Abstract
Monocyte chemoattractant protein-1 (MCP-1) is commercially available in a form of recombinant protein. This makes it more convenient to study the functions of MCP-1 and its involvement in many cell functions. However, when using MCP-1 in experimental studies, if the analysis is not performed immediately, the stability of recombinant MCP-1 may become an issue. In this study, the stability of recombinant MCP-1 at different concentrations and storage conditions was investigated. Results show that no significant loss of MCP-1 is observed when MCP-1 solutions were stored at non-freezing condition (4°C) for seven days. However, for storage at freezing conditions (−20°C or −81°C), it appears that the first freeze-thaw cycle may contribute to some loss of MCP-1 in the solutions, and such loss may be concentration and time dependent. The effect of multiple freeze-thaw cycles for storage at freezing conditions was also examined. Data reveal that the second freeze-thaw cycle causes approximately 50% loss of MCP-1 in the solutions. This finding confirms that multiple freeze-thaw cycles should be avoided. The findings of this study provide an outline of how storage can affect the stability of recombinant proteins and should be taken into account during the evaluation of the concentration of recombinant proteins.
Keywords: Monocyte chemoattractant protein-1, Recombinant proteins, Freeze-thaw cycle, Working concentration, Storage temperature
1. Introduction
Chemokines (chemotatic cytokines) are low molecular weight proteins (8–12 kDa) that play an important role in the migration of immune cells [1]. Their functions involve with homeostatic mechanisms, e.g., lymphocyte trafficking or immune surveillance, as well as inflammatory activities, such as recruiting lymphocytes to an injured area [2, 3]. Monocyte chemoattractant protein-1 (MCP-1 or CCL2) is a chemokine that is secreted by various cell types and involves in the progression of many inflammatory-related diseases including HIV, cancer, and atherosclerosis [4]. The key function of MCP-1 during inflammation is to mediate the recruitment, migration, and infiltration of monocytes to an infected site [5].
Since MCP-1 was first purified more than two decades ago [6–8], a number of studies have been done to characterize and relate it to the pathogenesis of many diseases [4, 5, 9]. With the advancements in technology, MCP-1 is now commercially available for laboratory use in the form of recombinant protein. Recombinant MCP-1 is frequently used in many in vitro studies [10–13] including those that focus on the transendothelial migration of monocytes [14, 15]. In our previous study, we used recombinant human MCP-1 to examine the formation of MCP-1 concentration gradients across the collagen matrix of a 3D in vitro vascular tissue model (Manuscript in review). Briefly, recombinant MCP-1 was added to the system, samples were collected from the system at various time points, and the concentration of MCP-1 in the samples were determined by ELISA. The gradients of MCP-1 in the collagen matrix were estimated based on the concentration data. To achieve an accurate estimation of the amount of MCP-1 in the system, it is important to measure accurately the concentration of MCP-1 in the samples collected from the system. Since the collected samples are stored at −81°C until ready for analysis, this raises a concern about the loss of MCP-1 during storage.
Generally, it is suggested that recombinant MCP-1 should be stored at high concentration (10 µg/ml or greater) to maintain the stability of the protein. During an experiment, recombinant MCP-1 is diluted to physiological relevant concentrations, with working concentrations in the pg/ml to ng/ml range [10–15]. Consequently, samples that are collected from such experimental systems contain recombinant MCP-1 at lower concentrations than the suggested value for storage. Thus, if those samples are not analyzed immediately, some MCP-1 molecules in the samples may become unstable over time.
In the present study, we investigated the effect of storing conditions on the loss of MCP-1 in samples containing recombinant human MCP-1 at the working concentrations. The objectives of this study include determining 1) the loss of MCP-1 at different storage conditions and 2) the effect of multiple freeze-thaw cycles to the stability of MCP-1 in the samples. Results of this study were aimed to provide a guideline to handle samples that contain recombinant human MCP-1, but can also be applied to other recombinant proteins used at low working concentrations.
2. Materials and methods
2.1 Materials
Recombinant human MCP-1 was purchased from R&D Systems (Minneapolis, MN). Dulbecco’s phosphate-buffered saline (D-PBS), Medium 199, and penicillin-streptomycin-glutamine (PSG) solution were purchased from Invitrogen (Carlsbard, CA). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT). Tween® 20 was purchased from Fisher Scientific (Pittsburgh, PA). Bovine serum albumin (BSA) was purchased from Sigma-Aldrich (St. Louis, MO). BD OptEIA™ Human MCP-1 ELISA Set was purchased from BD Biosciences (San Jose, CA).
2.2 Experiments
MCP-1 solutions were prepared from recombinant human MCP-1 and complete medium (Medium M199 containing 1% PSG and 10% FBS) at the following concentrations: 0.25, 5, 15, 25, and 50 ng/ml. The prepared solutions were stored at standard cell culture conditions (37°C and humidified atmosphere of 5% CO2 and 95% air), 4°C, −20°C, or −81°C for seven days. On the seventh day, samples were collected from the solutions and the concentration of MCP-1 was determined by ELISA, according to the manufacturers protocol. After collecting the samples, the solutions that were previously stored at freezing conditions (−20°C and −81°C) were frozen again at the same conditions for one more day before being thawed and analyzed again to determine the effect of multiple freeze-thaw cycles.
2.3 Statistical analysis
MCP-1 concentrations are expressed as mean ± SD of three samples. Significant differences in the results were determined using student’s t test. A value of p < 0.05 was considered significant.
3. Results
3.1 Storing conditions
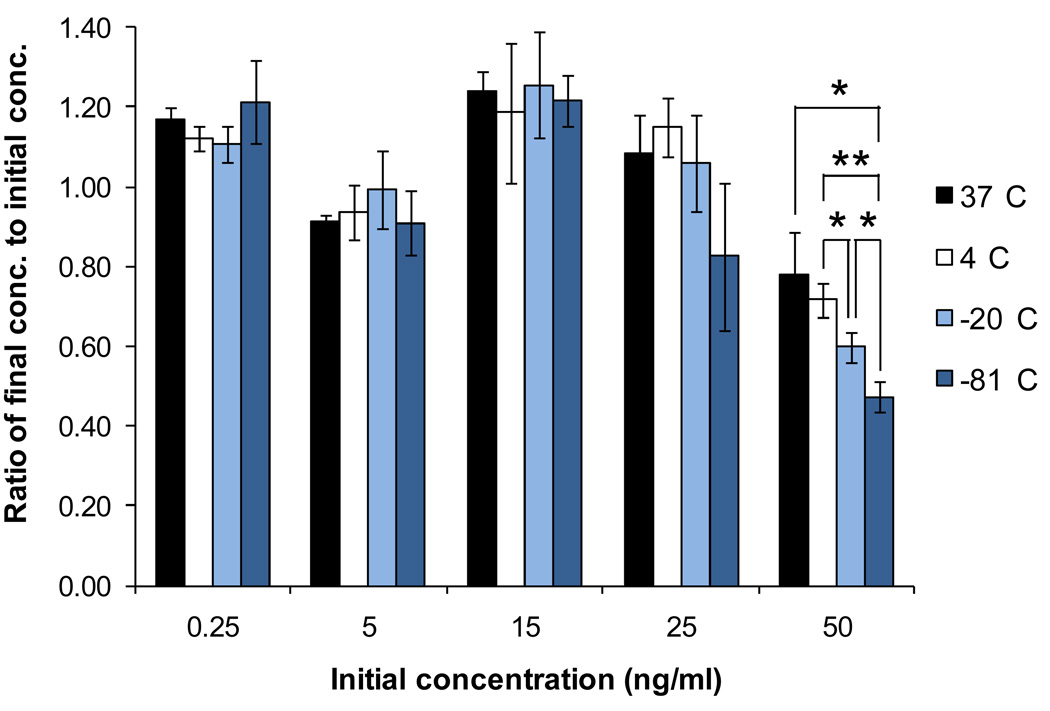
To determine the effect of storage conditions to the loss of MCP-1 in samples, MCP-1 solutions were prepared from recombinant human MCP-1 and complete medium, and stored at different conditions for seven days. ELISA was performed at the end of the storage time to determine the concentration of MCP-1. Results are represented in Figure 1. As shown in the figure, there is no significant change in the concentration of MCP-1 among samples with the same initial concentration, except for the 50 ng/ml samples in which the significant loss of MCP-1 is observed in samples that were stored at freezing conditions. Results also show that if the data from 50 ng/ml samples are excluded, the percent recovery of MCP-1 varies between 80% and 100%. These two findings suggest that for samples with low MCP-1 concentration (≤ 25 ng/ml), recombinant human MCP-1 in the samples is stable for all of the storage conditions tested for the seven days. Moreover, when comparing results from samples that were stored at non-freezing conditions to those stored at freezing conditions, it appears that the first freeze-thaw cycle does not affect the MCP-1 stability in the samples, as shown by no significant change in the concentration of the samples. However, the same conclusion does not apply to 50 ng/ml samples. Because the significant loss of MCP-1 is only found in 50 ng/ml samples that were stored at freezing conditions, we believe that the loss is due to the freeze-thaw process and is concentration dependent.
Figure 1.
The effect of storage conditions on MCP-1 stability. MCP-1 solutions (recombinant human MCP-1 in complete medium) were stored at standard conditions, 4°C, −20°C, or −81°C for seven days and analyzed by ELISA for the concentration of MCP-1. Values are presented as mean ± SD, * p < 0.05 and ** p < 0.01 for changes in the concentration.
3.2 Multiple freeze-thaw cycles
The test to determine the effect of multiple freeze-thaw cycles was carried out after the end of the seven-day storage. MCP-1 solutions that were previously stored at −20°C or −81°C were collected and frozen again at the same conditions for 24 hours. The samples were thawed for the second time and analyzed for the concentration of MCP-1. The concentration was found to decrease significantly after the second thaw for most of the samples that were stored at −20°C and −81°C ( Figure 2). The average percentage loss of MCP-1 due to the second thaw is 48% and 49% for the samples stored at −20°C and −81 °C, respectively. These results demonstrate that unlike the first freeze-thaw cycle, which affects only samples with high concentrations, the second freeze-thaw cycles results in approximately a 50% loss to recombinant human MCP-1 in all samples, regardless of the freezing conditions.
Figure 2.
The effect of freeze-thaw cycle(s) on MCP-1 stability. MCP-1 solutions that were stored at −20°C or −81°C, as described in Figure 1, were collected and stored at the same conditions for an additional day. The concentration of MCP-1 after the second thaw was determined by ELISA. Results from both −20°C (2A) and −81°C (2B) storage temperatures were compared to those shown in Figure 1. Values are represented as mean ± SD, * p < 0.05 and ** p < 0.01 for changes in MCP-1 concentration after the second thaw, compared to results from the first thaw.
4. Discussion
The use of recombinant MCP-1 in an in vitro study generally requires that the recombinant protein is diluted from the stock concentration in the µg/ml range to the working concentrations in the pg/ml to ng/ml range. For many studies, samples containing MCP-1 at the working concentrations are stored and analyzed at the end of an experiment to determine the concentration of MCP-1. The stability of recombinant MCP-1 in samples may not be an issue if the analysis can be performed immediately after the collection of the samples. However, it may be unavoidable that the samples must be stored for a period of time before being analyzed. In this case, MCP-1 stability needs to be confirmed in order to ensure that the analyzed concentration of MCP-1 represents the actual concentration in the samples at the time they were collected.
In the present study, the stability of recombinant human MCP-1 during storage was examined. MCP-1 solutions were stored at different conditions for seven days to evaluate the effect of short-term storage. Results show that for most of the solutions, recombinant MCP-1 is stable for at least seven days for all conditions tested. It can also be deduced from the same results that the first freeze-thaw cycle does not affect the concentration of MCP-1 in most of the solutions. However, the stability of recombinant MCP-1 seems to decrease as the concentration of MCP-1 in the solutions increases. Because the significant loss of MCP-1 was only observed in the solution that was stored at freezing conditions and contained the high level of MCP-1 (50 ng/ml), we believe that the first freeze-thaw cycle is a major contributor to such a loss and that the loss is concentration dependent.
In our previous study, we demonstrated that recombinant human MCP-1 is stable at standard conditions for at least 24 hours (Manuscript in review). Samples were collected every six hours from MCP-1 solutions that were incubated at standard conditions and stored at −81°C for one to four days before being analyzed for the concentration of MCP-1. As shown in Figure 3 there is no significant loss of MCP-1 of samples stored at 37°C for seven days (from the current study) and that of samples stored at standard conditions for 6–24 hours (from the previous study). This finding agrees with our conclusion that recombinant human MCP-1 is stable for at least seven days. Interestingly, it appears in the figure that the freeze-thaw cycle did not affect the stability of MCP-1 in the previous study as it did in the present study for the 50 ng/ml samples. Since in the previous study, samples were stored at −81°C for no more than four days, we question that time may be another factor that affects the loss of MCP-1 during the first freeze-thaw cycle.
Figure 3.
The comparison between results from previous and current studies. Data labeled as “Previous study” are the average concentration of MCP-1 solutions that were incubated at standard conditions for 6–24 hours, and subsequently stored at −81°C for one to four days. The concentration of MCP-1 solutions after stored at either standard conditions or −81°C for seven days is obtained from data in Figure 1 and presented as “Current study (37°C)” or “Curren t study (−81°C)”. Values are represented as mean ± S D, * p < 0.05 and ** p < 0.01 for difference in MCP-1 concentration.
The effect of multiple freeze-thaw cycles is another aspect being investigated in the present study. Experimental results show that for both freezing conditions (−20°C and −81°C), the second freeze-thaw cycle causes nearly a 50% loss to the concentration of MCP-1 in samples. This demonstrates that repeatedly freezing and thawing samples should be avoided. Since the loss to the samples is constant, the concentration of samples that have gone through two freeze-thaw cycles can still be estimated. The actual concentration of MCP-1 in the samples is approximately two times the concentration measured from an analysis after the second thaw.
In conclusion, the stability of recombinant human MCP-1 when it is stored at working concentrations was investigated in this study. Experimental results show that samples containing recombinant human MCP-1 can be stored at non-freezing condition for a short term without significant loss of MCP-1. However, we recommend that if samples are stored at freezing conditions, they should be analyzed as soon as possible because evidence shows that the loss of MCP-1 during the first freeze-thaw cycle may be time dependent. Also, in the case of storing the samples frozen, multiple freeze-thaw cycles can lead to a significant loss of MCP-1 in samples and should be avoided.
Acknowledgment
This work was supported by a grant from the National Institute of Biomedical Imaging and Bioengineering (1R15EB009527-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reape TJ, Groot PH. Chemokines and atherosclerosis. Atherosclerosis. 1999;147:213–225. doi: 10.1016/s0021-9150(99)00346-9. [DOI] [PubMed] [Google Scholar]
- 2.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469–499. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- 4.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melgarejo E, Medina MA, Sanchez-Jimenez F, Urdiales JL. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol. 2009;41:998–1001. doi: 10.1016/j.biocel.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Valente AJ, Graves DT, Vialle-Valentin CE, Delgado R, Schwartz CJ. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988;27:4162–4168. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- 7.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169:1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollins BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198–204. doi: 10.1016/1357-4310(96)88772-7. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Eckes B, Mauch C, Hartmann K, Krieg T. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1 alpha loop. J Immunol. 2000;164:6174–6179. doi: 10.4049/jimmunol.164.12.6174. [DOI] [PubMed] [Google Scholar]
- 11.Johrer K, Janke K, Krugmann J, Fiegl M, Greil R. Transendothelial migration of myeloma cells is increased by tumor necrosis factor (TNF)-alpha via TNF receptor 2 and autocrine up-regulation of MCP-1. Clin Cancer Res. 2004;10:1901–1910. doi: 10.1158/1078-0432.ccr-1053-03. [DOI] [PubMed] [Google Scholar]
- 12.Lee YR, Liu MT, Lei HY, Liu CC, Wu JM, Tung YC, et al. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol. 2006;87:3623–3630. doi: 10.1099/vir.0.82093-0. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, et al. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67:3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 14.Wain JH, Kirby JA, Ali S. Leucocyte chemotaxis: Examination of mitogen-activated protein kinase and phosphoinositide 3-kinase activation by Monocyte Chemoattractant Proteins-1, -2, -3 and -4. Clin Exp Immunol. 2002;127:436–444. doi: 10.1046/j.1365-2249.2002.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy LA, Booth TA, Lau EK, Handel TM, Ali S, Kirby JA. Examination of MCP-1 (CCL2) partitioning and presentation during transendothelial leukocyte migration. Lab Invest. 2004;84:81–90. doi: 10.1038/labinvest.3700007. [DOI] [PubMed] [Google Scholar]