Abstract
Mammalian ageing is accompanied by accumulation of genomic DNA damage and progressive decline in the ability of tissues to regenerate1. DNA damage activates the tumour suppressor p53, which leads to cell-cycle arrest, senescence or apoptosis. The stability and activity of p53 are induced by DNA damage through posttranslational modifications such as phosphorylation of Thr 21 and Ser 23 (refs 2–5). To investigate the roles of DNA damage and p53 in tissue-regenerative capability, two phosphorylation-site mutations (T21D and S23D) were introduced into the endogenous p53 gene in mice, so that the synthesized protein mimics phosphorylated p53. The knock-in mice exhibit constitutive p53 activation and segmental progeria that is correlated with the depletion of adult stem cells in multiple tissues, including bone marrow, brain and testes. Furthermore, a deficiency of Puma, which is required for p53-dependent apoptosis after DNA damage6, rescues segmental progeria and prevents the depletion of adult stem cells. These findings suggest a key role of p53-dependent apoptosis in depleting adult stem cells after the accumulation of DNA damage, which leads to a decrease in tissue regeneration.
The transcription factor p53 remains in an unstable and inactive state in unstressed cells. Once activated, p53 initiates the transcription of hundreds of genes, including p21, 14-3-3σ, Noxa and Puma6, but also suppresses the expression of a number of genes, such as the pluripotency factor Nanog in embryonic stem cells7. The transcriptional targets of p53 are responsible for mediating p53-dependent functions. For example, after DNA damage, p21 and 14-3-3σ are required to mediate cell-cycle arrest8 and Puma is essential for apoptosis6.
The stability and activity of p53 is regulated primarily through the modulation of the interaction between p53 and its negative regulators Mdm2 or MdmX, which suppress the transcriptional activities of p53 (ref. 3). Additionally, Mdm2 destabilizes p53 by promoting its ubiquitylation and degradation3. In response to DNA damage and other cellular stresses, the stability and activity of p53 are significantly increased. Accumulating evidence indicates that post-translational modifications of p53, such as phosphorylation, are important in regulating p53 stability and activity9. Both Thr 18 and Ser 21 of human p53 (corresponding to Thr 21 and Ser 23 of mouse p53) are located within the region that interacts with Mdm2/MdmX, and DNA damage-induced phosphorylation of these residues disrupts the interaction between p53 and Mdm2/MdmX, leading to p53 activation9.
Accumulation of DNA damage has been considered as a physiological inducer of ageing10. To investigate the role of p53 in the ageing process, we introduced two missense mutations (T21D and S23D) into the endogenous p53 gene in mice; the mutated protein then mimics the phosphorylated p53 induced by DNA damage. The strategy to generate the knock-in mutant mice, denoted as p53TSD mice, is described in Supplementary Information, Figure S1. Heterozygous mutant embryonic stem cells with the targeted allele that retained the PGK–Neor cassette were used to generate chimaeric mice for subsequent germline transmission. As it is well established that the PGK–Neor gene suppresses the expression of the targeted allele, we initially attempted to excise the LoxP-flanked PGK–Neor gene from the targeted allele by breeding the heterozygous mice with CMV–Cre transgenic mice that express Cre recombinase during the zygote stage. However, after screening hundreds of offspring, we were unable to identify any animals that had undergone deletion of the PGK–Neor gene. As the same strategy has been used previously to successfully delete the PGK–Neor gene from the targeted allele in p53S18A and p53S18A/S23A knock-in mice4,11, it seems that p53TSD could induce embryonic lethality when expressed at normal levels once the PGK–Neor gene is deleted. Furthermore, when the heterozygous mutant mice with the targeted allele containing the PGK–Neor cassette were intercrossed, homozygous mutant offspring were rarely obtained, indicating that two copies of the p53TSD allele induce embryonic lethality (Supplementary Information, Fig. S1e, top). To test this potential dosage effect, p53TSD/+ mice were bred with p53+/− mice, and p53TSD/− offspring were observed with a normal Mendelian ratio (Supplementary Information, Fig. S1e, bottom). The entire p53 cDNA derived from p53TSD/− mouse embryonic fibroblasts (MEFs) was sequenced to verify that only the T21D/S23D substitutions were introduced into the endogenous p53 gene of p53TSD/− mice. Additionally, p53 protein was expressed in p53TSD/− MEFs, but at much lower levels than in p53+/+ MEFs (Supplementary Information, Fig. S1f).
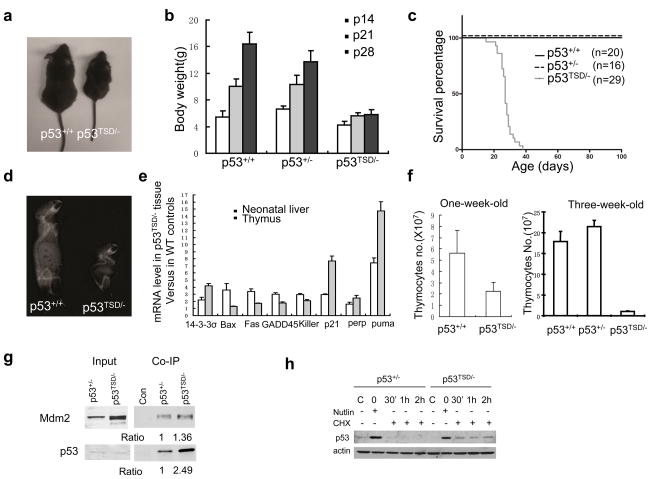
Although the p53TSD/− mice were born with an expected Mendelian frequency and were similar to their wild-type littermates by 2 weeks of age, these mice became runted and weighed about 50% of the sex- and age-matched p53+/+- and p53+/−-mice by 4 weeks of age (Fig. 1a, b). When compared with p53+/+ mice, p53TSD/− mice had a significantly shorter lifespan and died by 6 weeks of age (Fig. 1c). Furthermore, p53TSD/− mice exhibited a number of ageing-related phenotypes, including acute spine curvature (Fig. 1d), hypoplasia in the bone marrow (Fig. 2a) and lymphopenia and anaemia (Fig. 2c, d).
Figure 1.
p53TSD/− mice exhibit segmental progeria and constitutively active p53. (a) Representative image of male p53+/+ and p53TSD/− littermates at 30 days of age. (b) Growth rate of p53+/+, p53+/− and p53TSD/− mice between 14 and 28 days postnatal. Values represent means and s.d. (n = 11). (c) Survival curve of p53+/+-, p53+/−-and p53TSD/−-mice. P < 0.0001 between p53TSD/− mice, compared with p53+/+-and p53+/−-mice. (d) Representative X-ray image showing the spine curvature of 30-day-old p53+/+-and p53TSD/−-littermates. (e) mRNA levels of various p53-target genes in the liver of newborn, and thymus of three-week-old, p53TSD/− mice. The level of mRNA in p53TSD/− tissues was calculated by normalization to wild-type controls. Values represent means ± s.d. (n = 3). (f) The number of thymocytes in mice with the indicated phenotypes at one-week old and at three-weeks old. Values are means ± s.d. (n = 3). (g) The T21D and S23D mutation in p53 partially disrupts the interaction between Mdm2 and p53. To allow normal expression of p53 from the targeted allele, p53TSD/− and p53+/− MEFs were infected with retroviral-Cre to excise the PGK–Neor cassette from the targeted allele. p53 was immunoprecipitated, and the amount of p53 and Mdm2 in the immunoprecipitate was determined by western blotting. Nonspecific antibody (control) was used to indicate the specificity of immunoprecipitation. The ratio of the protein levels of p53 and Mdm2 in the immunoprecipitates from the different mice strains is indicated. (h) Nutlin, which inhibits the interaction between p53 and Mdm2, can stabilize p53TSD and make it more stable than wild-type p53. After deletion of PGK–Neor from the targeted allele, p53TSD/− and p53+/− MEFs were treated with nutlin (25 μM) for 24 h, and then further cultured in medium containing cycloheximide (CHX; 10 μM), but without nutlin. The protein levels of p53 in untreated samples, nutlin-treated samples before cycloheximide treatment (0 h), and samples at the indicated times after cycloheximide treatment, were analysed by western blotting.
Figure 2.
Progressive degeneration of the haematopoietic system in p53TSD/− mice. (a) Hypoplasia in the bone marrow of p53TSD/− mice. Representative images of sections from femurs of four-week-old p53+/+ and p53TSD/− mice stained with haematoxylin and eosin. Scale bar, 100μm. (b) LSK cells are progressively depleted in p53+/+ and p53TSD/− mice. Flow cytometry was used to analyse the percentage of LSK cells in the liver of p53+/+-and p53TSD/−-mice (fetal liver at embryonic day 12; E12, and the newborn liver), and in the bone marrow of p53+/+-, p53+/−-and p53TSD/−-mice at 14 days postnatal (P14). Values represent means ± s.d. (n = 3). P values are compared with p53+/+ mice and obtained from a two-tailed t-test with unpaired samples and unequal variance. (c) Lymphopenia in p53TSD/− mice. The number of T-and B-cells in the spleen of two-week-old p53TSD/− mice are calculated by normalization to wild-type control mice. Values represent means ± s.d. (n = 3 mice per strain). P values are indicated, compared with p53+/+ mice. (d) Progressive anaemia in p53TSD/− mice. The haemoglobin levels in p53+/+-and p53TSD/−-mice at 14, 21 and 28 days postnatal were determined. Values represent means ± s.d. (P14; n = 9 pairs of mice, P21; n = 14 pairs of mice and P28; n = 6 pairs of mice). P values, compared with p53+/+ mice, are obtained from a two-tailed t-test with paired samples.
p53-dependent gene expression was analysed in the liver of newborn, and the thymus of three-week-old, p53TSD/−- and wild-type-mice. The expression of most p53-target genes was increased in p53TSD/− cells, indicating that p53 is constitutively active (Fig. 1e and Supplementary Information, Fig. S2). In the thymus of p53TSD/− mice, the mRNA levels of p53-target genes (p21 and Puma) were markedly increased, compared with other p53-target cell-cycle genes (Bax and 14-3-3σ). Consistent with the increased expression of p53 targets, such as Puma in p53TSD/− thymocytes, the number of thymocytes was markedly reduced in p53TSD/− mice when compared with wild-type controls (Fig. 1f). The cause of thymocyte reduction is probably intrinsic because thymocyte number is already reduced in one-week-old p53TSD/− mice when no other ageing phenotypes are apparent.
To confirm that the increased p53 activity is not limited to the lymphoid tissues, the expression of p53-target genes p21 and Puma in different tissues of p53TSD/− and wild-type mice were compared, including in brain, kidney, small intestine, liver, lung, skin and spleen. The mRNA levels of Puma and p21 were increased in most tissues of p53TSD/− mice, indicating that p53 is constitutively active in p53TSD/− mice (Supplementary Information, Fig. S2). As expected from previous findings2,5, the TSD mutation partially disrupted the interaction between p53 and its negative regulator Mdm2, accounting for the increased p53 stability and activity in p53TSD/− cells (Fig. 1g, h and Supplementary Information, Fig. S1g).
Adult stem cells are important for tissue-regenerative capability in mammals1. To test whether the segmental progeria observed in p53TSD/− mice is due to the depletion of the adult stem/progenitor cells, we analysed adult stem cells in multiple tissues, including bone marrow, brain and testes. Histological sections of femurs derived from 30-day-old p53TSD/−- and wild-type-mice showed severe hypoplasia in the bone marrow of p53TSD/− mice (Fig. 2a). Additionally, haematopoietic stem/precursor cells in the fetal liver and bone marrow, identified as lineage-negative (Lin−), Sca1-positive and c-Kit-positive (Lin− Sca1+c-Kit+; LSK), were progressively depleted in p53TSD/− mice (Fig. 2b). When compared with wild-type- or p53+/−-mice, the percentage of the LSK population was similar in the fetal liver of p53TSD/− mice, decreased by about 40% in the newborn liver of p53TSD/− mice, and was essentially depleted in the bone marrow of 14-day-old p53TSD/− mice. Consistent with this, lymphopenia was apparent in two-week-old p53TSD/− mice (Fig. 2c). Furthermore, p53TSD/− mice became progressively anaemic (Fig. 2d). The defects in the haematopoietic stem cells (HSCs) of p53TSD/− mice were cell autonomous, because unlike wild-type mice, the fetal-liver cells of p53TSD/− mice failed to rescue lethally irradiated mice by repopulation of the haematopoietic system (data not shown). Therefore, bone-marrow failure is a probable cause of the shortened life span of p53TSD/− mice.
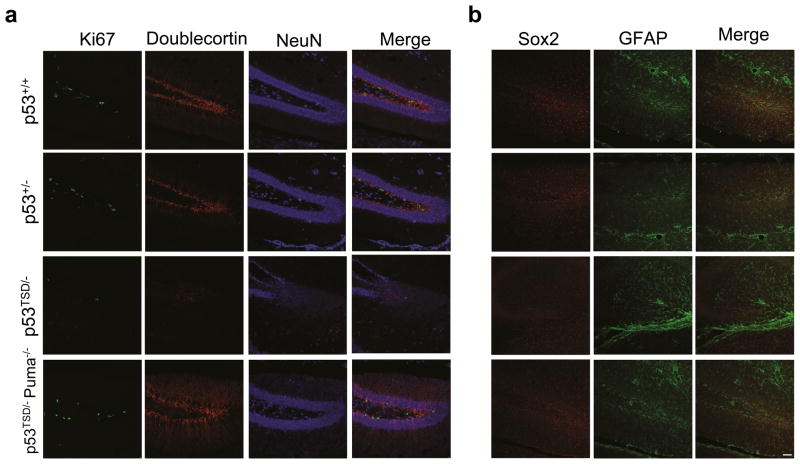
Adult neural stem cells/progenitor cells have been identified in the dentate gyrus of the hippocampus of mice and are progressively depleted during ageing12. To examine the effect of increased p53 activity on adult neural stem cells and their progeny, we employed the immature neuronal marker, doublecortin (DCX), and neuron-specific nucleic-acid marker, NeuN, to investigate neurogenesis in p53TSD/− mice. Furthermore, the cells undergoing proliferation in the dentate gyrus, another marker for self-renewing adult neural stem cells, were detected by an antibody specific for Ki67, a nuclear antigen associated with cellular proliferation. The depletion of Ki67-positive and DCX-positive cells in the dentate gyrus suggests a marked depletion of the adult neural stem cells and their progeny in the dentate gyrus of one-month-old p53TSD/− mice (Fig. 3a). Using Sox2, a marker specific for adult neural stem cells, we confirmed that neural stem cells are depleted in the dentate gyrus of one-month-old p53TSD/− mice (Fig. 3b).
Figure 3.
Depletion of adult neural stem cells in the dentate gyrus of p53TSD/− mice, which is suppressed by Puma-deficiency. (a) Representative images of sections taken from the brains of 30-day-old p53+/+-, p53+/−-, p53TSD/−-and p53TSD/− Puma−/−-littermates that were immunochemically stained for Ki67, doublecortin and NeuN. (b) Sections from a were immunochemically stained for Sox2 and Glial fibrillary acidic protein (GFAP) to identify the neural stem cells and neurons. Scale bar, 50 μm.
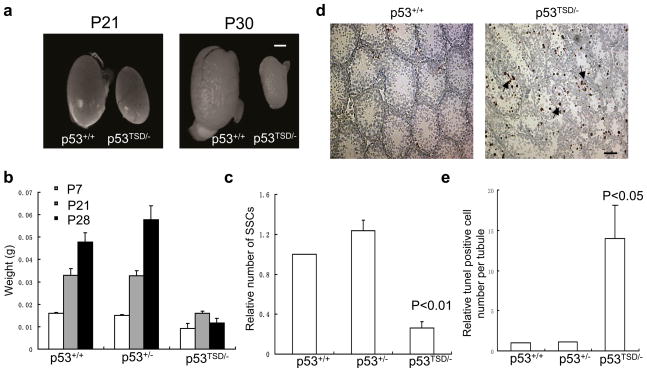
Spermatogenesis is mediated by continuous self-renewal and differentiation of spermatogonial stem cells (SSCs), a process compromised during ageing13. SSCs are a very small subpopulation of spermatogonia lining the inner membrane of the seminiferous tubules of the testes. Although the size of the testes in wild-type and p53+/− mice similarly increased with age (postnatal day 7 to 21), the size of the p53TSD/− mice testes became increasingly smaller (Fig. 4a, b). The impaired spermatogenesis was because of the decreased number of SSCs (Epcam+, α6-integrin+ and c-Kit−) in the testes of p53TSD/− mice (Fig. 4c). Additionally, the apoptosis of the spermatogonia in the seminiferous tubules of p53TSD/− mice was greatly increased when compared with that in wild-type- and p53+/−-mice (Fig. 4d, e). Therefore, SSCs are depleted in the testes of p53TSD/− mice probably as a result of increased apoptosis.
Figure 4.
Testicular atrophy and depletion of SSCs in p53TSD/− mice. (a) Testicular atrophy in p53TSD/− mice. Representative images of the testes derived from 21-or 30-day-old wild-type-and p53TSD/−-mice. Scale bar, 1 mm. (b) The weight of the testes derived from p53+/+-, p53+/−-, and p53TSD/−-mice of indicated ages. Values represent means ± s.d. (n = 3 pairs of mice). Scale bar, 20 μm. (c) The number of SSCs is significantly reduced in p53TSD/− mice. SSCs (Epcam+, integrin-α6+ and c-kit−) were analysed by flow cytometry. Values represent means ± s.d. (n = 3). P values, compared with p53+/+ mice were calculated using a two-tailed t-test with paired samples. (d) Representative images of increased spermatogonia apoptosis in the seminiferous tubules of p53TSD/− mice. The apoptotic cells were identified by TUNEL assay and are indicated with arrows. Scale bar, 20 μm. (e) Number of apoptotic cells per tubule in p53+/+-, p53+/−-and p53TSD/−-mice, normalized relative to p53+/+ mice. The number of apoptotic cells from 30 tubules is counted. Values represent means ± s.d. (n = 3 mice per strain). P values, compared with p53+/+ mice were calculated using a two-tailed t-test with paired samples.
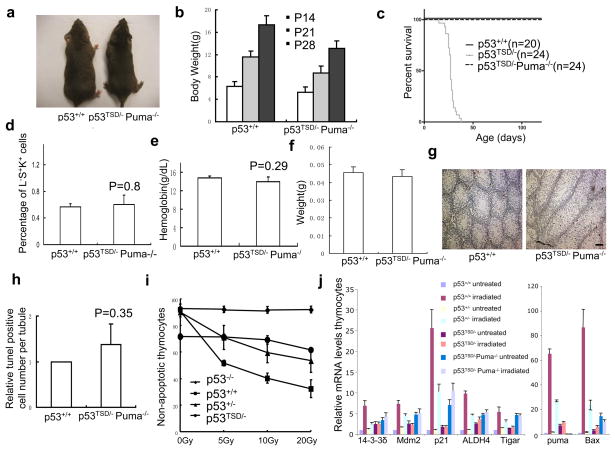
As the expression of p21 and Puma is increased in various tissues of p53TSD/− mice, which is required for p53-mediated cell-cycle arrest and apoptosis, p21−/−- and Puma−/−-mice were used to examine the importance of p53-dependent cell-cycle arrest and apoptosis in depleting adult stem cells. p21 deficiency fails to rescue the ageing-related phenotypes and early death of the p53TSD/− mice (Supplementary Information, Fig. S3). In contrast, Puma-deficiency rescues the segmental progeria phenotypes observed in p53TSD/− mice. Four-month-old p53TSD/− Puma−/− mice were phenotypically similar to wild-type mice (Fig. 5a). p53TSD/− Puma−/− mice are also similar to wild-type mice in growth rate (Fig. 5b), and all p53TSD/Puma−/− mice survived longer than 6 weeks of age, whereas all p53TSD/− mice died (Fig. 5c). Furthermore, Puma-deficiency rescued the atrophy of testes, and prevented the depletion of adult stem cells in the bone marrow, testes and brain of p53TSD/− mice (Figs 3a, b and 5d, f and Supplementary Information, Fig. S4). Puma-deficiency also prevented anaemia and partially rescued the thymocyte-reduction phenotype in p53TSD/− mice (Fig. 5e and Supplementary Information, Fig. S4d).
Figure 5.
Puma-deficiency rescues the segmental progeria phenotypes and inhibits the depletion of adult stem cells in p53TSD/− mice. (a) Representative image of 4-months-old p53TSD/−Puma−/−-and wild-type-mice. (b) Growth rate of p53TSD/− Puma−/− mice. Values represent means ± s.d. (n = 12 mice per strain at each time). (c) Survival curve of p53+/+-, p53TSD/−-and p53TSD/− Puma−/−-mice. (d) Effect of Puma deficiency on LSK population in the bone marrow of four-week-old p53TSD/− Puma−/−-and wild-type-mice. Values represent means ± s.d. (n = 3 per strain). P-value was calculated using a two-tailed t-test with paired samples. (e) Haemoglobin concentration in four-week-old p53TSD/− Puma−/− mice, compared with wild-type p53+/+ mice. Values represent means ± s.d. (n = 3 mice per strain). P values were calculated using a two-tailed t-test with paired samples. (f) Weight of testes in four-week-old p53+/+-and p53TSD/− Puma−/−-mice. Values represent means ± s.d. (n = 4 mice per strain). (g) Representative image of spermatogonia apoptosis in four-week-postnatal p53+/+-and p53TSD/− Puma−/−-mice, identified by TUNEL assay of testes paraffin sections. Scale bar, 20 μm. (h) The number of apoptotic spermatogonia per seminiferous tubule in four-week-old p53TSD/− Puma−/−mice relative to p53+/+ mice. Values represent means ± s.d. (n = 3 mice per strain). P values were calculated using a two-tailed t-test with paired samples. (i) p53-dependent apoptosis in p53−/−-, p53+/+-, p53+/−-and p53TSD/−-mice thymocytes 10 h after treating with 5, 10 and 20 Gy of irradiation. Values represent means ± s.d. (n = 3 mice per strain). (j) Expression of p53-target genes in p53+/+-, p53+/−-, p53TSD/−-and p53TSD/− Puma−/−-mice thymocytes without treatment or 6 h after irradiation (5 Gy). For each gene, level of mRNA was calculated by normalization to p53+/+ mice. Values represent means ± s.d. (n = 3 mice per strain).
Consistent with the critical roles of Puma in p53-dependent apoptosis, Puma-deficiency inhibited the apoptosis of spermatogonia in p53TSD/− mice (Fig. 5g, h). Confirming that p53 is constitutively active in p53TSD/− thymocytes, ionizing radiation failed to increase p53-dependent apoptosis and p53-dependent transcription in p53TSD/− thymocytes (Fig. 5i). The partial rescue of thymocytes by Puma-deficiency is probably due to the increased expression of other p53-target genes such as p21 and Bax in the thymus of p53TSD/−Puma−/− mice (Fig. 5j). Furthermore, the greatly increased apoptosis of cells in the small intestinal crypt that leads to the degeneration of small intestine in p53TSD/− mice is suppressed by Puma deficiency (Supplementary Information, Fig. S5). These findings suggest that p53-dependent apoptosis has an important role in depleting adult stem cells in response to the accumulation of DNA damage.
Although accumulation of DNA damage has been shown to be associated with normal ageing14–16, it remains unclear how DNA damage induces ageing processes. As a critical component in activating cellular responses to DNA damage, the role of p53 in ageing also remains unclear because of the conflicting data from previous transgenic mouse studies. Two studies have shown that mutant mice with increased p53 activity (by expression of a p53 mutant with a truncated amino terminus) develop accelerated ageing phenotypes17,18. However, normal ageing is observed in mice that have additional copies of the p53 or ARF gene, or a hypomorphic mutation in Mdm2, despite increased p53 activity19–21. Recent studies showed that transgenic mice with additional copies of both p53 and ARF genes live longer than wild-type mice22. The delayed ageing in these transgenic mice is correlated with decreased accumulation of DNA damage in aged mice. However, DNA damage accumulates during ageing in a physiological setting1. Therefore, the complex roles of p53 in DNA damage responses during mammalian ageing remain to be established.
By generating a knock-in mouse model with a genetically modified p53 that mimics DNA-damage-activated p53, we show that increased p53 activity leads to segmental progeria and depletion of adult stem cells, suggesting a role for p53 in coordinating tissue regenerative capability and accumulated DNA damage. Together with the findings that p53 inhibits the self-renewal of DNA-damaged embryonic stem cells and that p53-deficient embryonic stem cells are genetically unstable7,23, these findings suggest a universal role for p53 in maintaining genetic stability in stem cells by eliminating DNA-damaged cells from the self-renewing pool. These findings also have implications for the current effort to develop cancer therapies by activating p53 in human cancer cells that harbour wild-type, but inactive, p53. Considering the critical roles of Mdm2 in destabilizing and suppressing p53 activity, and the frequent overexpression of Mdm2 in human cancer cells, the disruption of the interaction between Mdm2 and p53 has been an extensively pursued therapeutic strategy to activate p53 in cancer cells24. Our findings indicate that it is important to evaluate the long-term toxicity of the potential anti-Mdm2 therapy resulting from the depletion of adult stem cells.
One of the key findings from this study is the discovery of the critical roles for Puma in the depletion of adult stem cells induced by constitutively active p53. As Puma is a p53 transcription target required for p53-dependent apoptosis after DNA damage6, this finding suggests that p53-dependent apoptosis could have an important role in ageing by depleting adult stem cells when DNA damage is accumulated in the organism. In support of this, apoptosis is increased with normal ageing20. As p53 is critical for tumour suppression in humans, it would be impractical to develop a strategy to maintain adult stem cells by directly targeting p53. Along with previous findings that Puma-deficiency does not increase the cancer risk in mice25,26, our results provide a feasible target to prevent depletion of adult stem cells in human patients with defective DNA repair pathways or patients undergoing radiation or chemotoxic cancer therapy.
Methods
Generation of p53TSD knock-in mice
A mouse p53 genomic fragment containing exon 2 was cloned into pBluescript, and the codons for Thr 21 and Ser 23 were simultaneously mutated to aspartic acid (from ACA and TCA to GAT). The LoxP site-flanked PGK-neomycin resistance gene was inserted into intron 4. The targeting vector was linearized with XbaI and electroporated into the mouse embryonic stem cells. Geneticin (G418)-resistant colonies were expanded and homologous recombinants were identified by Southern blotting analysis. The heterozygous mutant embryonic stem cells were used to generate chimaeric mice, which transmitted the mutant allele into mouse germline.
Cell culture conditions
Mouse embryonic fibroblasts were isolated from embryonic day 13 embryos as previously described21, and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS; Hyclone, Logan, Utah), 1% (w/v) antibiotics and 55 μM β-mecaptoethanol (Invitrogen). Saos-2 cells were maintained in DMEM supplemented with 10% (v/v) FBS (Hyclone), 55μM β-mercaptoethanol (Invitrogen) and 1% (w/v) antibiotics (Invitrogen) at 37 °C in a humidified incubator containing 5% CO2.
Histology analysis
Tissues were harvested from the euthanized mice, fixed in 10% (w/v) buffered formalin, embedded in paraffin and sectioned. Sections were stained with haematoxylin and eosin for histological assessment. X-ray images of euthanized animals were taken using a Faxitron X-ray system.
Statistical methods
For the survival curve, Prism5 software (GraphPad) was used to generate the Kaplan-Meier survival curve. For most presented data, mean values from multiple independently obtained data are shown with s.d. Two-tailed P values were obtained from a t-test with paired or unpaired samples using Excel (Microsoft).
Determination of haemoglobin concentration in mouse blood
Blood samples were collected by Micro-Hematocrit capillary tubes (Fisher Scientific) from the tail veil, and diluted 1:100 to determine the haemoglobin concentration. Mouse haemoglobin was determined by QuantiChrom Hemoglobin Assay Kit (BioAssay Systems) according to manufacturer’s instructions.
Western blotting analysis and co-immunoprecipitation
Protein extracts were resolved on SDS–PAGE gel and transferred to nitrocellulose membrane, which was probed with antibodies against p53 (1:1000; FL393, Santa Cruz Biotechnology), or actin (1:2000; Santa Cruz Biotechnology) or Mdm2 (1:500; Santa Cruz Biotechnology). The membrane was subsequently probed with a horseradish peroxidase-conjugated secondary antibody and developed with ECL PLUS (Amersham). For the co-immunoprecipitation assay, cells were harvested and lysed in lysis buffer (20mM Tris, pH 7.6, 1 mM EDTA, 150 mM NaCl, 0.5% (v/v) NP-40, 10 mM NaF, 1 mM NaVO4,10% (v/v) glycerol and proteinase inhibitor). Whole cell protein extracts (1–3 mg) were immunoprecipitated with polyclonal antibody against p53 (1:1000; FL393, Santa Cruz Biotechnology), followed by incubating with protein-G conjugated beads (Amersham) overnight at 4 °C. The beads were washed three times with lysis buffer and boiled with 1 × SDS loading buffer for 5 min. The amount of p53 and Mdm2 in the immunoprecipitate was analysed by western blotting. For detecting the immunoprecipitated p53, the secondary antibody is the Rabbit Trueblot (eBioscience) with 1:5,000 dilutions.
Quantitative real-time PCR
Total RNA from MEFs or mouse tissues was isolated using Trizol (Invitrogen) and RNAeasy Mini Kit (Qiagen). Total RNA (1 μg) was reverse-transcribed into cDNA using Superscript II RT kit (Invitrogen). Quantitative PCR was performed with an ABI Prism 7000 (Applied Biosystems) with Power SybrGreen PCR Master Mix (Applied Biosystems). PCR conditions were as follows: 10 min hot-start at 95 °C followed by 40 cycles of 30 s at 95 °C and 1 min at 60 °C. The levels of gene expression were normalized with the mRNA levels of GAPDH as previously described9. The sequence of the PCR primers are: p21, 5′-ATGTCCAATCCTGGTGATGT-3′ and 5′-TGCAGCAGGGCAGAGGAAGT-3′; killer5, 5′-CGTCTCATGCGGCAGTTG-3′ and 5′-TCGGCTTTGACCATTTGGA-3′; Puma 5′-CGACCTCAACGCGCAGTA-3′ and 5′-AGTCCCATGAAGAGATTGTACATGAC-3′; Bax, 5′-ATGCGTCCACCAAGAAGCTGA-3′ and 5′-AGCAATCATCCTCTGCAGCTCC- 3′; GAPDH, 5′-AATGTGTCCGTCGTGGATCTGA-3′ and 5′-GATGCCTGCTTCACCACCTTCT-3′; Noxa, 5′-CCACCTGAGTTCGCAGCTCAA-3′ and 5′-GTTGAGCACACTCGTCCTTCAA-3′; Mdm2, 5′-ATTGCCTGGATCAGGATTCAGTT-3′ and 5′-ACCTCATCATCCTCATCTGAGA-3′; Perp, 5′-TCATCCTGTGCATCTGCTTC-3′ and 5′-GGGTTATCGTGAAGCCTGAA-3′; 14-3-3δ, 5′-GCCGAACGGTATGAAGACAT-3′and 5′-CTCCTCGTTGCTCTTCTGCT- 3′; Fas, 5′-CCAAGACACAGCTGAGCAGAAA-3′ and 5′-CTCTTCCCATGAGATTGGTACCA-3′; GADD45, 5′-TGGTGACGAACCCACATTCAT-3′ and 5′-ACCCACTGATCCATGTAGCGAC-3′; Tigar, 5′-CTTCGCCTTGACCGTTAT-3′ and 5′-TGTATGGTCTGCTTAGTCCTC-3′; ALDH4, 5′-AGTCTACTGGGTCTGTGGTG-3′ and 5′-GGCTTATGAGTCTCCTTGAT-3′.
Flow cytometry analysis of haematopoietic and spermatogonia stem cells
Haematopoietic stem cells were analysed as previously described27. Briefly, bone marrow cells were isolated, filtered and incubated with biotinylated anti-lineage-marker antibodies (CD3, B220, CD11b, Ly-6G and TER119; 1:50; eBioscience). After 15 min of incubation on ice, the cells were washed twice and incubated with phycoerythrin–anti-c-kit antibody, FITC–anti-Sca-1 antibody (1:200) and APC-streptavidin (0.5 μg per million cells; eBioscience) for 15 min on ice, followed by washing twice with PBS supplemented with 3% (v/v) fetal bovine serum. The stained cells were analysed on a BD LSRII system (BD bioscience). For the isolation of haematopoietic stem/progenitor cells, the bone marrow cells were incubated with the biotinylated anti-lineage markers antibodies, followed by streptavidin-conjugated magnetic beads (Dynabeads) to eliminate lineage marker positive cells. Negatively selected cells were incubated with biotinylated anti-Sca-1 and anti-c-Kit antibodies (1:50; eBioscience), followed by incubation with streptavidin-conjugated magnetic beads to purify haematopoietic stem/progenitor cells.
Spermatogonia in the testes were isolated as previous described28. Briefly, testes were dissociated by digesting with 1 mg ml–1 collagenase (Sigma), followed by the treatment with trypsin containing 5 U ml–1 DNase I (Invitrogen). Total cell numbers were counted and 1 ×106 cells per 100 μl were suspended in PBS supplemented with 3% (v/v) fetal bovine serum, stained with biotin-conjugated anti-EpCAM antibody (Biolegend) for 30 min on ice, and washed twice with wash buffer. The cells were further incubated with FITC–anti-α6-integrin antibody (1:5; Biolegend), phycoerythrin-anti-c-kit antibody (1:200) and APC-conjugated streptavidin (eBioscience) for another 30 min on ice. Stained cells were washed twice, resuspended in 100 μl PBS with 3% (v/v) fetal bovine serum, and analysed by BD LSR system (BD bioscience).
Analysis of neural stem cells
Brains were removed from the skulls, postfixed with 4% (v/v) paraformaldehyde and incubated overnight at 4 °C. Brains were then transferred into a 30% (w/v) sucrose solution in PBS and incubated for 24 h at 4 °C. Brains were cut into 40 μm coronal sections with a sliding microtome and sections were collected in a 96-well plate. For immunohistochemistry, sections were washed with PBS and incubated with primary antibodies for 2 days at 4 °C. The primary antibodies were washed out of the sections with PBS and the sections were then incubated with secondary antibodies for 3 h at room temperature. The primary antibodies used were mouse anti-neuronal-specific nuclear protein (NeuN) antibody (1:200; Chemicon), goat anti-DCX (1:250; Santa Cruz Biotechnology), rabbit anti-Ki67 (1:500; Vector Laboratories), chicken anti-GFAP (1:1000; Chemicon) and rabbit anti-Sox2 (1:200; Chemicon). Immunostained sections were mounted on glass slides with a coverslip. For cresyl-violet staining, reagents and protocol was provided by the Cresyl Echt Violet Stain Kit (American Mastertech Scientific).
Ki67-positive and DCX-positive cells were counted using every 6th section (240 μm apart) of all brains, on a conventional epifluorescence microscope with a ×20 objective. The volume of the granule cell layer was measured by tracing the area of the NeuN-positive region and multiplying it by the thickness of the sections (40 μm). The granule-cell-layer was traced using a semi-automatic stereology system and a ×10 objective.
Analysis of apoptotic cells in tissue sections
The apoptotic cells in tissue sections were detected by TUNEL assay according to manufacturer’s instructions (Millipore). Briefly, deparaffinized tissue sections were digested with proteinase K for 15 min. After they were washed twice in dH2O, the slides were quenched in 0.3% hydrogen peroxide for 30 min, and rinsed with PBS twice, 5 min each. The slides were incubated in equilibration buffer for 10 min, followed by incubating in TdT (terminal deoxynucleotidyl transferase) enzyme solution for 1 h at 37 °C. The slides were agitated for 15 s and incubated for 10 min at room temperature. The anti-digoxin antibody was applied to the section for 30 min at room temperature in a humidified chamber. The positive signal was developed using peroxidase substrate AEC (3-amino-9-ethylcarbazole; Vector Laboratories) for 15–30 min. After washing with dH2O three times, the slides were counterstained with haematoxylin and mounted with coverslip.
p53-dependent apoptosis in mouse thymocytes
p53-dependent apoptosis was analysed as previously described9. Briefly, a single-cell suspension of thymocytes derived from 3–4-week-old mice was isolated and cultured i DMEM supplemented with 5% (v/v) FBS, 25 mM HEPES at pH 7, 55 μM 2-Mercaptoethanol (Invitrogen) and 1% (w/v) antibiotics at a density of 106 cells ml–1. The cells were exposed to increasing dosages of gamma irradiation (0, 5, 10 and 20 Gy). The percentage of apoptotic cells was analysed 10 h later by staining with Annexin V-FITC (BD bioscience, using a BD LSR system).
Supplementary Material
Acknowledgments
We thank Hua Tian and Olga Gaidarenko for technical support, and Dr. Nissi Varki for help with mouse pathology. This work is supported by a NIH grant to Y.X. (CA094254).
Footnotes
Author Contributions
D.P.L. and Y.X. designed and conducted experiments, and wrote the manuscript. L.O. C.C. and M.E.L. carried out experiments including the rescue experiments by p21-deficiency and puma-deficiency. G.D.C. Jr. and F.H.G analyzed the neural stem cells and revised the manuscript. G.P.Z. provided the Puma−/− mice, consulted on the experiments and was involved in writing of the manuscript.
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Schumacher B, Garinis GA, Hoeijmakers JHJ. Age to survive: DNA damage and aging. Trends Genet. 2008;24:77–85. doi: 10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Craig AL, et al. Novel phosphorylation sites of human tumour suppressor protein p53 at Ser20 and Thr18 that disrupt the binding of mdm2 (mouse double minute 2) protein are modified in human cancers. Biochem J. 1999;342:133–141. [PMC free article] [PubMed] [Google Scholar]
- 3.Kruse JP, Gu W. Modes of p53 Regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao C, Herr D, Chun J, Xu Y. Ser18 and 23 phosphorylation is required for p53- dependent apoptosis and tumor suppression. Embo J. 2006;25:2615–2622. doi: 10.1038/sj.emboj.7601167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito S, et al. Phosphorylation site interdependence of human p53 posttranslational modifications in response to stress. J Biol Chem. 2003;278:37536–37544. doi: 10.1074/jbc.M305135200. [DOI] [PubMed] [Google Scholar]
- 6.Michalak E, Villunger A, Erlacher M, Strasser A. Death squads enlisted by the tumour suppressor p53. Biochem Biophys Res Commun. 2005;331:786–798. doi: 10.1016/j.bbrc.2005.03.183. [DOI] [PubMed] [Google Scholar]
- 7.Lin T, et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- 8.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y. Regulation of p53 responses by post-translational modifications. Cell Death Differ. 2003;10:400–403. doi: 10.1038/sj.cdd.4401182. [DOI] [PubMed] [Google Scholar]
- 10.Garinis GA, van der Horst GTJ, Vijg J, Hoeijmakers JHJ. DNA damage and ageing: new-age ideas for an age-old problem. Nat Cell Biol. 2008;10:1241–1247. doi: 10.1038/ncb1108-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao C, et al. Cell type-and promoter-specific roles of Ser18 phosphorylation in regulating p53 responses. J Biol Chem. 2003;278:41028–41033. doi: 10.1074/jbc.M306938200. [DOI] [PubMed] [Google Scholar]
- 12.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316:404–405. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu T, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 15.Bahar R, et al. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- 16.Kujoth GC, et al. Mitochondrial DNA mutations, oxidative stress and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 17.Tyner SD, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 18.Maier B, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Cao I, et al. ‘Super p53’ mice exhibit enhanced DNA damage response, are tumor resistant and age normally. Embo J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matheu A, et al. Increased gene dosage of Ink4a/Arf results in cancer resistance and normal aging. Genes Dev. 2004;18:2736–2746. doi: 10.1101/gad.310304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendrysa SM, et al. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev. 2006;20:16–21. doi: 10.1101/gad.1378506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matheu A, et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 23.Song H, Chung SK, Xu Y. Modeling disease in human ESCs using an efficient BAC- based homologous recombination system. Cell Stem Cell. 2010;6:80–89. doi: 10.1016/j.stem.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7:979–987. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- 25.Jeffers JR, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 26.Villunger A, et al. p53-and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 27.Ikuta K, et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- 28.Takubo K, et al. Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell. 2008;2:170–182. doi: 10.1016/j.stem.2007.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.