Summary
An effective Th1 type cell-mediated immune response against cancer cells is critical in limiting cancer progression. Gadd45b, a signaling molecule highly upregulated during Th1 type responses, is studied for its role in limiting tumor growth. Mouse B16 melanoma cells implanted into Gadd45b−/− mice grew faster than those in wild type or Gadd45b +/− littermate controls. The defect of Gadd45b−/− mice in tumor immunosurveillance was attributed to the reduced expression of IFN-γ, granzyme B, and CCR5 in Gadd45b−/− CD8+ T cells at the tumor site. Activation of p38 MAP kinase, but not ERK or JNK, by either TCR-stimuli or IL-12 and IL-18 is diminished in Gadd45b−/− CD8+ T cells, resulting in reduced production of IFN-γ. In addition, mRNA of T-bet and Eomes were reduced in Gadd45b−/− CD8+ T cells, supporting a critical role of Gadd45b in shaping the Th1 fate. More importantly, the tumor vaccination which is effective in wild type mice failed in Gadd45b/Gadd45g doubly deficient mice. Collectively, these data demonstrate that members of the Gadd45 gene family are important for anti-tumor immune responses.
Introduction
IFN-γ is a hallmark of Th1 type immune responses [1]. It is produced primarily by Th1 cells, CD8+ T cells, NK cells, NKT cells, and γδ T cells [2]. IFN-γ is critical in mediating the tumor suppression activities of these cells, and therefore important for immune-editing of cancers [3]. Consistent with this notion, factors that drive Th1 responses are also involved in tumor suppression. IL-12 is important for the generation of Th1 responses and directly stimulates IFN-γ production in various cells [4]. The anticanceractivities of IL-12 have been extensively demonstrated in various murine cancer models [5]. In these preclinical models, IL-12 was shown to activate several subsets, including NK cells, CD4+ and CD8+ T cells, and NK-T cells expressing the Vα14 invariant T cellreceptor [5]. T-bet, a master regulator of Th1 responses is important for controlling metastasis in mouse prostate cancer model [6]. In human colorectal cancer, the expression of T-bet and Eomes in cancer tissues is associated with favorable clinical outcome [7, 8]. The presence of Th1 and CD8+ T cells within the tumor sites and patients’ blood are associated with a better prognosis of cancer patients [9]. Therefore, Th1 type responses are important for containing tumors and their metastasis. Th1 cells utilize unique signaling molecules for their biological functions. Members of Gadd45 protein family are preferentially expressed in Th1 cells and enhances MAP kinase signaling [10–14]. Gadd45b mRNA can be induced by TCR signaling in naïve as well as activated CD4+ T cells [11]. IL-12 further augments Gadd45b and Gadd45g in activated CD4+ T cells [10, 12, 13]. Both Gadd45b and Gadd45g mediate MAP kinase activation in Th1 cells and are important for IFN-γ production. In addition, Gadd45b and Gadd45g are important for the initiation of Th1 responses in vivo [11, 12]. However, it is unclear whether the Gadd45 pathway plays any significant role in the function of other IFN-γ-producing cells such as CD8+ T cells.
In this study, we demonstrate that Gadd45b plays an important role in inhibiting tumor growth by CD8+ T cells. Gadd45b is induced in CD8+ T cells by both TCR and IL-12 and mediates their signaling by potentiating p38 MAP kinase activities. Gadd45b is also required for the IFN-γ production by CD8+ T cells. Moreover, we demonstrate that Gadd45b increases the expression of Th1 lineage master regulators T-bet and Eomes in CD8+ T cells. Our study establishes the Gadd45 signaling pathway is important in containing cancer.
Results
Gadd45b is important for the immune surveillance of tumor
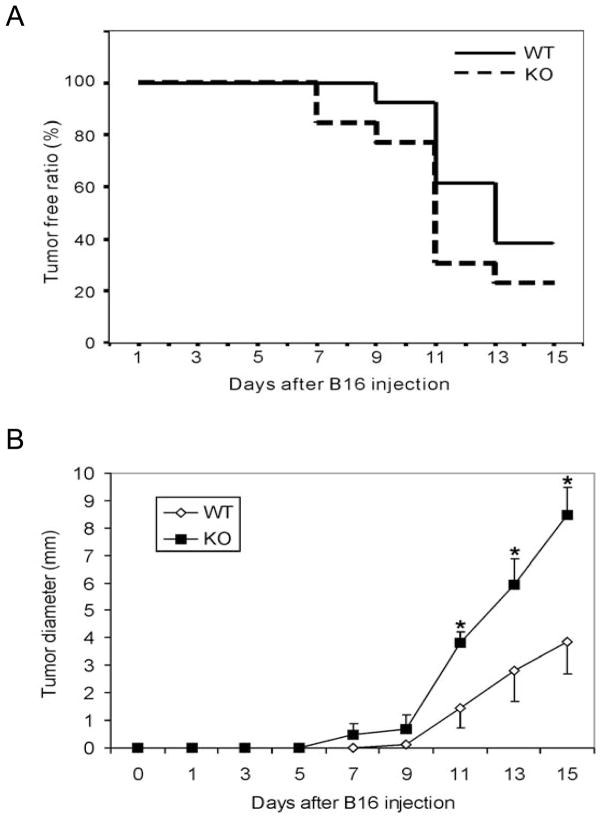
Because Gadd45b is important for the initiation of Th1 responses, which are important for tumor immune surveillance, we decided to examine whether Gadd45b is important for suppressing tumor growth. To define the role of Gadd45b in tumor immune surveillance, sex- and age-matched B6 WT or Gadd45b+/− (n = 13) and B6 Gadd45b−/− mice were inoculated with the B16 F0 melanoma cell line, and subsequent tumor growth was recorded every two days. Compared with WT mice, Gadd45b−/− mice showed only slightly earlier tumor development (Figure 1a), though the difference in the onset of observable tumor did not reach statistical significance. However, the tumor size of Gadd45b−/− mice was larger when compared to the size in wild type mice (Figure 1b). Therefore, Gadd45b is important for inhibiting tumor growth in mice, suggesting that Gadd45b is likely involved in host anti-cancer responses.
Figure 1.
Gadd45b is involved in suppressing tumor growth. Gadd45b−/− mice (KO) or wild type mice (n=13 per group) were injected subcutaneously with 2.5×104 B16F0 cells per mouse. (A) Percentage of tumour free mice after injection. (B) Tumour size was measured every 2 days. *p<0.05, Mann-Whitney test.
CD8+ T cell responses against tumor growth are mediated by Gadd45b
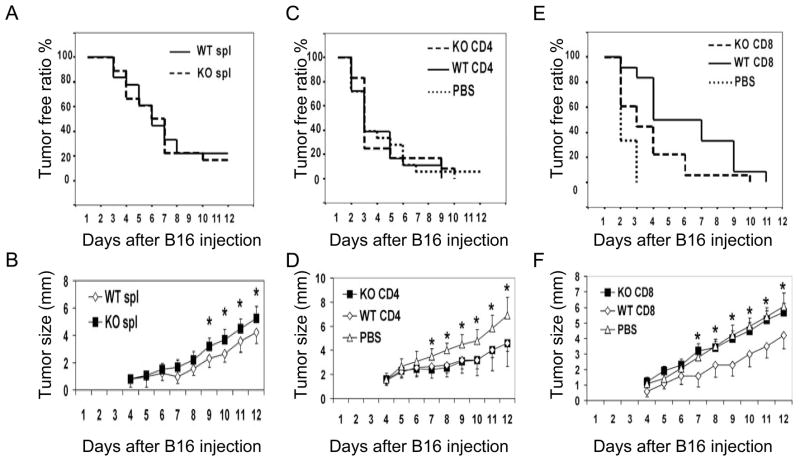
To further characterize subsets of lymphocytes that are responsible for the defect of anti-tumor responses in Gadd45b−/− mice, we performed adoptive transfer experiments using sub-lethally irradiated wild type mice as recipients. We first adoptively transferred B6 wild type and B6 Gadd45b−/− total spleen cells separately to B6 wild type mice which had been sub-lethally irradiated to eliminate the majority of peripheral lymphocytes. 24 h after the adoptive transfer, we inoculated about eight to ten mice receiving wild type cells and a similar number of mice receiving Gadd45b−/− cells with B16F0 melanoma cells. We found that tumors were much larger in mice which were adoptively transferred with Gadd45b−/− splenocytes as compared to mice transferred with wild type splenocytes (Figure 2b) although the tumor incidence was similar (figure 2a). These data suggest that the Gadd45b deletion diminishes the anti-tumor function of spleen cells.
Figure 2.
Gadd45b expression in CD8+ T cells is important for tumor surveillance. WT recipient mice were sub-lethally irradiated at 500 rad, and then adoptively transferred with donor cells within 6 hours of irradiation. 24 hour later, the recipient mice were inoculated with B16 F0 melanoma cells (1×105 cells/mouse). (A) and (B) Donar lymphocytes were isolated from spleens of donor Gadd45b−/− (KO) and WT mice. (C) and (D) Donor Gadd45b−/− (KO) and WT CD4 + T cells were purified, and then mixed with CD4-depleted spleen cells from B6 WT mice. (E) and (F) Donor Gadd45b−/− (KO) and WT CD8 + T cells were purified, and then mixed with CD8-depleted spleen cells from B6 WT mice. Percentage of tumor free mice was recorded (A) (C) (E). Average tumor size was recorded every two days (B) (D) (F). * p < 0.05. Statistical analysis was performed using one-way analysis of variance test (ANOVA) followed by Tukey’s post hoc t-test. Each group contained eight to ten animals.
To further examine whether Gadd45b mediates the anti-tumor function of CD4+ T cells, we adoptively transferred wild type and Gadd45b−/− CD4+ T cells mixed with CD4-depleted wild type splenocytes into sub-lethally irradiated B6 wild type. Compared with PBS controls, mice adoptively transferred with either wild type or Gadd45b−/− CD4+ T cells suppressed tumor growth with equal potency. Therefore, the Gadd45b deletion in CD4+ T cells did not affect tumor suppression by the immune system (Figure 2c and d).
To determine whether deficiency in CD8+ T cells was responsible for the faster tumor growth in Gadd45b−/− mice, we adoptively transferred wild type and Gadd45b−/− CD8+ T cells with CD8-depleted wild type splenocytes into sub-lethally irradiated B6 wild type mice. Compared with PBS controls, mice adoptively transferred with wild type, but not Gaddr45b−/− CD8+ T cells, suppressed tumor growth (Figure 2e and f). Therefore, Gadd45b expression in CD8+ T cells is critical for tumor immune surveillance.
Gadd45b deletion resulted in reduced IFN-γ production by CD8+ T cells in tumors
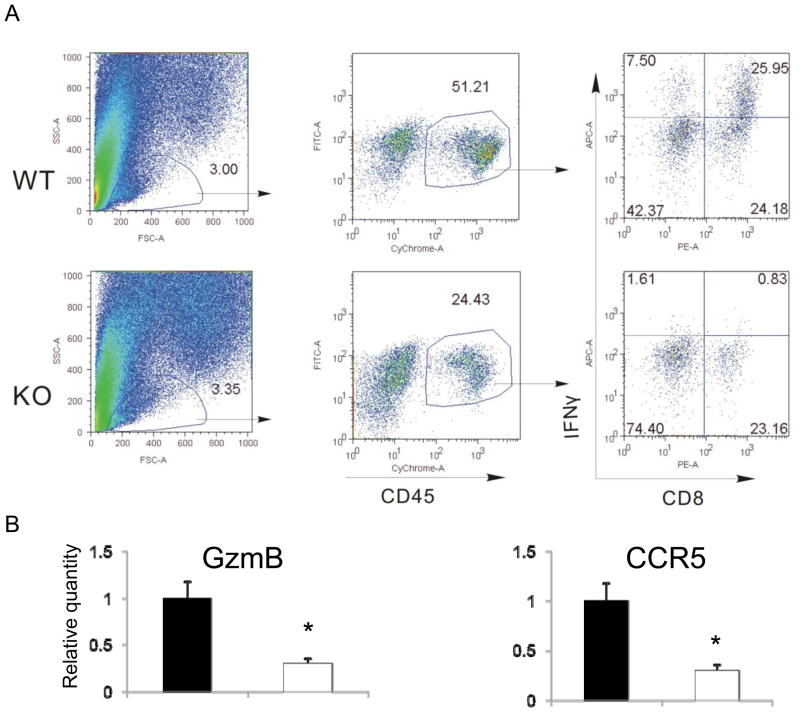
Our in vivo data suggest Gadd45b plays a role in CD8+ T cells in the setting of tumor surveillance. We have also found Gadd45b mRNA is expressed in CD8+ T cells at a high level (data not shown). Because Gadd45b is expressed in CD8+ T cells and involved in the anti-tumor function of these cells, we decided to examine whether it is required for the effector function of tumor infiltrating T cells. Tumors that have been growing for 13 days in wild type and Gadd45b−/− mice were dissected. Tumor infiltrating lymphocytes (TILs) were examined for their abilities to produce IFN-γ as an indicator of their effector function. We have found that the number of tumor infiltrating CD8 T cells was reduced in the Gadd45b deficient mice compared to WT mice (figure 3A), suggesting Gadd45b is likely important for the migration of CD8 T cells to tumor sites. A large percentage of wild type CD8+ T cells produced IFN-γ ex vivo. In contrast, much reduced numbers of Gadd45b−/− CD8+ T cells produced IFN-γ. Therefore, Gadd45b is required for IFN-γ production by CD8+ in the tumor site (Figure 3A). We also carried out real time PCR assays to examine mRNA levels of granzyme B and CCR5 in TILs and found that they are also regulated by Gadd45b (Figure 3B). Therefore, Gadd45b is important for both tumor cytotoxicity within the tumor microenvironment and T cell migration to tumor sites.
Figure 3.
Gadd45b deletion resulted in reduced CD8+ T cell IFN-γ production in the tumor site. Tumor masses were removed at day 13 after B16 F0 cells inoculation and single cells were made as described in the Materials and methods. Cells were stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 6 hours and incubated for the last 3 hours with Brefeldin A. Cells producing IFN-γ was examined with intracellular cytokine staining and flow cytometry (A). In some experiments tumor-associated CD8 T cells were purified by FACS. Total RNA was made from T cells and then subjected to real time PCR analysis of granzyme B and CCR5 (B). *p<0.05, Student’s t-test.
Gadd45b is required for optimal activation of the p38 MAP kinase and functions of effector CD8+ T cells
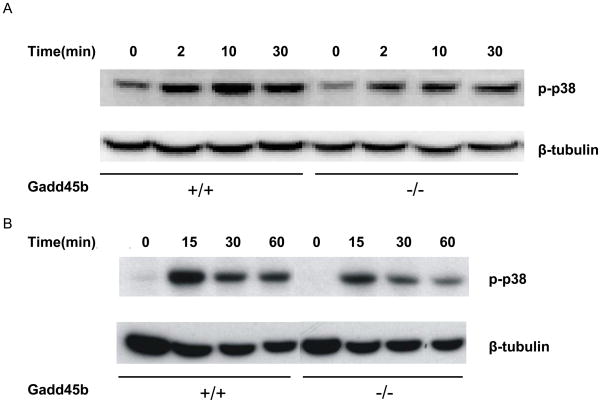
To further study the role of Gadd45b in the regulation of effector function of CD8+ T cells, we used an in vitro culture system in which wild type and Gadd45b−/− naïve CD8+ T cells were differentiated into effector CD8+ T cells. Gadd45b was shown to be required for activation of MAP kinases in Th1 cells [10–13]; we therefore decided to examine whether it is also required for activation of MAP kinases in effector CD8+ T cells. Using anti-phospho-antibodies as assays of MAP kinase activation, we found that the activation of the p38 MAP kinase was affected by Gadd45b deletion. In Gadd45b−/− CD8+ T cells, as compared to wild type CD8+ T cells, the level of TCR-stimulated phospho-p38 were diminished (Figure 4a). Similarly, levels of active form of p38 stimulated by IL-12 and IL-18 were diminished and failed to sustain in Gadd45b−/− CD8+ T cells when compared to wild type CD8+ T cells (Figure 4b). In contrast, the activation of JNK and ERK was not affected (data not shown). Therefore, Gadd45b specifically potentiates p38 activation in CD8+ T cells.
Figure 4.
Gadd45b mediates p38 activation by either TCR signaling or IL-12 plus IL-18 in CD8+ T cells. Effector CD8+ T cells were stimulated for various amounts of time as indicated with either (A) plate-bound anti-CD3 (10 μg/ml), or (B) IL-12 (10 ng/ml) and IL-18 (30 ng/ml). Cell extracts were analyzed by immunoblot with α-phospho-p38. p-, phosphorylated.
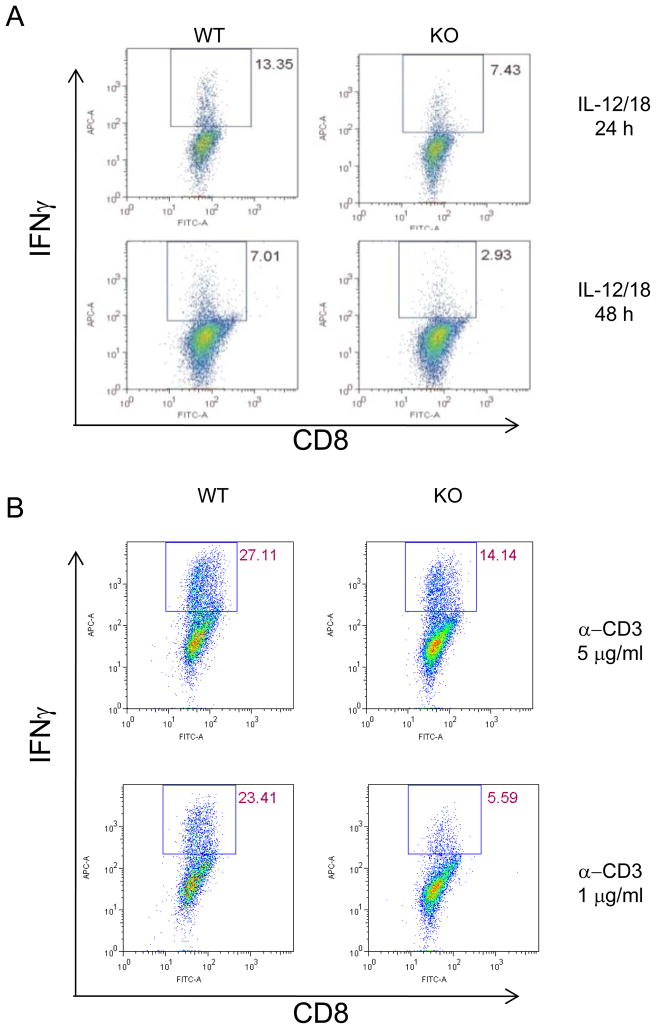
We have found that CD8+ T cells isolated from tumors grown in Gadd45b−/− mice produced much less IFN-γ when compared to those from wild type mice. However, we did not know whether this was due to intrinsic deficiencies of Gadd45b−/− CD8+ T cell. In order to determine whether Gadd45b is required for the IFN-γ production by CD8+ T cells, we cultured CD8+ T cells alone without antigen presenting cells and differentiated them to effector cells. Afterwards, we measured their production of IFN-γ upon either TCR stimulation or IL-12/IL-18 stimulation. We first labeled the naïve T cells with CFSE and examined clonal expansion after four day culture. We have found no difference in cell division and survival between WT T cells and Gadd45b deficient T cells (data not shown). The frequency of IFN-γ producing cells after stimulation with IL-12 and IL-18 were diminished in Gadd45b−/− effector CD8+ T cells when compared to wild type CD8+ T cells (Figure 5a). Similarly, Gadd45b−/− CD8+ T cells produced less IFN-γ protein than wild type CD8+ T cells (data not shown). In addition, in response to crosslinking CD3, which mimics cognate signaling, lower numbers of Gadd45b−/− effector CD8+ T cells produced IFN-γ than wild type CD8+ T cells (Figure 5b). In response to TCR signaling, Gadd45b−/− effector CD8+ T cells also secreted less IFN-γ protein than wild type effector CD8+ T cells (data not shown), likely due to the reduced number of IFN-γ positive cells. In contrast, when we used PMA/ionomycin to bypass TCR signaling, we did not find any difference in the frequency of IFN-γ-producers, suggesting, at least in vitro, Gadd45b is required for mediating TCR-triggered IFN-γ production.
Figure 5.
Gadd45b is important for either TCR or IL-12 plus IL-18 induced effector cytokine production in CD8+ T cells. (A) Naïve CD8+ T cells were stimulated with plate bound anti-CD3 (10 μg/ml) and anti-CD28 (5 μg/ml) plus Th1 culture media for four days, CD8+ T cells were then stimulated with IL12 (10 ng/ml) + IL18 (30ng/ml) for 24 hours or 48 hours. (B) Naïve CD8+ T cells were stimulated with different concentration of anti-CD3 and constant levels of anti-CD28 (5 μg/ml) and Th1 culture media for four days, and then re-stimulated with plate-bound anti-CD3 (10 mg/ml) for 6 hours. Data are representative of three independent experiments. Cells producing IFN-γ was identified by intracellular cytokine staining. KO, Gadd45b−/−. WT, Gadd45b+/+.
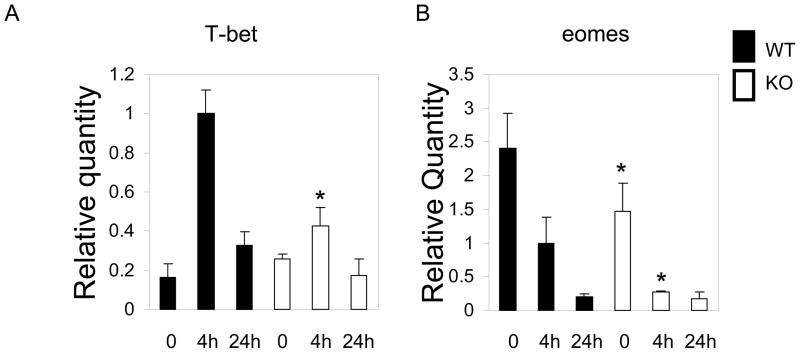
Although Gadd45b is highly expressed in CD8+ T cells cultured in the Th1 condition, it is not known whether it is required for Th1 lineage commitment. To address this question, we used real time PCR assay to examine the two Th1 lineage transcription factors, T-bet and Eomes [15, 16]. IL-12 is known to drive Th1 differentiation by promoting T-bet expression. IL-12 induced high levels of T-bet in wild type CD8+ T cells, whereas, it induced significantly lower levels of T-bet in Gadd45b−/− CD8+ T cells at 4 hours (Figure 6a). On the other hand, Eomes, another member of the T-box binding transcription factor, displayed similar results. Eomes expressed at very high levels in CD8+ T cells. We have shown that, compared with wild type CD8+ T cells, Gadd45b−/− CD8+ T cells expressed lower levels of Eomes in effector cells before and after IL-12 administration (Figure 6b). Therefore, Gadd45b is involved in the upregulation of T-bet. In addition, it also plays a significant role in maintaining the levels of eomes in effector CD8+ T cells.
Figure 6.
Gadd45b promotes mRNA levels of T-bet and Eomes in CD8+ T cells. Effector CD8+ T cells were stimulated at times indicated with IL-12 (10 ng/ml) and IL-18 (30 ng/ml). Total RNA were isolated and assayed by real time PCR for the levels of T-bet and Eomes. Relative quantities of mRNA were calculated by real time PCR software. *p<0.001, Student’s t-test, wild type vs. Gadd45b−/− at the same time point)
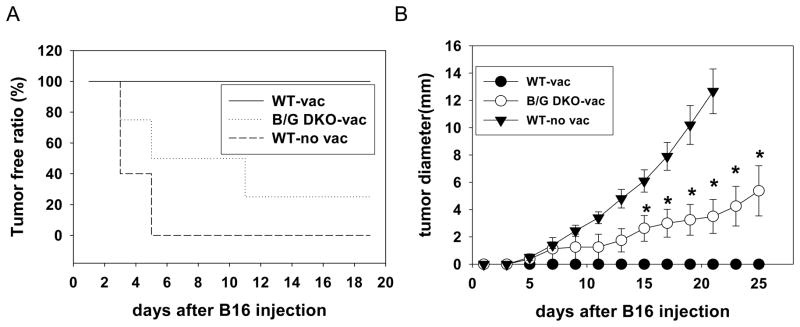
Our data suggest that Gadd45b is involved in anti-tumor activities in mice. However, whether Gadd45b is involved in the adaptive anti-tumor responses is not known. Besides Gadd45b, Gadd45g is also expressed in Th1 cells and CD8 T cells and likely play a similar role as Gadd45b. Gadd45a, the other member of the Gadd45 family is not expressed at appreciable level in peripheral T cells. We therefore decided to investigate whether Gadd45b and Gadd45g are required for adaptive immunity against cancer using newly generated Gadd45b/Gadd45g doubly deficient (B/G DKO) mice. WT and B/G DKO mice were vaccinated with irradiated B16 melanoma cells expressing GM-CSF. To further boost adaptive immune response to tumor cells, we depleted peripheral Tregs by anti-CD25 antibodies. We then challenged these mice with live B16 cells 90 days after vaccination. In unvaccinated WT mice, the challenged B16 cells quickly grew and formed tumor nodules. However, no tumor nodules were observed in WT mice that had been vaccinated (Fig. 7a, b). Strikingly, about 70% of the vaccinated B/G DKO mice grew tumors (Fig. 7a, b). Therefore, Gadd45b and Gadd45g mediate adaptive anti-tumor responses in mice.
Figure 7.
Gadd45b and Gadd45g are required for tumor vaccination. Gadd45b/Gadd45g DKO and WT mice were vaccinated as described in the Materials and methods. 90 days after immunization, mice were challenged with 5×105 B16F0 cells i.d. (A) Percentage of tumour free mice after injection. (B) Tumour size was measured every 2 days. At least six mice were used per group. *p<0.05, Mann-Whitney test.
Discussion
In this study, we have found that Gadd45b is required for anti-tumor activities of CD8+ T cells. Gadd45b potentiates activities of the p38 MAP kinase, leading to higher levels of IFN-γ in CD8+ T cells. Gadd45b is up-regulated by IL-12, enhances TCR-triggered activation of p38 MAP kinase, and therefore encodes a key signaling protein for effective anti-tumor immune responses. Moreover, we have shown that Gadd45b enhances the expression of Th1 lineage master regulators T-bet and Eomes. Our study therefore reveals Gadd45b as an important signaling modulator for cancer immune surveillance.
T cells infiltrating tumors must maintain their effector function and overcome local mechanisms of immune suppression in the tumor microenvironment in order to eradicate tumor [17]. However, due to the immune suppressive nature of the tumor microenvironment, tumor infiltrating T cells are largely anergic and downregulate their proximal signaling molecules such as CD3-ζ [18, 19]. IL-12 is the master cytokine for promoting the generation of Th1 cells and cytotoxic lymphocytes. Although systemic delivery of IL-12 failed in clinical trials due to severe side effects [20], various approaches have been tested to reduce toxicity based on the delivery of IL-12 locally and directly to the tumor site, as well as using small releasing particles to give low, but sustained doses of IL-12 [21]. All of these approaches have so far achieved efficacy in animal models. Delivery of IL-12 at the tumor site is believed to change tumor microenvironment to one favoring Th1 type adaptive immune response. One of the most interesting properties of IL-12 on TILs is its abilities to reverse T cell anergy [21]. TILs pretreated in vitro with IL-12, but not the T cells incubated with media only, translocated NFAT into the nucleus in response to TCR stimulation [21]. Therefore, the anergic state of TILs is not terminal but reversible when cultured in vitro with IL-12. We have demonstrated that in activated T cells, Gadd45b is induced by IL-12. More importantly, the induction of Gadd45b by IL-12 elevated the sensitivity of the TCR-triggered p38 MAP kinase pathway. This property of Gadd45b serves as a critical mechanism that enables IL-12 to potentiate TCR signaling, and therefore allows T cell to respond to lower dose of antigen and to prevent or even reverse anergy in the tumor microenvironment.
Gadd45b and Gadd45g were originally identified to be highly expressed in Th1 cells [10, 12]. Like many Th1 type genes, Gadd45b and Gadd45g are now found expressed also in CD8+ T cells. This finding supports the unique signaling property of CD8+ T cells. Th1 lineage transcription factors T-bet and Eomes have been shown to be expressed highly in CD8+ T cells [16]. Whether the Gadd45 pathway affects the Th1 lineage transcription factors was not known. We have now demonstrated that Gadd45b increases the levels of these transcription factors in CD8+ T cells, supporting that the Gadd45b signaling pathway influencing the Th1 fate decision in CD8+ T cells. Interestingly, although IL-12 was shown to suppress Eomes in effector CD8+ T cells [22], Gadd45b, on the contrary, promotes the expression of Eomes. Therefore, Gadd45b play an important role in promoting the lineage commitment in effector CD8+ T cells.
In this study, we provide evidence that Gadd45b is involved in tumor surveillance by CD8+ T cells. Therefore, in this case, Gadd45b is functionally a tumor-suppressor. Gadd45b is also reported down-regulated in certain cancer such as hepatocellular carcinoma [23]. Gadd45g was reported down regulated in a number of cancers [24]. Over-expression of members of Gadd45 gene family has been shown to inhibit the growth of transformed cells [25]. Therefore, Gadd45b and Gadd45g could serve as potential therapeutic targets for an immune thereapy aimed to boost Th1 responses and combine with chemotherapy to target cancer cells.
Materials and Methods
Mice
Gadd45b−/− mice were generated as described previously [11]. Gadd45b+/− mice were backcrossed onto C57Bl/6 background for more than ten generations. Gadd45b+/+ and Gadd45b +/− littermates were used as normal controls for Gadd45b−/− mice. All animals were maintained under specific pathogen-free conditions and used at 8–12 wk of age unless specifically mentioned. All animal experiments were approved by the Institution Animal Care and Use Committee (IACUC) of University of Pittsburgh.
Cell line and Reagents
Recombinant murine IL-12 and IL-18 were purchased from R&D Systems. Recombinant human IL-2 was obtained from NIH the BRB Preclinical Repository. Anti-phosphorylated p38, anti-phosphorylated JNK, anti-phosphorylated ERK antibodies were purchased from Cell Signaling Technology. Anti-β-tubulin and anti-rabbit IgG-HRP antibodies were purchased from Santa Cruz Biotechnology. Collagenase type I was purchased from Sigma. B16 F0 melanoma cells (kindly provided by Dr. Z. Yin, Yale University, School of Medicine, New Haven, CT) [26].
Analysis of CD8+ T cells cultured in Th1 conditions
Naïve CD8+ T cells were sorted from B6 WT and Gadd45b−/− mice (CD62LhighCD44low) and cultured for 48h with 10 μg/ml plate-bound anti-CD3 and 5 μg/ml anti-CD28 Abs in the presence of IL-2 (20 units/ml), IL-12 (3.4 ng/ml) plus anti-IL-4 (10 μg/ml, Clone 11B11, obtained from the BRB Preclinical Repository). After 48 hours, cells were re-plated to new wells without anti-CD3 or anti-CD28, and with freshly added IL-2 (5 units/ml) for another 48h. Cells were subsequently re-stimulated with plate-bound anti-CD3 (α-CD3; 10 μg/ml) or IL-12(10 ng/ml) plus IL-18 (30 ng/ml). Cells were then harvested for intracellular IFN-γ staining, and the supernatants were harvested for ELISA analysis. Extracts of 2×105 cells were used to analyze MAP kinase activation.
Preparation of single cell suspensions from tumor mass and measurement of IFN-γ production by tumor infiltrating T cells
Gadd45b−/− mice and wild type or Gadd45b+/− littermates at about 10 weeks old were inoculated with 1×105/mouse of B16F0 melanoma cells. On day 13 after inoculation, tumor masses were removed and digested with 0.29% trypsin at 37 °C for 2 hours, and then further incubated in collagenase and hyaluronidase digestion solution (0.27% collagenase type I, 0.025% hyaluronidase, 1% DNase, 0.01% HEPES in RPMI) for 2 hours at 37 °C. The digested tumor masses were filtered through a 40-mm cell strainer (Becton Dickinson). Cells were resuspended in RPMI1640 with 10% FCS at 1×106/ml, and then stimulated with phorbol 12-myristate 13-acetate (PMA) (50 ng/ml) and Ionomycin (500 ng/ml) for 6 hours in the presence of Brefeldin A for 5 hours. The cells were stained with anti-CD45 and anti-CD8, then fixed with 2% formaldehyde, permeablized by 0.5% saponin, and further stained with anti-IFN-γ for measuring intracellular IFN-γ.
Adoptive transfer experiments
Wild type host C57Bl/6 mice were irradiated with 500 rad 6 hours before adoptive transfer. Neomycin sulfate (Sigma-Aldrich) was administered through drinking water starting 4 days before irradiation. CD4+ T cells were purified from splenocytes and lymph nodes cells harvested from Gadd45b−/− mice or Wild type littermates, first by negatively enrichment by depletion using anti-MHC class I, anti-B220, Mac1, and anti-CD8 antibodies, and then followed by positive purification using biotinylated anti-CD4 antibody and streptavidin conjugated beads (Miltenyi Biotec). CD8+ T cells were first enriched by depletion using anti-MHC class I, anti-B220, Mac1, and anti-CD4 antibodies, and then followed by positive purification using biotin-anti-CD4 antibody and streptavidin conjugated beads (Miltenyi Biotec).
To deplete CD4+ T cells or CD8+ T cells from splenocytes, total spleen cells were incubated with anti-CD4 (GK1.5) or anti-CD8 (TIB105) antibodies for 30 min at 4°C. The cells were then treated with rabbit complement (Pel-Freez Biologicals, USA) at 37°C for 30 min.
For spleen cell adoptive transfer, splenocytes were harvested from Gadd45b−/− mice and Wild type littermates. A total of about 5×107 spleen cells were injected intravenously via the retro-orbital sinus. For adoptively transferring CD4+ T cells, 1.5×107 purified CD4+ T cells from Gadd45b−/− mice or wild type littermates were mixed with 5×107 spleen cells which have been depleted of CD4+ T cells, and then the mixed cells were transferred intravenously via the retro-orbital sinus. For adoptively transferring CD8+ T cells, 3×106 purified CD8+ T cells from Gadd45b−/− mice or wild type littermates were mixed with 5×107 spleen cells that have been depleted of CD8+ T cells, and then the mixed cells were transferred intravenously via the retro-orbital sinus. One day after the adoptive transfer, 1×105 B16 melanoma cells were inoculated subcutaneously into the host mice and tumor growth was observed.
Tumor vaccination
Depletion of peripheral Tregs was accomplished by injection of anti-CD25 (clone PC61, 350 μg, i.p.) on day 0 and day 7. B16-GM-CSF cells were irradiated at 35 Gy, washed, and resuspended in 100 μl PBS for intradermal (i.d.) administration of 5×105 per mouse at day 4 and 7 d. On the day indicated, mice were challenged with 5×105 B16F0 cells i.d. The size of intradermally growing tumors was measured every 2 days.
Gene expression analysis
Total RNA was extracted using the RNeasy mini kit (Qiagen) and reverse transcribed using Superscript II (Invitrogen). The real time PCR was performed on with SYBR green (Applied Biosystem). Cycling condition were 10 min at 95°C followed by 40 repeats of 95°C for 15 s and 60°C for 60 s. Analysis was performed by sequence detection software supplied with the instrument (Applied Biosystem). The primers included: GzmB sense, 5′-CCACTCTCGACCCTACATGG -3′; GzmB antisense, 5′-GGCCCCCAAAGTGACATTTATT-3′; CCR5 sense, 5′-AGCCGCAATTTGTTTCACAT-3′; CCR5 antisense, 5′-GAGACATCCGTTCCCCCTAC-3′; T-bet sense, 5′-CGGGAGAACTTTGAGTCCATGT-3′; T-bet antisense, 5′-GCTGGCCTGGAAGGTCG-3′; Eomes sense, 5′-CACGGCTTCAGAAAATGACA-3′; Eomes antisense, 5′-CTCTGTTGGGGTGAGAGGAC-3′; β-actin sense: 5′-TCCTTCGTTGCCGGTCCAC-3′; antisense 5′-ACCAGCGCAGCGATATCGTC-3′.
Acknowledgments
We thank Dr. Jeff Kovacs for editing the manuscript. B. Lu is supported by the Investigator award from Cancer Research Institute. This work is partly supported by Cancer and Aging Program (NIH grant #P20 CA103730) and NSFC grant 30528008 and 30400394. Dr. Zhang is supported by the National Key Basic Research Program of China 2007CB512402. L Liu is supported by NIH training grant 5T32CA082084-09.
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 3.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 4.O’Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994;6:458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 5.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 6.Peng SL, Townsend MJ, Hecht JL, White IA, Glimcher LH. T-bet regulates metastasis rate in a murine model of primary prostate cancer. Cancer Res. 2004;64:452–455. doi: 10.1158/0008-5472.can-03-3401. [DOI] [PubMed] [Google Scholar]
- 7.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 8.Atreya I, Schimanski CC, Becker C, Wirtz S, Dornhoff H, Schnurer E, Berger MR, Galle PR, Herr W, Neurath MF. The T-box transcription factor eomesodermin controls CD8 T cell activity and lymph node metastasis in human colorectal cancer. Gut. 2007;56:1572–1578. doi: 10.1136/gut.2006.117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM, Mueller-Berghaus J, Kirkwood JM, Kwok WW, Storkus WJ. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Zhu H, Murphy TL, Ouyang W, Murphy KM. IL-18-stimulated GADD45 beta required in cytokine-induced, but not TCR-induced, IFN-gamma production. Nat Immunol. 2001;2:157–164. doi: 10.1038/84264. [DOI] [PubMed] [Google Scholar]
- 11.Lu B, Ferrandino AF, Flavell RA. Gadd45beta is important for perpetuating cognate and inflammatory signals in T cells. Nat Immunol. 2004;5:38–44. doi: 10.1038/ni1020. [DOI] [PubMed] [Google Scholar]
- 12.Lu B, Yu H, Chow C, Li B, Zheng W, Davis RJ, Flavell RA. GADD45gamma mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity. 2001;14:583–590. doi: 10.1016/s1074-7613(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 13.Chi H, Lu B, Takekawa M, Davis RJ, Flavell RA. GADD45beta/GADD45gamma and MEKK4 comprise a genetic pathway mediating STAT4-independent IFNgamma production in T cells. Embo J. 2004;23:1576–1586. doi: 10.1038/sj.emboj.7600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Tran E, Zhao Y, Huang Y, Flavell R, Lu B. Gadd45 beta and Gadd45 gamma are critical for regulating autoimmunity. J Exp Med. 2005;202:1341–1347. doi: 10.1084/jem.20051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 16.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 17.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 18.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F, Viola A. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 20.Robertson MJ, Cameron C, Atkins MB, Gordon MS, Lotze MT, Sherman ML, Ritz J. Immunological effects of interleukin 12 administered by bolus intravenous injection to patients with cancer. Clin Cancer Res. 1999;5:9–16. [PubMed] [Google Scholar]
- 21.Broderick L, Yokota SJ, Reineke J, Mathiowitz E, Stewart CC, Barcos M, Kelleher RJ, Jr, Bankert RB. Human CD4+ effector memory T cells persisting in the microenvironment of lung cancer xenografts are activated by local delivery of IL-12 to proliferate, produce IFN-gamma, and eradicate tumor cells. J Immunol. 2005;174:898–906. doi: 10.4049/jimmunol.174.2.898. [DOI] [PubMed] [Google Scholar]
- 22.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 23.Qiu W, David D, Zhou B, Chu PG, Zhang B, Wu M, Xiao J, Han T, Zhu Z, Wang T, Liu X, Lopez R, Frankel P, Jong A, Yen Y. Down-regulation of growth arrest DNA damage-inducible gene 45beta expression is associated with human hepatocellular carcinoma. Am J Pathol. 2003;162:1961–1974. doi: 10.1016/s0002-9440(10)64329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying J, Srivastava G, Hsieh WS, Gao Z, Murray P, Liao SK, Ambinder R, Tao Q. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin Cancer Res. 2005;11:6442–6449. doi: 10.1158/1078-0432.CCR-05-0267. [DOI] [PubMed] [Google Scholar]
- 25.Vairapandi M, Balliet AG, Hoffman B, Liebermann DA. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol. 2002;192:327–338. doi: 10.1002/jcp.10140. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]