Abstract
We examined the ability of people 70 to 90 years old to apply global, configural and featural face-processing strategies. In addition we investigated age-related changes in the ability to categorize faces at basic, subordinate and individual levels. Using the N170 potential as index of early face processing and the P300 component as index of categorical decision making and effort, we found significant age-related perceptual changes which slowed and somewhat impaired face processing. Specifically, older participants had problems integrating face features into global structures, demonstrating enhanced dependence on distal global information. They did not apply configural computations by default while processing faces which suggests that, unless identification is required, they process faces only at a basic level. These perceptual changes could be the cause for slower and less accurate subordinate categorization, particularly when it is based on details. At the neural levels face processing was not right-lateralized, reflecting excessive involvement of the left hemisphere in perception leading to a more general reduction of inter-hemispheric asymmetry. In addition we found excessive but nonselective activation of frontal regions adding support to the view that executive control and particularly inhibition of irrelevant input are reduced in the elderly.
Evidence from human (Bian and Andersen, 2008; Fiorentini, et al., 1996; Habak and Faubert, 2000) and monkey research (e.g. Wang, et al., 2005; Zhang, et al., 2008) indicates that, though not dramatically (e.g. Norman, et al., 2008), the efficiency of visual perception declines with age even in healthy individuals. Within this framework age-associated changes in face recognition have been the focus of many studies using performance (e.g. Bartlett and Fulton, 1991; Boutet and Faubert, 2006; Habak, et al., 2008) as well as EEG (e.g. Pfutze, et al., 2002) and hemodynamic neuroimaging (e.g. Grady, 2002; Grady, et al., 2007) methods. Most of these studies revealed reduced face recognition ability in the elderly (e.g. Lott, et al., 2005; Searcy, et al., 1999), even more so than it is evident for object recognition (Boutet and Faubert, 2006). Notwithstanding the amply documented age-related impairment in memory functions, which obviously accounts at least in part for impaired face recognition and identification (e.g. Bartlett and Fulton, 1991; D'Argembeau and Van der Linden, 2004; Fulton and Bartlett, 1991), several studies explored the possibility that perceptual factors might also cause face recognition difficulty in the normally aging population (Lott, et al., 2005). Some of these studies associated this impairment to the deterioration of optical functions such as acuity (Tejeria, et al., 2002) and contrast sensitivity (Owsley, et al., 1981; for broader reviews see Sekular and Sekular, 2000; Spear, 1993). Yet, there is also evidence that age-related changes in low-level vision can hardly be the single (or even the main) account for face recognition impairments (Anstey, et al., 2002). Indeed, other studies suggested that face recognition difficulties might be caused by age-related impairments in higher-level perceptual processes, such as the inability to quickly transform perceptual representations into familiar templates (Habak, et al., 2008), reduced neural specialization for faces (Park, et al., 2004; Payer, et al., 2006), impaired categorical perception (Kiffel, et al., 2005), changes in face-scanning strategies (Firestone, et al., 2007), impaired spatiotemporal integration (Del Viva and Agostini, 2007; Norton, et al., 2009), reduced sensory processing speed (Salthouse, 1996; Salthouse, 2000), reduced attention/working memory capacity (Bopp and Verhaeghen, 2005; Lange and Verhaeghen, 2009), augmented distractibility (Healey, et al., 2008; Machado, et al., 2009), impaired pre-frontal inhibitory control (Chao and Knight, 1997; for reviews see Grady, 2008; Reuter-Lorenz, 2002), and reduced cognitive flexibility as evidenced by a general reduction in task switching performance (Reimers and Maylor, 2005). Given this wealth of studies suggesting that impaired face recognition might result from perceptual impairments, it is surprising that only a few studies attempted to directly investigate possible age-related changes in the perceptual processes involved in face recognition.
Four types of information can be extracted from faces and (arguably) involved in different stages of face processing. Since some ambiguity exists regarding their definition we will introduce each type as relevant for the present study. One type of information, which we termed global (Bentin, et al., 2006) refers to the general structure of the face that is sufficient to categorize it at a basic level. It includes the typically symmetric placement of the two eyes on both sides of a vertical axis above the nose and the mouth, all included in an elliptical contour. Ignoring the contour, Maurer and colleagues (2002) defined the face-characteristic global structure only on the basis of the typical arrangement of its inner components and coined this configuration as first-order relations. A second type of information extracted from faces results from what has been labeled holistic processing. Following others (e.g. Tanaka and Farah, 1993), by holistic processing we refer to the integration of the inner components and the face contour leading to the perception of the face as a unitary gestalt. This information seems to be essential during the individuation of the face, as best demonstrated in the composite effect, which shows a prevalence of the whole face structure over its constituent parts (Young, et al., 1987) (but see Richler, et al., 2008, for a different interpretation). The third type of information extracted from faces is featural. Resulting from analysis at a local level (Bentin, et al., 2006) this information refers to the shape of face components such as the roundness of the eyes, the thickness of the eye-brows, the curvature of the lips, etc., and is used in face identification as well as for subordinate level categorization (particularly by race Zhao & Bentin, unpublished). Finally, the fourth type of information processed during face recognition is labeled configural. These are the spatial metric computations relating the inner components one to the other and to the face contour. In Maurer et al.'s (2002) terms these are the second-order relations between the face components that, by many accounts, are most important for the individualization of a face and its identification (e.g. Rhodes, et al., 1993).
With only a notable exception (Boutet and Faubert, 2006), there are no studies that systematically investigated the ability of older participants to apply the perceptual processes necessary for extracting the above described types of information while porcessing faces. Moreover, even in that study the authors addressed only a part of the face-relevant processes. They found that older participants were sensitive to the face-inversion effect (FIE) as much as younger participants. Since the FIE allegedly reflects the reliance of the observer on configural information for face recognition (e.g. Freire, et al., 2000; Searcy and Bartlett, 1996), Boutet and Faubert concluded that the ability to extract configural information is not affected by aging. Similarly, finding an advantage in identifying face components in the context of the whole face than in isolation (cf., Tanaka and Farah, 1993) in older participants, the authors concluded that the ability to apply holistic processing is also spared in the elderly. However, the latter conclusion should be tempered by the fact that the older participants in that study did not show the composite effect, which is also a measure of holistic processing (Young, et al., 1987). The latter deficiency might, in fact, reflect a more general age-related decline in contour integration ability (Roudaia, et al., 2008).
As far as we know there are no studies testing perception of either global or local (features) information in faces in the elderly, but we may infer these abilities from studies that tested aging-related effects on global and local processing of other stimuli, such as hierarchical letters (Navon, 1977).1 Some studies found an age-related shift from global to local processing precedence (Lux et al., 2008, Oken et al., 1999), and interpreted the relative faster decline of global processing to a narrowed attentional field (cf., Kosslyn et al., 1999). Other studies, however, found preserved global precedence in the normal elderly albeit compared with younger participants, the older participants were overall slower, less accurate (Bruyer and Scailquin, 2000; Bruyer, et al., 2003; Georgiou-Karistianis, et al., 2006 (Experiment 1)), and could not easily switch from one processing level to the other (Georgiou-Karistianis, et al., 2006 (Experiment 2)).
Hence, on the basis of the current literature we might provisionally conclude that face recognition difficulties encountered in the elderly cannot be explained by age-related changes in the ability to use face-specific perceptual strategies and extract the relevant information for face recognition. However, such a conclusion might be premature. First, as evident in the above review not all the perceptual processes that are relevant to face recognition have been properly and systematically tested. Second, although studies showed that face inversion as well as other manipulations affecting holistic and global processing influence the performance of older participants in face recognition tasks like that of younger ones, in most those studies performance was overall slower and less accurate in the older than in the younger groups. Importantly, similar age-related effects were not found (when tested) for the recognition of other objects (Boutet and Faubert, 2006). Given this ambiguity, additional studies seem to be necessary for shedding more light on age-related changes in perceptual processing that may account for specific impairments in face recognition. In particular, it is important to explore face processing online in order to distinguish between face recognition processes, the completion of which might be particularly delayed or impaired, and processes that are not affected by age. Whereas reaction time (RT) and accuracy measures cannot provide an online and continuous measure for face processing, contemporary developments in electrophysiological neural manifestations of face processing demonstrated that event-related potentials (ERPs) could complement the RT studies in this respect.
Scalp-recorded ERPs are time continuous fluctuations of averaged EEG activity, time locked (directly or indirectly) to the onset of an event, such as the appearance of a stimulus in the observer's perceptual field. Ample research identified a series of potentials distinguished by peak latency and scalp distribution and putatively associated these ERP components with different cognitive mechanisms. Among those, relevant to the current study are the N170 and the P300.
The N170 is a negative ERP component normally peaking between 150 and 180 ms from stimulus onset with considerable higher amplitudes in response to faces than to any other stimulus categories and, therefore, has been associated with early perceptual processes elicited by the detection of faces in the visual field (Bentin, et al., 1996; for a recent review see Rossion and Jacques, 2008). This categorical difference between faces and non-face stimuli, labeled the N170-effect, is most conspicuous at posterior-temporal recording sites where its absolute amplitude is maximal. The lateral distribution distinguishes this face-sensitive neural response from the non-specific N1, which peaks at more medial sites in response to any visual stimulus, sensitive to spatial attention but not to the stimulus content (e.g., Clark & Hillyard, 1996).. Finally the N170 is typically larger over right than left hemisphere sites, which goes along with ample neuropsychological evidence for higher involvement of the right hemisphere in face processing.
P300 is a generic name for a variety of relatively late positive components with a centro-parietal or centro-frontal midline distribution (Donchin, 1981; Polich, 2007).2 While initially discovered in response to task-relevant oddball (infrequent) stimuli (Sutton, et al., 1965), and found sensitive to the subjective probability assigned to the occurrence of the eliciting event (Duncan-Johnson and Donchin, 1977), many theories about the cognitive mechanism(s) manifested by this neural event have been proposed (Donchin, 1987; Donchin and Coles, 1998; Verleger, 1988; Verleger, et al., 2005). Notwithstanding those controversies, it is unanimously agreed that the P300 latency reflects the length of stimulus evaluation processes when a two-choice RT is required (e.g. Kutas, et al., 1977; McCarthy and Donchin, 1981) and that, other factors being kept constant, its amplitude is largely determined by the stimulus relevance (Gray, et al., 2004), by the amount of attention allocated to the stimulus (Kok, 2001) and by the task complexity (Johnson, 1986).
In Experiment 1 we compared the N170-effect in elderly and younger groups of healthy participants while manipulating stimulus conditions to assess the sensitivity of each group to global, configural and featural processing. In Experiment 2 both the N170 and the P300 potentials were used to compare the efficiency of basic-level categorization of faces (distinguishing faces from cars), subordinate categorization (distinguishing between male and female faces) and individual face categorization (distinguishing between familiar and unfamiliar faces).
General Methods
Participants
Two groups of 16 participants with no history of psychiatric or neurological disorders were defined by age and tested in both experiments. The younger group included undergraduate students (10 females) with an age range between 19 and 33 years (Mean = 24.3). They were all healthy individuals that received either course credit or money payment for their participation. The older included 16 healthy individuals (10 females) with an age range between 70 and 90 years (Mean = 77.1). They live an independent life in the community and were paid for participation.
To ensure normal cognitive abilities they were tested with a Mini Mental State Examination (MMSE - Tombaugh and McIntyre, 1992) and only people who scored 27/30 and up were included in the study. Contrast sensitivity of all participants was tested using the Functional Acuity Contrast Test (Vision Sciences Research Corporation). Both groups were within normal ranges (corrected for age), there was no difference between the younger and older participants at low-spatial frequency (1.5 cycle/degree), while at high frequencies the contrast sensitivity was higher in the younger than in the older participants. Importantly, however, the analysis of the P1 component3 (which is modulated primarily by low-level vision factors) revealed no difference between the groups either for amplitude or latency. If anything the P1 tended to peak higher and earlier in the older than in the younger groups (4.2 μV (108 ms) and 2.5 μV (110 ms) for the older and younger groups, respectively; F(1,22) = 3.6, MSe = 1218.6, P = 0.07, and F(1,22) < 1.0, for amplitude and latency, respectively). Visual acuity was tested for each participant using a Snellen chart (Rosenbaum Pocket Vision Screener) and it was normal or corrected to normal in the older as well as the younger group (equally distributed between 20/20, 20/25 and 20/30). The experiment was approved by the Hebrew University ethical committee and all participants signed an informed consent form.
EEG recording
The EEG analog signals were recorded continuously (from DC up to a low-pass filter set at 100 Hz) by 64 Ag-AgCl pin-type active electrodes mounted on an elastic cap according to the extended 10–20 system (Biosemi™). Two additional electrodes were placed at the right and left mastoids and one on the tip of the nose. All electrodes were referenced during recording to a common-mode signal (CMS) electrode between POz and PO3 and were subsequently re-referenced digitally (see data processing below). Eye movements, as well as blinks, were monitored using bipolar horizontal and vertical EOG derivations via two pairs of electrodes, one pair attached to the external canthi, and the other to the infraorbital and supraorbital regions of the right eye. Both EEG an EOG were digitally amplified and sampled at 256 Hz using a Biosemi Active II system.
Data pre-processing
The data were processed using Brain Vision Analyzer software (Brain Products). Raw EEG data was initially high-pass filtered at 0.5 Hz (24 dB) with a notch filter at 50 Hz and re-referenced off-line to the tip of the nose. Eye movements were corrected using an ICA procedure (Jung, et al., 2000a; Jung, et al., 2000b) and remaining artifacts exceeding transitions of ±100 μV in amplitude during a period of 50 ms at the sites included in the analyses (see below) were rejected. Artifact-free data were then segmented into epochs ranging from −100 ms before to 600 ms after stimulus onset for the N170 analysis and from −100 ms to 1000 ms for the P300 analysis (see Experiment 2).
Experiment 1
The goal of this experiment was to assess possible age-related differences in perceptual processing of faces and its time course as expressed by modulations of the N170-effect during a relatively non demanding, target monitoring task. Notwithstanding current controversies about the particular face-processing mechanism reflected by the N170 (e.g. Carmel and Bentin, 2002; Gauthier and Curby, 2005; Rossion, et al., 2002) or its precise neural origin (Itier and Taylor, 2004), there is no serious argument that this potential is influenced primarily by the stimulus categorical content (mostly faces) rather than its low-level visual quality (see discussion in Rossion and Jacques, 2008). Based on previous studies and particularly on the fact that the amplitude of the N170 is higher for faces than other objects, in the present study we assumed that higher N170 amplitude reflects a better correspondence between the stimulus presented and the sensitivity of the perceptual mechanism involved in categorical distinction of faces. To this end, the N170 is a good candidate to explore possible aging effects on neural activity associated with categorical perception of faces in the visual system.
Previous studies demonstrated that for young participants the N170 is usually larger over the right than the left hemisphere (Bentin, et al., 1996; Rossion, et al., 2003), which is in line with ample evidence for the right-hemisphere superiority in face-processing (Levy, et al., 1972; Yovel, et al., 2008). Suggesting a special sensitivity to face features, the N170 is more robust when the inner components are presented outside of the face contour (Zion-Golumbic and Bentin, 2007) and larger (as well as somewhat delayed) in response to isolated eyes than full faces (Bentin et al., 1996; Itier, et al., 2007). Moreover, similarly large N170 potentials are elicited by intact and scrambled faces when the components are recognizable (Zion-Golumbic and Bentin, 2007), which suggests that the global face structure is not a necessary condition for activating this neural mechanism (albeit it is probably a sufficient condition). The sufficiency of a face global structure is supported by significant N170-effects in response to correctly configured schematic faces4 (Sagiv and Bentin, 2001) but not when the inner components of the schematic faces are scrambled (Bentin and Golland, 2002). Finally, the N170 amplitude is consistently delayed (and usually enhanced) by face inversion (Rossion and Gauthier, 2002) and, more importantly, the difference between the N170 elicited by inverted faces and non-face stimuli is significantly reduced. Whereas the enhancement of the N170 amplitude in response to inverted relative to upright faces is difficult to interpret, the delay of the N170 peak and particularly the reduction of the N170-effect might reflect the fact that face-specific computations are not easily applied to inverted faces (for a discussion see Rossion, et al., 2000). Assuming that the N170 manifests the activation of a mechanism apt to extract diagnostic features from faces (Bentin, et al., 1999), the significant reduction of the N170-effect in response to inverted faces suggests that inverted faces are processed like other objects. In other words, inverted faces do not benefit from perceptual processes tuned to extract face-specific diagnostic information that allows the distinction between individual faces. This might explain why inverted faces are not easily identified.
The few previous studies that compared the N170 in different age-groups reported larger N170 potentials in older than younger groups particularly when the faces were unattended (de Fockert, et al., 2009). Moreover, the N170-effect in the elderly is fairly robust, albeit not lateralized to the right hemisphere (Chaby, et al., 2001; Gao, et al., 2009; Pfutze, et al., 2002) and delayed relative to younger controls (Chaby, et al., 2003). Interestingly, the effect of face-inversion on the N170 potential, which is very reliable in the younger population, is absent in older participants (Gao, et al., 2009). Along with the above line of reasoning, the absence of the FIE on the N170 might indicate that elderly people do not automatically apply configural processes while seeing a face. Based on this outcome Gao and colleagues suggested that face identification is not the default level of processing in the elderly.
In addition to their scarcity, none of the existent studies compared the face-perception processes in younger and older participants systematically. To address this absence, in the present experiment, in addition to exploring the N170-effect in the two age groups by comparing ERPs elicited by upright faces to those elicited by wrist-watch panels, we also compared the sensitivity of the perceptual mechanism reflected by the N170 to global face analysis, to face features, and to configural processing. Global processing and the sensitivity to first-order relations were assessed comparing the response to scrambled faces with normally structured faces. The observed absence of difference between these two types of face stimuli in young participants might not replicate itself in elderly participants who might depend more than the young ones on the global face structure. If so, the N170 elicited by scrambled faces in the elderly group might be reduced relatively to the normally configured faces. Featural analysis was assessed comparing the ERPs elicited by isolated components with those elicited by full upright faces. If the aging perceptual system loses some of its ability to process stimulus details, it is possible that when the inner face components are seen outside of a face contour, the stimulus is not immediately categorized as a face. In that case, in contrast to the pattern which has been observed in younger participants, the N170 elicited by inner components outside the face contour should be smaller than that elicited by full faces and this reduction should be more conspicuous when the configuration of the isolated inner components is also scrambled. Finally, the face inversion effect was used to assess the sensitivity to configural processing. Since configural computations obviously require detailed analysis of the inner face components, we assume that elderly participants are less likely to apply this perceptual strategy than younger participants. Consequently, face inversion should not change the processing of the face much in the elderly group, which should result in similar N170 components to upright and inverted faces.
Methods
Stimuli
The initial stimulus set consisted of 80 photographs of different faces, 80 photographs of different watches (W) and 120 photographs of different flowers. The faces were edited to form five face conditions, with 80 stimuli in each condition: Regularly configured full faces (RF), Inverted Faces (IF), Scrambled Faces (SF), that is faces in which the spatial location of the inner components was scrambled, Inner Components (IC), that is, normally configured eyes nose and mouth without the face contour), and Scrambled Inner Components (SIC), that is, two isolated eyes, a nose and a mouth presented in random configuration and without the face contour (see examples of the stimuli in Figure 1).
Figure 1.
Examples of stimuli presented in Experiment 1: (A) Face, (B) Inverted Face-IF, (C) Scrambled Face-SF, (D) Inner components-IC, (E) Scrambled Inner Components-SIC, (F) watch.
All stimuli (including watches and flowers) were equated for luminance, so that, on the average, they were matched for luminance and brightness. The stimuli were presented at fixation and, seen from a distance of approximately 70 cm occupied 8.1° × 11.3° in the visual field (10 cm × 14 cm).
Task, Design and Procedure
Like in many previous N170 studies in our laboratory, the task was oddball target monitoring in which stimuli from different experimental categories were presented one after another and participants were requested to press a button each time a flower appeared on the screen. This procedure ensured that all stimulus categories of interest in this study were equally task-relevant; that is, they were all distracters that did not require any action or additional processing after classification. The 600 stimuli were fully randomized and presented in 6 blocks of 100 stimuli each, with a short break between blocks for refreshment.
The experiment was run in an acoustically treated and electrically isolated chamber. Following the mounting of the electrode-cap the participants sat in a comfortable reclining chair and the monitor was raised at their eyes level. Each trial began with a 500 ms long fixation mark, followed by a stimulus exposed for 200 ms. The inter-trial intervals varied between 1400 ms and 1600 ms.
N170 analysis and statistical evaluation
ERPs resulted from averaging the segmented trials separately in each condition. The averaged waveforms were smoothed by applying a low-pass filter of 17 Hz (24 dB) and were baseline-corrected by subtracting the mean amplitude during an epoch starting 100 ms before stimulus onset. The characteristic scalp distribution of the N170 in each condition was estimated by spherical spline interpolations with 4 levels. Based on previous studies and on scrutiny of the present N170 distribution, the statistical analysis was restricted to posterior lateral regions including sites P8, PO8, and P10 over the right hemisphere and the homologue sites over the left (Figure 2). For each subject the peak of the N170 was initially determined at these sites (based on the filtered waveforms) as the most negative peak between 130 and 230 ms. Subsequent visual scrutiny ensured that the most negative values represented real peaks rather than end points of the epoch. The N170 amplitudes at the 3 sites in each region of interest were averaged to determine the latency of the N170 component over each hemisphere. Then we measured again the amplitude of the N170 potential at these latencies separately for each recording site within the two regions of interest. Four major effects were compared within and across groups: (a) Basic sensitivity to faces as a distinct category was assessed comparing the N170 elicited by upright faces (RF) with that elicited by watches (W); (b) Configural processing was assessed by comparing the N170 elicited by upright faces (RF) with that elicited by inverted faces (IF); (c) Global processing (first order) was assessed by comparing the N170 elicited by upright faces (RF) with that elicited by faces with scrambled inner components (SF); (d) Feature processing effects were assessed comparing the N170 elicited by upright faces (RF) with that elicited by inner components presented in a normal configuration (IC) as well as scrambled inner components outside the face contour (SIC).
Figure 2.
The 64 recording sites. The N170 was analyzed at the dark-gray locations. The P300 was analyzed at the light-gray locations. The selection of sites was based on previous research in our laboratory as well as many previous published studies.
In addition, since across stimulus types the absolute amplitudes of the N170 were different in different participants and different in the old and young groups, a normalization procedure was necessary to allow a proper comparison of the effect sizes (see also Gao, et al., 2009).5 To this end, a normalized N170-effect on the amplitudes was calculated as the ratio (RF − W)/(RF + W). Since in some participants the amplitudes elicited by watches were positive, in order to avoid ratios larger than 1 (which would be meaningless) we elevated the baseline by subtracting the maximum positive amplitude observed across watches and faces and participants from all individual ERPs. Hence the normalized N170-effect ranged between 1 (when the W=0) and −1 (when RF=0). The same procedure was used to calculate the face-inversion effect as (IF−RF)/(IF+RF), the global effect as (RF−SF)/(RF+SF), and features processing effect as (IC−RF)/(IC+RF) and as (IC−SIC)/(IC +SIC).
Mixed-model ANOVAs were used to analyze the N170 amplitude and latency. The between-subjects factor was Group (young, elderly) and the within-subjects repeated factors were Hemisphere and a Stimulus-type factor reflecting the analyzed effect (see results). The analysis of the N170 amplitude also included the Site (P7/8, PO7/8, P9/10) as an additional factor. For factors with more than two levels, the degrees of freedom were corrected for non-sphericity using the Greenhouse-Geisser correction (for simplicity, the uncorrected degrees of freedom and G-G Epsilon (G-GE) are presented).
Results
Both young and older participants were at ceiling in detecting the target category (flowers) and, therefore, we did not analyze performance in this experiment.
Robust N170 components were elicited in both age groups and all experimental conditions. These data were analyzed separately for each a priori-defined effect.
Sensitivity to faces as a distinct category - Amplitude
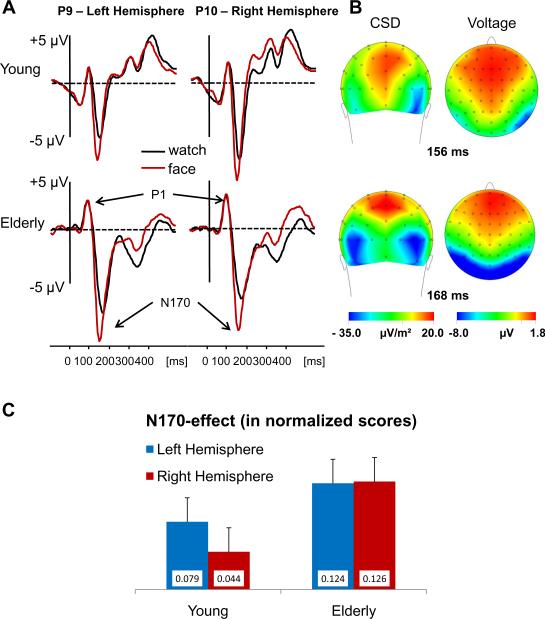
The data are presented in Figure 3. A Group (old, young) × Stimulus type (face, watch) × Hemisphere (left, right) × Site (P7/8, PO7/8, P9/10) ANOVA showed that all 4 main effects were significant. The N170 amplitude in the elderly group (−10.3 μV) was larger than in the young group (−7.0 μV), F(1,30) = 5.4, MSe = 205.0, p < .05; faces elicited larger N170 (−9.2 μV) than watches (−8.1 μV), F(1,30) = 24.9, MSe = 17.2, p < .001; the amplitudes were larger at right (−9.2 μV) than at left hemisphere sites (−8.1 μV), F(1,30) = 4.6, MSe = 28.2, p < .05; and the N170 amplitude differed across sites F(2,60) = 3.2, MSe = 14.5, p < .5. Post hoc contrasts showed that the Site effect was explained by bigger amplitudes at P9/10 (8.9 μV) and PO7/8 (−8.9 μV) than at P7/8 (−8.0 μV). Importantly, all the within-subject main effects were qualified by interactions with Group [F(1,30) = 4.9, MSe= 17.2, p < .05, F(1,30) = 5.1, MSe = 28.2, p < .05; F(2,60) =4.0, MSe = 14.5, p < .025, for Stimulus type, Hemisphere, and Site, respectively]. These interactions were further explored by separate analyses for each age group. These analyses revealed that: (a) In both groups faces elicited larger N170 than watches, but the difference was larger in the elderly group [3.0 μV; F(1, 15) = 30.2, MSe = 14.7 p < .001] than in the younger group [1.17 μV; F(1, 15) = 3.4, MSe = 19.6, p = .08]; (b) In the younger group the N170 was larger at right hemisphere (−8.1 μV) than at left hemisphere sites [−5.8 μV; F(1,15) = 12. 6, MSe = 21.7, p < .005], but there was no inter-hemispheric difference in the older group [−10.4 μV and −10.3 μV, for the left and right hemispheres, respectively; F(1,15) < 1.0]; (c) The main effect of Site was significant in the older group, F(2,30) = 5.3, MSe = 14.0, p < .025, but not in the younger group, F(2,30) = 2.0, MSe = 15.4 p = .15; (d) Finally t-tests revealed that although the N170 amplitudes were larger for elderly than for younger participants in response to both faces and watches the difference was significant for faces (−4.3 μV; t(30) = 2.621, p < .025) but only tended to be so for watches (−2.4 μV; t(30) = 1. 78,3 p = .085).
Figure 3.
ERPs elicited at right and left posterior temporal sites in the elderly and younger groups. (A) The N170 at P9 and P10 elicited by upright faces and watches. (B) The Current Source Density (CSD) seen from the back and scalp voltage distribution of the N170 seen from above. Both distributions and CSD were calculated at the peak latency of the N170 at P10 and P9 in each group Notice the more symmetrical sources and larger amplitudes in the elderly relative to the younger groups. (C) The normalized N170 effect (face – watches, see text). Notice that the difference between face and watches is actually slightly larger in the elderly group.
The analysis of the normalized N170 effect showed that this effect was numerically larger in the older than in the younger group albeit not significantly so [0.09 μV and 0.04 μV for older and younger participants, respectively; F(1,30) = 3.1, MSe = 0.33, p = .088]. There was no significant hemisphere difference [F(1,30) = 1.1]. Across groups, the N170-effect was larger at P9/10 (0.09 μV) and P7/8 (0.06 μV) than at the more medial sites PO7/8 [0.05 μV; F(2, 60) = 7.0, MSe = 0.08, p < .0025].
Sensitivity to faces as a distinct category – Latency
Across the other conditions the N170 peaked later for the older group (181 ms) than for the younger group [165 ms; F(1,30) = 8.0, MSe = 1063, p < .01]. Across groups and hemispheres the N170 latency elicited by faces was shorter (166 ms) than that elicited by watches [179 ms; F (1,30) = 38.6, MSe = 143, p < .001]. Importantly, there was no interaction between stimulus type and group, [F(1, 30) < 1], indicating that the stimulus type effect was similar for young and elderly subjects. Across stimuli and groups the N170 recorded at left hemisphere sites (173 ms) was similar to that recorded at the right hemisphere sites (172 ms) [F(1,30) < 1.0] and the Hemisphere effect did not interact with Stimulus type [F(1,30) < 1.0]. There were no significant interactions.
Summary of the face sensitivity effects
The present results suggest that the basic ability to detect faces as revealed by the N170 is similar for older and younger participants, albeit it takes longer in the older group. For both groups faces elicited higher amplitudes and shorter latencies than watches. In line with previous studies, across stimuli the N170 amplitudes were larger in the elderly group than in the younger group (albeit the difference was significant only for faces). Whereas the N170 amplitudes were higher at right than left hemisphere sites for young participants, no hemispheric differences were found for the elderly participants. In particular, it is important to notice that in addition to being significant in both groups the normalized N170 effect and its distribution across sites and hemispheres were similar for older and younger participants.
Configural processing - Amplitude
These data are presented in Figure 4. Despite the observed overall amplitude differences of the N170 between old (−11.1 μV) and young (−9.7 μV), this difference failed to reach significance, though a tendency is apparent [F(1, 30) = 3.1, MSe, = 273.8, p = .09]. Across groups and sites the N170 elicited by up-right faces was smaller (−9.7 μV) than by inverted faces [−11.0 μV; F (1.30) = 12.3, MSe = 13.8, p < .001 and, importantly, the face inversion effect significantly interacted with group [F(1,30) = 13.1, MSe = 13.8, p <.001]. Separate ANOVAs for each group revealed that inversion enhanced the amplitude of the N170 significantly in the young group [−7.5μV and −10.3 μV for upright and inverted faces, respectively; F(1,15) = 20.8, MSe = 16.8, p < .001], but not in the old group [−11.8 μV and −11.9 μV for upright and inverted faces, respectively; F(1, 15) < 1].
Figure 4.
ERPs elicited by inverted faces and upright faces in the two age groups. (A) The N170 components. Notice the slight delay of the N170 peak in response to inverted faces, the conspicuous amplitude differences between the two conditions in the young group and the absence of such a difference in the elderly group. (B) The Current Source Density (CSD) seen from the back and scalp voltage distribution of the N170 in response to inverted faces seen from above. Notice that, as for upright faces, the distribution of the response to inverted faces in the elderly group is symmetrical across hemispheres. (C) The normalized face-inversion effect (inverted – upright, see text) in the two age groups. Notice the absence of a face inversion effect in the elderly group.
Across stimuli and groups the N170 amplitude was larger at right hemisphere (−11.1 μV) than left hemisphere sites [−9.7 μV; F(1,30) = 4.6, MSe = 41.3, p <.05]. The laterality effect interacted with Stimulus type [F(1,30) = 5.4, MSe = 2.9, p < .05], and, as in the face detection analysis, the inter-hemispheric difference interacted with group [F(1,30) = 4.1, MSe = 41.3, p = .05]. Separate ANOVAs for each age-group revealed that across face orientation the N170 elicited in the elderly group was similar at left hemisphere (−11.8 μV) and right hemisphere sites (−11.9 μV); F(1, 15) < 1]. In contrast, for the younger participants, the N170 was larger at right hemisphere (−10.3 μV) than at left hemisphere sites [−7.5 μV); F(1, 15) = 11.4, MSe = 31.7, p < .005].
The N170 was different at the different sites [F(2,60) = 3.5, MSe = 25.2, p < .05]. Post hoc contrasts showed that it was significantly larger at P9/P10 (−11.0 μV) and PO7/8 (−10.5 μV) than at the more medial sites P7/P8 (−9.6 μV; p < .005). There was also an interaction between Site and Group [F(2, 60) = 3.7, MSe = 25.2, p < .05]. For the older group the largest amplitude was at PO7PO8 sites (−12.7μV), while for the younger group the largest amplitude was at P9/P10 (−10.2 μV). For the older group the lowest amplitude was at P7/P8 (−10.9 μV), while for the young group it was at PO7/PO8 (−8.1 μV) sites. There were no other significant interactions.
The analysis of the normalized inversion effect showed that it was significantly higher for younger participants (.07 μV) than for older participants [−0.01 μV; F(1,30) = 10.7, MSe = .031, p < .005]. No other main effects or interactions were found.
Configural processing - Latency
Across stimulus orientation, the N170 peak was delayed in older people (180 ms) relative to the younger participants [163 ms; F(1,30) = 8.1, MSe = 1028, p < .01] and, across groups, the N170 peak was delayed by face inversion [177 ms and 166 ms for inverted and upright faces, respectively; F(1,30) = 126.8, MSe = 28.5, p < .001]. There was no interaction between the face inversion effect and group [F(1, 30) = 1.0], indicating that the stimulus type effect was similar for young and elderly subjects.
Across stimuli and groups the latency of the N170 at left hemisphere sites (172 ms) was not significantly later than at right hemisphere sites [171 Ms; F(1,30) < 1.0] and there were no significant interactions
Summary of the configural processing effects
Like shown in previous studies, face inversion increased the amplitude and delayed the latency of the N170. However, this effect differed between young and old participants: Whereas face inversion significantly delayed the N170 peak similarly for both age groups, the amplitude of the N170 was augmented by face inversion in the younger group but not in the older group. Indeed, the normalized N170 effect on amplitudes was significant only for the younger group and, obviously, higher than the normalized face-inversion effect in the older group (Figure 4).
Global processing effects- amplitude
As evident in Figure 5, across scrambling conditions the amplitude of the N170 was larger for the elderly group (−11.5 μV) than for the younger group [−7.9 μV; F(1, 30) = 5.7, MSe = 213, p < .025]. Although as a main effect the N170 was not affected by scrambling the inner components in the face [F(1,30) < 1.0], a significant Stimulus type × Group interaction [F(1,30) = 5.1, MSe = 11.0, p < .05] revealed that this manipulation influenced the two groups differently. Separate ANOVA for each group showed that destroying the global structure reduced the N170 amplitude in the elderly group [−11.9 μV and −11.1 μV, for normally configured and scrambled faces, respectively; F(1,15) = 3.5, MSe = 7.5, p = .07], but slightly increased it in the younger group [−7.5 μV and −8.3 μV, for normally configured and scrambled faces, respectively; F(1,15) = 2.1, MSe = 14.5, p = .170].
Figure 5.
ERP elicited by scrambled faces compared with the upright, intact faces in the two groups. (A) The N170 components. Notice the slightly larger N170 amplitudes in response to intact relative to scrambled faces in the elderly group and the inverse tendency in the younger group. (B) The Current Source Density (CSD) seen from the back and scalp voltage distribution of the N170 in response to scrambled faces seen from above. Notice that, as for intact faces, the distribution of the N170 to scrambled faces in the elderly group is symmetrical across hemispheres. (C) The effect of destroying the global shape of the faces (scrambled – intact, see text). Notice the opposite trends in the young and elderly groups.
As in the previous comparisons, the N170 amplitude was larger over right hemisphere than left hemisphere sites in the younger group [−9.0 μV and −6.9 μV, respectively, F(1,15) = 9.8, MSe = 22.8, p < .01], while no such asymmetry was found in the elderly group [11.5 μV for both hemispheres; F(1,15) < 1.0]. In addition, there was a small effect of Site for both groups [F(2,30) = 3.5, MSe = 21.5, p < .05 and F(2,30) = 3.1, MSe = 16.0, p = 0.6, for the younger and older groups, respectively. As before, for the younger participants the N170 was largest at the most lateral sites P9/P10 (−8.9 μV) and smallest at the more medial sites PO7/PO8 (−7.3 μV); in contrast, for the elderly, the largest N170 amplitudes were recorded at the more medial sites (−12.3 μV). Neither the Hemisphere nor the Site effects interacted with Stimulus Type for any of the age-groups.
Global processing - latency
The N170 latency was longer in the elderly group (177 ms) than in the younger group [164 ms; F(1,30) = 4.9, MSe = 1076, p < .05] and, across groups, it was longer for scrambled (174 ms) than normally configured faces [166 ms; F(1,30) = 28.8, MSe = 73.1, p < .001]. A significant Stimulus type × Group interaction followed by separate ANOVA in each group showed that the N170 latency was significantly delayed by scrambling the face in both groups [F(1,15) =15.0, MSe = 16.3, p < .005 and F(1,15) =18.7, MSe = 130.7, p < .001, for the older and younger groups, respectively], but the delay was larger in the younger group (12 ms) than in the older group (3.9 ms). Across groups there was no effect of Hemisphere [F(1,3) < 1.0], and no Group × Hemisphere interaction [F(1,30) = 1.4, MSe = 181, p = .240]. Also, there was no interaction between Hemisphere and Stimulus type [F(1,30) <1.0], and no second order interaction (F(1,30) < 1.0].
Summary of the global processing effects
The disruption of the global face structure reduced the N170 amplitude in the elderly group but had no effect on the younger group. The latency of the N170 was delayed by scrambling the inner components within the face in both age groups, but more so in the younger one. There were no inter-hemispheric differences.
Feature processing - amplitude
The N170 elicited by inner face components and the normalized feature-processing effects are presented in Figure 6. Evidently, separating the features from the full global structure had a different effect in the younger and older groups. This pattern was analyzed by a mixed model ANOVA as above except that the Stimulus Type factor included three levels, RF, IC, and SIC. This ANOVA revealed that the main effect of Group was not significant [F(1,30) = 1.5, MSe = 325.6, p = .233], but it significantly interacted with all other main effects [F(2,60) = 18.5, MSe = 20.0, p <.001, F(1,30) = 4.9, MSe = 47.9, p < .05 and F(2,60) = 3.6, MSe = 30.1, p < .05, for Stimulus Type, Hemisphere and Site, respectively]. These interactions were explored post hoc by separate ANOVAs within each group.
Figure 6.
(A) ERPs elicited by normally configured and scrambled inner components compared with full (upright) faces. (B) The effect of excluding the contour from the faces (inner components – full faces, see text). (C) The effect of presenting spatially scrambled inner components (scrambled inner components – full faces). Notice the absence of an effect of eliminating the face contour from faces (indeed, the slightly reduced N170 amplitudes for inner components in the elderly group and the robust dissociation between the effects of presenting scrambled inner components to the two groups.
In the younger group, the Stimulus type effect on the N170 amplitude was significant [F(2,30) = 14.8, MSe = 20.9, p < .001], reflecting that both inner components (−10.2 μV) and scrambled inner components (−10.1 μV) were larger than full faces (−7.5 μV; for both comparisons p < .005), and there was no effect of scrambling (p = 1.0). The main effect of Stimulus type was significant also in the elderly group [F(2,30) = 10.4, MSe = 21.0, p < .0025]. However, in contrast to the younger group, in the elderly group the N170 elicited by inner components was the same as that elicited by full faces (−11.8 μV), and scrambling of the components significantly reduced the amplitude [−9.6 μV; p < .025]. As in previous analyses, in the younger group the N170 was larger at right hemisphere (−10.6 μV) than at left hemisphere sites [−8.0 μV; F(1,15) = 14.0, MSe = 35.5, p < .0025]. In contrast there was no inter-hemispheric asymmetry in the elderly group [−11.1 μV and −11.2 μV for the left and right hemispheres, respectively; F(1,15) < 1.0]. Finally, as previously, the Site effect was significant in both groups [F(2,30) = 3.8. MSe = 38.7, p < .05 and F(2,30) = 3.4, MSe = 22.6, p <.05 for the younger and older, groups, respectively], reflecting , however, a different distribution. Again, replicating the results of the previous comparisons in the younger group the N170 was largest at P9/10 (−10.4 μV; p < .05) than at either P7/8 or PO7/8 (−8.7 μV at both sites). In the older, group, however, the largest N170 amplitude was more medially, at PO7/8 (−11.9 μV) while there was no difference between the lateral sites P9/10 and P7/8 (−10.3 μV and −11.1 μV, respectively). None of the interactions were significant either as second order or as first order in the separate ANOVAs.
Feature processing - latency
As in all previous analyses the N170 peaked later in the elderly group (186 ms) than in the younger group [171 ms; F(1,30) = 5.7, MSe = 1883, p < .025]. There was a main effect of Stimulus type (F(2,60) = 82.6, MSe = 112.2, p < 001]. Which revealed that the latency of the N170 elicited by isolated inner components (182 ms) peaked later that that elicited by full faces (166 ms), and scrambling the inner components delayed the N170 peak even more (189 ms). However, in contrast to the amplitude, there was no Stimulus type × Group interaction [F(2,60) < 1.0]. The main effect of hemisphere was not significant (F(1,30) < 1.0). None of the other interactions were significant (all F values < 1.0).
Summary of the features processing effects
The effects of isolating and scrambling the inner components had conspicuously different patterns in elderly and younger groups. For younger participants the isolation of inner components yielded a larger N170 peak than full faces without further sensitivity to their spatial organization. In contrast, for the older participants the N170 amplitude elicited by isolated components was similar to that elicited by full faces if their spatial organization was normal and reduced if the isolate components were scrambled. The latency of the N170 was delayed by isolating the components in both groups, but only in the younger group this delay was reduced when the spatial organization of inner components was normal.
Discussion
The present experiment examined age-related differences in the application of face-characteristic perceptual strategies and their time course as revealed by the N170 component. The N170 was robust for the older as well as the younger participants, in fact larger for the former than the latter. The N170 peaks were delayed in the elderly for both faces and objects but, nevertheless, the N170-effect was similarly conspicuous in both groups. This pattern suggests that although the speed of visual processing is probably reduced by aging, the ability of the perceptual system to distinguish faces from other visual stimuli is preserved. To this end, the present data do not support an overall loss of neural specialization in the ventral extrastriate system as suggested by fMRI studies (Park, et al., 2004). Face inversion delayed the N170 peak similarly across groups but the older participants were apparently less sensitive than younger ones to face inversion, as suggested by the absence of the FIE effect on the N170 amplitude both when it was measured directly or when it was individually adjusted and normalized. To the extent that the FIE reflects, on the one hand, the impaired ability to apply configural computations to inverted faces and, on the other hand, the imposed focus on details, the absence of the N170 FIE effect in the elderly suggests that the neural mechanism which putatively sustains configural processing is not as efficient in the elderly. In other words, we interpret that the absence of the FIE on the N170 as evidence that older participants were less likely to apply configural computations even when faces were presented upright. Note that this interpretation does not preclude the possibility that detailed processing of face features is also impaired (see below). In fact, it stands to reason that the ability to process individual features is a pre-requisite of the ability to compute their relative spatial locations. Hence, it is possible that the apparently reduced tendency of elderly people to apply configural computations while processing faces is a result of a more basic impairment in processing details.
In contrast to the FIE, older participants were more sensitive than the younger ones to the global structure of the face, as disrupting this structure reduced the N170 in the older but not in the younger group. Yet, this manipulation delayed the N170 peak similarly in both groups. An enhanced reliance on global shapes might also reflect problems with processing details. Indeed, the current results revealed that older participants are particularly impaired in detecting faces when the physiognomic value of the stimuli is revealed only by components in the absence of the global face structure. This impairment is indicated by the considerable reduction of the N170 amplitude in the elderly group when the contour of the face was eliminated, and even more so, when the normal configuration of the isolated inner components was also disrupted. Note that presenting face components outside of the face contour augmented the N170 in the younger group, both when the components kept their normal face-like configuration and when they were spatially scrambled. The increase in the N170 amplitude in response to isolated face components has been amply documented in young participants (Itier, et al., 2007; Itier, et al., 2006; Zion-Golumbic and Bentin, 2007). This pattern suggests that young observers discover the physiognomic value of face components independently of their first-order relations. In contrast, it seems that in the elderly first-order relations (i.e., the global structure of the face) are more essential for detecting a face and for triggering face-characteristic perception mechanisms. It is interesting to consider this trend in light of the apparently increased involvement of the left hemisphere in processing faces by the elderly participants: As evident in Figure 3, the symmetrical distribution of the N170 across hemispheres in the elderly was not caused by a reduction of the amplitudes at RH sites but rather an enhancement of the amplitudes at LH sites (relative to the younger group). Given the traditional view that assigns detailed analysis to the left hemisphere and global analysis to the right (e.g. Bradshaw and Nettleton, 1983, Chapter 9; see also Hubner and Volberg, 2005; Martinez, et al., 1997; Weissman and Woldorff, 2005), the apparently greater involvement of the LH in processing faces is incompatible with our finding that elderly people rely more on global than local strategies while processing faces. It is also incompatible with studies that found aging-induced difficulties to attend to or process local elements (Pesce, et al., 2005).
On the other hand, the greater activation of the LH is compatible with studies that found local precedence in the elderly while processing Navon letters (Lux et al, 2008; Oken et al., 1999). A possible account for the seeming contradiction between the excessive reliance on the global shape while processing faces and local-processing precedence while discriminating between Navon hierarchical letters, is that aging entails a reduced cooperation between the hemispheres. Hence the verbal value of letters involves predominantly left hemisphere local processing strategies, while the right hemisphere lateralization for faces involves predominantly global processing strategies, without the ability to efficiently switch between strategies or compensate for a particular hemisphere-bound weakness. Although this hypothesis is post hoc and obviously calls for research aimed to testing inter-hemispheric transfer in the elderly, it is partly supported by studies that showed difficulties to switch between levels of processing in the elderly (Georgiou-Karistianis, et al., 2006). It also resonates findings showing reduced white-matter connectivity in the right hemisphere (Thomas et al., 2008). To this end, it is possible that the enhancement of the N170 amplitude at LH sites and, consequently, its symmetrical distribution, might not be related to face processing; in fact, a similar pattern has been also found while recording simple pattern-reversal evoked potentials (De Sanctis, et al., 2008).
To summarize, notwithstanding the above caveat, the symmetrical distribution of the N170 across the hemispheres in the elderly, which contrasts with the typical right hemisphere lateralization of the N170 evident in the younger group of participants, resonates with previous studies demonstrating reduced hemispheric asymmetry in older people (Cabeza, 2002; Dolcos, et al., 2002; Gao, et al., 2009; Pfutze, et al., 2002). The enhanced involvement of the left hemisphere in face processing goes along with the age-related right-hemisphere decline hypothesis (Bentin, et al., 1978; Brown and Jaffe, 1975; Cherry, et al., 2005). In addition, the results of Experiment 1 support previous studies indicating an overall ageing-related slowing of perceptual speed, which was found in performance (e.g. Salthouse, 1996; Salthouse, 2000) as well as in electrophysiological measurements (De Sanctis, et al., 2008; Rousselet, et al., 2009; Tobimatsu, 1995). The presently discovered age-related changes in face processing could partly account for the observed reduction in face recognition efficiency. In Experiment 2 we explored how these perceptual changes affect the categorization of faces at different levels.
Experiment 2
Following the pattern of age-related differences in the application of face-characteristic perceptual strategies observed in Experiment 1, the goal of the present experiment was to investigate if and how these differences affect the neural/electrophysiological manifestations that are associated with the categorization of faces at basic, subordinate and individual levels. To reach this goal, ERPs were recorded while the older and the younger participants were requested to distinguish between faces and cars (basic level categorization), between female and male faces (subordinate level categorization) and between famous and unfamiliar faces (individual level categorization). We focused on the P300 as well as on the N170 ERP component.
As mentioned in the general introduction, the latency of the P300 reflects the stimulus evaluation time (Kutas, et al., 1977) and it is independent of the participant response time, which in the elderly might be tainted by motor sluggishness (Duncan-Johnson, 1981; McCarthy and Donchin, 1981). The amplitude of the P300 is modulated by many factors, such as the probability of a stimulus from a particular category to occur (for a review see Donchin and Coles, 1988), mental workload, resource allocation and processing capacity (Donchin, 1987; Isreal, et al., 1980; for a review see Kok, 2001). A methodological but important factor that also affects the P300 amplitude is the inter-trial latency variability (“latency jitter”). Note, however, that the latency jitter is not without interest since it may correlate with task complexity and the ability to sustain attention. Indeed, it has been suggested that the latency jitter increases with age (McDowell, et al., 2003). Therefore, whereas the N170 reflects relative early perceptual processes, the P300 should unveil possible age-related changes in higher-level categorization of faces as well as decision making processes.
There are no previous studies investigating possible age effects on face-categorization at different levels. However, a large number of studies compared the P300 component elicited in younger and older groups and in most of these studies the participants made categorical decisions. The great majority of these studies documented normal ageing-related delayed latencies in a variety of auditory, visual and somato-sensory oddball detection paradigms (Brown, et al., 1983; Goodin, et al., 1978; Picton, et al., 1984; for a meta-analysis of 32 studies see Polich, 1996). The effects of aging on the P300 amplitude are not as consistent. Whereas some studies found lower amplitudes in older than younger groups (Pfefferbaum and Ford, 1988; Polich, 1997; Walhovd, et al., 2008), other studies did not find any amplitude modulations (Pfefferbaum, et al., 1984). Still other studies found reduced P300 amplitudes only in low-performing participants (Lorenzo-Lopez, et al., 2007). Some of this ambiguity might be/ accounted for by the level of cognitive performance of the older participants (see also Walhovd and Fjell, 2003). Moreover, additional confusion might stem from age-related changes in the scalp distribution of the P300 that have been consistently found. Most of these studies described a more pronounced and faster decline of the P300 amplitude recorded at posterior relative to frontal sites (Fjell and Walhovd, 2001), or even enhancement of the P300 amplitude at frontal sites in the elderly (Friedman, 2003). This pattern was explained as a non-selective involvement of frontal mechanisms when it should be typically inhibited (Friedman, et al., 1997; Lorenzo-Lopez, et al., 2008). Other studies, however, showed a reversed pattern with a more pronounced P300 reduction frontally (Anderer, et al., 2003).
The equivocal pattern of age-related changes in the amplitude of P300 is consistent with the multitude of factors that affect this measure, as well as with findings showing that this component combines the activity of several intracranial sources, each putatively associated with a slightly different cognitive system (Anderer, et al., 2003; Lorenzo-Lopez, et al., 2008). Nevertheless, regardless of age, the amplitude of the P300 should be reduced as a function of task complexity. Therefore, we predicted that for both younger and older participants the amplitude of the P300 would decrease from the basic level (face-car) to the subordinate level (male-female) and would be smallest in the individual-level categorization task (famous-unfamiliar). We also predicted that the latency of the P300 would increase in parallel with the decrease in amplitude and that the latency delay would be more pronounced in the older than the younger participants.
Methods
Stimuli
The stimulus set consisted of 500 photographs of different faces and 100 photographs of cars (photographed from an angle of 45°). The faces consisted of 200 unfamiliar male faces, 200 unfamiliar female faces, and 100 famous faces (48 males). The fame of the face was determined in a pilot survey which included participants of various ages rated the familiarity of 400 putatively famous faces downloaded from public internet websites. The famous faces selected for this experiment were recognized by at least 90% of the participants and consisted of political figures and TV personalities. Importantly, when debriefed at the end of the experiment, the elderly as well as the young participants recognized the names of at least 85% of the famous people used in this study, proving existence of semantic knowledge about them. All faces presented in the gender categorization task were without hair and edited to exclude any external visual cues except the inner face components. The use of cropped stimuli forced the participants to distinguish between male and female faces only on the basis of face physiognomy. The stimuli were presented in a range of gray tones and equated for contrast and luminance (see examples in Figure 7). All stimuli were presented at fixation and seen from a distance of 70 ms each face occupied 8.1° × 11.3° in the visual field (10 cm × 14 cm).
Figure 7.
Examples of stimuli used in the three categorization tasks. (A) Basic level categorization. (B) Subordinate (male/female) categorization. (C). Individual (familiar/unfamiliar) categorization.
Task, Design and Procedure
Three categorization tasks were designed to tap three levels of categorization. Each task presented two categories with 100 exemplars in each. The 200 stimuli were fully randomized and presented one at a time. An alternative response key was a priori mapped to the each category and the participant was instructed to select and press a key in each trial according to the category to which the exemplar belonged. At the basic level categorization the distinction was between (unfamiliar) faces and cars. The subordinate level categorization was between (unfamiliar) male and female faces. The individual level categorization was between famous faces (politicians, movie stars and TV personalities) and unfamiliar faces. The categorization tasks were presented in fixed order from basic to individual level. The reason for using a fixed order was to avoid subordinate categorization carry-over strategies that could have been automatically imposed on higher-level categorization, albeit not required. Note that the inverse effects are prevented by the task.
Each trial began with a 500 ms fixation mark, followed by a stimulus exposed for 200 ms. The SOA to the subsequent trial varied between 1600 ms and 1800 ms, during which the response was expected. Stimuli within level-of-categorization task were fully randomized and a short break was allowed after 100 stimuli. The participants were instructed to consider both speed and accuracy, but accuracy was emphasized more. All other procedures were identical to Experiment 1.
Data pre-processing
EEG data was collected and pre-processed as in Experiment 1, with the following changes. First, only EEG segments associated with correct answers were averaged. Second, we randomly selected trials in each condition for each participant so that the same number of trials would be averaged across conditions and participants. Combined with a priori exclusion of segments contaminated by artifacts this selection left us with 29 segments for the N170 analysis and 25 segments for the P300 analysis.6 Whereas averages based on 25–30 segments out of 100 might be questionable, note that the P300 component reaches stability at about 20 segments (Cohen and Polich, 1997). In addition, four elderly and two young participants identified less than 25 famous faces and were excluded from the P300 analysis, leaving us with 12 older and 14 younger participants.
ERP analysis and statistical evaluation
Categorization effects on the N170 potential and the possible interaction between this effect and age was assessed comparing the N170 elicited by unfamiliar faces in the three tasks using a mixed model ANOVA with Age as the between-subjects factor and Level-of-categorization (basic, subordinate, individual), Hemisphere and, for the amplitude analysis, Site as within-subjects factors. In addition we compared the N170 effects elicited by younger and older participants in the face/car categorization task and in the familiar/unfamiliar categorization task using the same ANOVA design as in Experiment 1. For the P300 analysis, we combined the two categories within each level of categorization and analyzed the data using a mixed-model ANOVA with Age-group as the between-subjects factor and Level-of-categorization and Site (Fz, Cz, Pz; see figure 2) as within-subject factors. (In a preliminary data screening we found no difference between the P300 elicited by the two categories within each categorization level.) In addition, since we assumed that the single-trials latency jitter was different in the two age groups we applied the Woody-filter (Harris and Woody, 1969; Woody, 1967) to assess the size of the single trials variability and compare the P300 amplitudes after correcting for this factor.
Analysis of Performance
The performance of the elderly and younger group was compared analyzing the RTs and the d-prime (d') using the same ANOVA design as for the P300. In addition we analyzed possible differences in categorization biases by comparing the “criterion” (c) set by each participant within and across the tasks.
Results
Performance
The categorization accuracy of each age group at each level was assessed by calculating the d' separately for the categorization of faces and cars, male and female faces and famous and unfamiliar faces. In addition we calculated the criterion used by each group in each task to assess possible biases in the categorization process. Finally we also collected the RTs and averaged them separately in each experimental condition using only correct responses. Individual RTs that were higher or lower than the mean +/- 2SDs within participant and experimental condition were also excluded from the analysis (less than 5%). These data were analyzed using mixed-model ANOVAs and are presented in Table 1.
Table 1.
Performance of the two age groups in the three face-categorization tasks
| Level of categorization | Young participants | Old participants | ||||
|---|---|---|---|---|---|---|
| d' (SD) | C (SD) | RT (SD) | d' (SD) | C (SD) | RT (SD) | |
| Basic | 3.3 (0.70) | −0.06 (0.30) | 384 (105) | 3.2 (0.78) | −0.03 (0.12) | 383 (36) |
| Subordinate | 2.5 (0.46) | −0.18 (0.12) | 498 (129) | 1.8 (0.75) | −0.14 (0.22) | 540 (77) |
| Individual | 1.8 (0.70) | 0.66 (0.48) | 577 (122) | 1.1 (0.93) | 0.64 (0.66) | 677 (88) |
The mixed-model ANOVA of the RTs revealed that, overall, the RTs in the elderly group (533 ms) were not significantly longer than the RTs in the young group [487 ms; F(1,26) = 1.9, MSe = 48948, p = .182]. There was a significant main effect of Level-of-categorization [F(2,52) = 219.0, MSe = 4077, p < .001], which interacted with the effect of Group [F(2,52) = 9.2, MSe = 4077, p < .001]. Across levels, there was no effect of category [F(1,26) <1.0] but there was an interaction between Level-of-categorization and Category [F(2,52) = 5.2, MSe = 2104, p < .01], which was further qualified by a second order interaction with Group [F(2,52) = 5.0, MSe = 2104, p < .025].
Separate ANOVA for each Age group showed that in the elderly, the only significant effect was that of Level-of-categorization [F(2,26) = 114.4, MSe = 5527, p < .001] with longer RTs in the familiarity categorization task than in the gender categorization, and shortest in the face/car categorization. In the younger group there was also a significant Level-of-categorization effect [F(2,26) = 113.5, MSe = 2667, p < .001] and, although across levels the Category effect was insignificant (F(1,13) < 1.0], there was a Level-of-categorization×Category interaction [F(2,26) = 10.5, MSe =1463,p < .001]. Post hoc analysis showed that cars were responded to faster than faces [371 ms vs. 397 ms; t(13) = 2.869, p < .025], male faces were responded to faster than female faces [485 ms vs. 512 ms; t(13) =2.380, p < . 05] and unfamiliar faces faster than famous faces [554 ms vs. 601 ms; t(13) = 2.619, p < .025].
Accuracy was overall better in the younger group (d' = 2.54) than in the older group [d' = 2.0; F(1,30) = 7.22, MSe = .85, p < .025], and significantly affected by the level of categorization [F(2,60) = 67.0 , MSe = .76, p < .001]. There was no Level-of-categorization×Group interaction [F(2,60) = 2.2, MSE = .76, p = .124]. Pairwise comparisons showed that categorization accuracy was best at the basic (car/face) level (d' = 3.23) followed by the subordinate (gender) level (d' = 2.16) and lowest at the individual (familiarity) level (d' = 1.5). There was a difference in the bias applied by the participants in each level [F(2,60) = 44.7, MSe = .21, p < .001]. This bias was very similar across age groups as revealed by the absence of a main effect of Group or a Level-of-categorization×Group interaction (both Fs < 1.0]. Compared with zero, t-tests revealed that there was no significant bias in the face/car categorization [c = −0.05; t(31) = 1.2, p = .25], a significant bias to categorize faces as “male” in the gender categorization task [c = −0.16; t(31) = 5.1, p < .001] and a strong tendency to categorize faces as unfamiliar in the familiarity categorization task [c = 0.65, t(31) = 6.5, p < .001].
Finally, a direct comparison between the two age groups separately for each categorical decision task was done on the basis of the Level-of-categorization×Group interactions found for both speed and accuracy. These analyses showed that the elderly participants were slower than the younger participants in the familiarity categorization task [t(30) = 2.153, p < .05], but not in the gender and basic level categorization tasks [t(30) = 1.227, p = .229 and t(30) < 1.0, respectively]. Comparing accuracy we found that the elderly people were less accurate than the younger ones in the familiarity task [t(30) =2.453, p < .025] and in the gender categorization task [t(30) = 3.073, p < .01], but not in the basic level categorization task [t(30) < 1.0]7
Summary of performance results
Across levels of categorization, age reduced the accuracy level and slightly delayed the response times. Across age groups, basic level categorization was fastest and most accurate, followed by the subordinate categorization, and the individual categorization was the slowest and least accurate. In uncertainty conditions participants categorized faces as male and unfamiliar.
N170- Amplitude
The mixed-model ANOVA showed that across all conditions the N170 elicited by elderly participants (−10.9 μV) was similar to that elicited by the younger participants [−10.4 μV; F(1,30) < 1.0]. All three within-subject factors yielded significant main effects [Figure 7; F(2,60) = 27.8, MSe = 31.1, p < .001, F(1,30 = 5.6, MSe = 36.0 p < .025, and F(2,60) = 3.9, MSe = 27.8 p < .025, for the Level-of-categorization, Hemisphere and Site, respectively]. However, both the Level-of-categorization and Hemisphere effects were qualified by interactions with Group [F(2,60) = 5.9, MSe = 31.1, p < .01 and F(1,30) = 6.3, MSe = 36.0, p < .025, respectively]. To explore these interactions we analyzed the data of each group separately by within-subject ANOVA.
The separate ANOVAs revealed that the effect of Level-of-categorization was significant in both groups [F(2,30) = 8.5 MSe = 34.3, p < .001 and F(2,30) = 26.9, MSe = 28.9, p < .001 for the elderly and the younger groups, respectively]. Paired comparisons, however, showed that whereas in the young group the N170 elicited by unfamiliar faces was largest in the basic level categorization task (−13.0 μV), intermediate in the gender categorization task (−10.6 μV) and smallest in the familiarity categorization task (−7.7 μV), in the elderly group there was no difference between the N170 amplitudes in the gender and familiarity tasks (−9.6 μV and −10.2 μV, respectively, both smaller than the N170 elicited in the basic level categorization task (−12.8 μV). Planned t-tests (Bonferroni corrected) showed, however that, across sites and hemispheres, in none of the tasks was the difference between the N170 elicited by unfamiliar faces in the younger and older groups significant.
As in Experiment 1, the N170 elicited by the younger participants was larger over right hemisphere sites (−11.6 μV) than over left hemisphere sites (−9.2 μV; F(1,15) = 13.1 MSe = 32.7, p < .005], whereas that elicited by the older participants was equally large across hemispheres [−10.8 μV and −10.9 μV, for the left and the right hemispheres, respectively F(1,15) < 1.0].
The analysis of the normalized N170 effect in the basic level categorization task showed that this effect was larger for younger participants (0.1 μV ) than for older participants [0.04 μV; F(1,30) = 5.6, MSe = 0.089, p = .025] and, across groups, the N170-effect was larger at P9/10 (1.0 μV) than P7/8 (0.03 μV) and PO7/8 sites [0.03 μV; F(2, 60) = 11.0, MSe = 0.01, p < .001]. In addition, although across groups there was no Hemisphere effect [F(1,30 < 1.0], there was a Group×Hemisphere interaction [F(1,30) = 4.3, MSe = 0.032, p < 0.5] which revealed that while for the elderly group the N170 effect was larger in the left hemisphere (0.02 μV than in the right hemisphere (−0.01 μV), for the younger group, it was larger in the right hemisphere (0.14 μV) than in the left hemisphere (0.07 μV).
The analysis of the N170 elicited in the familiarity categorization task revealed no effect of Group [F(1,26) = 2.1, MSe = 238.1, p = .160]. The N170 elicited by famous and unfamiliar faces was similar [−8.5 μV and −8.8 μV, respectively; F(1,26) < 1.0] and there was no interaction between Stimulus familiarity and Group [F(1,26) < 1.0]. Overall the N170 was slightly larger over right (−9.5 μV) than left hemisphere sites [−8.2 μV; F(1,26) = 3.4, MSe = 38.7, p = .07] and, as before, the Hemisphere×Group interaction was significant [F(1,27) = 3.5, MSe = 38.7, p < .07], indicating a reliable difference between the N170 elicited at right hemisphere than at left hemisphere sites in the younger group (2.5 μV) but not in the elderly group (−0.01 μV). The Site effect was significant [F(2,52) = 3.5, MSe = 16.3, p < .05], and there was no Site×Group interaction [F(2,52) = 1.8, MSe = 16.3, p = .172].
Finally, in order to assess the effects of task relevance (which could affect the amount of attention allocated to the stimuli), we compared the N170 elicited by faces and non-face stimuli in Experiment 1 (in which both stimulus types were distracters in the flower-monitoring task) with those elicited in Experiment 2 (in which the basic-level categorization task turned both faces and non-face stimuli task relevant). Reflecting the effect of task relevance, the N170 amplitudes were larger in experiment 2 (−12.1 μV) than in Experiment 1 [−8.6 μV; F(1,30) = 82.2, MSe = 27.9, p < .001]. However, more important for the purpose of the present study, this main effect was different in the two groups (Experiment×Group interaction F(1,30) = 14.3, MSe = 27.9, p < .001]. Whereas for the younger participants task relevance enhanced the N170 amplitudes by 4.9 μV, in the older group the difference between experiments was only 2.0 μV. Post hoc comparisons showed that across the N170 elicited by faces and the corresponding N1 elicited by non-face stimuli (hereafter N170/N1) the amplitudes were larger for older than younger participants in Experiment 1 [(−10.3 μV vs. −6.9 μV, respectively; t(30) = 2.322, p < .05], but not in Experiment 2 [−12.4 μV vs. −11.8 μV, respectively; t(30) = <1.0]
N170- Latency
The N170 latency was analyzed using the same approach and ANOVA design as its amplitude, except that there was no Site factor. The mixed-model ANOVA showed that the N170 latency was delayed in the elderly group (177 ms) relative to the younger group (164 ms; F(1,30) = 4.5, MSe = 1662, p < .05]. There were no main effect of Level-of-categorization [F(2,60) = 2.5, MSe = 240, p = .09], but a significant interaction between this effect and Group [F(2,60) = 5.2, MSe = 240, p < .01]. Further investigation of this interaction showed that although the pattern was slightly different in each group, none of the simple comparisons were significant. There was no main effect of Hemisphere [F(1,30) < 1.00], but the Hemisphere effect interacted with both Group [F(1,30) = 7.8, MSe = 123, p < .01] and Level-of-categorization [F(2,60) = 8.6, MSe = 122, p < .005]. The Hemisphere × Group interaction indicated that whereas in the younger group the N170 latency was slightly shorter at right hemisphere sites (162 ms) than at left hemisphere sites (167 ms), in the older group there was tendency in the opposite direction (179 ms and 175 ms for the right hemisphere and left hemisphere, respectively. The Hemisphere × Level-of-categorization interaction showed that whereas the latency of the N170 was slightly longer over the right than the over the left hemisphere in the face/car and male/female categorization tasks (172 ms vs. 171 ms and 170 ms vs. 166 ms, respectively), it was slightly longer over the left than the right hemispheres in the familiarity categorization task (177 ms vs. 169 ms). However, pairwise comparisons showed that none of these differences were significant. The second order, Level-of-categorization × Hemisphere × Group interaction was not significant [F(2,60) = 1.6, MSe = 122, p = .214].
Comparison of the age groups in the basic level categorization task showed, again, that the N170 in the elderly group (183 ms) was delayed relative to the younger group [165 ms; F(1,30) = 9.2, MSe = 1144, p < .005], and faces faster than cars [171 ms vs. 177 ms; F(1,30) = 12.8, MSe = 94.8, p = .001]. No other main effects or interactions were significant. Interestingly, there were no age effects on the N170 latency in the familiarity categorization task. Indeed, no significant effects were found in this ANOVA.
Summary of N170 results
Across levels of categorization the N170 was delayed in the elderly compared with the younger groups but its amplitude was not affected by age. Moreover, the normalized N170 effect in the face/car categorization task was similar across groups, and so was the N170 elicited by familiar and unfamiliar faces. In neither of the two age groups was the N170 affected by face familiarity. The major outcome of this analysis was that the N170 was significantly affected by the level of categorization, and this effect was slightly different in each Age-group. Whereas in both groups the largest N170 was elicited in the basic level categorization task, in the younger group the N170 was smaller in the individual level categorization that in the gender categorization. In contrast, in parallel with performance, in the elderly group the N170 elicited in the gender categorization task was as low as in the individual level categorization task.
P300-amplitude
Relative to oddball paradigms the P300 amplitudes in the current categorization tasks were low (Figure 8). The mixed-model ANOVA showed that although the P300 amplitude was overall larger in the younger group (8.5 μV) than in the older group (6.6 μV), the difference was not significant [F(1,24) = 3.0, MSe = 72.5, p = .09]. However both within-subject factors yielded significant main effects [F(2,48) = 15.9, MSe = 9.7, p < .001 and F(2,48) = 24.0, MSe = 4.1, p < .001, for Level-of-categorization and Site, respectively] and both these main effects were qualified by interaction with the Age-group effect [F(2,48) = 11.3, MSe = 9.7, p < .001 and F(2,48) = 11.8, MSe = 4.1, p < .001, for Group × Level and Group × Site, respectively. Given these interactions we further analyzed each age group separately.
Figure 8.
N170 components elicited by unfamiliar faces in the three categorization tasks. Notice that whereas in the younger group the amplitude of the N170 reflects the level of categorization with higher amplitudes in the subordinate than in the individual levels and largest in the basic level tasks, in the elderly the N170 elicited in the gender (subordinate) categorization task was similar to that elicited in the familiarity (individual) task, reflecting the specific difficulty that older people had to distinguish between male and female faces on the basis of inner components.
Separate ANOVAs in each age group showed that the effect of Level-of-categorization was highly significant in the younger group [F(2,26) = 23.0, MSe =11.1, p < .001] with a higher P300 amplitude in the face/car categorization task (10.7 μV) than in the male/female categorization task (8.7 μV) and lowest amplitudes in the famous/unfamiliar categorization task (6.2 μV). In addition, a Level-of-categorization × Site interaction [F(4,52) = 3.5, MSe = 5.1, p < ..025] showed that although the Level-of-categorization effect was significant at all three sites, it was smaller at Fz [F(2,26) = 14.2] than at Cz [F(2,26) = 16.9], and largest at Pz [F(2,26) = 26.4]. In the elderly group, the Level-of-categorization effect was not quite significant [F(2,22) = 3.2, MSe = 8.1 p = .06], with slightly higher P300 amplitude in the male/female categorization task (7.5 μV) than in the face/car or familiarity categorization tasks (6.3 μV and 6.0 μV, respectively), and there was no Level-of-categorization × Site interaction [F(4,44) = 1.4, MSe = 3.6, p = .26].
The effect of Site was highly significant in the younger group [F(2,23) = 27.0, MSe = 6.1, p < .001], revealing the typical enhancement of the P300 amplitude from Fz (6.6 μV) to Cz (8.9 μV) and highest at Pz (10.1 μV). In contrast, in the elderly group the P300 amplitude did not differ significantly across sites [6.4 μV, 6.4 μV, and 7.0 μV, at Fz, Cz, and Pz, respectively; F(2,22) = 2.7, MSe = 2.7, p = .089].
P300-Latency
The mixed-model ANOVA showed that across all tasks, the P300 latencies were significantly longer in the elderly (625 ms) than in the younger group [551 ms; F(1,24) = 8.3, MSe = 38249, p < .01]. There was a significant effect of Level-of-categorization [F(2,48) = 50.4, MSe = 9346, p < .001], which interacted significantly with Group [F(2,48) = 4.5, MSe = 9346, p < .025]. There were no other main effects or interactions. Planned comparisons revealed that the latency was significantly longer in the elderly group for the gender categorization [t(24) = 3.857, p < .01] while in the face-car and familiarity categorization tasks the difference was not significant [t(24) = 1.961, p = 0.062, and t(24) = 0.860, p = 0.402, respectively].
Separate ANOVAs showed that for both groups the Level-of categorization effect was significant [F(2,22) = 7.7, MSe = 14603, p < .005, and F(2,26) = 88.3, MSe = 6082, p < .001]. However, whereas in the younger group the P300 latency was shorter in the face/car categorization task (450 ms) than in the gender categorization task (554 ms) and longest in the familiarity categorization task (649 ms), in the elderly group the P300 latency was similar in the gender and familiarity categorization tasks (640 ms and 672 ms), both longer than in the face/car categorization task (563 ms).
Woody filter latency-jitter correction effects
In order to investigate the possible age differences between the within-subject variance among single trials (latency jitter), we applied a Woody filter (Woody, 1967), implemented in Matlab. The template was the average ERP within each participant for each condition. This template was cross-correlated with the individual trials along the whole segment length (1100 ms). The procedure was repeated until there was no significant difference (p > .01) between two consecutive iterations. The latency-jitter for each participant at each level of categorization and the post-correction P300 amplitudes at Pz were analyzed using a mixed-model ANOVA with Age-group as the between-subjects factor and Level-of-categorization as the within-subjects factor.
This analysis showed that Group did not influence the size of the latency jitter either as a main effect [540 ms and 552 ms for the older and younger groups, respectively; F(1,24) < 1.0], or as an interaction with Level-of-categorization [F(2,48) < 1.0]. The main effect of Level-of-categorization was significant [F(2,48) = 20.9, MSe = 1988, p < .001]. Pairwise comparisons revealed that the latency jitter was smallest in the basic level categorization task (420 ms), larger in the gender categorization task (544 ms) and largest in the familiarity categorization task (676 ms]. The amplitude at Pz after the Woody correction was larger for young (17.1 μV) than older participants [12.5 μV; F(1, 24) = 5.7, MSe = 72.0, p < .05]. There was also a significant effect of Level-of categorization [F(2,48) = 6.0, MSe = 25.0, p < .01] indicating lower amplitudes in the familiarity task (12.3 μV) than in the gender task (16.7 μV), which did not differ from the basic-level categorization task (15.4 μV). There was no interaction between Level-of-categorization and Group effects [F(2,48) = 1.3].
Summary of the P300 effects
The P300 peak was significantly affected by a latency jitter among single trials, but the overall pattern of fairly similar before and after Woody filter correction. The P300 was delayed and its amplitude reduced (particularly after correction) in the older relative to the younger group. Importantly, since the latency jitter was similar across the two Age groups, the difference in amplitude cannot be accounted by this factor. Overall, the pattern of P300 effects in the younger group was as expected: Its amplitude grew from the frontal to parietal sites and it was larger and peaked sooner in the basic categorization task, followed by the gender categorization and the lower (and longer) in the familiarity categorization task. In contrast, in the older group the P300 amplitude was as high at frontal sites as at parietal sites and, while large in the basic level categorization, was similar for the gender and familiarity categorization conditions. Yet, like for the younger group, the P300 latency was shortest in the basic level categorization task and longest in the familiarity categorization task.
Discussion
The present experiment investigated whether normal aging affects the ability of healthy participants to categorize faces at increasingly specific levels. Older participants were less accurate and slightly slower than the younger ones except for the simplest, basic level categorization between faces and cars. This categorization was equally fast and accurate (indeed, both close to ceiling) for both age groups. The preserved ability to efficiently distinguish between faces and cars in the elderly was also corroborated by the similar N170-effect in the two age groups: according to most authors the N170-effect is an index for categorical distinction of faces from other stimulus types (Rossion and Jacques, 2008).
Interestingly, the reduced accuracy of the older relative to younger participants in subordinate categorization tasks was similar for gender and familiarity. Since a memory component should be more influential while attempting to determine the familiarity of a face than while categorizing a face as female or male, the similar effect of ageing in these two tasks suggests that the reduction in subordinate categorization performance reflects impaired perceptual processing more than it reflects mnemonic factors. Yet, the RT difference between the two age-groups was twice as large in the familiarity relative to the gender categorization task. While perceptual and mnemonic factors may be confounded in RT, finding that the latency of the N170 (which manifests early perceptual processes) was also longer in the elderly than in the younger group supports a perceptual impairment in the elderly, which might explain, at least partly, the delayed responses.
In contrast to its peak latency, the amplitude of the N170 elicited by faces in the three categorization tasks was not affected by age. In fact, the only difference between the two age groups was that whereas in the younger group the N170 was right-hemisphere lateralized (that is, larger at right hemisphere than at left hemisphere sites), in the elderly-group the amplitude of the N170 was equal bilaterally. Across age groups, the most intriguing finding in the N170 amplitude analysis was its modulation by the level of categorization. Although some authors suggest that the N170 amplitude is modulated by selective attention (e.g. Crist, et al., 2008; Eimer, 2000) the current effect can hardly be explained by task-related differences in selective-attention because in all tasks the faces were at the focus of the participant's attention. Alternatively, it is possible that the attentional load or, in other words, the amount of effort invested in each categorization task affected the N170 so that its amplitude negatively correlated with task difficulty. Supporting this hypothesis, for both groups the largest N170 amplitude was found in the basic level categorization task, where accuracy was at ceiling and the RTs were the fastest. Also conforming to this view, for younger participants, who were more accurate and faster while categorizing faces by gender than by familiarity, the N170 amplitude was larger in the gender than in the familiarity tasks. Likewise, for the older participants, who were equally accurate and slow at both subordinate categorization levels, the N170 amplitudes in the gender and familiarity tasks were equal, both smaller than that elicited in basic level categorization task. This interpretation is also compatible with previous studies which reported that reducing resources allocated to processing of (unattended) faces while increasing the attentional load imposed by competing non-face stimuli, reduced the amplitude of the N170 elicited by the face (e.g. de Fockert, et al., 2009; Mohamed, et al., 2009). Finally, the sensitivity of the N170 amplitude to task difficulty despite equal attention to the face is compatible with a study suggesting that different mechanisms account for the modulation of the N170 amplitude by selective attention and sensory competition (Jacques and Rossion, 2007).
Unlike the N170, both the amplitude and the latency of the P300 were affected by age. Interestingly, although across all tasks, the latency was longer in the older than in the younger participants, this effect was statistically significant only for the gender categorization task. For basic level categorization, which was easy for both groups, the difference was large but only approached significance. Similarly, for the familiarity categorization task, which was presumably difficult for both young and older participants, the latency of the P300 component did not differ between the age groups. It is noteworthy that the P300 latency difference between groups was larger than the N170 latency difference and revealed a different pattern across tasks; therefore the N170 effect cannot be the sole account for the P300 effect. Rather, as suggested by many studies in the past (see review in the introduction to this experiment), the longer latency of the P300 elicited in the older group probably reflects the reduced ability of older people to make categorical decisions in general and distinguish between male and female faces, in particular. This pattern suggests a combination of perceptual and decision making age-related difficulties. We will develop this suggestion in the General Discussion.
Additional insight is provided by the latency jitter differences between tasks and the absence of significant differences in this measure between groups. Assuming that, indeed, the P300 latency is largely determined by the time needed to reach a categorical decision (McCarthy and Donchin, 1981; Verleger, 1988), it stands to reason that the size of the latency-jitter should positively correlate with task difficulty. Supporting this hypothesis the latency jitter was larger in the familiarity task than in the gender categorization task and smallest in the basic level categorization task. Given this pattern, the absence of a difference between the elderly and the younger group is intriguing indicating that although the inter-trials variance correlated with task difficulty the delayed categorical decision at subordinate levels in the elderly group was consistent across the different trials within each task. This supports a view associating the occasionally observed increase in RT variance in the elderly with response selection and execution rather than stimulus evaluation and perceptual processes (Fjell, et al., 2009). Furthermore these data corroborate a previous report, which demonstrated that the well documented reduction of the P300 amplitude in the elderly is not entirely explained by increased inter-trials response variability (Walhovd, et al., 2008). Conforming to these findings, the P300 amplitude elicited in the current older group was smaller than that elicited in the younger group even after the correction of the latency-jitter by the Woody filter. Indeed, only after this correction the main effect of age-group was statistically significant.
For both groups the P300 amplitude was smaller in the familiarity categorization than in the gender and basic-level categorization tasks. This pattern goes along with the lower performance of all participants in the familiarity categorization task along with the putatively additional demands of the individual level recognition task. It is not immediately clear, however, whether the attenuation of the P300 amplitude reflected additional perceptual difficulty in identifying individual persons or decision making difficulties imposed by the task. Whereas some studies emphasized the influence of perceptual difficulty on the amplitude of the P300 (e.g. Wickens, et al., 1984), other studies expended this view suggesting that the P300 amplitude negatively correlates with the amount of work-load and need for resources imposed by a task (Kramer, et al., 1983; Sirevaag, et al., 1989; Strayer and Kramer, 1986). Finally, other studies emphasized the link between the P300 amplitude and decision making processes (Philiastides, et al., 2006) or/and response selection (Verleger, et al., 2005). Obviously, the different explanations are not mutually exclusive: Perceptual vagueness should most probably induce decision making difficulties. Since the decrease in the P300 amplitude when the participants attempted to determine the familiarity of a face relative to the higher-level categorizations was similar in the elderly and younger groups, we believe that it reflected primarily the additional load on the stimulus evaluation process and decision making, whereas the effect of pure perceptual processes entailed by task was evident primarily in the modulation of the N170 latency, which, indeed, differed between the two age groups (Philiastides and Sajda, 2006; van Rijsbergen and Schyns, 2009).
Finally, whereas in the younger group the anterior-posterior distribution of the P300 was typical, systematically increasing from Fz to Cz and Pz, in the elderly group the P300 at the frontal site was as large as at the central and posterior sites. This higher than usual frontal activity has been documented before and several accounts for this age-related change have been offered. We will return to this point in the general discussion, to which we turn now.
General Discussion
In the present study we explored if and how normal aging affects face perception. Whereas previous studies focused mainly on face identification, showing a link between age-related reduction in episodic memory and increasing difficulty in face recognition with advancing age (e.g. Bartlett, et al., 1989), our research focused on perceptual factors. Changes in face processing strategies might hint to more general age-related changes in visual perception, and may account, at least in part, for impairments in face recognition (Behrmann, et al., 2005; Bentin, et al., 2007). Based on a theoretical analysis of the perceptual processes which putatively explain the superior human expertise in face recognition, in Experiment 1 we manipulated stimulus qualities affecting global, configural and featural processing. We compared the sensitivity to these manipulations as reflected by modulations of the N170 ERP testing older and younger groups of healthy volunteers. The results showed that the perceptual distinction between faces and non-face stimuli as indexed by the N170-effect is not influenced by age, albeit the process seemed a bit slower in the elderly group. Yet, even at this early stage of perception, important processing differences were found between the two age-groups. First, the absolute amplitude of the N170 in the elderly group was larger than in the younger group, a result that resembles a previous report of larger N1 components in the elderly population (Talsma, et al., 2006). A provisional support for a link between the non-specific N1 and the increased N170 amplitude with age comes from the more medial peak amplitude of the N170 in the elderly group, relative to the typical lateral maxima found in the younger group. Along the same line, it is intriguing that the N170 enhancement in the elderly was more pronounced at left hemisphere sites leading to an atypical symmetry between the hemispheres; this symmetry contrasts the usual right-dominant pattern of the N170 amplitude that was also found in the current younger group. The apparent enhanced involvement of the left hemisphere in processing faces suggests that the relative larger decline of visuo-spatial compared to verbal abilities, as frequently found in the elderly (e.g. Goldstein and Shelly, 1981), manifests factors other than faster aging of the right hemisphere. Hence, this study joins fMRI based evidence (for a review see Dolcos, et al., 2002) supporting an overall age-related reduction in inter-hemispheric asymmetry (see Cabeza, 2002; Cherry, et al., 2005).
An additional insightful outcome of the analysis of the N170 in response to upright faces and non-face stimuli is the difference between the N170/N1 amplitudes in Experiment 1 and those recorded in Experiment 2. In both experiments the participants made categorical decisions at the basic level. However, whereas in Experiment 2 the subjects actually distinguished between face and non-face stimuli, in Experiment 1 both stimulus types formed one category which did not require a differential response. Interestingly, the N170 to faces and the N1 to non-face stimuli were larger in the elderly than in the younger group in Experiment 1, while no such differences were found in Experiment 2. Assuming that the amplitude of these components reflects to some extent the amount of attention and effort during stimulus processing (for recent evidence see Mulert, et al., 2007), this pattern suggests that younger participants modulated the amount of attention allocated to the stimuli according to their task-relevance while the attention allocated to faces by older participants was mostly stimulus driven. These data add to a growing body of evidence suggesting that cognitive impairments associated with aging could have sources in loose cognitive control (e.g. Hasher, et al., 2007), and reduced resistance to irrelevant distracters (e.g. Andres, et al., 2006; Georgiou-Karistianis, et al., 2006; Machado, et al., 2009).
A second conspicuous characteristic of face processing in the elderly was the reduced sensitivity to face inversion on the one hand, and the augmented sensitivity to the global structure of the face, on the other hand. The reduced sensitivity to face inversion as documented by the N170 modulation was also found in a previous study (Gao, et al., 2009). Apparently, older individuals are less affected by manipulations that impede the computation of second order relations among face features. This might suggest that, in contrast to younger observers, elderly individuals do not apply configural computations by default even while seeing a face. This is not to say, however, that they are unable to do so. Indeed, as demonstrated by Boutet and Faubert (2006), when required to recognize individual faces in a study-test episodic memory paradigm, inversion affected performance of elderly participants as much as of younger ones. Since second-order relational information is considered essential for face individuation and recognition, the absence of an inversion effect on the N170 in our study, in which the faces were task-defined distracters, along with the occurrence of an inversion effect in studies in which faces had to be recognized, fit with the performance in the current familiarity categorization task. In Experiment 2 we found that whereas the accuracy of discriminating between famous and unfamiliar faces was only slightly reduced in the elderly relative to the younger group, the stimulus evaluation time, as reflected in the latency of P300 as well as in RTs, was significantly longer in the elderly.
The augmented sensitivity to the global face structure in the elderly was indicated by the slightly reduced amplitude of the N170 in response to faces with scrambled inner components, while not such reduction was found in the younger group. Moreover whereas, as in many previous studies (e.g. Zion-Golumbic and Bentin, 2007) the amplitude of the N170 was considerably larger when the inner components were presented without the face contour to the younger group, no such effect was found in the elderly participants. Since the global face structure naturally includes the contour, we consider the reduced response to inner components in the elderly relative to the younger group as evidence that despite the preserved normal configuration of the components, the elderly participants were troubled by the absence of a normal global face structure in this condition. The augmented sensitivity to the global face shape is in line with findings of larger global-to-local interference for older relative to younger participants while processing incongruent “Navon letters” (Roux and Ceccaldi, 2001).
The third, and probably the most striking difference between the younger and the older participants emerged in the features processing condition. Recall that the stimuli in this condition were scrambled face features presented outside the face contour. Hence, although clearly recognized as face components, unlike in the “inner components” condition, these stimuli did not resemble a face in any way. Yet, demonstrating the particular sensitivity of the N170 to face components in general and to eyes in particular (Bentin, et al., 1996; Itier, et al., 2007) the N170 amplitude elicited by these stimuli in the younger group was actually larger than that elicited by full upright faces. In contrast, in the older group the N170 elicited by scrambled inner face components was considerably smaller than that elicited by full faces. A possible interpretation of the reduction of the N170 amplitude in the elderly when face features are randomly distributed in the image space is that older people are impeded while attempting to mentally form global structures from fragments. This interpretation might also explain the age-related reduced ability to detect faces from fuzzy line drawings (Norton, et al., 2009) as well as the increased dependence on global information at input.
Interestingly, the difference in perceptual strategies discussed above had no consequence in the ability of elderly individual to categorize faces at basic level and only minor consequences in their ability to recognize familiar faces (albeit they were somewhat less accurate and clearly slower in the latter task). Indeed, the only interesting and unexpected difference between the two age groups in performance was found in the gender categorization task where elderly participants were considerably less accurate than the younger ones. The special case of gender categorization can be traced to early stages of processing, as manifested by modulations of the N170 (cf., Rousselet, et al., 2009). In younger participants, the N170 amplitude paralleled the task complexity with highest amplitudes for the face-car categorization, lowest in the individual familiarity level and an in-between size for the male-female categorization. In contrast, in the elderly group the N170 amplitude for the male-female categorization was as low as for the familiarity categorization task. Similarly, although the latency of the P300 was larger in the elderly than the younger group overall, a separate analysis for each task revealed that the age-group difference was significant only for the gender categorization. Although we did not predict this pattern we can tentatively account for it post hoc. Recall that all faces presented in the gender categorization task were without hair and similarly shaped in an oval template. Hence the distinction between male and female faces had to be based on extracting detailed information from the shape of the inner components. Since, as we have established in Experiment 1, elderly people have problems with processing the face details, the gender categorization based on the present stimuli was very difficult for them.
Finally, the more frontal distribution of the P300 component in the elderly group contrasts with the typical centro-parietal maxima of this component as amply documented and also found in the current group of younger participants. Since the P300 is an index of updating working memory (Donchin and Coles, 1988) and influenced by work load (Donchin, 1987), the more anterior distribution of this components indicates a higher involvement of the frontal lobes in mental activity in older relative to younger people. Such “over-recruitment” of regions in the pre-frontal cortex (PFC) have been documented primarily in memory and executive function tasks (for a recent review see Grady, 2008), but also during performance of perceptual tasks (Grady, et al., 1994; Madden, et al., 2004). While some authors have suggested that the higher activity in the PFC is compensatory re-organization of brain structure-function coupling (Cabeza, et al., 2002; Grady, et al., 2005) others have argued that the increased prefrontal activity in the elderly may reflect impaired and nonselective executive functioning (Logan, et al., 2002), which might account for increased distractibility and impaired sustained attention in the elderly (Chao and Knight, 1997).
In conclusion, based on a comprehensive and theoretically motivated study of face processing in the elderly, we found that although face recognition performance is not dramatically changed in aged population, there are significant age-related perceptual changes which slow and somewhat impair face processing. In a nutshell, the present data suggest that older people might have problems integrating face features into global structures, hence becoming more dependent on distal global information. In addition, they might not apply configural computations by default while processing faces, perhaps because, unless required, they process faces only at a basic level. These changes in perceptual strategy might be the cause of slower and less accurate subordinate categorization, particularly when it is based on details. At the neural level, face processing is not right-lateralized, probably reflecting a more general reduction in inter-hemispheric asymmetry, and involve excessive but nonselective involvement of frontal regions which might explain the reduced ability to control attention.
Figure 9.
(A) P300 potential elicited in the three categorization tasks, before and after latency jitter correction (Woody filter). Notice that although correcting the latency jitter the amplitude increased in both groups the trends and differences between category levels and groups did not change. (B) The distribution of P300 along the sagittal midline in both groups, Notice the atypical higher involvement of the frontal lobes in the elderly group.
Acknowledgements
This study was supported by a National Institute of Mental Health grant to S.B. (RO1 MH 64458). The authors thank Dr. Ani Flevaris for proof reading this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Hierarchical letters are (global) letters formed by an arrangement of smaller (local) letters that can be the same or different from the global letter (for example an “E” composed from a bunch of small “s”.
A major distinction is made between a P3a and P3b components, with the former preceding the later in time. Although both components are elicited by attended rare events, only the P3b is associated with processing pre-designated task-related targets. Therefore, we will use the generic term P300 while referring to the P3b component.
The P1 component was defined as the most positive peak between 80–120 ms, and measured across O1, O2, P07 and P08). Given the complexity of the results we will not present P1 analyses any further.
Note that the inner components of schematic faces are not recognized as face parts when the global face configuration is disrupted.
For example, a difference of 2 μV between faces and watches might be more significant if the N170 elicited by faces is −4 μV and that elicited by watches −2 μV than if the N170 elicited by faces is −12 μV and by watches −10 μV.
The different number of valid segments averaged for the N170 and P300 analysis reflects the longer segments analyzed for the P300, which increased the probability for EEG and EOG artifact rejections.
Bonferroni corrected for multiple repetitions.
Disclosure Statement We declare no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence (bias) our work.
The study has been approved by the Ethics Committee of the Hebrew University (IRB) and all participants signed informed consent according to the requirements of that committee.
REFERENCES
- Anderer P, Saletu B, Semlitsch HV, Pascual-Marqui RD. Non-invasive localization of P300 sources in normal aging and age-associated memory impairment. Neurobiol Aging. 2003;24(3):463–79. doi: 10.1016/s0197-4580(02)00058-1. [DOI] [PubMed] [Google Scholar]
- Andres P, Parmentier FBR, Escera C. The effect of age on involuntary capture of attention by irrelevant sounds: A test of the frontal hypothesis of aging. Neuropsychologia. 2006;44(12):2564–8. doi: 10.1016/j.neuropsychologia.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Dain S, Andrews S, Drobny J. Visual abilities in older adults explain age-differences in Stroop and fluid intelligence but not face recognition: Implications for the vision-cognition connection. Aging Neuropsychol Cognit. 2002;9(4):253–65. [Google Scholar]
- Bartlett JC, Fulton A. Familiarity and recognition of faces in old-age. Mem Cognition. 1991;19(3):229–38. doi: 10.3758/bf03211147. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Leslie JE, Tubbs A, Fulton A. Aging and memory for pictures of faces. Psychol Aging. 1989;4(3):276–83. doi: 10.1037//0882-7974.4.3.276. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Marotta JJ, Kimchi R. Detailed exploration of face-related processing in congenital prosopagnosia: 1. Behavioral findings. J Cognitive Neurosci. 2005;17(7):1130–49. doi: 10.1162/0898929054475154. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cognitive Neurosci. 1996;8(6):551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, DeGutis JM, D'Esposito M, Robertson LC. Too many trees to see the forest: Performance, event-related potential, and functional magnetic resonance imaging manifestations of integrative congenital prosopagnosia. J Cognitive Neurosci. 2007;19(1):132–46. doi: 10.1162/jocn.2007.19.1.132. [DOI] [PubMed] [Google Scholar]
- Bentin S, Deouell LY, Soroker N. Selective visual streaming in face recognition: evidence from developmental prosopagnosia. Neuroreport. 1999;10(4):823–7. doi: 10.1097/00001756-199903170-00029. [DOI] [PubMed] [Google Scholar]
- Bentin S, Golland Y. Meaningful processing of meaningless stimuli: The influence of perceptual experience on early visual processing of faces. Cognition. 2002;86(1):B1–B14. doi: 10.1016/s0010-0277(02)00124-5. [DOI] [PubMed] [Google Scholar]
- Bentin S, Golland Y, Flevaris A, Robertson LC, Moscovitch M. Processing the trees and the forest during initial stages of face perception: Electrophysiological evidence. J Cognitive Neurosci. 2006;18(8):1406–21. doi: 10.1162/jocn.2006.18.8.1406. [DOI] [PubMed] [Google Scholar]
- Bentin S, Silverberg R, Gordon HW. Asymmetrical cognitive deterioration in demented and Parkinsonian patients. Cortex. 1981;17:533–544. doi: 10.1016/s0010-9452(81)80060-3. [DOI] [PubMed] [Google Scholar]
- Bian Z, Andersen GJ. Aging and the perceptual organization of 3-D scenes. Psychol Aging. 2008;23(2):342–52. doi: 10.1037/0882-7974.23.2.342. [DOI] [PubMed] [Google Scholar]
- Bopp KL, Verhaeghen P. Aging and verbal memory span: A meta-analysis. J Gerontolo Series B-Psychological Sciences and Social Sciences. 2005;60(5):223–33. doi: 10.1093/geronb/60.5.p223. [DOI] [PubMed] [Google Scholar]
- Boutet I, Faubert J. Recognition of faces and complex objects in younger and older adults. Mem Cognition. 2006;34(4):854–64. doi: 10.3758/bf03193432. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Nettleton NC. Human cerebral asymmetry. Prentice-Hall, New Jersey: 1983. [Google Scholar]
- Brown JW, Jaffe J. Hypothesis on cerebral dominance. Neuropsychologia. 1975;13(1):107–10. doi: 10.1016/0028-3932(75)90054-8. [DOI] [PubMed] [Google Scholar]
- Brown WS, Marsh JT, Larue A. Exponential electrophysiological aging: P3 latency. Electroen Clin Neuro. 1983;55(3):277–85. doi: 10.1016/0013-4694(83)90205-5. [DOI] [PubMed] [Google Scholar]
- Bruyer R, Scailquin JC. The fate of global precedence with age. Exp Aging Res. 2000;26(4):285–314. doi: 10.1080/036107300750015705. [DOI] [PubMed] [Google Scholar]
- Bruyer R, Scailquin JC, Samson D. Aging and the locus of the global precedence effect: A short review and new empirical data. Exp Aging Res. 2003;29(3):237–68. doi: 10.1080/03610730303724. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Carmel D, Bentin S. Domain specificity versus expertise: factors influencing distinct processing of faces. Cognition. 2002;83(1):1–29. doi: 10.1016/s0010-0277(01)00162-7. [DOI] [PubMed] [Google Scholar]
- Chaby L, George N, Renault B, Fiori N. Age-related changes in brain responses to personally known faces: an event-related potential (ERP) study in humans. Neurosci Lett. 2003;349(2):125–9. doi: 10.1016/s0304-3940(03)00800-0. [DOI] [PubMed] [Google Scholar]
- Chaby L, Jemel B, George N, Renault B, Fiori N. An ERP study of famous face incongruity detection in middle age. Brain Cognition. 2001;45(3):357–77. doi: 10.1006/brcg.2000.1272. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex. 1997;7(1):63–9. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- Cherry BJ, Adamson M, Duclos A, Hellige JB. Aging and individual variation in interhemispheric collaboration and hemispheric asymmetry. Aging Neurops Cog. 2005;12(4):316–39. doi: 10.1080/17444128.2005.10367004. [DOI] [PubMed] [Google Scholar]
- Clark VP, Hillyard SA. Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. J Cogn Neurosci. 1996;8(5):387–402. doi: 10.1162/jocn.1996.8.5.387. [DOI] [PubMed] [Google Scholar]
- Cohen J, Polich J. On the number of trials needed for P300. Int J Psychophysiol. 1997;25(3):249–55. doi: 10.1016/s0167-8760(96)00743-x. [DOI] [PubMed] [Google Scholar]
- Crist RE, Wu C-T, Karp C, Woldorff MG. Face processing is gated by visual spatial attention. Front Hum Neurosci. 2008;1 doi: 10.3389/neuro.09.010.2007. Article No.: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A, Van der Linden M. Identity but not expression memory for unfamiliar faces is affected by ageing. Memory. 2004;12(5):644–54. doi: 10.1080/09658210344000198. [DOI] [PubMed] [Google Scholar]
- de Fockert JW, Ramchurn A, van Velzen J, Bergstroem Z, Bunce D. Behavioral and ERP evidence of greater distractor processing in old age. Brain Res. 2009;1282:67–73. doi: 10.1016/j.brainres.2009.05.060. [DOI] [PubMed] [Google Scholar]
- De Sanctis P, Katz R, Wylie GR, Sehatpour P, Alexopoulos GS, Foxe JJ. Enhanced and bilateralized visual sensory processing in the ventral stream may be a feature of normal aging. Neurobiol Aging. 2008;29(10):1576–86. doi: 10.1016/j.neurobiolaging.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Del Viva MM, Agostini R. Visual spatial integration in the elderly. Invest Ophth Vis Sci. 2007;48(6):2940–6. doi: 10.1167/iovs.06-0729. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neurosci Biobehav R. 2002;26(7):819–25. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise … Surprise. Psychophysiology. 1981;18(5):493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E. The P300 as a metric for mental workload. EEG Cl N Su. 1987;39:338–43. [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11(3):357–74. [Google Scholar]
- Donchin E, Coles MGH. Context updating and the P300. Behav Brain Sci. 1998;21(1):152–+. [Google Scholar]
- Duncan-Johnson CC. P300-latency - A new metric for information processing. Psychophysiology. 1981;18(3):207–15. doi: 10.1111/j.1469-8986.1981.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Donchin E. Quantifying surprise - Variation of Event Related Potentials with subjective probability. Psychophysiology. 1977;14(5):456–67. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Eimer M. Attentional modulations of event-related brain potentials sensitive to faces. Cognitive Neuropsych. 2000;17(1–3):103–16. doi: 10.1080/026432900380517. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Porciatti V, Morrone MC, Burr DC. Visual ageing: Unspecific decline of the responses to luminance and colour. Vision Res. 1996;36(21):3557–66. doi: 10.1016/0042-6989(96)00032-6. [DOI] [PubMed] [Google Scholar]
- Firestone A, Turk-Browne NB, Ryan JD. Age-related deficits in face recognition are related to underlying changes in scanning behavior. Neuropsychol Dev Cogn B Aging Neurops Cog. 2007;14(6):594–607. doi: 10.1080/13825580600899717. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Rosquist H, Walhovd KB. Instability in the latency of P3a/P3b brain potentials and cognitive function in aging. Neurobiol Aging. 2009;30(12):2065–79. doi: 10.1016/j.neurobiolaging.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. P300 and neuropsychological tests as measures of aging: Scalp topography and cognitive changes. Brain Topogr. 2001;14(1):25–40. doi: 10.1023/a:1012563605837. [DOI] [PubMed] [Google Scholar]
- Freire A, Lee K, Symons LA. The face-inversion effect as a deficit in the encoding of configural information: Direct evidence. Perception. 2000;29(2):159–70. doi: 10.1068/p3012. [DOI] [PubMed] [Google Scholar]
- Friedman D. Cognition and aging: A highly selective overview of event-related potential (ERP) data. J Clin Exp Neuropsyc. 2003;25(5):702–20. doi: 10.1076/jcen.25.5.702.14578. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski V, Fabiani M. An over-view of age-related changes in the scalp distribution of P3b. Evoked Potential - Electroen Clin Neuro. 1997;104(6):498–513. doi: 10.1016/s0168-5597(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Fulton A, Bartlett JC. Young and old faces in young and old heads: The factor of age in face recognition. Psychol Aging. 1991;6(4):623–30. doi: 10.1037//0882-7974.6.4.623. [DOI] [PubMed] [Google Scholar]
- Gao L, Xu J, Zhang BW, Zhao L, Harel A, Bentin S. Aging effects on early-stage face perception: An ERP study. Psychophysiology. 2009;46(5):970–83. doi: 10.1111/j.1469-8986.2009.00853.x. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Curby KM. A perceptual traffic jam on highway N170 - Interference between face and car expertise. Curr Dir Psychol Sci. 2005;14(1):30–3. [Google Scholar]
- Georgiou-Karistianis N, Tang J, Mehmedbegovic F, Farrow M, Bradshaw J, Sheppard D. Age-related differences in cognitive function using a global local hierarchical paradigm. Brain Res. 2006;1124:86–95. doi: 10.1016/j.brainres.2006.09.070. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Shelly C. Does the right hemisphere age more rapidly than the left? J Clin Neuropsychol. 1981;3:65–78. doi: 10.1080/01688638108403114. [DOI] [PubMed] [Google Scholar]
- Goodin DS, Squires KC, Henderson BH, Starr A. Age-related variations in evoked-potentials to auditory stimuli in normal subjects. Electroen Clin Neuro. 1978;44(4):447–58. doi: 10.1016/0013-4694(78)90029-9. [DOI] [PubMed] [Google Scholar]
- Grady CL. Age-related differences in face processing: A meta-analysis of three functional neuroimaging experiments. Can J Exp Psychol. 2002;56(3):208–20. doi: 10.1037/h0087398. [DOI] [PubMed] [Google Scholar]
- Grady CL. Year in Cognitive Neuroscience 2008. Blackwell Publishing; Oxford: 2008. Cognitive neuroscience of aging; pp. 127–44. [DOI] [PubMed] [Google Scholar]
- Grady CL, Keightley M, Hongwanishkul D, Lee W, Hasher L. The effect of age on memory for emotional faces. Neuropsychology. 2007;21(3):371–80. doi: 10.1037/0894-4105.21.3.371. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood-flow activation during visual processing of faces and location. J Neurosci. 1994;14(3):1450–62. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FIM. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43(10):1466–81. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Gray HM, Ambady N, Lowenthal WT, Deldin P. P300 as an index of attention to self-relevant stimuli. J Exp Soc Psychol. 2004;40(2):216–24. [Google Scholar]
- Habak C, Faubert J. Larger effect of aging on the perception of higher-order stimuli. Vision Res. 2000;40(8):943–50. doi: 10.1016/s0042-6989(99)00235-7. [DOI] [PubMed] [Google Scholar]
- Habak C, Wilkinson F, Wilson HR. Aging disrupts the neural transformations that link facial identity across views. Vision Res. 2008;48(1):9–15. doi: 10.1016/j.visres.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EK, Woody CD. Use of an adaptive filter to characterize signal-noise relationships. Comput Biomed Res. 1969;2(3):242–&. doi: 10.1016/0010-4809(69)90005-6. [DOI] [PubMed] [Google Scholar]
- Hasher L, Lustig C, Zacks RT. Inhibitory mechanisms and the control of attention. In: Conway A, Jarrold C, Kane M, Towse J, editors. Variation in working memory. Oxford University Press; New York: 2007. pp. 227–49. [Google Scholar]
- Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: costs and potential benefits. Prog Brain Res. 2008;169:353–63. doi: 10.1016/S0079-6123(07)00022-2. [DOI] [PubMed] [Google Scholar]
- Hubner R, Volberg G. The integration of object levels and their content: A theory of global/local processing and related hemispheric differences. J Exp Psychol Human. 2005;31(3):520–41. doi: 10.1037/0096-1523.31.3.520. [DOI] [PubMed] [Google Scholar]
- Isreal JB, Wickens CD, Chesney GL, Donchin E. The event-related brain potentials as an index of display-monitoring workload. Hum Factors. 1980;22(2):211–24. doi: 10.1177/001872088002200210. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Alain C, Sedore K, McIntosh AR. Early face processing specificity: It's in the eyes! J Cognitive Neurosci. 2007;19:1815–26. doi: 10.1162/jocn.2007.19.11.1815. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Latinus M, Taylor MJ. Face, eye and object early processing: What is the face specificity? Neuroimage. 2006;29(2):667–76. doi: 10.1016/j.neuroimage.2005.07.041. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Source analysis of the N170 to faces and objects. Neuroreport. 2004;15(8):1261–5. doi: 10.1097/01.wnr.0000127827.73576.d8. [DOI] [PubMed] [Google Scholar]
- Jacques C, Rossion B. Electrophysiological evidence for temporal dissociation between spatial attention and sensory competition during human face processing. Cereb Cortex. 2007;17(5):1055–65. doi: 10.1093/cercor/bhl015. [DOI] [PubMed] [Google Scholar]
- Johnson R. A triarchic model of P300 amplitude. Psychophysiology. 1986;23(4):367–84. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000a;37(2):163–78. [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin Neurophysiol. 2000b;111(10):1745–58. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kiffel C, Campanella S, Bruyer R. Categorical perception of faces and facial expressions: The age factor. Exp Aging Res. 2005;31(2):119–47. doi: 10.1080/03610730590914985. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38(3):557–77. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Brown HD, Dror IE. Aging and the scope of visual attention. Gerontology. 1999;45(2):102–9. doi: 10.1159/000022071. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Wickens CD, Donchin E. An analysis of the processing requirements of a complex perceptual-motor task. Hum Factors. 1983;25(6):597–621. doi: 10.1177/001872088302500601. [DOI] [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: P300 as a measure of stimulus evaluation time. Science. 1977;197(4305):792–5. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Lange EB, Verhaeghen P. No Age Differences in Complex Memory Search: Older Adults Search as Efficiently as Younger Adults. Psychol Aging. 2009;24(1):105–15. doi: 10.1037/a0013751. [DOI] [PubMed] [Google Scholar]
- Levy J, Sperry RW, Trevarthen C. Perception of bilateral chimeric figures following hemispheric disconnection. Brain. 1972;95:61–8. doi: 10.1093/brain/95.1.61. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron. 2002;33(5):827–40. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Lopez L, Amenedo E, Pascual-Marqui RD, Cadaveira F. Neural correlates of age-related visual search decline: A combined ERP and sLORETA study. Neuroimage. 2008;41(2):511–24. doi: 10.1016/j.neuroimage.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Lopez L, Amenedo E, Pazo-Avarez P, Cadaveira F. Visual target processing in high- and low-performing older subjects indexed by P3 component. Clin Neurophysiol. 2007;37(2):53–61. doi: 10.1016/j.neucli.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Lott LA, Haegerstrom-Portnoy G, Schneck ME, Brabyn JA. Face recognition in the elderly. Optometry Vision Sci. 2005;82(10):874–81. doi: 10.1097/01.opx.0000180764.68737.91. [DOI] [PubMed] [Google Scholar]
- Lux S, Marshall JC, Thimm M, Fink GR. Differential processing of hierarchical visual stimuli in young and older healthy adults: Implications for pathology. Cortex. 2008;44(1):21–8. doi: 10.1016/j.cortex.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Machado L, Devine A, Wyatt N. Distractibility with advancing age and Parkinson's disease. Neuropsychologia. 2009;47(7):1756–64. doi: 10.1016/j.neuropsychologia.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Provenzale JM, Huettel SA. Age-related changes in neural activity during visual target detection measured by fMRI. Cereb Cortex. 2004;14(2):143–55. doi: 10.1093/cercor/bhg113. [DOI] [PubMed] [Google Scholar]
- Martinez A, Moses P, Frank L, Buxton R, Wong E, Stiles J. Hemispheric asymmetries in global and local processing: Evidence from fMRI. Neuroreport. 1997;8(7):1685–9. doi: 10.1097/00001756-199705060-00025. [DOI] [PubMed] [Google Scholar]
- Maurer D, Le Grand R, Mondloch CJ. The many faces of configural processing. Trends Cogn Sci. 2002;6(6):255–60. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: A comparison of P300 latency and reaction-time. Science. 1981;211(4477):77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- McDowell K, Kerick SE, Santa Maria DL, Hatfield BD. Aging, physical activity, and cognitive processing: an examination of P300. Neurobiol Aging. 2003;24(4):597–606. doi: 10.1016/s0197-4580(02)00131-8. [DOI] [PubMed] [Google Scholar]
- Mohamed TN, Neumann MF, Schweinberger SR. Perceptual load manipulation reveals sensitivity of the face-selective N170 to attention. Neuroreport. 2009;20(8):782–7. doi: 10.1097/WNR.0b013e32832b7e24. [DOI] [PubMed] [Google Scholar]
- Mulert C, Leicht G, Pogarell O, Mergl R, Karch S, Juckel G, Moller HJ, Hegerl U. Auditory cortex and anterior cingulate cortex sources of the early evoked gamma-band response: Relationship to task difficulty and mental effort. Neuropsychologia. 2007;45(10):2294–306. doi: 10.1016/j.neuropsychologia.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Navon D. Forest before trees. Precedence of global features in visual perception. Cognitive Psychol. 1977;9(3):353–83. [Google Scholar]
- Norman JF, Norman HF, Craft AE, Walton CL, Bartholomew AN, Burton CL, Wiesemann EY, Crabtree CE. Stereopsis and aging. Vision Res. 2008;48(23–24):2456–65. doi: 10.1016/j.visres.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Norton D, McBain R, Chen Y. Reduced Ability to Detect Facial Configuration in Middle-Aged and Elderly Individuals: Associations With Spatiotemporal Visual Processing. J Gerontol B-Psycholog. 2009;64(3):328–34. doi: 10.1093/geronb/gbp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Kishiyama SS, Kaye JA, Jones DE. Age-related differences in global-local processing: Stability of laterality differences but disproportionate impairment in global processing. J Geriatr Psychiatry Neurol. 1999;12(2):76–81. doi: 10.1177/089198879901200207. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Boldt C. Aging and low-contrast vision - Face perception. Invest OphthVis Sci. 1981;21(2):362–5. [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. P Natl Acad Sci U S A. 2004;101(35):13091–5. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer D, Marshuetz C, Sutton B, Hebrank A, Welsh RC, Park DC. Decreased neural specialization in old adults on a working memory task. Neuroreport. 2006;17(5):487–91. doi: 10.1097/01.wnr.0000209005.40481.31. [DOI] [PubMed] [Google Scholar]
- Pesce C, Guidetti L, Baldari C, Tessitore A, Capranica L. Effects of aging on visual attentional focusing. Gerontology. 2005;51(4):266–76. doi: 10.1159/000085123. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM. ERPs to stimuli requiring response production and inhibition. Effects of age, probability and visual noise. Electroen Clin Neuro. 1988;71(1):55–63. doi: 10.1016/0168-5597(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Wenegrat BG, Roth WT, Kopell BS. Clinical application of the P3 component of event-related potentials. 1. Normal aging. Electroen Clin Neuro. 1984;59(2):85–103. doi: 10.1016/0168-5597(84)90026-1. [DOI] [PubMed] [Google Scholar]
- Pfutze EM, Sommer W, Schweinberger SR. Age-related slowing in face and name recognition: Evidence from event-related brain potentials. Psychol Aging. 2002;17(1):140–60. doi: 10.1037//0882-7974.17.1.140. [DOI] [PubMed] [Google Scholar]
- Philiastides MG, Ratcliff R, Sajda P. Neural representation of task difficulty and decision making during perceptual categorization: A timing diagram. J Neurosci. 2006;26(35):8965–75. doi: 10.1523/JNEUROSCI.1655-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philiastides MG, Sajda P. Temporal characterization of the neural correlates of perceptual decision making in the human brain. Cereb Cortex. 2006;16(4):509–18. doi: 10.1093/cercor/bhi130. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Champagne SC, Nelson RF. The effects of age on human event-related potentials. Psychophysiology. 1984;21(3):312–25. doi: 10.1111/j.1469-8986.1984.tb02941.x. [DOI] [PubMed] [Google Scholar]
- Polich J. Meta-analysis of P300 normative aging studies. Psychophysiology. 1996;33(4):334–53. doi: 10.1111/j.1469-8986.1996.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Polich J. EEG and ERP assessment of normal aging. Evoked Potentials-Electroen Clin Neuro. 1997;104(3):244–56. doi: 10.1016/s0168-5597(97)96139-6. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating p300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers S, Maylor EA. Task switching across the life span: Effects of age on general and specific switch costs. Dev Psychol. 2005;41(4):661–71. doi: 10.1037/0012-1649.41.4.661. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA. New visions of the aging mind and brain. Trends in Cogn Sci. 2002;6(9):394–400. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Brake S, Atkinson AP. Whats lost in inverted faces. Cognition. 1993;47(1):25–57. doi: 10.1016/0010-0277(93)90061-y. [DOI] [PubMed] [Google Scholar]
- Richler JJ, Gauthier I, Wenger MJ, Palmeri TJ. Holistic processing of faces: Perceptual and decisional components. J Expl Psychol Learn. 2008;34(2):328–42. doi: 10.1037/0278-7393.34.2.328. [DOI] [PubMed] [Google Scholar]
- Rossion B, Curran T, Gauthier I. A defense of the subordinate-level expertise account for the N170 component. Cognition. 2002;85(2):189–96. doi: 10.1016/s0010-0277(02)00101-4. [DOI] [PubMed] [Google Scholar]
- Rossion B, Gauthier I. How does the brain process upright and inverted faces? Behav Cogn Neurosci Rev. 2002;1(1):63–75. doi: 10.1177/1534582302001001004. [DOI] [PubMed] [Google Scholar]
- Rossion B, Gauthier I, Tarr MJ, Despland P, Bruyer R, Linotte S, Crommelinck M. The N170 occipito-temporal component is delayed and enhanced to inverted faces but not to inverted objects: an electrophysiological account of face-specific processes in the human brain. Neuroreport. 2000;11(1):69–74. doi: 10.1097/00001756-200001170-00014. [DOI] [PubMed] [Google Scholar]
- Rossion B, Jacques C. Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170. Neuroimage. 2008;39(4):1959–79. doi: 10.1016/j.neuroimage.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Rossion B, Joyce CA, Cottrell GW, Tarr MJ. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage. 2003;20(3):1609–24. doi: 10.1016/j.neuroimage.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Roudaia E, Bennett PJ, Sekuler AB. The effect of aging on contour integration. Vision Res. 2008;48(28):2767–74. doi: 10.1016/j.visres.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Rousselet GA, Husk JS, Pernet CR, Gaspar CM, Bennett PJ, Sekuler AB. Age-related delay in information accrual for faces: Evidence from a parametric, single-trial EEG approach. BMC Neurosci. 2009;10 doi: 10.1186/1471-2202-10-114. Article 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Ceccaldi M. Does aging affect the allocation of visual attention in global and local information processing? Brain Cognition. 2001;46(3):383–96. doi: 10.1006/brcg.2001.1296. [DOI] [PubMed] [Google Scholar]
- Sagiv N, Bentin S. Structural encoding of human and schematic faces: Holistic and part-based processes. J Cognitive Neurosci. 2001;13(7):937–51. doi: 10.1162/089892901753165854. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–28. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54(1–3):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Searcy JH, Bartlett JC. Inversion and processing of component and spatial-relational information in faces. J Exp Psychol Human. 1996;22(4):904–15. doi: 10.1037//0096-1523.22.4.904. [DOI] [PubMed] [Google Scholar]
- Searcy JH, Bartlett JC, Memon A. Age differences in accuracy and choosing in eyewitness identification and face recognition. Mem Cognition. 1999;27(3):538–52. doi: 10.3758/bf03211547. [DOI] [PubMed] [Google Scholar]
- Sekular R, Sekular AB. Visual perception and cognition. In: Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, editors. Oxford textbook of geriatric medicine. Oxford University Press; Oxford: 2000. pp. 874–80. [Google Scholar]
- Sirevaag EJ, Kramer AF, Coles MGH, Donchin E. Resource reciprocity: An event-related brain potential analysis. Acta Psychol (Amst) 1989;70(1):77–97. doi: 10.1016/0001-6918(89)90061-9. [DOI] [PubMed] [Google Scholar]
- Spear PD. Neural bases of visual deficits during aging. Vision Res. 1993;33(18):2589–609. doi: 10.1016/0042-6989(93)90218-l. [DOI] [PubMed] [Google Scholar]
- Strayer DL, Kramer AF. Psychophysiological indexes of automaticity and attentional resources. Psychophysiology. 1986;23(4):466. [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-Potential correlates of stimulus uncertainty. Science. 1965;150(3700):1187–&. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Talsma D, Kok A, Ridderinkhof KR. Selective attention to spatial and non-spatial visual stimuli is affected differentially by age: Effects on event-related brain potentials and performance data. Int J Psychophysiol. 2006;62(2):249–61. doi: 10.1016/j.ijpsycho.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Farah MJ. Parts and wholes in face recognition. Q J Exp Psychol - A. 1993;46(2):225–45. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- Tejeria L, Harper RA, Artes PH, Dickinson CM. Face recognition in age related macular degeneration: perceived disability, measured disability, and performance with a bioptic device. Brit J Ophthalmol. 2002;86(9):1019–26. doi: 10.1136/bjo.86.9.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, Humphreys K, Jung KJ, Peterson MA, Behrmann M. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. J Cogn Neurosci. 2008;20(2):268–84. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobimatsu S. Aging and patteren visual-evoked potentials. Optometry Vision Sci. 1995;72(3):192–7. doi: 10.1097/00006324-199503000-00007. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The mini-mental state examination - A comprehensive review. J Am Geriatr Soc. 1992;40(9):922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- van Rijsbergen NJ, Schyns PG. Dynamics of trimming the content of face representations for categorization in the brain. PLoS Comput Biol. 2009;5(11):e1000561. doi: 10.1371/journal.pcbi.1000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleger R. Event-Related Potentials and memory - A critique of the context updating hypothesis and an alternative interpretation of P3. Behav Brain Sci. 1988;11(3):343–56. [Google Scholar]
- Verleger R, Jaskowski P, Wascher E. Evidence for an integrative role of P3b in linking reaction to perception. J Psychophysio. 2005;19(3):165–81. [Google Scholar]
- Walhovd KB, Fjell AM. The relationship between P3 and neuropsychological function in an adult life span sample. Biol Psychol. 2003;62(1):65–87. doi: 10.1016/s0301-0511(02)00093-5. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Rosquist H, Fjell AM. P300 amplitude age reductions are not caused by latency jitter. Psychophysiology. 2008;45(4):545–53. doi: 10.1111/j.1469-8986.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Wang YC, Zhou YF, Ma YY, Leventhal AG. Degradation of signal timing in cortical areas V1 and V2 of senescent monkeys. Cereb Cortex. 2005;15(4):403–8. doi: 10.1093/cercor/bhh143. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Woldorff MG. Hemispheric asymmetries for different components of global/local attention occur in distinct temporo-parietal loci. Cereb Cortex. 2005;15(6):870–6. doi: 10.1093/cercor/bhh187. [DOI] [PubMed] [Google Scholar]
- Wickens CD, Kramer AF, Donchin E. The event-related potential as an index of processing demands of a complex target acquisition task. Ann NY Acad Sci. 1984;425:295–9. doi: 10.1111/j.1749-6632.1984.tb23550.x. [DOI] [PubMed] [Google Scholar]
- Woody CD. Characterization of an adaptive filter for the analysis of variable latency meuroelectric signals. Med Biol Eng. 1967;5:539–53. [Google Scholar]
- Young AW, Hellawell D, Hay DC. Configural information in face perception. Perception. 1987;16(6):747–59. doi: 10.1068/p160747. [DOI] [PubMed] [Google Scholar]
- Yovel G, Tambini A, Brandman T. The asymmetry of the fusiform face area is a stable individual characteristic that underlies the left-visual-field superiority for faces. Neuropsychologia. 2008;46(13):3061–8. doi: 10.1016/j.neuropsychologia.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang XS, Wang YC, Fu Y, Liang Z, Ma YY, Leventhal AG. Spatial and temporal sensitivity degradation of primary visual cortical cells in senescent rhesus monkeys. Eur J Neurosci. 2008;28(1):201–7. doi: 10.1111/j.1460-9568.2008.06300.x. [DOI] [PubMed] [Google Scholar]
- Zion-Golumbic E, Bentin S. Dissociated neural mechanisms for face detection and configural encoding: Evidence from N170 and induced gamma-band oscillation effects. Cereb Cortex. 2007;17(8):1741–9. doi: 10.1093/cercor/bhl100. [DOI] [PubMed] [Google Scholar]