Abstract
Pharmaceutical solid oral dosage forms must undergo dissolution in the intestinal fluids of the gastrointestinal tract before they can be absorbed and reach the systemic circulation. Therefore, dissolution is a critical part of the drug-delivery process. The rate and extent of drug dissolution and absorption depend on the characteristics of the active ingredient as well as properties of the dosage form. Just as importantly, characteristics of the physiological environment such as buffer species, pH, bile salts, gastric emptying rate, intestinal motility, and hydrodynamics can significantly impact dissolution and absorption. While significant progress has been made since 1970 when the first compendial dissolution test was introduced (USP Apparatus 1), current dissolution testing does not take full advantage of the extensive physiologic information that is available. For quality control purposes, where the question is one of lot-to-lot consistency in performance, using nonphysiologic test conditions that match drug and dosage form properties with practical dissolution media and apparatus may be appropriate. However, where in vitro – in vivo correlations are desired, it is logical to consider and utilize knowledge of the in vivo condition. This publication critically reviews the literature that is relevant to oral human drug delivery. Physiologically relevant information must serve as a basis for the design of dissolution test methods and systems that are more representative of the human condition. As in vitro methods advance in their physiological relevance, better in vitro - in vivo correlations will be possible. This will, in turn, lead to in vitro systems that can be utilized to more effectively design dosage forms that have improved and more consistent oral bioperformance.
Keywords: dissolution, absorption, physiologic, physiological, absorption, gastrointestinal, bioperformance, oral drug delivery, physicochemical properties
Introduction
Pharmaceutical solid oral dosage forms directed to the systemic circulation must dissolve in the intestinal fluids of the gastrointestinal (GI) tract prior to absorption, making dissolution vital to drug delivery. Pharmaceutical scientists must understand dissolution to efficiently develop robust dosage forms and ensure that drug products consistently meet critical performance criteria. The rate and extent of drug dissolution and absorption depend on characteristics of the active ingredient such as pKa, crystal form, and solubility, as well as properties of the dosage form1. Just as importantly, characteristics of the physiological environment such as buffer species, pH, bile salts, gastric emptying rate, intestinal motility, hydrodynamics, and shear rates significantly impact dissolution and absorption2.
To understand the complicated process of in vivo drug dissolution, scientists have attempted to replicate it using a variety of in vitro test methods. Numerous methodologies have been developed that are routinely used for quality control purposes (e.g., USP tests) and as tools to understand the effects of formulation and processing changes3. While these methodologies have existed for many years and have been used extensively, none accurately reflect in vivo conditions. Conventional USP testing methods employ simple, non-physiologic buffers (e.g., phosphate, acetate, maleate) and hydrodynamic conditions (e.g., single-chambered glass vessels) that do not accurately reflect dynamic in vivo conditions. To bridge the gap between in vitro and in vivo dissolution and absorption, the Biopharmaceutics Classification System (BCS) provides some guidance for predicting in vivo performance based on a drug’s solubility, permeability, and in vitro testing results4. The BCS has had a significant effect on the regulatory environment as the FDA and WHO consider biowaivers for some drugs, particularly those considered to be BCS Class 1 (high solubility, high permeability) and BCS Class III (high solubility, low permeability)5.
While significant progress has been made since 1970, when the first compendial dissolution test was introduced (USP Apparatus 1), current dissolution testing does not take full advantage of the extensive physiologic information that is available. For quality control purposes, where the question is one of lot-to-lot consistency in performance, utilizing nonphysiologic test conditions that match drug and dosage form properties with practical dissolution media and apparatus may be appropriate. However, where in vitro – in vivo correlations (IVIVCs) are desired, it is logical to consider and utilize our knowledge of the in vivo condition. Strides have been made in making dissolution testing methods more biologically based. Dressman et al. developed several biorelevant dissolution media designed to better reflect compositions and physicochemical characteristics of the fasted and fed states in the stomach and small intestine6. In addition, several authors have developed dissolution apparatuses that better capture aspects of the physiological environment compared to USP tests7–9.
Several good reviews of human GI physiology are available2,10–11 but none provide a comprehensive review of the physiological parameters that influence oral absorption in the context of dosage form performance and drug dissolution. The focus of this publication is to critically review the literature that is relevant to oral human drug delivery. This physiologically relevant information should serve as a basis for the design of dissolution test methods and systems that are more representative of the human gastrointestinal tract. As in vitro methods advance in their physiological relevance, better in vitro - in vivo correlations will be possible, leading to improved oral bioperformance of dosage forms.
Factors Affecting Dissolution and Absorption
Absorption is what ultimately carries orally administered drugs into the intestinal membrane to be transferred to the blood stream. However, the drug must dissolve before absorption can occur or the drug can act locally in the GI tract. Therefore, it is important to have a fundamental understanding of the key drug properties affecting both dissolution and absorption. These principles have taken a variety of mathematical forms over the years. According to Amidon et al., for example, the fraction of drug absorbed is a function of drug solubility, dose, and GI permeability4. According to Equation 1, the flux of drug across the intestinal wall, Jw, is dependent on the intestinal wall permeability, Pw (an effective permeability), and the concentration of drug at the wall, Cw. The equation applies to each point along the membrane, assumes that each parameter is dependent upon time and position, and assumes the concentration of drug in the epithelial cell to essentially equal to zero. Assuming no luminal reactions, the absorption rate is given by Equation 2, where A is the area available for absorption (i.e., membrane surface in contact with the drug) and m is mass.
| (Eq. 1) |
| (Eq. 2) |
Factors that affect dissolution can be understood by examining the simple Noyes-Whitney equation, which describes the mass of drug dissolving as a function of time. The equation, for dissolution from a planar surface, is given in Equation 3, where M is mass, D is drug diffusion coefficient, A is drug surface area available for dissolution, h is empirical thickness of the hydrodynamic boundary layer, Cs is the solubility at the solid liquid interface, and Cb is the bulk drug concentration12.
| (Eq. 3) |
Each of the parameters in Equation 2, describing absorption, and Equation 3, describing dissolution, is influenced by properties of the drug substance, drug product, and GI tract.
From the above description it is clear that in vivo dissolution and absorption are dependent on properties of the physiological environment and properties of the drug itself. Key physiological parameters include the dimensions of the GI tract, the volume and composition of fluid, the fluid hydrodynamics (i.e., flow rate, gastric-emptying rate, shear rate), and the properties of the intestinal membrane. Important drug properties include dose, solubility, pKa, diffusion coefficient, permeability, and particle size. A more complete list of drug properties and physiologic properties that influence oral drug dissolution and absorption is provided in Table 1.
Table 1.
Drug properties and physiological properties that influence oral drug dissolution and absorption
| Parameter | Drug properties | Physiological parameters |
|---|---|---|
| Drug diffusion coefficient, D | Radius, mass, volume | Solute concentration, temperature, fluid viscosity |
| Drug surface area, A | Particle size, size distribution, shape, state of particle aggregation | Fluid hydrodynamics |
| Length of hydrodynamic boundary layer (stagnant diffusion layer), h | Particle size, diffusion coefficient | Fluid velocity, viscosity, diffusion coefficients of diffusing species |
| Saturated solubility, Cs | Intrinsic solubility (molecular size, crystal properties, chemical groups), pKa | Buffer species, buffer concentration, buffer capacity, pH, presence of lipolytic products, bile salts, and phospholipids, temperature |
| Bulk concentration, Cb | Dose, intrinsic solubility (molecular size, crystal properties, chemical groups), pKa, intestinal permeability | Fluid volume (fluid ingested, gastric-emptying rate, transit time), absorption in GI membrane, buffer species, buffer concentration, buffer capacity, pH, presence of lipolytic products, bile salts, and phospholipids, temperature |
| Intestinal wall permeability, Pw | Absorption mechanism (Simple diffusion: lipophilicity, charge, polarity. Facilitated diffusion or active transport: affinity for membrane channels or pumps) | Intestinal segment, Composition of intestinal wall, number of channels or transporters, apparent permeability to mass transport (turbulence due to intestinal wall contractions) |
| Concentration at the intestinal wall, Cw | Dose, intrinsic solubility (molecular size, crystal properties, chemical groups), pKa, permeability, diffusion coefficient | Hydrodynamics, viscosity, shear, transit time |
Composition of the Gastrointestinal Fluid
Gastrointestinal fluid is a complex, dynamic mixture of components from a number of different sources within the gastrointestinal tract. Gastric fluid is made up of saliva, gastric secretions, dietary food and liquid, and refluxed liquid from the duodenum. The gastric fluid composition changes as the fluid is mixed and delivered to the duodenum. Some major components of gastric fluid important for drug disposition include hydrogen ion concentration, bile salts, lipase, and the protein-digesting enzyme pepsin (Refer to Tables 2 & 3 for a summary of components and concentrations.). The concentration of hydrogen ions affects the pH and thus the dissolution of some ionizable drugs. Pepsin may interfere with the stability of proteins and peptides, while lipase may affect drug release from lipid-based dosage forms2. Bile salts can combine with lipids to form mixed micelles, enhancing the solubility of some drugs and may also decrease surface tension and thus enhance wetting13.
Table 2.
Literature values for concentrations of some major components of fluid in the fasted and fed stomach and small intestine. The designation (e) indicates a value that was measured early in the post-prandial phase (between 0 and 60 minutes), (m) denotes a value measured in the mid post-prandial phase, and (l) denotes a value that was measured late in the post-prandial phase (greater than 100 minutes). Unless indicated next to the value, units are noted next to the name of the component.
| Stomach | Duodenum | Jejunum | Ileum | |||
|---|---|---|---|---|---|---|
| Bicarbonate (mEq L−1) | Fasted | Mean | 7.3a | 2.7105, 6.7106, 15b | 17105, 30b, 30c, 8.2±5 mMd | 40d, 50107, 70b, 74108, 75c, 30±11mMd |
| Range | 9–20g | 2–20e, 5–10109, 6–20f | ||||
| Fed | Mean | 10h | ||||
| Bile salts (mM) | Fasted | Median | 0.100j | 2.7k, 2.6l | ||
| Mean | 0.08±0.03l, 0.275110, 0.081m | 6.4±1.3n, 4.3±1.2n, 5.90±1.8o | 2±0.2p | |||
| Range | 1–5.3q, 0.6–5.1r, 0.3–9.6j | 0.8–5.5r, 0.1–13.3i, 5–6n, 0–17n | 2–10s | |||
| Fed | Median | 3.6j, 5.2j, 8.3 (e)j, 11.9 (e)j, 11.2(e)k, 5.2(l)k | 1.0t, 0.5t | |||
| Mean | 0.0620 | 14.5(e)u, 5.2(m)u, 16.2±1.5111, 9.7±1111, 9.1m | 8112, 15112, 8±0.1p, 6.5±0.9111 | |||
| Range | 1.6–6.2j, 3.2–6.8j, 6.7–13.4o | 0.5–40t(graph), 3–34112 | 0.5–30t, 0.2–1.3t | |||
| Lipids (mg/mL) | Fasted | Median | 0.5j | |||
| Mean | 0.56o | 0.6o | 0.1±0.01mMp | |||
| Range | 0–1.8j | |||||
| Fed | Median | 1.8j, 2.6j | ||||
| Mean | 22±1mMp | |||||
| Range | 50 (l)o, 150(e)o | 0.5–4.6j, 1.1–3.6j, 55–100o | ||||
| Phospholipids (mM) | Fasted | Median | 0.6j | |||
| Mean | 0.2±0.07p | |||||
| Range | 0.1–1.5j, 0.03–0.06q | |||||
| Fed | Median | 1.8j, 1.2j | ||||
| Mean | 3±0.3p | |||||
| Range | 1.3–2.4j, 0.8–1.6j | |||||
| Pepsin (mg/mL) | Fasted | Median | 0.11 (e)k, 0.22 (m)k | |||
| Mean | 0.87v | |||||
| Range | 0.83–1.27w | |||||
| Fed | Median | |||||
| Mean | 1.25v, 1.68v | |||||
| Range | 0.26–0.58w, 0.56–1.72w | |||||
| Lipase | Fasted | Mean | ~0.1mg/mLx | |||
| Range | ||||||
| Fed | Range | 11.4–43.9 U/mLo | ||||
| Potassium (mM) | Fasted | Mean | 13.4±3.0i | 5.4±2.1i, 4.8±0.543 | 4.9±1.543 | |
| Sodium (mM) | Fasted | Mean | 68±29i | 142±13i, 142±743 | 140±643 | |
| Fed | Mean | 106±15t, 101±17t | 139±11t, 133±8t | |||
| Chloride (mM) | Fasted | Mean | 102±28i | 126±19i, 135±843 | 125±1243 | |
| Calcium (mM) | Fasted | Mean | 0.6±0.2i | 0.5±0.3i | ||
From reference 21.
From reference 40.
From reference 42.
From reference 43.
From reference 39.
From reference 41.
From reference 22.
From reference 44.
From reference 50.
From reference 33.
From reference 14.
From reference 20.
From reference 19.
From reference 2.
From reference 18.
From reference 35.
From reference 34.
From reference 53.
From reference 38.
From reference 36.
From reference 37.
From reference 15.
From reference 16.
From reference 17.
Table 3.
Literature values for properties of fluids in the fasted and fed stomach and small intestine. The designation (e) indicates a value that was measured early in the post-prandial phase (between 0 and 60 minutes), (m) denotes a value measured in the mid post-prandial phase, and (l) denotes a value that was measured late in the post-prandial phase (greater than 100 minutes). Unless indicated nest to the value, units are noted next to the name of the component.
| Stomach | Duodenum | Jejunum | Ileum | |||
|---|---|---|---|---|---|---|
| Buffer capacity (mmol L−1 pH−1 | Fasted | Median | 7 (e)a, 18a | 5.6a | ||
| Mean | 3.2359 | 6.4b | ||||
| Range | 4–13c | 2.4–2.8d | ||||
| Fed | Range | 14–28a | 18–30a | 13.2–14.6d | ||
| Osmolality (mOsm kg−1) | Fasted | Median | 98 (e)a, 140 (l)a | 178a, 224e | ||
| Mean | 29f, 191±36g, 33.6±5.9h 221±15h |
142f, 137±54c | 271±15g, 200±68c, 278±16h | |||
| Range | 171–276i | 124–266e | ||||
| Fed | Median | 559 (e)a, 217 (l)a | 287e, 276e, >287 (e)a, 287 (l)a | |||
| Range | 250–367e, 268–304e | |||||
| Surface tension (mN m−1) | Fasted | Median | 32.3a, 41.2e | |||
| Mean | 28±1d, 33.7±2.8h | |||||
| Range | 41.9–45.7a | 33.3–46.0e | ||||
| Fed | Median | 34.2e, 35.4e | ||||
| Mean | 27±1d | |||||
| Range | 30–31a | 32.2–36.7e, 33.7–36.0e | ||||
| Viscosity (cP) | Fasted | Range | ||||
| Fed | Range | 10–2000j | ||||
| pH | Fasted | Median | 1.7k, 2.4 (e)a, 1.7 (l)a, 1.8143 | 6.1, 6.2a, 6.6e, 5.63113 | 7.2k | |
| Mean | 2.9 ± 1.97g | 6.71±0.44l, 7.0±0.4c, 4.9m, 6.4±0.6n | 6.8±0.4c, 7.5d, 7.1± 0.60g | 6.5±0.2o | ||
| Range | 1–2.5p, 1.4–2.1k, 1.23–7.36a, 1.4–7.5g | 5.8–6.5k, 4.00–5.39m, 5.17–6.10n | 4.4–6.5114, 5.3–8.1k, 5.3–8.1g | 6.8–8.0q | ||
| Fed | Median | 5.0k, 6.4 (e)a, 2.7 (l)a | 5.4k, 6.6 (e)a, 5.2 (l)a, 5.9e, 6.1e, 5.35r | |||
| Mean | 5.2 (e)m, 4.2 (l)m | 6.2±0.2 (e)115, 5.4 ± 0.2 (l)115, 6.1d | 7.5116 | |||
| Range | 4.3–5.4k | 3.1–6.7k, 4.5–5.5 (e)m, 3.9–4.8 (l)m, 5.1–5.7 (e) 117, 5.3–6.1 (l)117, 4.6–6.358 | 5.2–6.0 (e)m | 6.8–7.8118, 6.8–8.0s | ||
From reference 14.
From reference 59.
From reference 53.
From reference 35.
From reference 33.
From reference 61.
From reference 50.
From reference 63.
From reference 62.
From reference 67.
From reference 51.
From reference 52.
From reference 57.
From reference 54.
From reference 55.
From reference 49.
From reference 56.
From reference 58.
From reference 58.
Kalantzi et al. found median pepsin levels in the fasted state to range from 0.11–0.22mg/mL14, while other researchers have found them to be between 0.1 and 1.3 mg/mL15–16. Pepsin in the fed state is typically higher and has been shown to range from 0.26 to 1.72 mg/mL14, 16. The concentration of hydrogen ions, which are secreted by the stomach in the form of hydrochloric acid is reflected in the pH, which is typically 1–2 in the fasted state (0.01–0.1 M) and ranges from about 3–7 in the fed state (10−3 – 10−7 M). Vertzoni and co-workers state that gastric lipase is probably not important in the fasted state since it is active in the pH range of 3–6 and is thought to be present at concentrations of 0.1mg/mL17. Lipase activity in the fed stomach has been shown to range from 11.4–43.9 U/mL18. Bile salt levels have been found to be about 0.08 to 0.275 mM in the fasted stomach17, 19 and 0.06 mM in the fed stomach20. Vertzoni and co-workers recently measured the relative amounts of individual bile salts in the fasted stomach and found glycochenodeoxycholate and glycocholate to predominate19. Bicarbonate concentrations in the fasted stomach have been shown to range from 7 to 20 mEq/L21–22.
The composition of the fluid in the upper small intestine is made up of chyme from the stomach, as well as secretions from the liver, the pancreas, and the wall of the small intestine. Composition is affected by fluid compartmentalization, mixing patterns, absorption of fluid into the intestinal wall, and transit down the intestinal tract. Secretions from the pancreas include bicarbonate as well as proteases (the major ones are trypsin and chymotrypsin), amylases, and lipases23. The liver secretes bile, which contains bile salts, phospholipids, bicarbonate, cholesterol, bile pigments, and organic wastes. The wall of the small intestine secretes mineral ions such as bicarbonate, sodium, and chloride, as well as water. Bicarbonate is secreted to neutralize gastric secretion in the GI lumen and by the duodenal epithelial cells to protect the duodenal epithelium from acid-related damage24. The buffer species in the gastrointestinal media can significantly affect the dissolution rates of ionizable drugs25.
As food intake triggers many of the secretions in the small intestine, the composition of fed state intestinal fluid can vary greatly from fasted state intestinal fluid. This difference in composition can be partially responsible for differences in bioavailability seen when drug is administered in the fed versus the fasted state. For some lipophilic drugs, coadministration with a meal has been shown to increase bioavailability compared to the fasted state. Sunesen et al. showed that the oral bioavailability of the poorly soluble drug danazol was three-fold higher when taken with a high-lipid meal compared with 200 mL of water26. However, in some cases the oral bioavailability can be negatively affected due to chelation of a drug with food components27.
The increased bioavailability seen for some drugs in the fed state can be attributed to the enhanced solubilizing capacity of intestinal fluids due to bile and pancreatic secretions and the presence of exogenous lipid products28. For instance, dietary triglycerides are hydrolyzed into free fatty acids and monoglycerides in the duodenum mainly due to pancreatic lipase, and the free fatty acids combine with bile salts to form mixed micelles, which can be transported to the intestinal membrane29. Many instances of enhanced solubility and dissolution due to mixed micelles formed by bile secretions, and lipolysis products formed in the fed state exist in the literature30–32.
Concentrations of lipolytic products, bile salts, and phospholipids in the upper small intestine tend to show high variability with time and between study subjects14, 33. Lipolytic product concentrations have ranged from 0–1.8 mg/mL in the fasted and 0.5–100 mg/mL in the fed upper small intestine18, 33. After administration of Ensure Plus® (fed), and Scandishake Mix® (fat-enriched fed) Clarysse et al. found the dominant lipolytic products in the duodenum to be monoglycerides, which accounted for 5–88% of total lipids, followed by free fatty acids33. Phospholipid concentrations have ranged from 0.03–0.6 mM in the fasted33–34 and 0.8–3 mM in the fed state 33, 35. Bile salt concentrations have ranged from 0.6–17 mM 2, 33 and 1.6–40 mM36–37 in the fasted and fed states, respectively. Clarysse et al. found duodenal bile salts to be made up of cholate and chenodeoxycholate (which comprised about 65%) as well as deoxycholate and ursodeoxycholate33, while Vertzoni found the major bile salts in the duodenum to be glycodeoxycholate, glycochenodeoxycholate, and glycocholate in the fed state19. Concentrations of lipolytic products and phospholipids in the ileum are unavailable, but bile salt concentrations have ranged from 2–10 mM and 0.2–30 mM in the fasted and fed states, respectively36, 38.
The concentration of bicarbonate in the small intestine is dynamic and depends on location and prandial state. The bicarbonate concentration in the fasted state has ranged from about 2 to 30 mM in the duodenum and jejunum and 30–75 mM in the ileum39–43. Values in the fed state are less abundant. Rune and co-workers reported a value of 10 mEq L−1 in the fed duodenum44.
Properties of the Gastrointestinal Fluid
pH
The pH of the GI fluids in the local region of the intestine will influence a drug’s dissolution rate and possibly its permeability4. The pH strongly influences the solubility of weak electrolytes by determining their ionization state. When the pH is such that a drug is in its ionic form, the drug behaves like a strong electrolyte and solubility is usually high compared to its nonionized form45. The pH thus has a strong effect on the dissolution of drug products, especially those with pKa values within the physiological range. This phenomenon has been demonstrated for different types of dosage forms such as immediate- and modified-release46–48.
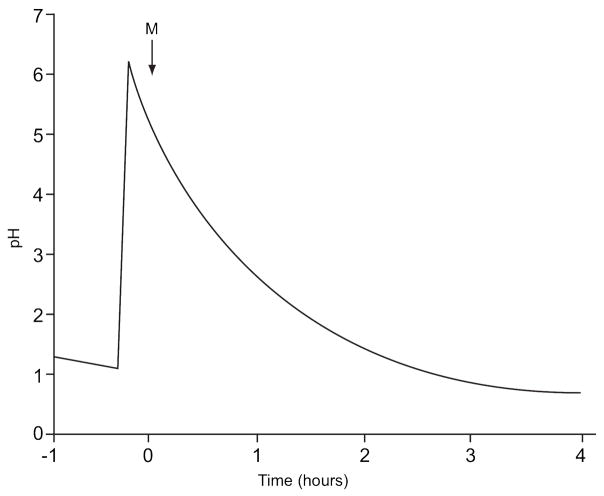
The pH in the gastrointestinal tract is a function of many variables including prandial condition, time, meal volume and content, and volume of secretions, and it varies along the length of the GI tract (Refer to Table 3 for a summary of pH values in the stomach, duodenum, jejunum and ileum.). The gastric pH in the fasted state has been recorded between 1 and 8 for individuals49–50, with typical median values falling between about 1 and 214, 51. Dressman et al. found gastric pH to remain below pH 2 68% of the time and below pH 3 90% of the time51. Shortly after ingestion of a meal, the pH has been shown to rise to about 6.0–7.0, and decreases back to fasting levels after approximately one to four hours, depending on factors such as meal composition, amount, and pH14. Gastric pH values in the fed state have ranged from 2.7–6.414, 51. An approximation of a typical gastric pH profile as measured by Dressman et al. 51 is shown in Figure 1.
Figure 1.
Approximation of a typical pH profile in the stomach. The letter “M” denotes food intake (Redrawn from reference 51).
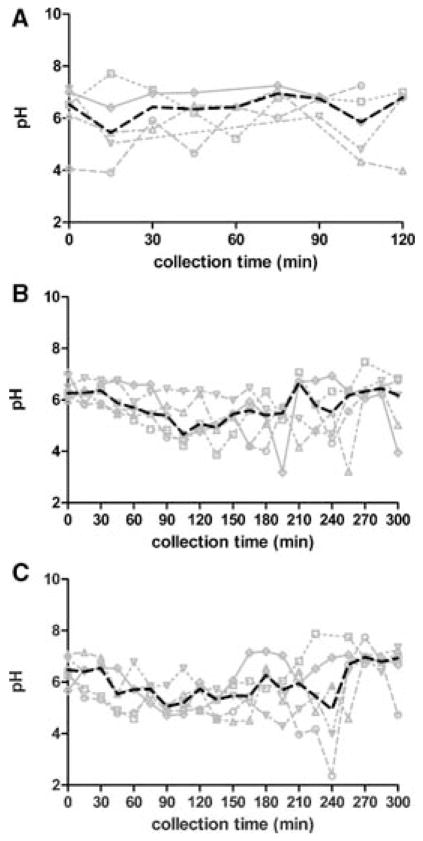
Average pH values in the fasted upper small intestine have been reported to range from about 4 to 852, 50, with typical values around 6.552–54. Clarysse et al. found duodenal pH in the fasted state to display considerable intra- and inter-subject variability as shown in Figure 233. In the ileum pH has be reported as 6.5–8 in the fasted state 55–56.
Figure 2.
Individual and median pH versus time in fasted (A), fed (B), and fat-enriched fed (C) state human duodenal fluid for five healthy subjects. Darkened lines represent median values33. (Reprinted from reference 33 with permission.)
The pH in the upper small intestine tends to be lower in the fed compared to the fasted state. As is found in the fed stomach, the pH in the upper small intestine tends to rise after meal intake and slowly decreases over time. Average values have been shown to vary from about 3 to 714, 51, with typical median values around 5 during the later post-prandial stage56–57. Kalantzi et al. found the pH in the distal duodenum to decrease from 6.6 to 5.2 over the first 210 min following administration of Ensure Plus®14. Fed pH values in the ileum have been reported in the range of 6.8–858. Clarysse et al. found the pH of the administered meal to have a strong impact on local pH, leading to decreased intersubject variability compared to the fasted state during the first 3 hours after meal intake33. They found the pH to decrease with time, with minimum individual values of 3.9–4.9, returning to fasting values after about 300 min after meal ingestion. Plots of individual and median pH versus time for the five healthy volunteers in the fasted and fed states as measured by Clarysse et al. are given in Figure 2.
Buffer capacity
The buffer capacity of the gastrointestinal fluid can affect the dissolution rate, particularly for ionizable drugs. The higher the buffer capacity, the more the buffer will influence pH changes at the drug-liquid interface (i.e., the surface pH)25. The buffer capacity depends on the pH of the fluid, the pKa of the buffer, and the buffer concentration.
Kalantzi et al. found the median buffer capacity in the stomach to be 7 mmol L−1 ΔpH−1 20 min after administration of water and 18 mmol L−1 ΔpH−1 at later time points (fasted-state conditions)14. In the fed state (after ingesting 500 mL Ensure plus), they found median values of gastric buffer capacity to increase from 14 to 28 mmolL−1 ΔpH−1 over a 30- to 210-min sampling period. They also found intersubject variability to increase with time after meal administration. Values for buffer capacity in the small intestine have ranged from 2–13 mmol/L/pH in the fasted state35, 53, and 13–30 mmol/L/pH in the fed state14, 35. While buffer capacity in the fed ileum is not available, Fadda and co-workers reported buffer capacity in the fasted state to be 6.4 mmol/L/pH59. Buffer capacity values found in the literature are summarized in Table 3.
Osmolality
Osmolality can affect drug release and excipient performance6. Delayed dissolution of 5-aminosalicylic acid from Eudragit L coated tablets was shown at higher osmolality60. Gastric osmolality in the fasted state has been shown to range from 29–276 mOsm/kg61–62. Kalantzi et al. found gastric contents in the fasted state to be hypoosmotic, with lower values of 98 mOsm/kg at early time points, plateauing to 140 mOsm/kg at later times. After a meal, Kalantzi et al. found the median value in the stomach to be 559 mOsm/kg after 30 min and 217 mOsm/kg after 210 min, with variability decreasing with time after the meal14.
In the upper small intestine, osmolality values range from 124–278 mOsm/kg in the fasted state33, 63, and 250–367 in the fed state33. Clarysse et al. found variability in osmolality to be higher in the fed compared to the fasted state, with high fed state fluctuations until 240 min after food intake33. They found fasted state values to be hypoosmotic or close to isoosmotic, with an overall median value of 224 mOsm/kg. In the fed- and fat-enriched-fed states they found values to be hyperosmotic during the first three hours post-prandially, with isoosmotic overall median values of 285 and 278 mOsm/kg, respectively. Jantratid and co-workers also state that osmolality in the distal duodenum increases slightly during the first 120 min after meal intake, and then gradually equilibrates to isoosmotic6. Osmolality values in the stomach and upper small intestine are provided in Table 3. Literature values of osmolality in the ileum could not be found.
Surface tension
Surface tension can affect dissolution by influencing wetting of the dosage form13, with a higher surface tension leading to decreased wetting. Gastric surface tension values in the fasted and fed states range from about 41–46 and 30–31 mN/m, respectively14. In the upper small intestine, surface tension values range from 28–46 mN/m in the fasted state, and 27–37 mN/m in the fed state33, 35. Surface tension values in the ileum are not available.
Viscosity
Measurement of the viscosity of fluids can be complex. Simple fluids such as water, tea, coffee, simple syrups and edible oils behave as Newtonian fluids where viscosity is constant (i.e., shear rate is proportional to shear stress)64. However, many liquefied foods and biological fluids demonstrate non-Newtonian flow behavior meaning that viscosity is dependent upon shear rate, often exhibiting decreased viscosity with increased shear rate (i.e., shear thinning)64 For non-Newtonian fluids it is therefore important to know the shear rate at which the viscosity is measured. In part for these reasons, measured values of GI fluid viscosity for humans in the fed and fasted states are very limited. The viscosity of water at 37°C is 0.691 cP (1cP = 1 mPa-s), while the viscosity of various test meals consisting of dietary fibers (e.g., methylcellulose, bran, psyllium, and guar gum) are often administered in solutions with viscosities that range from 10 to >10,000 cP64–66. Typical meals have therefore been characterized to have viscosities in the range of 10 to 2000 cP65, 67. Marciani and coworkers utilized echo-planar Magnetic Resonance Imaging (MRI) in humans to monitor changes in viscosity of viscous meals and demonstrated significant and rapid reductions in viscosity with time due to dilution by gastric fluids64. Viscosity is also influenced by pH in addition to soluble meal content and concentration. Increased viscosity has been shown to generally decrease stomach emptying and prolong GI transit and has been shown to influence blood glucose and cholesterol levels65, 68.
Temperature
The temperature of GI fluids also affects dissolution and absorption. It can affect the diffusion coefficients of the drug and buffer species, the drug solubility, and the bulk drug concentration. The average GI temperature is generally considered to be 37°C. Several researchers have found 37°C to be an accurate resting temperature, but temperature can increase slightly after exercise. Chin Leong Lim and co-workers used an ingestible telemetric temperature sensor to measure GI temperature during rest and exercise and found the average GI temperature of nine healthy male runners to increase from 37.6°C at rest to 39.3°C after running outside for 45 minutes69.
Volume
The volume of liquid in the gastrointestinal tract affects the amount and potentially the concentration of dissolved drug. If the volume of liquid is such that the potential bulk concentration of drug exceeds the solubility of the drug, then only a small fraction of the original dose may go into solution. Like other GI parameters, the volume of liquid in the various compartments can vary within and between individuals as well as with time and prandial state. It is affected by the amount of liquid ingested, the volume of gastric and pancreatic secretions, gastric-emptying rate, intestinal transit time, as well as uptake and efflux of liquids along the GI membrane.
Volume of liquid in the stomach depends on the amount of liquid ingested, the rate and amount of secretions, and the rate at which it empties into the small intestine. Using MRI, Steingoetter and co-workers measured liquid volumes in the fasted stomach before and after ingesting 300 mL of water and found them to be 28 (18–54) mL before water and 296 (279–323) mL after water70. However, in another study when Kwiatek et al. examined the ratio of the initial postprandial liquid volume in the stomach to the volume of the infused meal (nutrient drink), they found it to decrease as a function of infused meal volume (ratios of 1.25, 0.95, 0.92, and 0.83 for 200-, 400-, 600-, and 800-mL meal volumes, respectively)71. They attributed this progressive decrease in initial gastric volume as a function of meal volume to a larger proportion of liquid nutrient passing into the small intestine during a rapid, early emptying phase. After their measurements of initial volume, they also found the gastric volumes to increase further (due to gastric secretions) before volumes started to decline. They found this increase to be independent of caloric load and greater for the smaller rather than the larger infused meal volumes, demonstrating a slower rate of emptying compared to rate of secretion for the smaller volumes, but a faster rate of emptying compared to rate of secretion for larger volumes. For study participants in a seated position, Steingoetter and co-workers found the contents to be distributed throughout the proximal and distal portions of the stomach, with a distal-to-proximal ratio of 0.23 upon ingestion of the water and 0.58 after 30 min.
Liquid volume in the small intestine depends on the amount of liquid emptying from the stomach, absorption of fluid through the intestinal wall, and intestinal transit time. Volume in the fasted small intestine has been shown to range from 30–420 mL72, with average values tending to fall near 100 mL in several studies73–75. It seems that fasting volumes in the small intestine are less dependent on the amount of liquid ingested than fasting volumes in the stomach. Volume in the fed small intestine has been recorded in the range of about 18 to 660 mL73–74, and is more highly dependent on the amount and contents of the meal. Sutton recently modeled the mean plasma concentration profiles of four solubility-limited compounds using literature values of small and large intestinal liquid volumes76. On average a small intestinal liquid volume of about 130 mL (range of 10–150 mL) provided the best fits to the data, which is in agreement with the average small intestinal liquid volumes reported in the literature. Measured human gastric and intestinal liquid volumes from the literature are provided in Table 4.
Table 4.
Literature values for liquid volumes and geometry in the fasted and fed stomach and small intestine. Values for the small intestine are for the entire small intestine unless the value is contained in an individual column.
| Stomach | Small Intestine | |||||
|---|---|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | ||||
| Volume (mL) | Fasted | Mean | 28a 296 (300mL water)a 27119 |
86b, 81b, 112±27c, 109 ± 36c, 165±22d, 105±72e | ||
| Range | 18–54a 279–323(300mL water)a 21–33119 |
34–46b 37–130b 45–319e |
||||
| Fed | Mean | 250±23 (200mL) f, 380±25 (400mL)f, 555±30 (600mL)f, 664±34(800mL)f | 47b 381b 590±73c 54±41e |
|||
| Range | 18–78b, 343–491b, 20–156e | |||||
| Surface area (cm2) | Absorbing120 | Mean | 525.58 ± 24.143g, 1100h | 104-1.2 X 104 (considering valves of keckring), 105 (considering villi), 2 X 106 (considering microvilli) | ||
| 900h | 600000h | 600000h | ||||
| Geometric | Mean | 942i | 393i, 197–490j | 4712i, 825–1319j 3300120 |
4712i, 980–1862j | |
| Length (cm)120, k | Anatomical | Mean | 680 | |||
| Range | 255–1128 | |||||
| Mean | 260 | 395 | ||||
| Range | 25–30 | |||||
| Physiological | Mean | 282 | ||||
| Range | 229–337 | |||||
| Mean | 21 | 105 | 156 | |||
| Range | 18–26 | |||||
| Diameter (cm) | Absolute | Mean | 15h | 5h, 4120 | 5h | 5h |
| Range | 3.5–6120 | 2.5–4120 | 2–3.8120 | |||
| Cranial to caudal120 | Mean | 37 | ||||
| Range | 29.5–49.5 | |||||
| Greatest diameter120 | Mean | 15 | ||||
| Range | 6.5–21.5 | |||||
| Body120 | Mean | 11 | ||||
| Range | 4–19 | |||||
| Pyloric antrum120 | Range | 4–5 | ||||
From reference 70.
From reference 73.
From reference 74.
From reference 75.
From reference 72.
From reference 71.
Surface area of gastric mucosa.
From reference 95.
Calculated using length and diameter from reference 95 assuming cylindrical geometry.
Calculated using absolute diameter and physiological length from reference 120 assuming cylindrical geometry.
Anatomical lengths measured at autopsy or from material recovered from surgery and physiological lengths measured from living persons.
Schiller et al. used MRI to show that the GI lumen does not represent a continuous watery compartment72. Instead, they found the free water content to exist as fluid pockets. In the fasted small intestine they found the mean number of fluid pockets to be equal to 4, with a median volume of 12 mL per fluid pocket (Refer to Table 5.). In the fed small intestine the mean number of fluid pockets was 6, with a 4-mL median volume per pocket. In addition, they found the volume of free liquid to be lower in the fed than in the fasted state. Schiller et al. also showed that non-disintegrating capsules ingested prior to MRI acquisition were not completely surrounded by fluid in both the stomach and small intestine in the fasted and fed states. In the fasted small intestine only fifty-percent of ingested capsules (14 out of 28 capsules across multiple subjects) were completely surrounded by fluid. In the fed small intestine 1 out of 5 capsules were completely surrounded by fluid.
Table 5.
Total volume, number and volume of liquid pockets, and proximity of capsules to liquid-filled regions in the fasted and fed small intestine (Reproduced from reference 72.). Fasting conditions and 1 hour after a meal (n=12)72.
| Condition | Fasted | Fed | |
|---|---|---|---|
| Total volume of liquid (mL) | Mean±s.d | 105±72a | 54±41a |
| Range | 45–319 | 20–156 | |
| Median | 83 | 39 | |
| Individual (approx.)b | 45, 48, 69, 73, 77, 81, 85, 94, 113, 115, 130, 319 | 20, 22, 26, 28, 30, 38, 44, 50, 70, 75, 101, 156 | |
| Number of liquid pockets | Mean | 4c | 6c |
| Individual (approx.)b | 2, 3, 4, 5, 8 | 2, 5, 6, 7, 11 | |
| Volume of liquid pocket (mL) | Median | 12d | 4d |
| Number of capsules surrounded by liquid | No./Total | 14/28 | 1/5 |
| Number of capsules partially surrounded by liquid | No./Total | 6/28 | 1/5 |
| Number of capsules not in contact with liquid | No./Total | 8/28 | 3/5 |
P<0.01.
Approximate values read from graph.
P<0.05.
P<0.001
Based on these results, it is possible that the volume of water a dosage form is in contact with is less than the volumes shown in Table 4. In addition, a dosage form may not be exposed to fluid during the entire time it spends in the GI tract. Both scenarios could decrease the solubility and dissolution rate and could lead to an inhomogeneous concentration of drug in the GI lumen. Consequently, the absorption rate of the drug into the GI membrane may not be adequately predicted, as the drug concentration at the intestinal wall may not be similar to the bulk drug concentration.
Hydrodynamics
GI hydrodynamics are partially dependent on contractions in the stomach and small intestine, as well as the amount of liquid and solids present. Layers of smooth muscle contract in a coordinated, rhythmic motion. The contractions cause motility that propels food through the GI tract in a peristaltic motion, mixes chyme within the GI lumen, and juxtaposes chyme with the brush border of the enterocytes. Smooth muscle also causes intestinal villi to undulate, agitating the unstirred layer of fluid associated with the brush border of the enterocytes11. Contractile activity typically initiates in the antrum and migrates distally through the duodenum of the small intestine. The autonomic nervous system and various digestive system hormones control the contractions.
Contractility in the fasted state is characterized by cyclical fluctuations. The cycle comprises three well-defined phases, including a quiescent phase (phase I), a phase of intermittent and irregular contractions that gradually increase in strength (phase II), and a short period of intense contractions (phase III)77. This cyclical contractility pattern is called the Migrating Motility Complex (MMC). The MMC can initiate not only in the stomach, but also at various points along the esophagus and small intestine, with the incidences varying in the different segments10. The total cycle typically lasts approximately 90–120 min, but has been shown to range from 15–180 min78.
In the fed state, the MMC is replaced by regular, tonic contractions that propel food toward the antrum and mix it with gastric secretions79. During these contractions fine particles and liquids pass from the stomach to the duodenum, while larger particles are retro-pulsed back into the body of the stomach. Once the meal has been emptied from the stomach, the MMC resumes. Gastrointestinal motility influences the gastric emptying rate, intestinal transit time, and mixing patterns of solids and liquids in the stomach and intestine80–83.
Gastric-emptying rate and forces
The gastric emptying rate defines the rate at which liquids and solids empty from the stomach into the upper small intestine. It determines the residence time of a drug in the stomach as well as the rate at which the drug is introduced into the small intestine. As most drugs are absorbed primarily in the small intestine, the rate and extent to which dissolved drug is presented to this segment influences drug absorption, and thus onset of the desired therapeutic response. Gastric emptying can be the rate-limiting step in absorption for rapidly dissolving, immediate-release BCS I drugs84.
In the fasted state, the MMC greatly regulates gastric emptying rate, while in the fed state gastric emptying is influenced by low-amplitude contractions as well as pyloric resistance and duodenal feedback mechanisms77. In both the fasted and fed states, emptying rate also depends on the amount of liquid or solid ingested, the size/nature of the liquid or solid ingested, and the phase of contraction during which the liquid or solid was ingested (Refer to Table 6 for a summary of gastric residence times from the literature).
Table 6.
Literature values for residence time in the stomach, residence time in the small intestine and small intestinal flow rates.
| Time for half-emptying - stomach (min) | Fasted | Mean | 15.8 (300mL water)a, 12 (saline)b, 75 (glucose)c | ||
| Range | 11.5–17.0 (300mL water)a | ||||
| Fed | Mean | 44±15 (liquids)121, 105±21 (solids) 121, 40±13121, 32±7 (liquids)122, 46±9 (liquids)122, 67±9 (liquids)122, 76±6 (liquids)122, 72d, 69d | |||
| Range | 69–93d, 50–76d | ||||
| Time for complete emptying - stomach (min) | Fasted | Mean | 25a | ||
| Fed | Mean | 40d | |||
| Transit time - entire small intestine (min) | Fasted | Mean | 192 (coated pellets)e | ||
| Range | 90–324 (coated pellets)e, 132–354 (pellets)f, 54–372 (tablets)f | ||||
| Fed | Mean | 276±99 h (liquids) 121, 342±120 hg | |||
| Range | |||||
| Transit time - duodenum to jejunum (min)123, | Fed | Mean | 32±3 (40kcal/h) 30±1 (90kcal/h), 32±2 (160kcal/h) | ||
| Transit time - duodenum to ileum (min)123 | Fed | Mean | 59±2 (160kcal/h), 47±3 (40kcal/h), 47±2 (90kcal/h) | ||
| Flow rate - jejunum (mL/min)h | Fasted | Mean | 0.73 | ||
| Fed | Mean | 3.0 | |||
| Flow rate – ileum (mL/min)h | Fasted | Mean | 0.33 | ||
| Fed | Mean | 2.35 | |||
Non-nutrient liquids do not normally interrupt the MMC and are typically emptied in an exponential pattern70, 79. Granger and co-workers showed that the half-time for saline emptying from the human stomach is 12 min85, and Steingoetter and co-workers found the half-time for emptying 300 mL of water to be 15.8 min70.
Gastric emptying postprandially is largely dependent on meal size and composition79. When nutrient liquids or solid meals are ingested, the MMC can be interrupted due to feedback mechanisms in the duodenum. A 25% glucose solution has been shown to empty in 75 min in humans79. Kwiatek and co-workers found gastric emptying half time to decrease with increasing nutrient liquid volume and increase with increasing calorie load71 as shown in Table 7. Dressman et al. summarized typical solid-meal half-emptying rates in humans from the literature and found them to range from 70–130 min79.
Table 7.
Effects of meal volume and caloric load on the half-emptying time of gastric contents (Reproduced from reference 71.). Data and standard error between any 2 volumes (in parenthesis) were estimated from mixed-effects model. The standard errors for differences between 2 volumes are given in parenthesis71.
| Meal Volume (mL) | ||||
|---|---|---|---|---|
| Caloric load (kcal) | 200 | 400 | 600 | 800 |
| 200 | 56 (7) | 41 (8) | 42 (8) | 38 (8)a |
| 300 | 74 (7)+ | 59 (8)b | 60 (8)b | 56 (8)a,b |
| 400 | 92 (7)+ | 77 (8)b | 78 (8) | 74 (8)a,b |
P ≤ 0.05 vs. 200 mL
P < 0.01 vs. 200 kcal71.
It is thought by many researchers that beyond a size of 2–7 mm, gastric emptying of solid dosage forms or solid particulates differs from that of liquids and occurs mainly during phase II and III of the MMC84. Bass showed that single tablets ranging in diameter from about 5–13 mm typically left the stomach between 5 and 120 min (the average MMC cycle time), although times ranged from 5 to over 200 min, with high intrasubject and intersubject variability77. Rhie et al. demonstrated that gastric emptying of 0.7 mm caffeine pellets happened during the fed state, while 3.6 mm acetaminophen pellets emptied following the onset of phase II contractions in the fasted state86. Using modeling, Higaki et al. found gastric emptying of 0.7 mm caffeine pellets in the fed state to be regulated by gastric motor activity, with absorption kinetics closely related to the gastric-emptying profiles. Podczeck et al. showed that 3-mm- and 10-mm-diameter tablets emptied after food (dextrose solution, beef solution, or shepherd’s pie) had left the stomach, and that the influence of tablet diameter on median emptying time was significantly less than the influence of administering solid food (shepherd’s pie) compared to liquid meals (dextrose or beef solutions)87.
The forces to which tablets are exposed in the stomach were evaluated in both the fed and fasted states by Kamba and coworkers88. They utilized specially designed Teflon tablets with predetermined crushing strengths to evaluate these forces. They found that tablets with a crushing strength of 1.5 N were crushed in all four subjects under fed conditions and two of five subjects under fasting conditions. Tablets with a higher crushing strength of 1.89 N were crushed in two of six subjects under fed conditions and zero of five subjects under fasting conditions. The authors reasoned that the lower crushing forces in the fasted state occurred because of the open pylorus, resulting in lower overall forces being applied to the stomach contents. Laulicht and coworkers also investigated gastric forces using a magnetic tracking system89. The average human gastric emptying force was 414±194 dyn in the fasted state, which was statistically insignificantly lower than the 657±84 dyn measured in the fed state. Corresponding area normalized gastric emptying pressures were approximately 600 dyn/cm2 in the fasted state and 960 dyn/cm2 in the fed state.
Intestinal transit time and flow rate
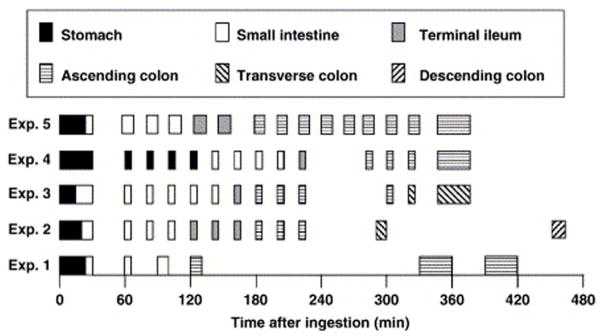
The transit time (i.e., residence time) of a drug in the intestinal tract is a strong determinant of dissolution and absorption. It affects the amount of time a drug has to dissolve and absorb in the GI tract. The transit time of a dosage form in different segments of the GI tract is dependent upon factors such as gastric emptying rate and flow rate, and can vary significantly for even a single individual. Weitschies et al. performed a study on one individual in which they administered a non-disintegrating capsule to a volunteer on several separate occasions and monitored it using magnetic marker monitoring90. As shown in Figure 3, the variability in residence times in different segments of the GI tract was high even for a single individual. Refer to Table 6 for a summary of intestinal residence times from the literature.
Figure 3.
Gastrointestinal transit of magnetically marked non-disintegrating capsules in a single volunteer after ingestion with 150 mL of water. Capsule taken after 8 h of fasting. Lunch served 240 min after ingestion of the capsule in experiments 1–490 (Reprinted from reference 90, with permission.).
Transit time in the small intestine is often quoted to be 3–4 h. McConnell and co-workers found times to range from 0.5–5.4 h with a mean of 3.2 h for a single individual given a 1–1.4-mm ethylcellulose –coated pellet on eight separate occasions10. Based on a review of the literature they stated that food has generally not been associated with changes in transit time in the small intestine.
Davis et al. completed a meta-analysis of transit data and found no difference in the intestinal transit times of tablets, pellets, and liquids91. Coupe et al. found transit times in the small intestine to range from 2.2 to 5.9 h for pellets and 0.9–6.2 h for 11.5-mm tablets92.
The mean intestinal flow rate during fasting for all three phases of the MMC was shown to be 0.73 mL/min in the jejunum and 0.33 mL/min in the ileum (the flow rate in the duodenum was too fast to measure)93. The flow rates were shown to increase postprandially, with a value of 3.0 mL/min in the jejunum and 2.35 mL/min in the ileum93. Granger and co-workers stated that chyme traverses the small intestine in humans at a rate of 1–4 cm/min, with the velocity being faster in the duodenum and proximal jejunum compared to the ileum85. Table 6 includes a summary of intestinal transit times and flowrates from the literature.
Intestinal transit time is especially important for dosage forms that are not fully absorbed, as a change in contact time with the absorption area will result in a change in the fraction absorbed. While in general an increase in transit time will lead to an increase in the absorption of poorly or incompletely absorbed drugs, absorption can be decreased in cases where transit time is slowed because of an inhibition of smooth muscle motility due to a decrease in agitation of the unstirred layer11.
Geometry & Composition of Intestinal Membrane
Surface area
Absorption rate is a function of the gastrointestinal surface area over which the drug is exposed. Generally speaking, a larger surface area would lead to a greater absorption rate. Drugs are rarely absorbed in the stomach due to its small surface area and short residence times94. The small intestine is the major site of drug absorption due to its large surface area and longer residence times. The mucosal surface of the small intestinal lumen is convoluted. Finger-like projections called villi extend from the luminal surface, and each villus is covered with smaller microvilli. Together, the convoluted mucosa along with the villi and microvilli increase the surface area of the small intestine approximately 600-fold above that of a flat tube of the same overall length and diameter23. These anatomical modifications increase the surface area of the duodenum and upper jejunum to a greater extent than the ileum, with the majority of surface area in the small intestine found in the jejunum11.
While the absolute surface area in the small intestine is quite large as described above, the geometric surface area (calculated solely based on the overall length and diameter of the intestine) may be a better estimate of the area of exposure for a dosage form, as it more accurately reflects the surface area of the unstirred layer which is a barrier to drug absorption. Absolute and geometric surface areas, as well as geometries are included in Table 4.
Nature of intestinal membrane and absorption mechanisms
Absorption of drugs in the GI tract occurs mainly in the intestine. Several positive factors help drive absorption, including a concentration gradient, electrochemical potential difference, and hydrostatic pressure gradient between the intestinal lumen and the membrane95. In addition, several other factors deter drug absorption, including the physical barrier of the intestinal mucosa as a result of tight junctions and the lipid composition of the membrane, as well as biochemical barriers such as the presence of metabolizing enzymes and efflux transporters95.
The pathways for drug absorption include carrier-mediated transcellular transport, vesicular transport, passive paracellular transport, and passive transcellular transport. In carrier-mediated transcellular transport, influx transporters expressed on the mucosa actively carry drugs across the membrane. The vesicular transport route includes fluid-phase endocytosis, receptor-mediated endocytosis, and transcytosis. In the passive paracellular route, drug absorption occurs through an extracellular route across the epithelium. Diffusion is regulated by electrochemical potential gradients derived from concentration differences and by electrical and hydrostatic pressure gradients between the two sides of the epithelium95. Tight junctions are the main barriers to this type of absorption. Finally, passive transcellular transport occurs when drugs move across the apical membrane, through the cytoplasm, and across the basolateral membrane. The surface area available for this type of transport makes up 99.9% versus 0.01% for the passive paracellular pathway95.
As mentioned above, enzymes expressed on enterocytes can metabolize some drugs, causing a decrease in absorption. In addition, drugs can be metabolized or degraded in the GI lumen. In addition, efflux transporters mediate the transfer of some compounds from the cytoplasm back into the intestinal lumen. These factors all decrease the net absorption of drugs in the intestinal membrane and thus lower the potential bioavailability.
Physiological dissolution methodologies
Simulated gastric and intestinal fluids are media designed to mimic the major characteristics of in vivo fluids. Simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were described in the USP as early as 195596. As our knowledge of GI physiology has increased over the years, these fluids have been updated to more closely mimic in vivo characteristics. The most recent update by Jantratid and co-workers presents the most up-to-date fluids (Refer to Tables 8 and 9.) and summarizes some of the changes made over the years6. Jantratid and co-workers have proposed the use of “snapshot media” to simulate both gastric and intestinal fluids during different stages after meal consumption. Despite some potential drawbacks, simulated gastric and intestinal fluids make dissolution testing more physiological compared to using simple buffers and a number of successful IVIVCs have been generated using these fluids97–98.
Table 8.
Evolution of fasted and fed simulated gastric fluids.
| Fluid name | USP SGF, TS96 | FaSSGFa | N/Ab | FeSSGFb | N/Ab |
|---|---|---|---|---|---|
| Prandial state | Fasted | Fasted | Fed (early) | Fed (middle) | Fed (late) |
| Year | 1955 | 2005 | 2008 | 2008 | 2008 |
| Buffer type | - | - | - | Acetate | Phosphate |
| Buffer concentration (mM) | - | - | - | 46.9 | 37.5 |
| pH | ~1.2 | 1.6 | 6.4 | 5.0 | 3 |
| Buffer capacity (mmol/L/pH) | - | - | 21.33 | 25 | 25 |
| Osmolality (mOsm/kg) | Not available | 120.7 ± 2.5 | 559 | 400 | 300 |
| Surface tension (mN/m) | 50.81124 | 42.6 | 49.7 ± 0.3 | 52.3 ± 0.3 | 58.1 ± 0.2 |
| Composition | Hydrochloric acid, 70 mM Pepsin, 3.2 g/L Sodium chloride, 34.2 mM | Sodium taurocholate, 80 μM Lecithin, 20 μM Pepsin, 0.1 mg/mL Sodium Chloride, 34.2 mM Hydrochloric acid, q.s. | Sodium chloride, 148 mM Milk:buffer, 1:0 Hydrochloric acid/Sodium hydroxide, q.s. | Sodium chloride, 237.02 mM Acetic acid, 17.12 mM Sodium acetate, 29.75 mM Milk:buffer, 1:1 Hydrochloric acid/Sodium hydroxide, q.s. | Sodium chloride, 122.6 mM Ortho-phosphoric acid, 5.5 mM Sodium dihydrogen phosphate, 32 mM Milk:buffer, 1:3 Hydrochloric acid/Sodium hydroxide, q.s. |
Table 9.
Evolution of fasted and fed simulated intestinal fluids.
| Fluid name | USP SIF, TSa | USP SIF, TSb | FaSSIF125 | FaSSIFm126 | FaSSIF-V2c | FeSSIF125 | FeSSIFc126 | FeSSIF-V2c |
|---|---|---|---|---|---|---|---|---|
| Prandial state | Not specified | Fasted | Fasted | Fasted | Fasted | Fed | Fed | Fed (combin ed early, middle, late) |
| Year | 1960d | 1996 | 1998 | 2004 | 2008 | 1998 | 2004 | 2008 |
| Buffer type | Phosphate | Phosphate | Phosphate | Maleate | Maleate | Acetate | Citrate | Maleate |
| Buffer concentration (mM) | 50.0d | 50.0 | 28.7 | 25.0 | 19.1 | 144 | 84 | 55.0 |
| pH | 7.5 | 6.8 | 6.5 | 6.5 | 6.5 | 5.0 | 5.0 | 5.8 |
| Buffer capacity (mmol/L/pH) | Not available | 18.4 ± 0.2 (w/o pancreatin) | 12 | 12 | 10 | 76 | 76 | 25 |
| Osmolality (mOsm/kg) | Not available | 113 | 270±10 | 270±10 | 180±10 | 635 ± 10 | 635 ± 10 | 390 ± 10 |
| Surface tension (mN/m) | Not available | Not available | Not available | Not available | 54.3 | Not available | Not available | 40.5 ± 2 |
| Composition | Monobasic potassium phosphate,50.0 mMd Sodium hydroxide, ~15.4 mMd Pancreatin, 10.0g/L Hydrochloric acid/Sodium hydroxide, q.s. | Monobasic potassium phosphate, 50.0 mM Sodium hydroxide, ~15.4 mM Pancreatin, 10.0g/L Hydrochloric acid/Sodium hydroxide, q.s. | Sodium taurocholate, 3 mM Egg phosphatidyl choline, 0.75 mM Sodium dihydrogen phosphate, 28.66 mM Sodium hydroxide, ~13.8 mM Sodium chloride, 106 mM | Sodium taurocholate, 3 mM Egg phosphatidyl choline, 0.75 mM Maleic anhydride, 25.01 mM Sodium hydroxide, ~45 mM Sodium chloride, 109 mM | Sodium taurocholate, 3 mM Lecithin, 0.2 mM Maleic acid, 19.12 Sodium Hydroxide, 34.8 mM Sodium Chloride, 68.62 mM | Sodium taurocholate, 15 mM Egg phosphatidylcholine, 3.75 mM Acetic acid, 144 mM Sodium hydroxide, ~101 mM Sodium chloride, 173 mM | Sodium taurocholate, 15 mM Egg phosphatidylcholine, 3.75 mM Citric acid, 84 mM Sodium hydroxide, ~200 mM Sodium chloride, 206mM | Sodium taurocholate, 10 mM Lecithin, 2 mM Glyceryl monooleate, 5 mM Maleic acid, 55.02 mM Sodium oleate, 0.8 mM Sodium Hydroxide, 81.65 mM Sodium Chloride, 125.5 mM |
While existing in vitro systems partially address some of the major fluid components by utilizing simulated fluids, existing dissolution and dosage form testing methodologies generally fail to adequately address physiologically relevant hydrodynamics of fluid flow, shear and viscosity2, 6, 67. New, innovative dissolution methodologies that are more reflective of in vivo hydrodynamics and fluid content in the human intestinal tract are needed. Current dissolution methodologies produce variable and generally extremely high fluid velocities and thus “unrealistic” fluid flow (e.g., 5000<Re<10000)99–102, while current information on fluid flow in the human stomach and intestine indicate Re in the range of 1 to 3067, 82–83, 103–104. Novel dissolution methodologies that characterize dissolution under low Re and fluid shear are required to better simulate dissolution in vivo.
Conclusions
Pharmaceutical solid oral dosage forms must undergo dissolution in the intestinal fluids of the gastrointestinal tract before they can be absorbed and reach the systemic circulation. Therefore, dissolution is a critical part of the drug-delivery process. The characteristics of the physiological environment such as buffer species, pH, bile salts, gastric emptying rate, intestinal motility, and hydrodynamics will significantly impact dissolution and absorption. While significant progress has been made since 1970, when the first compendial dissolution test was introduced, current dissolution testing does not take full advantage of the extensive physiologic information that is available. For quality control purposes, where the question is one of lot-to-lot consistency in performance, utilizing nonphysiological test conditions that match drug and dosage form properties with practical dissolution media and apparatus may be appropriate. However, where IVIVCs are desired, it is logical to consider and utilize knowledge of the in vivo situation. Physiologically relevant information must serve as a basis for the design of dissolution test methods and systems that are more representative of the human condition. As in vitro methods advance in their physiological relevance, better IVIVCs will be possible. In vitro systems can then be more effectively utilized to design dosage forms that have improved and consistent oral bioperformance.
Acknowledgments
This project was supported in part by Abbott Laboratories and grant number GM007767 from NIGMS. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS.
Footnotes
Supporting Information Available. This manuscript does not contain supporting information.
References
- 1.Abdou HM. Effect of the Physicochemical Properties of the Drug on Dissolution Rate. In: Gennaro A, Migdalof B, Hassert GL, Medwick T, editors. Dissolution, Bioavailability and Bioequivalence. 1. Mack Publishing; Easton, PA: 1989. pp. 56–72. [Google Scholar]
- 2.Dressman JB, Amidon GL, Reppas C, Shah VP. Dissolution Testing as a Prognostic Tool for Oral Drug Absorption: Immediate Release Dosage Forms. Pharm Res. 1998;15:11–22. doi: 10.1023/a:1011984216775. [DOI] [PubMed] [Google Scholar]
- 3.USP. The United States Pharmacopeia USP 31, the National Formulary NF 26. The United States Pharmacopeial Convention, Inc; Rockville: 2008. [Google Scholar]
- 4.Amidon GL, Lennernas H, Shah VP, Crison JR. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm Res. 1995;12:413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 5.FDA, Guidance for Industry. Waiver of the in Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. U.S. Department of Health and Human, Food and Drug Administration (FDA), Center for Drug Evaluation and Research; Washington, DC: 2000. pp. 1–13. [Google Scholar]
- 6.Jantratid E, Janssen N, Reppas C, Dressman J. Dissolution Media Simulating Conditions in the Proximal Human Gastrointestinal Tract: An Update. Pharm Res. 2008;25:1663–1676. doi: 10.1007/s11095-008-9569-4. [DOI] [PubMed] [Google Scholar]
- 7.Carino SR, Sperry DC, Hawley M. Relative Bioavailability Estimation of Carbamazepine Crystal Forms Using an Artificial Stomach-Duodenum Model. J Pharm Sci. 2006;95:116–125. doi: 10.1002/jps.20495. [DOI] [PubMed] [Google Scholar]
- 8.Grassi M, Grassi G, Lapasin R, Colombo I. Understanding Drug Release and Absorption Mechanisms, a Physical and Mathematical Approach. CRC Press; Boca Raton: 2007. Drug Dissolution and Partitioning; pp. 249–327. [Google Scholar]
- 9.Vangani S, Li X, Zhou PMDB, Chiu R, Cauchon N, Gao P, Medina C, Jasti B. Dissolution of Poorly Water-Soluble Drugs in Biphasic Media Using Usp 4 and Fiber Optic System. Clinical Research and Regulatory Affairs. 2009;26:8–19. [Google Scholar]
- 10.McConnell EL, Fadda HM, Basit AW. Gut Instincts: Explorations in Intestinal Physiology and Drug Delivery. Int J Pharm. 2008;364:213–226. doi: 10.1016/j.ijpharm.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 11.DeSesso JM, Jacobson CF. Anatomical and Physiological Parameters Affecting Gastrointestinal Absorption in Humans and Rats. Food Chem Toxicol. 2001;39:209–228. doi: 10.1016/s0278-6915(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 12.Florence AT, Attwood D. Physicochemical Principles of Pharmacy. 2. Chapman and Hall; New York: 1988. Properties of the Solid State; pp. 21–46. [Google Scholar]
- 13.Dahan AS, Amidon GL. Gastrointestinal Dissolution and Absorption of Class II Drugs. In: van de Waterbeemd H, Testa B, editors. Drug Bioavailability, Estimation of Solubility, Permeability, Absorption, and Bioavailability. 2. Wiley-VCH; Weinheim: 2009. pp. 33–51. [Google Scholar]
- 14.Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Reppas C. Characterization of the Human Upper Gastrointestinal Contents under Conditions Simulating Bioavailability/Bioequivalence Studies. Pharm Res. 2006;23:165–176. doi: 10.1007/s11095-005-8476-1. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt HA, Fritzlar G, Dolle W, Goebell H. Comparative Studies on the Histamine and Insulin Stimulated Acid Pepsin Secretion in Patients Suffering from Ulcus Duodeni and Control Persons. Dtsch Med Wochenschr. 1970;95:2011–2006. doi: 10.1055/s-0028-1108771. [DOI] [PubMed] [Google Scholar]
- 16.Lambert R, Martin F, Vagne M. Relationship between Hydrogen Ion and Pepsin Concentration in Human Gastric Secretion. Digestion. 1968;1:65–77. doi: 10.1159/000196835. [DOI] [PubMed] [Google Scholar]
- 17.Vertzoni M, Dressman J, Butler J, Hempenstall J, Reppas C. Simulation of Fasting Gastric Conditions and Its Importance for the in Vivo Dissolution of Lipophilic Compounds. Eur J Pharm Biopharm. 2005;60:413–417. doi: 10.1016/j.ejpb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Armand M, Borel P, Pasquier B, Dubois C, Senft M, Andre M, Peyrot J, Salducci J, Lairon D. Physicochemical Characteristics of Emulsions During Fat Digestion in Human Stomach and Duodenum. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1996;34:G172–G183. doi: 10.1152/ajpgi.1996.271.1.G172. [DOI] [PubMed] [Google Scholar]
- 19.Vertzoni M, Archontaki H, Reppas C. Determination of Intralumenal Individual Bile Acids by HPLC with Charged Aerosol Detection. J Lipid Res. 2008;49:2690–2695. doi: 10.1194/jlr.D800039-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes J, Barnardo DE, Phillips SF, Rovelsta Ra, Hofmann AF. Increased Reflux of Bile into Stomach in Patients with Gastric Ulcer. Gastroenterology. 1969;57:241–252. [PubMed] [Google Scholar]
- 21.Kristensen M. Titration Curves for Gastric-Secretion - Study on Duodenal-Ulcer and Gastric-Ulcer with Particular Reference to Effect of Glycopyrronium. Scand J Gastroenterol. 1975;10:1–148. [PubMed] [Google Scholar]
- 22.Rees WD, Botham D, Turnberg LA. A Demonstration of Bicarbonate Production by the Normal Human Stomach in Vivo. Dig Dis Sci. 1982;27:961–966. doi: 10.1007/BF01391739. [DOI] [PubMed] [Google Scholar]
- 23.Widmaier EP, Raff H, Strang KT. Vander’s Human Physiology: The Mechanisms of Body Function. 10. McGraw-Hill; New York: 2006. The Digestion and Absorption of Food; pp. 575–614. [Google Scholar]
- 24.Konturek PC, Konturek SJ, Hahn EG. Duodenal Alkaline Secretion: Its Mechanisms and Role in Mucosal Protection against Gastri Acid. Dig Liver Dis. 2004;36:505–512. doi: 10.1016/j.dld.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Sheng JJ, McNanara DP, Amidon GL. Toward an in Vivo Dissolution Methodology: A Comparison of Phosphate and Bicarbonate Buffers. Mol Pharmaceutics. 2009;6:29–39. doi: 10.1021/mp800148u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sunesen VH, Vedelsdal R, Kristensen HG, Christrup L, Mullertz A. Effect of Liquid Volume and Food Intake on the Absolute Bioavailability of Danazol, a Poorly Soluble Drug. Eur J Pharm Sci. 2005;24:297–303. doi: 10.1016/j.ejps.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Leyden JJ. Absorption of Minocycline Hydrochloride and Tetracycline Hydrochloride - Effect of Food, Milk, and Iron. J Am Acad Dermatol. 1985;12:308–312. doi: 10.1016/s0190-9622(85)80041-4. [DOI] [PubMed] [Google Scholar]
- 28.Clarysse S, Psachoulias D, Brouwers J, Tack J, Annaert P, Duchateau G, Reppas C, Augustijns P. Postprandial Changes in Solubilizing Capacity of Human Intestinal Fluids for Bcs Class II Drugs. Pharm Res. 2009;26:1456–1466. doi: 10.1007/s11095-009-9857-7. [DOI] [PubMed] [Google Scholar]
- 29.Evans DF, Micellar HW. The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet. 2. VCH Publishers Inc; New York: 1999. Solutions Play a Key Role in Many Industrial and Biological Processes; pp. 198–216. [Google Scholar]
- 30.Rosoff M, Serajuddin ATM. Solubilization of Diazepam in Bile-Salts and in Sodium Cholate-Lecithin-Water Phases. Int J Pharm. 1980;6:137–146. [Google Scholar]
- 31.Mithani SD, Bakatselou V, TenHoor CN, Dressman JB. Estimation of the Increase in Solubility of Drugs as a Function of Bile Salt Concentration. Pharm Res. 1996;13:163–167. doi: 10.1023/a:1016062224568. [DOI] [PubMed] [Google Scholar]
- 32.Cai XH, Grant DJW, Wiedmann TS. Analysis of the Solubilization of Steroids by Bile Salt Micelles. J Pharm Sci. 1997;86:372–377. doi: 10.1021/js9602148. [DOI] [PubMed] [Google Scholar]
- 33.Clarysse S, Tack J, Lammert F, Duchateau G, Reppas C, Augustijns P. Postprandial Evolution in Composition and Characteristics of Human Duodenal Fluids in Different Nutritional States. J Pharm Sci. 2009;98:1177–1192. doi: 10.1002/jps.21502. [DOI] [PubMed] [Google Scholar]
- 34.Brouwers J, Tack J, Lammert F, Augustijns P. Intraluminal Drug and Formulation Behavior and Integration in in Vitro Permeability Estimation: A Case Study with Amprenavir. J Pharm Sci. 2006;95:372–383. doi: 10.1002/jps.20553. [DOI] [PubMed] [Google Scholar]
- 35.Persson EM, Gustafsson AS, Carlsson AS, Nilsson RG, Knutson L, Forsell P, Hanisch G, Lennernas H, Abrahamsson B. The Effects of Food on the Dissolution of Poorly Soluble Drugs in Human and in Model Small Intestinal Fluids. Pharm Res. 2005;22:2141–2151. doi: 10.1007/s11095-005-8192-x. [DOI] [PubMed] [Google Scholar]
- 36.Ladas SD, Isaacs PE, Murphy GM, Sladen GE. Comparison of the Effects of Medium and Long Chain Triglyceride Containing Liquid Meals on Gall Bladder and Small Intestinal Function in Normal Man. Gut. 1984;25:405–411. doi: 10.1136/gut.25.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fausa O. Duodenal Bile Acids after a Test Meal. Scand J Gastroenterol. 1974;9:567–570. [PubMed] [Google Scholar]
- 38.Northfield TC, McColl I. Postprandial Concentrations of Free and Conjugated Bile Acids Down the Length of the Normal Human Small Intestine. Gut. 1973;14:513–518. doi: 10.1136/gut.14.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGee LC, Hastings AB. The Carbon Dioxide Tension and Acid-Base Balance of Jejunal Secretions in Man. J Biol Chem. 1942;142:893–904. [Google Scholar]
- 40.Hardman JG. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. 10. McGraw-Hill; New York: 2001. [Google Scholar]
- 41.Davenport HW. Physiology of the Digestive Tract. Year Book Medical Publishers, Inc; Chicago: 1982. Digestion and Absorption; pp. 179–235. [Google Scholar]
- 42.White A, Handler P, Smith EL. Principles of Biochemistry. 4. McGraw-Hill; New York: 1968. Specialized Extracellular Fluids; pp. 806–827. [Google Scholar]
- 43.Banwell JG, Gorbach SL, Pierce NF, Mitra R, Mondal A. Acute Undifferentiated Human Diarrhea in Tropics 2. Alterations in Intestinal Fluid and Electrolyte Movements. J Clin Invest. 1971;50:890–900. doi: 10.1172/JCI106561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rune SJ. Acid-Base Parameters of Duodenal Contents in Man. Gastroenterology. 1972;62:533–539. [PubMed] [Google Scholar]
- 45.Sinko PJ. Solubility and Distribution Phenomena. In: Troy D, editor. Martin’s Physical Pharmacy. 5. Lippincott: Williams & Wilkins; 2006. pp. 231–266. [Google Scholar]
- 46.Sheng JJ, Kasim NA, Chandrasekharan R, Amidon GL. Solubilization and Dissolution of Insoluble Weak Acid, Ketoprofen: Effects of pH Combined with Surfactant. Eur J Pharm Sci. 2006;29:306–314. doi: 10.1016/j.ejps.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Li SF, Wong SM, Sethia S, Almoazen H, Joshi YM, Serajuddin ATM. Investigation of Solubility and Dissolution of a Free Base and Two Different Salt Forms as a Function of pH. Pharm Res. 2005;22:628–635. doi: 10.1007/s11095-005-2504-z. [DOI] [PubMed] [Google Scholar]
- 48.Phaechamud T, Ritthidej GC. Sustained-Release from Layered Matrix System Comprising Chitosan and Xanthan Gum. Drug Dev Ind Pharm. 2007;33:595–605. doi: 10.1080/03639040601015521. [DOI] [PubMed] [Google Scholar]
- 49.Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of Gastrointestinal pH Profiles in Normal Ambulant Human Subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindahl A, Ungell AL, Knutson L, Lennernas H. Characterization of Fluids from the Stomach and Proximal Jejunum in Men and Women. Pharm Res. 1997;14:497–502. doi: 10.1023/a:1012107801889. [DOI] [PubMed] [Google Scholar]
- 51.Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, Jarvenpaa KM. Upper Gastrointestinal (GI) pH in Young, Healthy Men and Women. Pharm Res. 1990;7:756–761. doi: 10.1023/a:1015827908309. [DOI] [PubMed] [Google Scholar]
- 52.Annaert P, Brouwers J, Bijnens A, Lammert F, Tack J, Augustijns P. Ex Vivo Permeability Experiments in Excised Rat Intestinal Tissue and in Vitro Solubility Measurements in Aspirated Human Intestinal Fluids Support Age-Dependent Oral Drug Absorption. Eur J Pharm Sci. 2010;39:15–22. doi: 10.1016/j.ejps.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Perez de la Cruz Moreno M, Oth M, Deferme S, Lammert F, Tack J, Dressman J, Augustijns P. Characterization of Fasted-State Human Intestinal Fluids Collected from Duodenum and Jejunum. J Pharm Pharmacol. 2006;58:1079–1089. doi: 10.1211/jpp.58.8.0009. [DOI] [PubMed] [Google Scholar]
- 54.Benn A, Cooke WT. Intraluminal pH of Duodenum and Jejunum in Fasting Subjects with Normal and Abnormal Gastric or Pancreatic Function. Scand J Gastroenterol. 1971;6:313–317. doi: 10.3109/00365527109181126. [DOI] [PubMed] [Google Scholar]
- 55.Youngberg CA, Berardi RR, Howatt WF, Hyneck ML, Amidon GL, Meyer JH, Dressman JB. Comparison of Gastrointestinal pH in Cystic-Fibrosis and Healthy Subjects. Dig Dis Sci. 1987;32:472–480. doi: 10.1007/BF01296029. [DOI] [PubMed] [Google Scholar]
- 56.Watson BW, Meldrum SJ, Riddle HC, Brown RL, Sladen GE. pH Profile of Gut as Measured by Radiotelemetry Capsule. Br Med J. 1972;2:104–106. doi: 10.1136/bmj.2.5805.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ovesen L, Bendtsen F, Tage-Jensen U, Pedersen NT, Gram BR, Rune SJ. Intraluminal Ph in the Stomach, Duodenum, and Proximal Jejunum in Normal Subjects and Patients with Exocrine Pancreatic Insufficiency. Gastroenterology. 1986;90:958–962. doi: 10.1016/0016-5085(86)90873-5. [DOI] [PubMed] [Google Scholar]
- 58.Bown RL, Sladen GE, Clark ML, Dawson AM. The Production and Transport of Ammonia in the Human Colon. Gut. 1971;12:863. [PubMed] [Google Scholar]
- 59.Fadda HM, Sousa T, Carlsson A, Abrahamsson B, Kumar D, Basit AW. Drug Solubility in Luminal Fluids from Different Regions of the Small and Large Intestine of Humans. AAPS Annual Meeting and Exposition; Los Angeles, Los Angeles: 2009. p. AAPS2009-003733. [DOI] [PubMed] [Google Scholar]
- 60.Rudolph MW, Klein S, Beckert TE, Petereit H, Dressman JB. A New 5-Aminosalicylic Acid Multi-Unit Dosage Form for the Therapy of Ulcerative Colitis. Eur J Pharm Biopharm. 2001;51:183–190. doi: 10.1016/s0939-6411(01)00134-5. [DOI] [PubMed] [Google Scholar]
- 61.Gisolfi CV, Summers RW, Lambert GP, Xia T. Effect of Beverage Osmolality on Intestinal Fluid Absorption During Exercise. J Appl Physiol. 1998;85:1941–8. doi: 10.1152/jappl.1998.85.5.1941. [DOI] [PubMed] [Google Scholar]
- 62.Davenport HW. Physiology of the Digestive Tract. Year Book Medical Publishers, Inc; Chicago: 1982. Secretion; pp. 101–178. [Google Scholar]
- 63.Pedersen BL, Mullertz A, Brondsted H, Kristensen HG. A Comparison of the Solubility of Danazol in Human and Simulated Gastrointestinal Fluids. Pharm Res. 2000;17:891–894. doi: 10.1023/a:1007576713216. [DOI] [PubMed] [Google Scholar]
- 64.Dikeman CL, Fahey GC. Viscosity as Related to Dietary Fiber: A Review. Crit Rev Food Sci Nutr. 2006;46:649–663. doi: 10.1080/10408390500511862. [DOI] [PubMed] [Google Scholar]
- 65.Marciani L, Gowland PA, Spiller RC, Manoj P, Moore RJ, Young P, Al-Sahab S, Bush D, Wright J, Fillery-Travis AJ. Gastric Response to Increased Meal Viscosity Assessed by Echo-Planar Magnetic Resonance Imaging in Humans. J Nutr. 2000;130:122–127. doi: 10.1093/jn/130.1.122. [DOI] [PubMed] [Google Scholar]
- 66.Dikeman CL, Murphy MR, Fahey GC. Dietary Fibers Affect Viscosity of Solutions and Simulated Human Gastric and Small Intestinal Digesta. J Nutr. 2006;136:913–919. doi: 10.1093/jn/136.4.913. [DOI] [PubMed] [Google Scholar]
- 67.Abrahamsson B, Pal A, Sjoberg M, Carlsson M, Laurell E, Brasseur JG. A Novel in Vitro and Numerical Analysis of Shear-Induced Drug Release from Extended-Release Tablets in the Fed Stomach. Pharm Res. 2005;22:1215–1226. doi: 10.1007/s11095-005-5272-x. [DOI] [PubMed] [Google Scholar]
- 68.Malkki Y. Physical Properties of Dietary Fiber as Keys to Physiological Functions. Cereal Foods World. 2001;46:196–199. [Google Scholar]
- 69.Lim CL, Byrne C, Lee JKW. Human Thermoregulation and Measurement of Body Temperature in Exercise and Clinical Settings. Annals Academy of Medicine Singapore. 2008;37:347–353. [PubMed] [Google Scholar]
- 70.Steingoetter A, Fox M, Treier R, Weishaupt D, Marincek B, Boesiger P, Fried M, Schwizer W. Effects of Posture on the Physiology of Gastric Emptying: A Magnetic Resonance Imaging Study. Scand J Gastroenterol. 2006;41:1155–1164. doi: 10.1080/00365520600610451. [DOI] [PubMed] [Google Scholar]
- 71.Kwiatek MA, Menne D, Steingoetter A, Goetze O, Forras-Kaufman Z, Kaufman E, Fruehauf H, Boesiger P, Fried M, Schwizer W, Fox MR. Effect of Meal Volume and Calorie Load on Postprandial Gastric Function and Emptying: Studies under Physiological Conditions by Combined Fiber-Optic Pressure Measurement and Mri. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;297:G894–G901. doi: 10.1152/ajpgi.00117.2009. [DOI] [PubMed] [Google Scholar]
- 72.Schiller C, Frohlich CP, Giessmann T, Siegmund W, Monnikes H, Hosten N, Weitschies W. Intestinal Fluid Volumes and Transit of Dosage Forms as Assessed by Magnetic Resonance Imaging. Aliment Pharmacol Ther. 2005;22:971–979. doi: 10.1111/j.1365-2036.2005.02683.x. [DOI] [PubMed] [Google Scholar]
- 73.Marciani L, Cox EF, Hoad CL, Pritchard S, Totman JJ, Foley S, Mistry A, Evans S, Gowland PA, Spiller RC. Postprandial Changes in Small Bowel Water Content in Healthy Subjects and Patients with Irritable Bowel Syndrome. Gastroenterology. 2010;138:469–U90. doi: 10.1053/j.gastro.2009.10.055. [DOI] [PubMed] [Google Scholar]
- 74.Placidi E, Hoad CL, Marciani L, Gowland PA, Spiller RC. Effects of an Osmotic Laxative on the Distribution of Water between the Small and Large Intestine in Humans; British Society of Gastroenterology Annual Scientific Meeting; 2010. [Google Scholar]
- 75.Marciani L, Foley S, Hoad C, Campbell E, Totman J, Armstrong A, Manby P, Gowland PA, Spiller R. Effects of Ondansetron on Small Bowel Water Content: A Magnetic Resonance Imaging Study. United European Gastroenterology Week (UEGW); Paris, Paris: 2007. [Google Scholar]
- 76.Sutton SC. Role of Physiological Intestinal Water in Oral Absorption. AAPS J. 2009;11:277–285. doi: 10.1208/s12248-009-9087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bass P. Capsugel Symposium Series. 1993. Gastric Emptying; Differences among Liquid, Fiber, Polymer and Solid Dosage Forms of Medications; pp. 21–33. [Google Scholar]
- 78.Oberle RL, Chen TS, Lloyd C, Barnett JL, Owyang C, Meyer J, Amidon GL. The Influence of the Interdigestive Migrating Myoelectric Complex on the Gastric Emptying of Liquids. Gastroenterology. 1990;99:1275–1282. doi: 10.1016/0016-5085(90)91150-5. [DOI] [PubMed] [Google Scholar]
- 79.Dressman JB. Comparison of Canine and Human Gastrointestinal Physiology. Pharm Res. 1986;3:123–131. doi: 10.1023/A:1016353705970. [DOI] [PubMed] [Google Scholar]
- 80.Pal A, Indireshkumar K, Schwizer W, Abrahamsson B, Fried M, Brasseur JG. Gastric Flow and Mixing Studied Using Computer Simulation. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:2587–2594. doi: 10.1098/rspb.2004.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Indireshkumar K, Brasseur JG, Faas H, Hebbard GS, Kunz P, Dent J, Feinle C, Li MJ, Boesiger P, Fried M, Schwizer W. Relative Contributions Of “Pressure Pump” And “Peristaltic Pump” To Gastric Emptying. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2000;278:G604–G616. doi: 10.1152/ajpgi.2000.278.4.G604. [DOI] [PubMed] [Google Scholar]
- 82.Pal A, Williams RB, Cook IJ, Brasseur JG. Intrabolus Pressure Gradient Identifies Pathological Constriction in the Upper Esophageal Sphincter During Flow. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2003;285:G1037–G1048. doi: 10.1152/ajpgi.00030.2003. [DOI] [PubMed] [Google Scholar]
- 83.Pal A, Brasseur JG, Abrahamsson B. A Stomach Road Or “Magenstrasse” For Gastric Emptying. Journal of Biomechanics. 2007;40:1202–1210. doi: 10.1016/j.jbiomech.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 84.Higaki K, Choe SY, Lobenberg R, Welage LS, Amidon GL. Mechanistic Understanding of Time-Dependent Oral Absorption Based on Gastric Motor Activity in Humans. Eur J Pharm Biopharm. 2008;70:313–325. doi: 10.1016/j.ejpb.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 85.Granger DN, Barrowman JA, Kvietys PR. Clinical Gastrointestinal Physiology. WB. Saunders; Philadelphia: 1985. [Google Scholar]
- 86.Rhie JK, Hayashi Y, Welage LS, Frens J, Wald RJ, Barnett JL, Amidon GE, Putcha L, Amidon GL. Drug Marker Absorption in Relation to Pellet Size, Gastric Motility and Viscous Meals in Humans. Pharm Res. 1998;15:233–238. doi: 10.1023/a:1011962501270. [DOI] [PubMed] [Google Scholar]
- 87.Podczeck F, Mitchell CL, Newton JM, Evans D, Short MB. The Gastric Emptying of Food as Measured by Gamma-Scintigraphy and Electrical Impedance Tomography (EIT) and Its Influence on the Gastric Emptying of Tablets of Different Dimensions. J Pharm Pharmacol. 2007;59:1527–1536. doi: 10.1211/jpp.59.11.0010. [DOI] [PubMed] [Google Scholar]
- 88.Kamba M, Seta Y, Kusai A, Ikeda M, Nishimura K. A Unique Dosage Form to Evaluate the Mechanical Destructive Force in the Gastrointestinal Tract. Int J Pharm. 2000;208:61–70. doi: 10.1016/s0378-5173(00)00552-4. [DOI] [PubMed] [Google Scholar]
- 89.Laulicht B, Tripathi A, Schlageter V, Kucera P, Mathiowitz E. Understanding Gastric Forces Calculated from High-Resolution Pill Tracking. Proc Natl Acad Sci U S A. 2010;107:8201–8206. doi: 10.1073/pnas.1002292107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weitschies W, Kosch O, Monnikes H, Trahms L. Magnetic Marker Monitoring: An Application of Biomagnetic Measurement Instrumentation and Principles for the Determination of the Gastrointestinal Behavior of Magnetically Marked Solid Dosage Forms. Adv Drug Delivery Rev. 2005;57:1210–1222. doi: 10.1016/j.addr.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 91.Davis SS, Hardy JG, Fara JW. Transit of Pharmaceutical Dosage Forms through the Small-Intestine. Gut. 1986;27:886–892. doi: 10.1136/gut.27.8.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coupe AJ, Davis SS, Wilding IR. Variation in Gastrointestinal Transit of Pharmaceutical Dosage Forms in Healthy-Subjects. Pharm Res. 1991;8:360–364. doi: 10.1023/a:1015849700421. [DOI] [PubMed] [Google Scholar]
- 93.Kerlin P, Zinsmeister A, Phillips S. Relationship of Motility to Flow of Contents in the Human Small-Intestine. Gastroenterology. 1982;82:701–706. [PubMed] [Google Scholar]
- 94.Mayersohn M. Modern Pharmaceutics. Marcel Dekker; New York: 1996. Principles of Drug Absorption; pp. 21–71. [Google Scholar]
- 95.Grassi M, Grassi G, Lapasin R, Colombo I. Understanding Drug Release and Absorption Mechanisms, a Physical and Mathematical Approach. 1. CRC Press; Boca Raton: 2007. Part 1: Gastrointestinal Tract; pp. 29–68. [Google Scholar]
- 96.USP. The Pharmacopeia of the United States of America Xv. Mack Publishing Company; Easton: 1955. [Google Scholar]
- 97.Jantratid E, De Maio V, Ronda E, Mattavelli V, Dressman JB, et al. Application of Biorelevant Dissolution Tests to the Prediction of in Vivo Performance of Diclofenac Sodium from an Oral Modified-Release Pellet Dosage Form. Eur J Pharm Sci. 2009;37:434–441. doi: 10.1016/j.ejps.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 98.Jung H, Milan RC, Girard ME, Leon F, Montoya MA. Bioequivalence Study of Carbamazepine Tablets: In Vitro in Vivo Correlation. Int J Pharm. 1997;152:37–44. [Google Scholar]
- 99.Baxter JL, Kukura J, Muzzio FJ. Hydrodynamics-Induced Variability in the USP Apparatus II Dissolution Test. Int J Pharm. 2005;292:17–28. doi: 10.1016/j.ijpharm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 100.Baxter JL, Kukura J, Muzzio FJ. Shear-Induced Variability in the United States Pharmacopeia Apparatus 2: Modifications to the Existing System. AAPS J. 2005;7:E857–E864. doi: 10.1208/aapsj070483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kukura J, Arratia PE, Szalai ES, Muzzio FJ. Engineering Tools for Understanding the Hydrodynamics of Dissolution Tests. Drug Dev Ind Pharm. 2003;29:231. doi: 10.1081/ddc-120016731. [DOI] [PubMed] [Google Scholar]
- 102.Kukura J, Baxter JL, Muzzio FJ. Shear Distribution and Variability in the USP Apparatus 2 under Turbulent Conditions. Int J Pharm. 2004;279:9–17. doi: 10.1016/j.ijpharm.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 103.Sjoberg M, Laurell E, Carlsson M, Abrahamsson B, Pal A, Brasseur JG. A New in Vitro Dissolution Method Providing in Vivo Relevant Gastric Shear Stress. Eur J Pharm Sci. 2004;23:S45–S45. [Google Scholar]
- 104.Sheng JJ, Sirois PJ, Dressman JB, Amidon GL. Particle Diffusional Layer Thickness in a Usp Dissolution Apparatus II: A Combined Function of Particle Size and Paddle Speed. J Pharm Sci. 2008;97:4815–4829. doi: 10.1002/jps.21345. [DOI] [PubMed] [Google Scholar]
- 105.Bucher GR, Flynn JC, Robinson CS. The Action of the Human Small Intestine in Altering the Composition of Physiological Saline. J Biol Chem. 1944;155:305–313. [Google Scholar]
- 106.Repishti M, Hogan DL, Pratha V, Davydova L, Donowitz M, Tse CM, Isenberg JI. Human Duodenal Mucosal Brush Border Na+/H+ Exchangers Nhe2 and Nhe3 Alter Net Bicarbonate Movement. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001;281:G159–G163. doi: 10.1152/ajpgi.2001.281.1.G159. [DOI] [PubMed] [Google Scholar]
- 107.West JB. Best and Taylor’s Physiological Basis of Medical Practice. 11. Williams & Wilkins; Baltimore: 1985. Absorption; pp. 751–790. [Google Scholar]
- 108.Johnson LR. Fluid and Electrolyte Absorption. In: Johnson LR, editor. Gastrointestinal Physiology. 6. Mosby; St. Louis: 2001. pp. 143–154. [Google Scholar]
- 109.Rune SJ, Henriksen FW. Carbon Dioxide Tensions in the Proximal Part of the Canine Gastrointestinal Tract. Gastroenterology. 1969;56:758–762. [PubMed] [Google Scholar]
- 110.Efentakis M, Dressman JB. Gastric Juice as a Dissolution Medium: Surface Tension and pH. Eur J Drug Metab Pharmacokinet. 1998;23:97–102. doi: 10.1007/BF03189322. [DOI] [PubMed] [Google Scholar]
- 111.Rautureau M, Bisalli A, Rambaud JC. Bile Salts and Lipids in Aqueous Intraluminal Phase During the Digestion of a Standard Meal in Normal Man. Gastroenterol Clin Biol. 1981;5:417–425. [PubMed] [Google Scholar]
- 112.Tangerman A, van Schaik A, van der Hoek EW. Analysis of Conjugated and Unconjugated Bile Acids in Serum and Jejunal Fluid of Normal Subjects. Clin Chim Acta. 1986;159:123–132. doi: 10.1016/0009-8981(86)90044-6. [DOI] [PubMed] [Google Scholar]
- 113.Bratten J, Jones MP. Prolonged Recording of Duodenal Acid Exposure in Patients with Functional Dyspepsia and Controls Using a Radiotelemetry pH Monitoring System. J Clin Gastroenterol. 2009;43:527–533. doi: 10.1097/MCG.0b013e31818e37ab. [DOI] [PubMed] [Google Scholar]
- 114.Maxwell JD, Ferguson A, Watson WC. The Effect of Gastric Secretory Status on Jejunal pH Measured by Radiotelemetering. Digestion. 1971;4:345–352. doi: 10.1159/000197139. [DOI] [PubMed] [Google Scholar]
- 115.Zentler-Munro PL, Fine DRFFWJ, Northfield TC. Effect of Intrajejunal Acidity on Lipid Digestion and Aqueous Solbilisation of Bile Acids and Lipids in Health, Using a New Simple Method of Lipase Inactivation. Gut. 1984;25:491–499. doi: 10.1136/gut.25.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Borgstrom B, Dahlqvist A, Lundh G, Sjovall J. Studies of Intestinal Digestion and Absorption in the Human. J Clin Invest. 1957;36:1521–1536. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Malagelada JR, Longstreth GF, Summerskill WHJ, Go VLW. Measurement of Gastric Functions During Digestion of Ordinary Solid Meals in Man. Gastroenterology. 1976;70:203–210. [PubMed] [Google Scholar]
- 118.Fordtran JS, Locklear TW. Ionic Constituents and Osmolality of Gastric and Small-Intestinal Fluids after Eating. Am J Dig Dis. 1966;11:503–521. doi: 10.1007/BF02233563. [DOI] [PubMed] [Google Scholar]
- 119.Lobo DN, Hendry PO, Rodrigues G, Marciani L, Totman JJ, Wright JW, Preston T, Gowland P, Spiller RC, Fearon KCH. Gastric Emptying of Three Liquid Oral Preoperative Metabolic Preconditioning Regimens Measured by Magnetic Resonance Imaging in Healthy Adult Volunteers: A Randomised Double-Blind, Crossover Study. Clin Nutr. 2009;28:636–641. doi: 10.1016/j.clnu.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 120.Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH. Report of the Task Group on Reference Man. Pergamon Press; New York: 1975. Anatomical Values for Reference Man; pp. 8–46. [Google Scholar]
- 121.Coleman NS, Marciani L, Blackshaw E, Wright J, Parker M, Yano T, Yamazaki S, Chan PQ, Wilde K, Gowland PA, Perkins AC, Spiller RC. Effect of a Novel 5-Ht3 Receptor Agonist Mkc-733 on Upper Gastrointestinal Motility in Humans. Aliment Pharmacol Ther. 2003;18:1039–1048. doi: 10.1046/j.1365-2036.2003.01797.x. [DOI] [PubMed] [Google Scholar]
- 122.Marciani L, Gowland PA, Spiller RC, Manoj P, Moore RJ, Young P, Fillery-Travis AJ. Effect of Meal Viscosity and Nutrients on Satiety, Intragastric Dilution, and Emptying Assessed by MRI. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001;280:G1227–G1233. doi: 10.1152/ajpgi.2001.280.6.G1227. [DOI] [PubMed] [Google Scholar]
- 123.Holtmann G, Kelly DG, Sternby B, DiMagno EP. Survival of Human Pancreatic Enzymes During Small Bowel Transit: Effect of Nutrients, Bile Acids, and Enzymes. Am J Physiol. 1997;273:G553–G558. doi: 10.1152/ajpgi.1997.273.2.G553. [DOI] [PubMed] [Google Scholar]
- 124.Anwar S, Fell JT, Dickinson PA. An Investigation of the Disintegration of Tablets in Biorelevant Media. Int J Pharm. 2005;290:121–127. doi: 10.1016/j.ijpharm.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 125.Galia E, Nicolaides E, Horter D, Lobenberg R, Dressman JB. Evaluation of Various Dissolution Media for Predicting in Vivo Performance of Class I and II Drugs. Pharm Res. 1998;15:698–705. doi: 10.1023/a:1011910801212. [DOI] [PubMed] [Google Scholar]
- 126.Vertzoni M, Fotaki N, Kostewicz E, Stippler E, Leuner C, Nicolaides E, Dressman J, Reppas C. Dissolution Media Simulating the Intralumenal Composition of the Small Intestine: Physiological Issues and Practical Aspects. J Pharm Pharmacol. 2004;56:453–462. doi: 10.1211/0022357022935. [DOI] [PubMed] [Google Scholar]
- 127.USP. The Pharmacopeia of the United States of America XVI. Mack Publishing Company; Easton: 1960. [Google Scholar]
- 128.Gray VA, Dressman JB. Change of Ph Requirements for Standard Intestinal Fluid Ts. Pharmacopeial Forum. 1996;22:1943–1945. [Google Scholar]