial disclosure of all authors for the past year:
|
Stock Ownership in medically- related fields None |
Intellectual Property Rights Licenses None |
|
Consultancies None |
Expert Testimony None |
|
Advisory Boards None |
Employment None |
|
Partnerships None |
Contracts None |
|
Honoraria None |
Royalties None |
|
Grants NIH/NINDS R01NS058714 (PI: Perlmutter) NIH R01NS058797 (PI: Hershey) NIH RO1 1RO1NS41509 (PI: Perlmutter) |
Other None |
|
Stock Ownership in medically- related fields None |
Intellectual Property Rights Licenses
Chemicon International Inc. (Millipore) 2000 Santa Cruz 2005 Affinity BioReagents (ABR) 2006 Novus Biological 2007 Invitrogen (D1a only) 2007 |
|
Consultancies None |
Expert Testimony none |
|
Advisory Boards None |
Employment none |
|
Partnerships None |
Contracts none |
|
Honoraria None |
Royalties See licenses approx $1,100 |
|
Grants NIH/NIDA: R01 DA024675-01A2 Development Of Dopamine D3 Ligands As Medications For Psychostimulant Addicton R.R. Luedtke Co-Investigator from 12/09 to 11/12 |
Other None |
|
Stock Ownership in medically- related fields None |
Intellectual Property Rights Licenses None |
|
Consultancies None |
Expert Testimony None |
|
Advisory Boards None |
Employment None |
|
Partnerships None |
Contracts Amgen Subcontract (MJ Welch PI) |
|
Honoraria None |
Royalties None |
|
Grants None |
Other None |
|
Stock Ownership in medically- related fields None |
Intellectual Property Rights Licenses None |
|
Consultancies None |
Expert Testimony None |
|
Advisory Boards None |
Employment None |
|
Partnerships None |
Contracts None |
|
Honoraria None |
Royalties None |
|
Grants NIH P01 NS035966 NIH P50 NS006833 NIH P50 NS055977 NIH R01 NS041509 NIH R01 NS042167 NIH R01 NS050425 NIH R01 NS051631 NIH R01 NS051631 NIH R01 NS058714 |
Other None |
|
Stock Ownership in medically- related fields None |
Intellectual Property Rights Licenses None |
|
Consultancies None |
Expert Testimony None |
|
Advisory Boards None |
Employment None |
|
Partnerships None |
Contracts None |
|
Honoraria None |
Royalties None |
|
Grants None |
Other None |
|
Stock Ownership in medically- related fields None |
Intellectual Property Rights Licenses None |
|
Consultancies None |
Expert Testimony None |
|
Advisory Boards None |
Employment None |
|
Partnerships None |
Contracts |
|
Honoraria None |
Royalties None |
|
Grants R 21 NS050658-01A1 |
Other None |
|
Stock Ownership in medically- related fields None |
Intellectual Property Rights Licenses None |
|
Consultancies None |
Expert Testimony None |
|
Advisory Boards None |
Employment None |
|
Partnerships None |
Contracts None |
|
Honoraria None |
Royalties None |
|
Grants NIH RO1 1RO1NS41509, NIH RO1 NS050425, NIH P30 NS057105, NIH/NCRR RR024992, NIH R01ES013743, NIH R01 NS039821, NIH R01NS058797, NIH NS065701 |
Other None |
Reference List
- 1.Fahn S, Bressman SB, Marsden CD. Classification of dystonia. Adv Neurol. 1998;78:1–10. [PubMed] [Google Scholar]
- 2.Wooten GF, Trugman JM. The dopamine motor system. Mov Disord. 1989;4 Suppl. 1:S38–S47. doi: 10.1002/mds.870040506. [DOI] [PubMed] [Google Scholar]
- 3.Schneider SA, Bhatia KP, Hardy J. Complicated recessive dystonia parkinsonism syndromes. Mov Disord. 2009;24(4):490–499. doi: 10.1002/mds.22314. [DOI] [PubMed] [Google Scholar]
- 4.Poewe WH, Lees AJ, Stern GM. Dystonia in Parkinson's disease: clinical and pharmacological features. Ann Neurol. 1988;23(1):73–78. doi: 10.1002/ana.410230112. [DOI] [PubMed] [Google Scholar]
- 5.Nygaard TG. Dopa-responsive dystonia. Curr Opin Neurol. 1995;8(4):310–313. doi: 10.1097/00019052-199508000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Brancati F, Valente EM, Castori M, Vanacore N, Sessa M, Galardi G, et al. Role of the dopamine D5 receptor (DRD5) as a susceptibility gene for cervical dystonia. J Neurol Neurosurg Psychiatry. 2003;74(5):665–666. doi: 10.1136/jnnp.74.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Playford ED, Fletcher NA, Sawle GV, Marsden CD, Brooks DJ. Striatal [18F]dopa uptake in familial idiopathic dystonia. Brain. 1993;116:1191–1199. doi: 10.1093/brain/116.5.1191. [DOI] [PubMed] [Google Scholar]
- 8.Balcioglu A, Kim MO, Sharma N, Cha JH, Breakefield XO, Standaert DG. Dopamine release is impaired in a mouse model of DYT1 dystonia. J Neurochem. 2007;102(3):783–788. doi: 10.1111/j.1471-4159.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- 9.Tabbal SD, Mink JW, Antenor JV, Carl JL, Moerlein SM, Perlmutter JS. MPTP-induced acute transient dystonia in monkeys associated with low striatal dopamine. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.04.072. (In Press) [DOI] [PubMed] [Google Scholar]
- 10.Perlmutter JS, Stambuk MK, Markham J, Black KJ, McGee-Minnich L, Jankovic J, et al. Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. J Neurosci. 1997;17(2):843–850. doi: 10.1523/JNEUROSCI.17-02-00843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, et al. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology. 2005;64(2):347–349. doi: 10.1212/01.WNL.0000149764.34953.BF. [DOI] [PubMed] [Google Scholar]
- 12.Garver DL, Davis DM, Dekirmenjian H, Ericksen S, Gosenfeld L, Haraszti J. Dystonic reactions following neuroleptics: time course and proposed mechanisms. Psychopharmacologia. 1976;47(2):199–201. doi: 10.1007/BF00735822. [DOI] [PubMed] [Google Scholar]
- 13.Kolbe H, Clow A, Jenner P, Marsden CD. Neuroleptic-induced acute dystonic reactions may be due to enhanced dopamine release on to supersensitive postsynaptic receptors. Neurology. 1981;31(4):434–439. doi: 10.1212/wnl.31.4.434. [DOI] [PubMed] [Google Scholar]
- 14.Rupniak NM, Jenner P, Marsden CD. Acute dystonia induced by neuroleptic drugs. Psychopharmacology (Berl) 1986;88(4):403–419. doi: 10.1007/BF00178501. [DOI] [PubMed] [Google Scholar]
- 15.Perlmutter JS, Tempel LW, Black KJ, Parkinson D, Todd RD. MPTP induces dystonia and parkinsonism: Clues to the pathophysiology of dystonia. Neurology. 1997;49(5):1432–1438. doi: 10.1212/wnl.49.5.1432. [DOI] [PubMed] [Google Scholar]
- 16.Perlmutter JS, Moerlein SM, Huang D-R, Todd R. Non-steady-state measurement of in vivo radioligand binding with positron emission tomography: specificity analysis and comparison with in vitro binding. J Neurosci. 1991;11:1381–1389. doi: 10.1523/JNEUROSCI.11-05-01381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moerlein SM, Perlmutter JS, Markham J, Welch MJ. In vivo kinetics of [18F](N-methyl)benperidol: a novel PET tracer for assessment of dopaminergic D2-like receptor binding. J Cereb Blood Flow Metab. 1997;17:833–845. doi: 10.1097/00004647-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moerlein SM, Banks WR, Parkinson D. Production of fluorine-18 labeled (3-N-methyl)benperidol for PET investigation of cerebral dopaminergic receptor binding. Applied Radiation and Isotopes: including data, instrumentation and methods for use in agriculture, industry and medicine. 1992;43:913–917. doi: 10.1016/0883-2889(92)90155-8. [DOI] [PubMed] [Google Scholar]
- 21.Moerlein SM, Perlmutter JS, Cutler PD, Welch MJ. Radiation dosimetry of [18F](N-methyl) benperidol as determined by whole-body PET imaging of primates. Nucl Med Biol. 1997;24:311–318. doi: 10.1016/s0969-8051(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 22.Wienhard K, Dalhbom M, Eriksson L, Michel C, Bruckbauer T, Pietrzyk U, et al. The ECAT EXACT HR: performance of a new high resolution positron scanner. J Comput Assist Tomogr. 1994;18:110–118. [PubMed] [Google Scholar]
- 23.Ollinger JM. Model-based scatter correction for fully 3D PET. Phys Med Biol. 1996;41(1):153–176. doi: 10.1088/0031-9155/41/1/012. [DOI] [PubMed] [Google Scholar]
- 24.Karimi M, Golchin N, Tabbal SD, Hershey T, Videen TO, Wu J, et al. Subthalamic nucleus stimulation-induced regional blood flow responses correlate with improvement of motor signs in Parkinson disease. Brain. 2008 doi: 10.1093/brain/awn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talairach J. Co-planar stereotaxic atlas of the human brain. New York: Theime Verlag; 1988. [Google Scholar]
- 26.Andersson JL, Sundin A, Valind S. A method for coregistration of PET and MR brain images. J Nucl Med. 1995;36(7):1307–1315. [PubMed] [Google Scholar]
- 27.Pluim JP, Maintz JB, Viergever MA. Image registration by maximization of combined mutual information and gradient information. IEEE Trans Med Imaging. 2000;19(8):809–814. doi: 10.1109/42.876307. [DOI] [PubMed] [Google Scholar]
- 28.Antenor-Dorsey JA, Markham J, Moerlein SM, Videen TO, Perlmutter JS. Validation of the reference tissue model for estimation of dopaminergic D2-like receptor binding with [18F](N-methyl)benperidol in humans. Nucl Med Biol. 2008;35(3):335–341. doi: 10.1016/j.nucmedbio.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Luedtke RR, Freeman RA, Boundy VA, Martin MW, Huang Y, Mach RH. Characterization of (125)I-IABN, a novel azabicyclononane benzamide selective for D2-like dopamine receptors. Synapse. 2000;38(4):438–449. doi: 10.1002/1098-2396(20001215)38:4<438::AID-SYN9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Magar ME. Data Analysis in Biochemistry and Biophysics. New York: Academic Press; 1972. p. 93. [Google Scholar]
- 32.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 33.Carbon M, Niethammer M, Peng S, Raymond D, Dhawan V, Chaly T, et al. Abnormal striatal and thalamic dopamine neurotransmission: Genotype-related features of dystonia. Neurology. 2009;72(24):2097–2103. doi: 10.1212/WNL.0b013e3181aa538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perlmutter JS, Stambuk MK, Markham J, Black KJ, McGee-Minnich L, Jankovic J, et al. Decreased [18F]spiperone binding in putamen in dystonia. In: Fahn S, Marsden CD, DeLong MR, editors. Dystonia 3. Philadelphia: Lippincot-Raven; 1998. pp. 161–168. [PubMed] [Google Scholar]
- 35.Beukers RJ, Booij J, Weisscher N, Zijlstra F, van Amelsvoort TA, Tijssen MA. Reduced striatal D2 receptor binding in myoclonus-dystonia. Eur J Nucl Med Mol Imaging. 2009;36(2):269–274. doi: 10.1007/s00259-008-0924-9. [DOI] [PubMed] [Google Scholar]
- 36.Berger HJ, van der Werf SP, Horstink CA, Cools AR, Oyen WJ, Horstink MW. Writer's cramp: Restoration of striatal D2-binding after successful biofeedback-based senorimotor training. Parkinsonism & Related Disorders. 2007;13(3):170–173. doi: 10.1016/j.parkreldis.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Catafau AM, Suarez M, Bullich S, Llop J, Nucci G, Gunn RN, et al. Within-subject comparison of striatal D2 receptor occupancy measurements using [123I]IBZM SPECT and [11C]Raclopride PET. Neuroimage. 2009;46(2):447–458. doi: 10.1016/j.neuroimage.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Hassanzadeh B, Chu W, Tu Z, Jones LA, Luedtke RR, et al. [(3)H]4-(dimethylamino)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl) butyl)benzamide: A selective radioligand for dopamine D(3) receptors. II. Quantitative analysis of dopamine D(3) and D(2) receptor density ratio in the caudate-putamen. Synapse. 2010;64(6):449–459. doi: 10.1002/syn.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain. 1985;108:463–483. doi: 10.1093/brain/108.2.463. [DOI] [PubMed] [Google Scholar]
- 40.Augood SJ, Hollingsworth Z, Albers DS, Yang L, Leung JC, Muller B, et al. Dopamine transmission in DYT1 dystonia: a biochemical and autoradiographical study. Neurology. 2002;59(3):445–448. doi: 10.1212/wnl.59.3.445. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto M, Hidaka K, Tada S, Tasaki Y, Yamaguchi T. Low levels of mRNA for dopamine D4 receptor in human cerebral cortex and striatum. J Neurochem. 1996;66(3):915–919. doi: 10.1046/j.1471-4159.1996.66030915.x. [DOI] [PubMed] [Google Scholar]
- 42.Landwehrmeyer B, Mengod G, Palacios JM. Dopamine D3 receptor mRNA and binding sites in human brain. Molecular Brain Research. 1993;18:187–192. doi: 10.1016/0169-328x(93)90188-u. [DOI] [PubMed] [Google Scholar]
- 43.Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20(1):60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 44.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 45.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 46.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15(4):133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- 47.Beck S, Richardson SP, Shamim EA, Dang N, Schubert M, Hallett M. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci. 2008;28(41):10363–10369. doi: 10.1523/JNEUROSCI.3564-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 49.Keefe KA, Gerfen CR. D1–D2 dopamine receptor synergy in striatum: effects of intrastriatal infusions of dopamine agonists and antagonists on immediate early gene expression. Neuroscience. 1995;66(4):903–913. doi: 10.1016/0306-4522(95)00024-d. [DOI] [PubMed] [Google Scholar]
- 50.Sciamanna G, Bonsi P, Tassone A, Cuomo D, Tscherter A, Viscomi MT, et al. Impaired striatal D2 receptor function leads to enhanced GABA transmission in a mouse model of DYT1 dystonia. Neurobiol Dis. 2009;34(1):133–145. doi: 10.1016/j.nbd.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres-Russotto D, Perlmutter JS. Task-Specific Dystonias: A Review. Annals of the New York Academy of Sciences. 2008 doi: 10.1196/annals.1444.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quartarone A, Rizzo V, Terranova C, Morgante F, Schneider S, Ibrahim N, et al. Abnormal sensorimotor plasticity in organic but not in psychogenic dystonia. Brain. 2009;132(Pt 10):2871–2877. doi: 10.1093/brain/awp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanger TD, Merzenich MM. Computational model of the role of sensory disorganization in focal task-specific dystonia. J Neurophysiol. 2000;84(5):2458–2464. doi: 10.1152/jn.2000.84.5.2458. [DOI] [PubMed] [Google Scholar]
- 54.Pisani A, Centonze D, Bernardi G, Calabresi P. Striatal synaptic plasticity: implications for motor learning and Parkinson's disease. Mov Disord. 2005;20(4):395–402. doi: 10.1002/mds.20394. [DOI] [PubMed] [Google Scholar]
- 55.Horvitz JC. Stimulus-response and response-outcome learning mechanisms in the striatum. Behav Brain Res. 2009;199(1):129–140. doi: 10.1016/j.bbr.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23(10 Suppl):S71–S77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- 57.Peterson DA, Sejnowski TJ, Poizner H. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiol Dis. 2010;37(3):558–573. doi: 10.1016/j.nbd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotter R. Postsynaptic integration of glutamatergic and dopaminergic signals in the striatum. Progress in Neurobiology. 1994;44:163–196. doi: 10.1016/0301-0082(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 59.Rice ME. Distinct regional differences in dopamine-mediated volume transmission. Prog Brain Res. 2000;125:277–290. doi: 10.1016/S0079-6123(00)25017-6. [DOI] [PubMed] [Google Scholar]
- 60.Venton BJ, Michael DJ, Wightman RM. Correlation of local changes in extracellular oxygen and pH that accompany dopaminergic terminal activity in the rat caudate-putamen. J Neurochem. 2003;84(2):373–381. doi: 10.1046/j.1471-4159.2003.01527.x. [DOI] [PubMed] [Google Scholar]
- 61.Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27(11):662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321(5890):848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasim S, Blake BL, Fan X, Chartoff E, Egami K, Breese GR, et al. The role of dopamine receptors in the neurobehavioral syndrome provoked by activation of L-type calcium channels in rodents. Dev Neurosci. 2006;28(6):505–517. doi: 10.1159/000095113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 66.Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, et al. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3(3):226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- 67.Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5(1):25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- 68.Heidbreder C. Selective antagonism at dopamine D3 receptors as a target for drug addiction pharmacotherapy: a review of preclinical evidence. CNS Neurol Disord Drug Targets. 2008;7(5):410–421. doi: 10.2174/187152708786927822. [DOI] [PubMed] [Google Scholar]
- 69.Fiorentini C, Busi C, Spano P, Missale C. Dimerization of dopamine D1 and D3 receptors in the regulation of striatal function. Curr Opin Pharmacol. 2010;10(1):87–92. doi: 10.1016/j.coph.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, et al. Identification of dopamine D1–D3 receptor heteromers. Indications for a role of synergistic D1–D3 receptor interactions in the striatum. J Biol Chem. 2008;283(38):26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, Kuraguntla D, et al. Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem. 2007;282(49):35842–35854. doi: 10.1074/jbc.M611758200. [DOI] [PubMed] [Google Scholar]