Abstract
Background
The sensitivity of the tuberculin skin test is impaired in HIV-1 infected persons. ELISpot-based detection of immune sensitisation may be less affected. Furthermore, the quantitative response can be related to the CD4 count, potentially improving specificity for active disease.
Methods
T-SPOT.TB was performed on HIV-1 infected participants, 85 with active tuberculosis (TB) and 81 healthy patients (non-TB). The ratio of summed ESAT-6 and CFP-10 response to the CD4 count (SFC/CD4) was calculated.
Results
Using manufacturer’s guidelines, active TB was diagnosed with 76% sensitivity and 53% specificity. Using a SFC/CD4 ratio of 0.12, sensitivity (80%) and specificity (62%) improved.
The quantitative T-cell response increased with increasing smear positivity in the active TB group (p=0.0008). In the non-TB group, the proportion of persons scored positive by T-SPOT.TB was lower in the CD4<200 group (p=0.029).
Conclusion
The ratio of summed T cell response to CD4 count improved diagnostic accuracy of the T-SPOT.TB assay in HIV-infected persons and ratio of SFC/CD4>0.12 should prompt investigation for active disease. A strong association between the degree of sputum positivity and T-SPOT.TB score was found. The sensitivity of T-SPOT.TB in active disease may be less impaired by advanced immunosuppression.
Keywords: Mycobacterium tuberculosis, HIV-1, interferon-gamma, diagnosis, management
INTRODUCTION
The World Health Organisation’s strategy to halt the spread of tuberculosis (TB) outlines amongst its targets, the improvement of TB diagnosis in HIV-infected persons. Africa accounts for more than 31% of the global burden of TB and has an estimated 1.39 million HIV-infected TB cases and 480,000 deaths in 2005 (1). It is well recognised that CD4 T-cell depletion increases the risk of developing HIV-associated TB (2, 3) with the risk of incident TB shown to double within the first year after seroconversion (4). The performance of widely used TB diagnostic tests such as the tuberculin skin test (TST) and sputum microscopy in HIV-infected patients is compromised (5-7).
Interferon gamma release assays (IGRA) are in vitro immuno-diagnostic tests that measure T-cell interferon-gamma response to M.tb-specific antigens. Studies have assessed the power of IGRA-based analysis in detecting latent TB infection (LTBI) in HIV-1 (HIV) co-infected persons in endemic TB and HIV settings (8, 9). A head to head comparison of IGRA demonstrated that the T-SPOT.TB scored a higher proportion of positive results, when compared to QuantiFERON® TB Gold (QTF) in HIV-infected adults (10). A larger comparison by Rangaka et al in a high TB endemic setting also suggested the T-SPOT.TB assay was less impaired in advanced immunosuppression (11). However, these tests, as presently interpreted, do not allow distinction between LTBI and active disease.
A pilot study by our laboratory suggested a method of detecting active TB in HIV-infected patients by summing the ELISpot response to TB specific antigens (ESAT-6 and CFP-10) and dividing by the CD4 cell count (12). A ratio of >1 strongly suggested active disease. As these preliminary findings employed an in-house IGRA, we designed a larger study using the ratio of the summed ELISpot count from the T-SPOT.TB assay divided by the CD4 count to diagnose active TB, and included a robust group of non-TB, HIV-infected patients as controls.
METHODS
Study location and design
The study site at Ubuntu TB/HIV clinic is located in Khayelitsha, a peri-urban township near Cape Town with a population of over 400,000. Khayelitsha has an exceptionally high burden of HIV and TB (1612 per 100,000 in 2005) (12), with approximately 67% of TB being HIV related. A cross-sectional study design was employed, sampling HIV-infected patients with active TB and HIV-infected persons without evidence of active TB as controls.
Participants
Written informed consent was obtained from all participants and the study was approved by the University of Cape Town Research Ethics Committee (REC 012/2007). All 166 participants were antiretroviral therapy (ART) naïve at the time of recruitment. 85 HIV-infected TB patients with culture positive TB disease were recruited from the clinic prior to starting anti-TB chemotherapy. These patients had presented to the clinic with signs and symptoms of TB. 81 HIV-infected healthy participants were enrolled from the pre-ART HIV clinic with no symptoms of active TB using a symptom-screen (any cough, night sweats, loss of weight and loss of appetite). All healthy participants (non-TB group) were induced-sputum smear and TB culture negative and had no radiological features of TB. Persons enrolled into this group received TST using 2 TU of tuberculin PPD RT23 injected intradermally into the volar aspect of the forearm. All persons with a skin induration diameter of ≥5 mm were offered, and commenced on isoniazid preventive therapy (IPT) after whole blood was collected for IGRA. No participant had ever received IPT. A history of previous TB within 3 months of recruitment was an exclusion criterion.
At the point of recruitment, questionnaires were completed and blood samples were collected for CD4 count and T-SPOT.TB assay. Persons with CD4<200/mm3 were referred to the ART clinic to start treatment as per national guidelines. The ratio of summed ESAT-6 and CFP-10 response to CD4 count was calculated and Receiver Operating Characteristic (ROC) curve analysis conducted on results.
PBMC preparation
Peripheral blood mononuclear cells (PBMC) were extracted from heparinised whole blood within four hours of collection. PBMC were separated from the whole blood by Ficoll-Paque™ gradient technique and stored in liquid nitrogen for batched T-SPOT.TB analysis.
ELISpot
Laboratory workers were blinded to the clinical status of participants. The ELISpot assay was performed using the T-SPOT.TB kit according to manufacturer’s instructions (13). Viable PBMC (2.5 × 105 cells /well) in serum free media were added to the pre-coated plates and stimulated with the provided antigens and controls for 16-20 hours at 37°C with 5% CO2. After incubation, the plates were developed and the number of spots were counted using ImmunoSpot 3.2 reader and verified manually. A positive response was defined, irrespective of the phytohaemaglutinin (PHA) positive control response, as greater than 6 spot forming cells (SFC) in either ESAT-6 or CFP-10 wells above the SFC in the negative control (if Nil control had 0-5 spots) or at least double the nil control spot count where the nil control had 6-10 SFC. A negative response was recorded if the above criteria were not met and the positive control was valid. An indeterminate response was recorded in the presence of less than twenty SFC in the PHA well when ESAT-6 / CFP-10 wells were non-reactive (less than six SFC) or in the presence of a nil control count greater than ten SFC.
TB Microscopy and culture
The sputum collection, microscopy and culture were performed as part of the national TB programme at a national reference laboratory. The non-TB participants had study-specific sputum collection and processing done.
Statistical methods
With a sample size of 166, we aimed to detect a 30% increase in the proportion of positive T.SPOT-TB result in the active versus non-TB group with 95% power and a 5% level of significance. Predictors of disease status were analysed by logistic regression using culture positivity or negativity as the response variable. The model was built both manually and using the step-wise method and we included variables considered to be associated with active tuberculosis including age, sex, and previous TB history. Confounding variables, outlying and influential observations were identified and possible effect-modification assessed. ROC analysis was conducted to determine sensitivity and specificity at varying cut-off values for T-SPOT.TB. The ROC curves of the T-SPOT.TB and the ratio of spot forming cells (SFC) to CD4 count were compared by assessing equivalence of areas under the curve (AUCs). Post-hoc analysis was done comparing the sputum smear microscopy to the quantitative T-cell response using scatter plots and the Kruskal-Wallis test to compare medians. All p-values were two-sided with α=0.05. All data were analysed using STATA 10.0 (StataCorp, College Station, Texas).
RESULTS
Baseline characteristics of study participants
Table 1 summarises the baseline characteristics of participants. Of the 166 participants, 63% were female. TB patients were significantly more likely to be older and male with a previous history of TB. All TB patients were TB culture-positive with 34 (40%) smear-negative (median time to culture positivity 17 days). Stratification by CD4 showed that 44% of TB patients with CD4<200 were smear negative compared to 32% of patients with CD4>200 although not statistically significant (p=0.3).
Table 1.
Baseline characteristics
| NON-TB n (%) |
ACTIVE TB n (%) |
P values | OVERALL N (%) |
|
|---|---|---|---|---|
| SEX: Female | 59 (73) | 46 (54) | 0.012 | 105 (63) |
| Male | 22 (27) | 39 (46) | 61 (37) | |
| Previous TB: No | 72 (89) | 51 (60) | <0.0001 | 123 (74) |
| Yes | 8 (10) | 30 (35) | 38 (23) | |
| Missing data | 1 (1) | 4 (5) | 5 (3) | |
| BCG scar: No | 56 (69) | 55 (65) | 0.272 | 111 (67) |
| Yes | 24 (30) | 25 (29) | 49 (29) | |
| Missing data | 1 (1) | 5 (6) | 6 (4) | |
| TB contact: No | 64 (79) | 61 (72) | 0.446 | 125 (75) |
| Yes | 11 (14) | 13 (15) | 24 (15) | |
| Missing data | 6 (7) | 11 (13) | 17 (10) | |
| CD4 count: <100 | 6 (8) | 25 (30) | <0.0001 | 31 (19) |
| 100-199 | 14 (17) | 32 (37) | 46 (28) | |
| 200-299 | 23 (28) | 11 (13) | 34 (20) | |
| ≥300 | 36 (44) | 17 (20) | 53 (32) | |
| Missing data | 2 (3) | 0 (0) | 2 (1) | |
| Medians (IQR) | ||||
| Age | 30 (26-36) | 35 (29-41) | 0.0002 | 33 (28-39) |
| CD4 count | 291 (189-436) | 155 (73-259) | <0.0001 | 221.5 (116.6-364.5) |
| BMI | 24.2 (21.6-29) | 21 (19-23) | <0.0001 | 22.7 (19.8-26.2) |
T-SPOT.TB results
Of the 166 participants, the T-SPOT.TB assay was positive in 92 (55.4%) overall. The median SFC/million was higher in the active (56, IQR 24-184) versus non-TB group (20, IQR 4-60), p<0.0001. There were a significantly higher proportion of T-SPOT.TB positive results in the active TB versus non-TB group (68% vs. 42% respectively, p=0.001) as shown in Figure 1A (left panel). There was a 5% indeterminate result rate (n=8, median CD4 154.5, IQR 80.5-209.5), all due to nil control spot counts>10, with a non-significant trend towards an increased rate in those with CD4 counts<200 (6.5% vs. 3.3%, p=0.349). 71% of the smear negative, culture positive TB patients were T-SPOT.TB positive.
Figure 1.
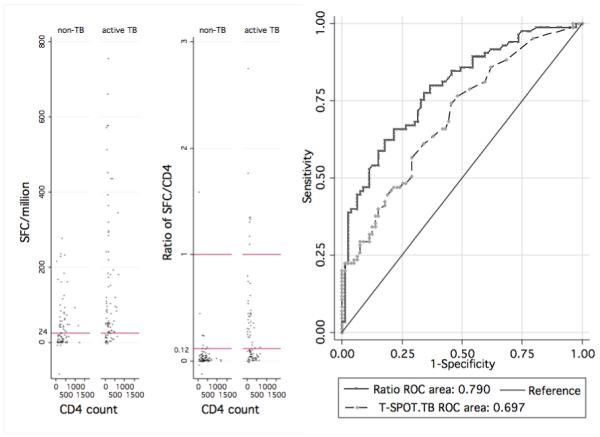
A (left) shows scatter plots of SFC/CD4 ratio and T-SPOT.TB quantitative data. At the manufacturer’s cut-off of 24 SFC/million, the T-SPOT.TB test was noted to have a high proportion of positive results compared to the SFC/CD4 ratio in the non-TB group (figure 1A-left panel). The right panel of figure 1A highlights an increase in sensitivity in distinguishing active disease from latent infection when the cut-off for the SFC/CD4 is lowered from 1 to 0.12. Reference horizontal lines show cut-off values at the manufacturer’s cut-off for the T-SPOT.TB (left panel) and at a ratio of 0.12 and 1 (right panel); spots above reference lines test positive using T-SPOT.TB and SFC/CD4 ratio tests respectively.
B (right) shows ROC curves of T-SPOT.TB and SFC/CD4 ratio showing Area Under the Curve (AUC) values
Summed ESAT-6+CFP-10 response as a ratio of the CD4 count (SFC/CD4)
Using the T-SPOT.TB assay, the SFC/CD4 was calculated by dividing the total number of spot-forming cells above the negative control by the CD4 count. Overall values ranged from −0.48 to 78. The median ratio in the active TB group (0.42, IQR 0.15-1.67) was significantly higher (p<0.001) than in the non-TB group (median 0.079, IQR 0.02-0.21) as shown in Figure 1A (right panel).
ROC Analysis
Receiver operating characteristic curve analysis was conducted on results, including those with indeterminate values using the TB and non-TB arms as comparator groups. Using the manufacturer’s cut-off for the T-SPOT.TB assay, the sensitivity for diagnosis of active TB was 76% (95%CI 70-83%) with a specificity of 53% (95%CI 46-61%) and a positive likelihood ratio of 1.63. Using a cut-off of 0.12 for the SFC/CD4, the sensitivity was 80% (95% CI 74%-86%) and specificity 62% (95% CI 56%-73%) with a likelihood ratio of 2.1. Lowering the T-SPOT.TB cut-off to achieve a similar sensitivity resulted in a lower specificity (see tables 2 and 3). The likelihood ratios (LR) of both the SFC/CD4 and the T-SPOT.TB tests were low at the chosen cut-off values although at any given sensitivity or specificity, the SFC/CD4 had superior likelihood ratios. The positive predictive value (PPV) was greater using the SFC/CD4 compared to the T-SPOT.TB (70% vs. 63%). Importantly, the negative predictive value (NPV) for the SFC/CD4 was also higher than the T-SPOT.TB test (75% vs. 67%). The area under the curve was significantly greater using the SFC/CD4 compared to the T-SPOT.TB (p=0.001) as shown in Figure 1B.
Table 2.
ROC curve analysis at varying cut-off values for T-SPOT.TB results
| POSITIVE T-SPOT | ||||
|---|---|---|---|---|
| CUT-OFF | SENSITIVITY(%) | SPECIFICITY(%) | LIKELIHOOD RATIO | |
| (SFC/million) | POSITIVE | NEGATIVE | ||
| 12 | 85.9 | 39.5 | 1.4 | 0.4 |
| 20 | 78.8 | 48.2 | 1.5 | 0.4 |
| 24 | 76.5 | 53.1 | 1.6 | 0.4 |
| 96 | 40.0 | 85.2 | 2.7 | 0.7 |
| 220 | 23.5 | 95.1 | 4.8 | 0.8 |
Table 3.
ROC curve analysis at varying cut-off values for ratio of summed T-cell response: CD4 count
| POSITIVE SFC/CD4 | ||||
|---|---|---|---|---|
| RATIO CUT-OFF | SENSITIVITY(%) | SPECIFICITY(%) | LIKELIHOOD RATIO | |
| (SFC/million: CD4) | POSITIVE | NEGATIVE | ||
| .04 | 91.8 | 38.0 | 1.5 | 0.2 |
| .08 | 85.9 | 50.6 | 1.7 | 0.3 |
| .12 | 80.0 | 62.0 | 2.1 | 0.3 |
| 1 | 38.8 | 97.5 | 15.3 | 0.6 |
| 1.793 | 22.4 | 98.7 | 17.7 | 0.8 |
Risk factors for Active Tuberculosis and T-SPOT.TB positivity
In univariate analysis, there was a positive association between active TB and older age, lower BMI, male sex, lower CD4, history of previous TB, a positive T-SPOT.TB result and SFC/CD4 above 0.12. In multivariate analysis CD4> 200 (OR 0.24, p=0.001), body mass index (OR 0.85, p=0.001), older age (OR 1.04, p=0.021) and the SFC/CD4 ratio (OR 5.99, p<0.0001) were associated with active TB (table 4). Significant predictors of a positive T-SPOT.TB test were higher age (RR 2.2, p=0.04), history of previous TB (RR 2.8, p=0.033), and culture positive TB (RR 3.2,p=0.024). There was no significant confounding or interaction between variables.
Table 4.
Univariate and Multivariate logistic regression analysis for culture positive TB disease
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
||||
| OR | P values | OR | P values | |
| Age (years) | 1.08 (1.04-1.13) |
<0.0001 |
1.038
(0.98-1.1) |
0.021 |
| Male sex | 2.27 (1.19-4.35) |
0.013 | 0.75 (0.29-1.96) |
0.562 |
| BMI | 0.84 (0.77-0.91) |
<0.0001 |
0.85
(0.78-0.93) |
0.001 |
| CD4>200 | 0.16 (0.08-0.32) |
<0.0001 |
0.24
(0.11-0.54) |
0.001 |
| History of previous TB |
4.19 (1.95-8.97) |
<0.0001 | 1.78 (0.68-4.62) |
0.239 |
| Positive T.SPOT | 2.72 (1.52-4.86) |
0.001 | 0.81 (0.31-2.11) |
0.660 |
| Positive ratio | 6.9 (3.42-13.91) |
<0.0001 |
5.99
(2.61-13.77) |
<0.0001 |
| BCG scar presence |
1.35 (0.77-2.36) |
0.291 | 1.00 (0.42-2.41) |
1.000 |
| Contact with TB | 1.3 (0.81-2.11) |
0.282 | 1.43 (0.68-2.99) |
0.346 |
T-cell response and degree of immunosuppression
Overall there was no significant correlation between the IFN-γ SFC response and the degree of immunosuppression as measured by the CD4 count (Spearman correlation coefficient r=−0.022, p=0.78). However when results were stratified by CD4 category and TB disease status, a CD4>200 was found to be associated with a positive T-SPOT.TB result (p=0.029) in the non-TB group implying that advanced immunosuppression does impair the performance of the T-SPOT.TB test in this group. Figure 2 shows an increasing proportion of T-SPOT.TB positive results with increasing CD4 counts. Interestingly, this was not the case in the active TB disease group (p=0.939) implying that the sensitivity of the T-SPOT.TB test is impaired by advanced immunosuppression in the non-TB group but less affected in active TB.
Figure 2.
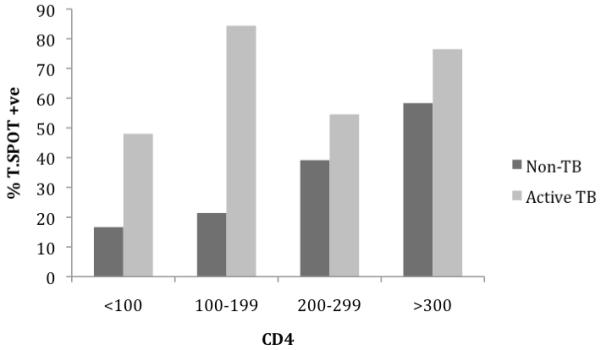
Bar graphs showing increasing proportion of T-SPOT.TB positive results in the non-TB but not in the TB group
Secondary Analysis
Bacillary load and T-cell response
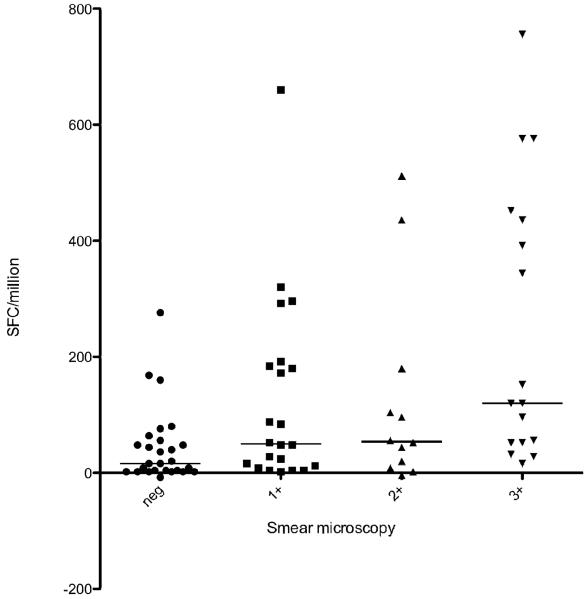
We conducted a post-hoc analysis relating the degree of sputum positivity in active TB to the quantitative T-SPOT.TB assay response. We found a significant association with the bacillary load as estimated by the degree of sputum smear microscopy positivity (Figure 3). There was a positive bacillary load-immune response relationship with a significant increase in quantitative T-cell response with increasing smear positivity (Kruskal-Wallis test p=0.0008). To further explore this relationship, we examined the correlation between the immune response and the number of days to culture positivity (CP) as an alternative surrogate marker of bacillary load. This showed a non-statistically significant negative correlation between SFC and days to culture positivity (Spearman’s co-efficient r=−0.12, p=0.29) although notably 90% of specimens that became culture positive after 11 days (median days to CP) had SFC/million counts less than 290 (the 90th percentile of SFC) as shown in figure 4.
Figure 3.
Scatter plot showing bacillary load-immune response relationship: quantitative T-cell response shown to increase with increasing smear positivity. Median values and IQR shown (Kruskal Wallis p=0.0008)
Figure 4.

Scatter plot of spot forming cells (SFC) / million and number of days to culture positivity in active TB patients. X-axis reference line shows median days to culture positivity. Y-axis reference line shows the 90th percentile of total SFC/million.
DISCUSSION
We have assessed a novel ratio technique of diagnosing active TB in an HIV-1 co-infected population in a highly TB endemic setting by relating the total number of spot forming cells from the T-SPOT.TB test to CD4 count. Other diagnostic studies assessing the diagnosis of active TB were conducted using the QuantiFERON-TB Gold-In-Tube test, non-commercial in-house assays, and non-commercial ELISpot assays in predominantly HIV negative populations, or in low TB endemicity settings (12, 14-20). We used the T-SPOT.TB test as previous studies have reported the T-SPOT.TB to have a better sensitivity than the QuantiFERON-TB Gold-In-Tube test in HIV-1 infected persons in high prevalence settings such as ours (10, 11).
The T-SPOT.TB diagnosed active TB disease with a sensitivity of 76% and a specificity of 53%. The SFC/CD4 diagnosed TB disease with a higher sensitivity with a ratio above 0.12 indicating a 6-fold higher risk of TB disease in multivariate analysis. The sensitivity of the SFC/CD4 ratio in diagnosing active TB remained high in advanced immunosuppression. Therefore in practice, in an HIV-infected patient with a SFC/CD4>0.12, active TB should be strongly suspected and investigated while a positive T-SPOT.TB should prompt consideration of latent infection treatment. In those with a SFC/CD4<0.12, although active TB is not excluded, they should be considered for treatment of latent infection. However, we acknowledge a NPV of 75% means that 25% of active TB cases would test negative using this method (33% using the T-SPOT.TB) potentially limiting its use in clinical practice.
In the smear-negative, culture-positive patients, the median time to culture positivity was 17 days. Importantly, the time to diagnosis could potentially have been shortened by 2 weeks using the T-SPOT.TB test with public health implications including reducing infectivity and transmission rates as well as potentially decreasing morbidity and mortality.
In the non-TB group, a CD4 count<200/mm3 was associated with impaired test performance. A possible reason for this is that the higher bacillary load associated with having TB disease drives a positive TB-specific antigen response regardless of CD4 count resulting in relatively less impaired test performance. By contrast, the positive responses in the non-TB group would be less intense due to lower bacillary load making test accuracy susceptible to impairment in advanced immunosuppression. This hypothesis is supported in our data, both by the presence of a strong bacillary load-immune response association and the raw data showing higher responses in the active TB group. To our knowledge, this is one of the clearest bacillary load-immune response relationships to be demonstrated in humans, using sputum smear microscopy to estimate bacillary load. Our results showed a negative correlation between the immune response and number of days to culture positivity that, although we did not find statistical significance, is worthy of further investigation.
Previous studies have suggested that the infectious load is associated with the magnitude of T-cell response. Hill et al showed that the quantitative ELISpot response reflected the likely infecting dose of M.tb, measured by gradient of exposure to smear and culture positive TB patients (21). Ribeiro et al also hypothesized that IFN gamma-producing T cells might be related to bacterial load, determined using surrogate disease severity markers of low BMI and presence of cavitatory disease (22). In these two studies, participants were predominantly HIV-uninfected. These, and our, data suggest that the T-cell response could be used as a surrogate marker for treatment response, disease relapse or monitoring progression from latent to active disease. A small pilot study conducted in Uganda by Goletti et al showed potential utility by demonstrating that RD1 T-cell responses decrease in HIV/TB patients after successful therapy for TB (23). Aiken et al achieved a similar conclusion in a predominantly HIV-uninfected cohort (24). Our results suggest that the same might be true for HIV-infected persons. However Ribeiro et al found a high inter-patient variability in quantitative results potentially limiting the use of post-treatment immune response assessment in practice (22).
The SFC/CD4 cut-off value used in this study is lower than found in a similar pilot study conducted by our laboratory (12). To further investigate this we conducted a parallel comparison of the in-house assay to the T-SPOT.TB and found the in-house assay to score a greater proportion positive with a higher number of spot forming units (data not shown). This factor contributes to the difference in ratios.
This study has several strengths. The definition of the control non-TB group, who were both asymptomatic and had two sputum TB culture negative results, is very robust. In this HIV-infected population known to have a high prevalence of asymptomatic, sub-clinical TB, we felt the definition of non-TB persons had to be actively sought through comprehensive screening. In addition, this is the largest study to assess the ability of the T-SPOT.TB test and the ratio of the summed T-cell response over the CD4 count to differentiate active from latent TB in an HIV-infected population in a highly TB endemic setting.
Limitations
The relatively low specificity of the SFC/CD4 ratio, although higher than the T-SPOT.TB test alone, means that it would be more suitable as a ‘rule-in’ test to seek a diagnosis of active TB than as a rule-out test to exclude TB. A higher proportion of active TB patients had a history of previous TB, potentially biasing this group towards a higher response rate and ratio compared to the non-TB group. However, as the T-SPOT.TB assay is a short overnight assay, the T-cell response is thought to predominantly constitute effector, and not memory, T-cells. Therefore, a previous history of TB, which would induce memory T-cells, should not significantly influence our results.
Active TB patients were significantly older, with lower CD4 counts and BMI as well as a higher proportion of males compared to the non-TB group. In multivariate analysis, age, BMI and a low CD4 count were significant risk factors for active TB. This difference in baseline characteristics between the 2 groups could be a potential source of bias.
In relating smear microscopy results to the T-cell response, our analysis was post-hoc and smear microscopy was performed at reference laboratories as per programmatic guidelines and not study-specific. As a result, performer-related variability could affect the validity of results. Nonetheless, the findings are interesting and warrant further investigation under more controlled conditions.
In addition, frozen cells were used instead of fresh as recommended by the manufacturer, due to logistic constraints of the study. As a result, we conducted a direct comparison of fresh and frozen cells from 11 patients (frozen for a mean of 2.5 days) and showed no difference in proportion of non-responders between fresh and frozen samples (data not shown).
In conclusion, in this high TB burden setting, a PPV of 70% and NPV of 75% could limit its clinical use as 30% of persons testing positive using the ratio would be unnecessarily treated and 25% of those testing negative will have a TB diagnosis missed. As a result, our findings are more easily translated into practice in low TB prevalence areas where the pre-test probability of latent infection is lower than in our setting. Nonetheless, that the novel ratio technique could aid the clinical decision to start TB treatment, particularly in advanced immunosuppression, shortening the time to TB treatment merits further evaluation.
ACKNOWLEDGEMENTS
RJW is funded by the Wellcome Trust (072070, 084323, 088316) and MRC (UK).
Grant support from the European Union (EU Sante 121404C/G/Multi).
Grant support from the National Institutes of Health (NIH 1RO1HD058791-01)
JP received travel support from Oxford Immunotec.
T-SPOT.TB kits were supplied by Oxford Immunotec.
MM receives funding support from PEPFAR/USAID.
Oxford Immunotec had no part in the design of the study or the decision to publish the findings of this study.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
T-SPOT.TB kits were supplied by Oxford Immunotec. J Patel received travel expenses from Oxford Immunotec.
REFERENCES
- 1.Global tuberculosis control : epidemiology, strategy, financing : WHO report 2009. 2009. Report No.: 978 92 4 156380 2. [Google Scholar]
- 2.Wood R, Maartens G, Lombard CJ. Risk factors for developing tuberculosis in HIV-1-infected adults from communities with a low or very high incidence of tuberculosis. J Acquir Immune Defic Syndr. 2000 Jan 1;23(1):75–80. doi: 10.1097/00126334-200001010-00010. [DOI] [PubMed] [Google Scholar]
- 3.Elliott AM, Hodsdon WS, Kyosiimire J, Quigley MA, Nakiyingi JS, Namujju PB, et al. Cytokine responses and progression to active tuberculosis in HIV-1-infected Ugandans: a prospective study. Trans R Soc Trop Med Hyg. 2004 Nov;98(11):660–70. doi: 10.1016/j.trstmh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005 Jan 15;191(2):150–8. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 5.Elliott AM, Namaambo K, Allen BW, Luo N, Hayes RJ, Pobee JO, et al. Negative sputum smear results in HIV-positive patients with pulmonary tuberculosis in Lusaka, Zambia. Tuber Lung Dis. 1993 Jun;74(3):191–4. doi: 10.1016/0962-8479(93)90010-U. [DOI] [PubMed] [Google Scholar]
- 6.Huebner RE, Schein MF, Bass JB., Jr. The tuberculin skin test. Clin Infect Dis. 1993 Dec;17(6):968–75. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 7.Duncan LE, Elliott AM, Hayes RJ, Hira SK, Tembo G, Mumba GT, et al. Tuberculin sensitivity and HIV-1 status of patients attending a sexually transmitted diseases clinic in Lusaka, Zambia: a cross-sectional study. Trans R Soc Trop Med Hyg. 1995 Jan-Feb;89(1):37–40. doi: 10.1016/0035-9203(95)90649-5. [DOI] [PubMed] [Google Scholar]
- 8.Clark SA, Martin SL, Pozniak A, Steel A, Ward B, Dunning J, et al. Tuberculosis antigen-specific immune responses can be detected using enzyme-linked immunospot technology in human immunodeficiency virus (HIV)-1 patients with advanced disease. Clin Exp Immunol. 2007 Nov;150(2):238–44. doi: 10.1111/j.1365-2249.2007.03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman AL, Munkanta M, Wilkinson KA, Pathan AA, Ewer K, Ayles H, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS. 2002 Nov 22;16(17):2285–93. doi: 10.1097/00002030-200211220-00008. [DOI] [PubMed] [Google Scholar]
- 10.Mandalakas AM, Hesseling AC, Chegou NN, Kirchner HL, Zhu X, Marais BJ, et al. High level of discordant IGRA results in HIV-infected adults and children. Int J Tuberc Lung Dis. 2008 Apr;12(4):417–23. [PubMed] [Google Scholar]
- 11.Rangaka MX, Wilkinson KA, Seldon R, Van Cutsem G, Meintjes GA, Morroni C, et al. Effect of HIV-1 infection on T-Cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007 Mar 1;175(5):514–20. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- 12.Rangaka MX, Diwakar L, Seldon R, van Cutsem G, Meintjes GA, Morroni C, et al. Clinical, immunological, and epidemiological importance of antituberculosis T cell responses in HIV-infected Africans. Clin Infect Dis. 2007 Jun 15;44(12):1639–46. doi: 10.1086/518234. [DOI] [PubMed] [Google Scholar]
- 13.T-SPOT® Oxford Immunotec. Oxford UK: [Accessed 6 July 2009]. www.oxfordimmunotec.com. [Google Scholar]
- 14.Chen X, Yang Q, Zhang M, Graner M, Zhu X, Larmonier N, et al. Diagnosis of active tuberculosis in China using an in-house gamma interferon enzyme-linked immunospot assay. Clin Vaccine Immunol. 2009 Jun;16(6):879–84. doi: 10.1128/CVI.00044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chee CB, Gan SH, Khinmar KW, Barkham TM, Koh CK, Liang S, et al. Comparison of sensitivities of two commercial gamma interferon release assays for pulmonary tuberculosis. J Clin Microbiol. 2008 Jun;46(6):1935–40. doi: 10.1128/JCM.02403-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura T, Hasegawa N, Mori M, Takebayashi T, Harada N, Higuchi K, et al. Accuracy of an interferon-gamma release assay to detect active pulmonary and extra-pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008 Mar;12(3):269–74. [PubMed] [Google Scholar]
- 17.Kang YA, Lee HW, Hwang SS, Um SW, Han SK, Shim YS, et al. Usefulness of whole-blood interferon-gamma assay and interferon-gamma enzyme-linked immunospot assay in the diagnosis of active pulmonary tuberculosis. Chest. 2007 Sep;132(3):959–65. doi: 10.1378/chest.06-2805. [DOI] [PubMed] [Google Scholar]
- 18.Dewan PK, Grinsdale J, Kawamura LM. Low sensitivity of a whole-blood interferon-gamma release assay for detection of active tuberculosis. Clin Infect Dis. 2007 Jan 1;44(1):69–73. doi: 10.1086/509928. [DOI] [PubMed] [Google Scholar]
- 19.Syed Ahamed Kabeer B, Sikhamani R, Swaminathan S, Perumal V, Paramasivam P, Raja A. Role of interferon gamma release assay in active TB diagnosis among HIV infected individuals. PLoS ONE. 2009;4(5):e5718. doi: 10.1371/journal.pone.0005718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, Andersen P, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003 Apr 5;361(9364):1168–73. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 21.Hill PC, Fox A, Jeffries DJ, Jackson-Sillah D, Lugos MD, Owiafe PK, et al. Quantitative T cell assay reflects infectious load of Mycobacterium tuberculosis in an endemic case contact model. Clin Infect Dis. 2005 Jan 15;40(2):273–8. doi: 10.1086/427030. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro S, Dooley K, Hackman J, Loredo C, Efron A, Chaisson RE, et al. T-SPOT.TB responses during treatment of pulmonary tuberculosis. BMC Infect Dis. 2009;9:23. doi: 10.1186/1471-2334-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goletti D, Carrara S, Mayanja-Kizza H, Baseke J, Mugerwa MA, Girardi E, et al. Response to M. tuberculosis selected RD1 peptides in Ugandan HIV-infected patients with smear positive pulmonary tuberculosis: a pilot study. BMC Infect Dis. 2008;8:11. doi: 10.1186/1471-2334-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aiken AM, Hill PC, Fox A, McAdam KP, Jackson-Sillah D, Lugos MD, et al. Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases. BMC Infect Dis. 2006;6:66. doi: 10.1186/1471-2334-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]