Abstract
The Id3 gene has been shown to play important roles in the development and function of broad tissue types including B and T cells. Id3 deficient mice develop autoimmune disease similar to human Sjögren’s syndrome. Both B and T lymphocytes have been implicated to contribute to the disease phenotype in this disease model. In order to gain a better understanding of individual cell types in this disease model, we generated an Id3 conditional allele. An LckCre transgene was used to induce Id3 deletion in developing T cells. We showed that the Id3 gene was efficiently disrupted in early thymocyte development prior to T cell receptor (TCR)-mediated positive selection. Consequently, thymocyte maturation was impaired in the conditional knockout mice. These mice developed exocrinopathy starting at two months of age and subsequently exhibited high incidence of lymphocyte infiltration to salivary glands between eight and 12 months of age. This progressive feature of disease development is very similar to those observed in Id3 germline knockout mice. This study establishes a new model for investigating the relationship between T cell development and autoimmune disease. Our observation provides an experimental case that autoimmune disease may be induced by acquired mutation in developing T cells.
Keywords: Id3, thymocyte development, Sjögren’s syndrome, lymphocyte infiltration, saliva secretion
Introduction
T cell development occurs in the thymus where a diverse repertoire of T cell receptors (TCR) is generated and clonally selected. The expression of either αβTCR or γδTCR heterodimers results in developmentally and functionally distinct αβ or γδT cell population, respectively. While a small fraction of thymocytes develop to γδT cells, the majority of thymocytes enters the αβT cell fate and undergoes further selection and lineage differentiation. These freshly produced αβTCR positive thymocytes also express the CD4 and CD8 co-receptors, and thus named CD4CD8 double positive (DP) cells. The DP thymocytes undergo a rigorous selection, which eliminates more than 95% of the population and allows the remaining ones to become either CD4 helper or CD8 cytotoxic single positive (SP) T cells [1,2]. This selection process is critical for T cells to acquire the ability of providing antigen specific immune function while maintaining tolerance to self-antigens.
Id3 is known to play important roles in mediating TCR signals during DP to SP selection [3]. The Id3 gene encodes a 13 kD helix-loop-helix protein which primarily functions as a competitive inhibitor of E-protein transcription factors such as E2A and HEB [4–6]. Id3 was first isolated from cultured fibroblasts as an immediate early gene responding to serum stimulation [7]. It has been confirmed in many tissue types that Id3 up regulation promotes cell proliferation partially through inhibition of E-protein activities. Although Id3 is expressed in broad tissue types, Id3 seems to play a particularly important role in the lymphoid system. Id3−/− mice exhibit multiple defects in both B and T cell compartments. Humoral immune responses to T cell dependent antigens and type 2 T cell independent antigens were impaired in Id3−/− mice [8]. B cell proliferation upon BCR cross-linking by IgM treatment was also perturbed in Id3−/− mice. Analysis of Id3−/− mice also revealed a reduction of both CD4 and CD8 single positive T cell population and an enhancement of γδT cell population in the thymus [9,10]. Introduction of the AND or HY transgenic TCR into the Id3−/− background confirmed a necessary role for Id3 in both positive selection and negative selection [10].
In addition to the developmental defects, Id3−/− mice develop primary Sjögren’s syndrome-like symptoms [11]. Sjögren’s syndrome is a common rheumatic autoimmune disease with a prevalence of about 0.6% in the general population [12–14]. The main clinical features are persistent dry eyes and mouth, pathological score of focal infiltrates in salivary gland biopsy, and serum positive for multiple autoantibodies such as anti-Ro and anti-La. Id3−/− mice were found to exhibit a reduction of tear and saliva as early as two months of age, prior to any detectable sign of lymphocyte infiltration in the gland tissues. Lymphocyte infiltration in salivary and lachrymal glands can be detected at a later time around six months of age. Anti-Ro and anti-La auto-antibodies were detected in two third of mice after they have reached one year of age. This study also identified a T cell intrinsic role for Id3 contributing to disease development. However, depletion of B cells in Id3−/− by CD20 antibody treatment could also ameliorate the disease symptoms, indicating B cell involvement in disease pathology [15]. Given the broad expression pattern of Id3 in development and in most somatic tissues, it remains to be determined unambiguously whether loss function of Id3 in T cells accounts for the initial trigger of the autoimmune symptoms observed in Id3 knockout mice.
A recent study of human Id3 gene revealed no evidence of any germline mutations or unique polymorphisms in over 200 patients clinically diagnosed with primary Sjögren’s syndrome [16]. However, this study is not sufficient to rule out the possibility that acquired mutations or epigenetic changes in the lymphoid lineage may play a greater role than germline mutations in human patients. It is not known whether loss function of Id3 in lymphocytes could have played a primary role in the development of Sjögren’s syndrome. To further understand the role of Id3 in lymphocyte development and immune tolerance, we developed an Id3 floxed allele for conditional removal of Id3 in specific cell lineage. Studies presented here demonstrated that conditional disruption of Id3 in the developing T cells leads to impaired thymocyte selection and development of autoimmune symptoms similar to Sjögren’s syndrome.
Material and methods
Generation of Id3 conditional deletion allele
The targeting vector is composed of a 5.7 kb long arm and a 2.5 kb short arm sequences from the Id3 genomic sequence. The 5’ and 3’ loxP sequences were inserted upstream of the first exon and in the second intron, respectively. Pgk-Neo and Pgk-TK were used as the positive and negative selection marker, respectively (Fig.1A). The linearized targeting vector was introduced into W4/129S6 embryonic stem cells (Taconic). Genotyping PCR screen with primers Id3-1 and YZ29 (Table S1) identified 14 positive clones from 94 G418-resistant ES clones. These positive clones were subsequently confirmed by Southern analysis and their qualities were verified by karyotyping. Germ line transmission of the targeting allele was obtained from two independent ES clones. The Pgk-Neo cassette was then deleted by crossing the positive offspring to Actin-FLPe mice [17]. The resulting Id3 floxed allele (labeled Id3f) was crossed with LckCre transgenic mice for T lineage specific Id3 deletion [18]. Primer pairs Id3-2/Id3-3, Tek-F/Tek-B, and Flp-F/Flp-B were used for the genotyping PCR of Id3 loxP sites, Cre, and FLPe, respectively (Table S1). The details of genotyping PCR was described previously [19]. Mice were bred and maintained in the mouse facility of the Institute of Developmental Biology and Molecular Medicine following the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care and institutional regulations.
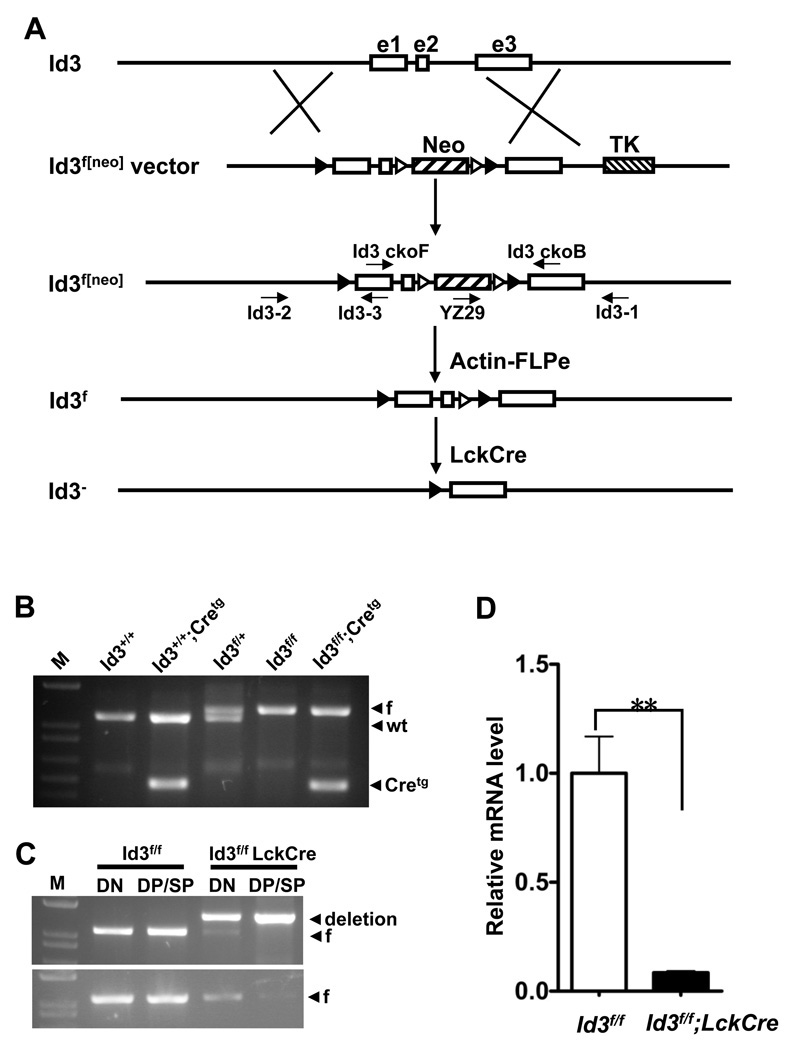
Figure 1. Conditional deletion of the Id3 gene in developing thymocytes.
(A) Generation of the Id3 conditional deletion allele. Open box: exon. Hatch box: selection markers. Solid arrowhead: loxP. Open arrowhead: FRT. Arrows: primers. (B) Representative genotyping results of the Id3f allele. DNA samples were prepared from mouse toes. 4 primers were used: Id3-2 and Id3-3 for the loxP (1.25 kb) and the wild type (1.15 kb) Id3 allele, Tek-F and Tek-R for the LckCre transgene (0.48 kb). M: 1 kb plus DNA ladder. (C) Deletion of Id3 in thymocytes sorted from 7-week-old mice. Thymocytes were separated into the DN (CD4−CD8−) and DP/SP (CD4+CD8+, CD4+CD8−, and CD4−CD8+) groups by magnetic beads. For the upper panel, the floxed and deletion alleles were determined by competitive PCR using three primers, Id3-2, Id3ckoF and Id3ckoB shown in (A). It produced a 1.07-kb band for the Id3f allele and a 1.34-kb band for the deleted allele. For the bottom panel, only two primers (Id3ckoF and Id3ckoB) were used to detect the floxed allele. (D) Real-time quantitative PCR analysis of Id3 expression. RNA was prepared from total thymocytes of 7-week old Id3f/f (open bar, n=4) and Id3f/f;LckCre (filled bar, n=3) mice. **P=0.006. The graphed results are means with SEM.
Antibody staining, FACS analysis and cell sorting
Single cell suspension from thymus, spleen and lymph node samples was prepared in PBS with 5% bovine calf serum. Approximately 106 cells were used for antibody staining. FACS antibodies include FITC-conjugated anti-CD4 (Caltag, 83-RM2501-1), PE-conjugated anti-CD8b (Caltag, 83-RM510-3), APC-conjugated anti-TCRβ (Caltag, 83-HM3605), FITC-conjugated anti-TCR γδ (Caltag, 83-HM3801), FITC-conjugated CD69 (Caltag, 83-HM4001), PE-conjugated anti-TCRβ (eBioscience, 85-12-5961-81), PE-conjugated CD44 (Caltag, 83-RM5704), PE-conjugated CCR7 (eBioscience, 85-12-1971-82), APC-conjugated anti-CD8b (Caltag, RM-5015), APC-conjugated anti-CD25 (Caltag, 83-RM6005), APC-conjugated CD62L (eBioscience, 17-0621), and APC-conjugated anti-B220 (Caltag, 83-RM2605). Seven-amino-actinomycin D (7AAD) (Invitrogen, A1310) was used to exclude dead cells. For CCR7 staining, the binding of CCR7 was in 37°C water bath for 30 min. Co-staining with other antibodies was carried out at 4°C for 30 min. FACS analysis was performed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.). For cell sorting, about 107 cells were stained by rat anti mouse CD4 and CD8 antibodies, then washed and resuspended. Magnetic beads conjugated-anti rat antibody (Invitrogen Cat. #. 110.35) were applied to the cell suspension for separation of DN cells from DP/SP cells.
Histological and pathological analysis
Tissues were embedded in OCT, frozen in liquid nitrogen-cooled isopentane. Slides of 8-µm tissue sections were stained according to the standard H&E staining protocol. Images were analyzed by a Leica DM RXA2 microscope, and photographs were taken by a Leica DC350F camera. Infiltration number was scored using the method described previously [11]. Basically, one infiltrate is defined as 50 or more nucleated cells in the cluster. The score for each mouse is the total infiltrates present in two histological sections prepared from one facial side including one parotid gland and one submandibular gland.
Quantitative real-time RT-PCR
Total RNA was prepared with TRIzol (Invitrogen, Cat#. 15596-018). DNase I treatment and reverse transcription were carried out with TaKaRa RNA PCR Kit (AMV) according to the manufacturer’s instructions. Quantitative RT-PCR was performed and analyzed on an Mx3000P Quantitative PCR System (Stratagene) using the ABsolute QPCR SYBR Green Mixes (ABgene, Cat#. AB-1166/b) according to the standard protocol. The primers of Id3 were mId3QPCRF and mId3QPCRB. The primers for the β-actin internal control were Actb-L1 and Actb-R1 (Table S1)
Saliva secretion test
Saliva secretion test was performed according to the procedures previously described [11]. In brief, mice were anesthetized by intraperitoneal injection of Avertin (Sigma cat#. T4, 840-2). Pilocarpine hydrochloride (Sigma, Cat#. P-6503) was administrated to mice intraperitoneally with 0.5 µg/g of body weight. Saliva was collected for 9 min and measured using 100µl-sized microcapillary pipet (VWR, cat#. 53432-921). The volume of saliva was normalized by body weight.
Statistical analysis
Statistical significance was determined by the unpaired student’s t-test.
Results
Establishing the Id3 conditional knockout mice
Mice carrying a germline mutation in the Id3 gene develop autoimmune symptoms similar to human primary Sjögren’s syndrome [11]. This study also indicated a dominant role for Id3 deficient T cells in disease development. However, the study of T cell function is complicated by the fact that functions of other cells such as B cells are also affected by Id3 disruption. To further investigate the lineage and stage specific function of Id3 in this disease model, we generated a floxed Id3 allele using Cre-loxP and FRT-FLPe systems. Briefly, two loxP sequences were introduced into the Id3 locus flanking the first two exons, which contain the entire coding region of the Id3 protein. A Pgk-Neo expression cassette flanked by two FRTs was inserted in the second intron to provide a positive selection during transfection of ES cells (Figure 1A). After successful germ-line transmission of the targeted ES clones, the Pgk-Neo cassette was deleted by Flp/FRT mediated recombination in mice expressing an Actin-Flpe transgene [17]. The resulting Id3f strain carried the Id3 floxed allele to be used for subsequent conditional knockout studies. Id3f/f homozygous mice were viable and phenotypically indistinguishable from wild type or heterozygous littermates.
To delete the Id3 gene specifically in developing T cells, we crossed the Id3f mice with LckCre transgenic mice [20]. The earliest activation phase for the LckCre transgene was mapped to the DN2 stage and the expression of Cre was restricted within T cell lineage [18]. The Id3f/f mice were used as controls in the following experiments. The primer pair Id3-2 and Id3-3 was used to detect the 5’ loxP site and the primer pair Tek-F and Tek-R was used to detect the LckCre transgene (Table S1). These primers were used in the same PCR reaction to yield a 1.25 kb and a 1.15 kb product representing the Id3f and the wild type allele, respectively. The PCR product of the LckCre transgene was 0.48 kb (Figure 1B). To examine the efficiency of LckCre induced deletion of Id3, we designed a three-primer competitive PCR assay (Id3ckoF, Id3ckoB and Id3-2) to reveal both the floxed and the deleted allele simultaneously. PCR test of total thymocytes isolated from Id3f/f and Id3f/f;LckCre mice showed exclusively the floxed band and the deletion band, respectively (Figure S1). A mix of equal amount of thymocyte DNA from Id3f/f;LckCre mice and Id3f/f mice resulted in approximately equal amount of the deletion and floxed bands in the three-primer reaction (Figure S1). Thus, the two alleles were detected with similar efficiency in this PCR test. Thymocytes from Id3f/f;LckCre mice and Id3f/f mice were separated into DN (CD4−CD8−) and DP/SP (CD4+CD8+, CD4+CD8−, and CD4−CD8+) fractions by Dynal bead sorting. DN cells were enriched from less than 5% to more than 90% after bead depletion (Supplemental Figure 1B). Using the three-primer PCR test, we found that deletion efficiency increased as thymocytes mature from the DN stage to the DP/SP stage (Figure 1 C upper panel). PCR reaction with primer pairs exclusive for the floxed allele further confirmed a substantial loss of the floxed allele among the DP/SP cells in the presence of the LckCre transgene (Figure 1C, bottom panel). Because DP cells represent approximately 80% of the DP/SP fraction, we concluded that the deletion of Id3 was completed in a large fraction of cells reaching the DP stage. To verify the inactivation of Id3 at the transcriptional level, mRNA from total thymocytes of Id3f/f;LckCre mice and the Cre negative controls was prepared for quantitative RT-PCR analysis of Id3 transcripts. Consistent with the deletion in the Id3 gene from the genomic sequence, the expression level of Id3 mRNA in LckCre transgenic background was found to be decreased to less than 10% of control littermates (Figure 1 D).
Deletion of Id3 in developing T cells perturbs T cell selection and maturation
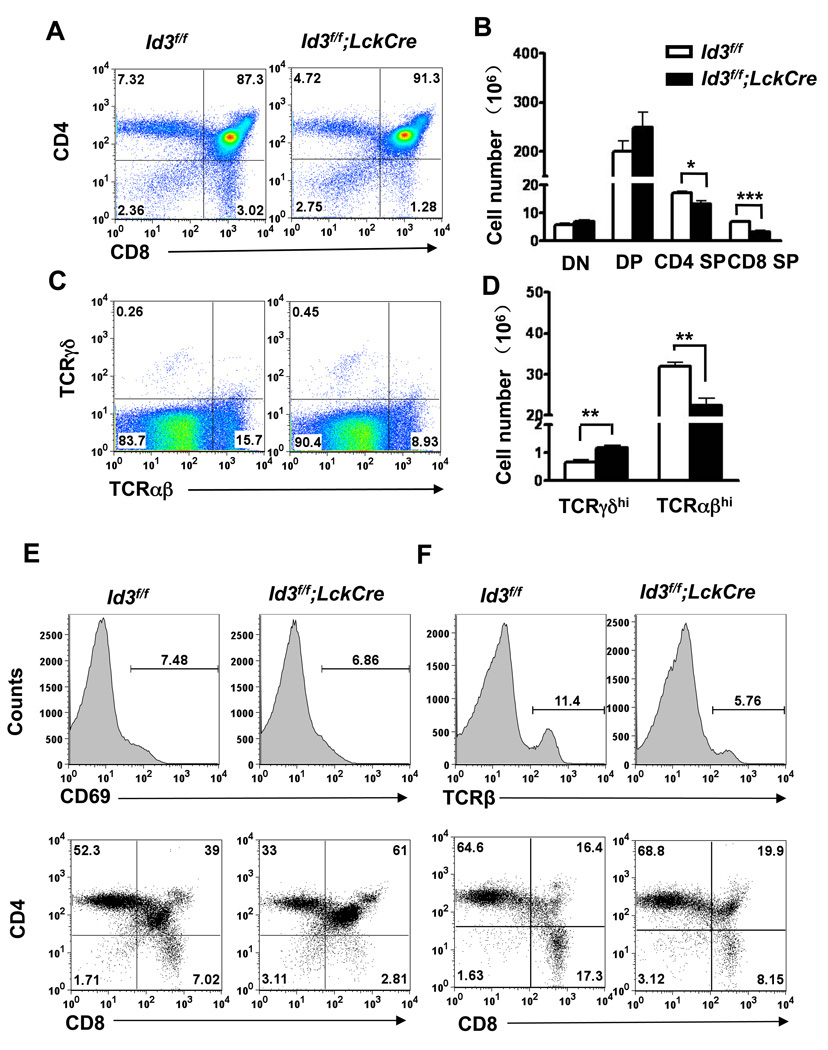
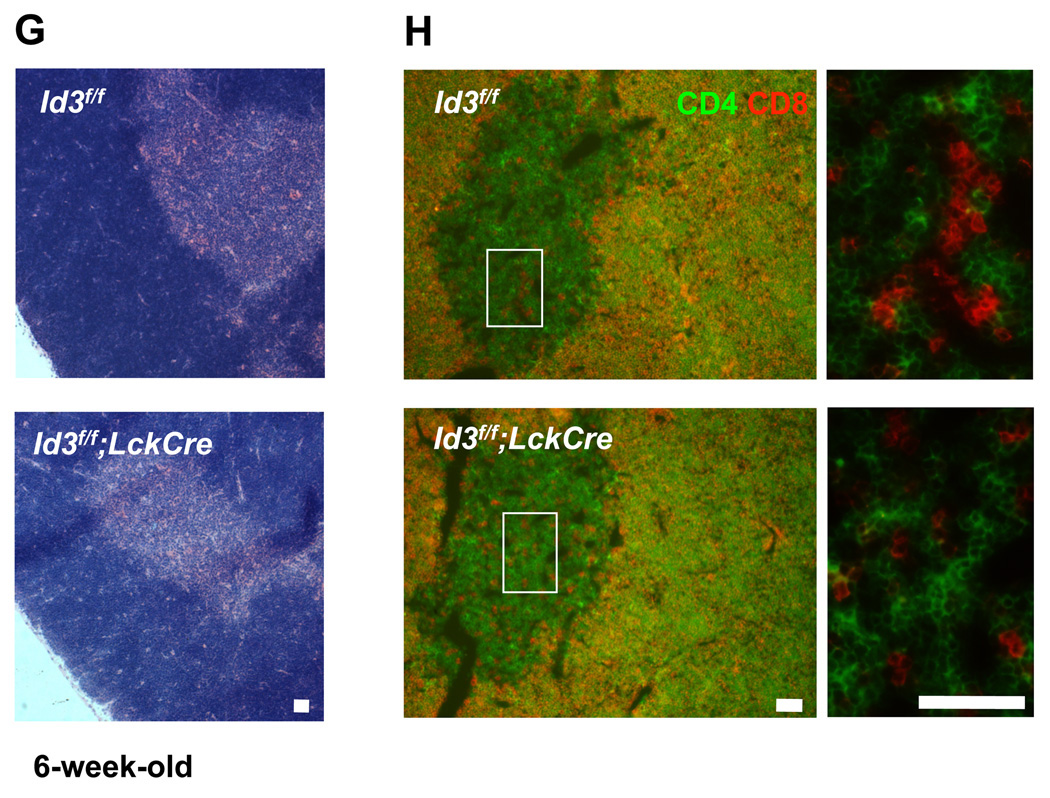
Id3 has been reported to play an important role during T cell selection and maturation [10]. More recently, we have reported that Id3 deficiency promotes γδT cell development in the C57Bl/6 background [9]. Analysis of thymocytes collected from Id3f/f;LckCre mice showed that CD4 SP cells and CD8 SP cells were decreased both in total cell numbers and relative proportion to the DP cells (Figure 2 A and B). The FACS analysis also revealed a moderate increase of γδ T cells and a decrease of mature αβ T cells in the thymus (Figure 2 C and D). DP cells undergoing positive selection are accompanied by increased expression of CD69 and TCRβ. As SP cells further mature into naïve T cells, CD69 is down regulated whereas high level TCRβ expression is maintained. Thus, an accumulation of CD69 positive and TCRβ positive cells at the DP stage would indicate a delay in positive selection [10]. Analysis with CD69 and TCRβ gated population revealed a proportional increase in CD69 positive and TCRβ positive DP cells in Id3f/f;LckCre mice, suggesting a delay in T cell development from the DP to the SP stage (Figure 2 E and F). This result is consistent with the notion that Id3 plays a T cell intrinsic role in DP to SP selection [10]. Histological analysis of thymus section showed a grossly normal structure with a clear separation of the cortex and medulla areas (Figure 2 G). CD4 and CD8 SP cells were clearly visible in the medulla of Id3f/f;LckCre mice (Figure 2 H). Therefore, Id3 deficient SP cells are able to enter medulla where negative selection takes place.
Figure 2. Disturbed γδT and αβT cell development in the thymus of Id3f/f;LckCre mice.
(A) Representative FACS analysis of αβ thymocyte development by CD4 and CD8 expression. Thymocytes were isolated from 4–6 weeks old Id3f/f;LckCre (n=3) and the Id3f/f littermate (n=4) controls. Dead cells were excluded by 7AAD staining. (B) Summary of the absolute numbers of DN (CD4−CD8−), DP (CD4+CD8+), CD4 SP (CD4+CD8−), and CD8 SP (CD4−CD8+) thymocytes. *P=0.027. ***P=0.0001. The statistical significance was assessed by unpaired student’s t test. (C) Representative FACS plots of γδ and αβ thymocyte population in Id3f/f;LckCre mice and Id3f/f mice. (D) Summary of the absolute numbers of mature γδ and αβ thymocytes. **P=0.009 (γδT cells) and 0.003 (αβT cells). The graphed results are means with SEM. (E) The CD69hi cells in total thymocytes were gated for two-D plot analysis of CD4 and CD8 expression. (F) The TCRβhi thymocytes were gated for analysis of CD4 and CD8 expression. Results of (E) and (F) were representatives of four littermate pairs. (G) H&E staining of thymus section from Id3f/f;LckCre mice and Id3f/f mice. (H) Two-color immunofluorescence analysis of thymus section with CD4 and CD8 antibodies. The green and red signals are CD4 and CD8 SP thymocytes, respectively. The yellow signals are CD4+CD8+ DP thymocytes resulting from CD4 and CD8 double staining. The enlarged views of medulla are shown on the right. Scale bar equals 50 µm.
Id3f/f;LckCre mice develop Sjögren’s syndrome-like phenotypes
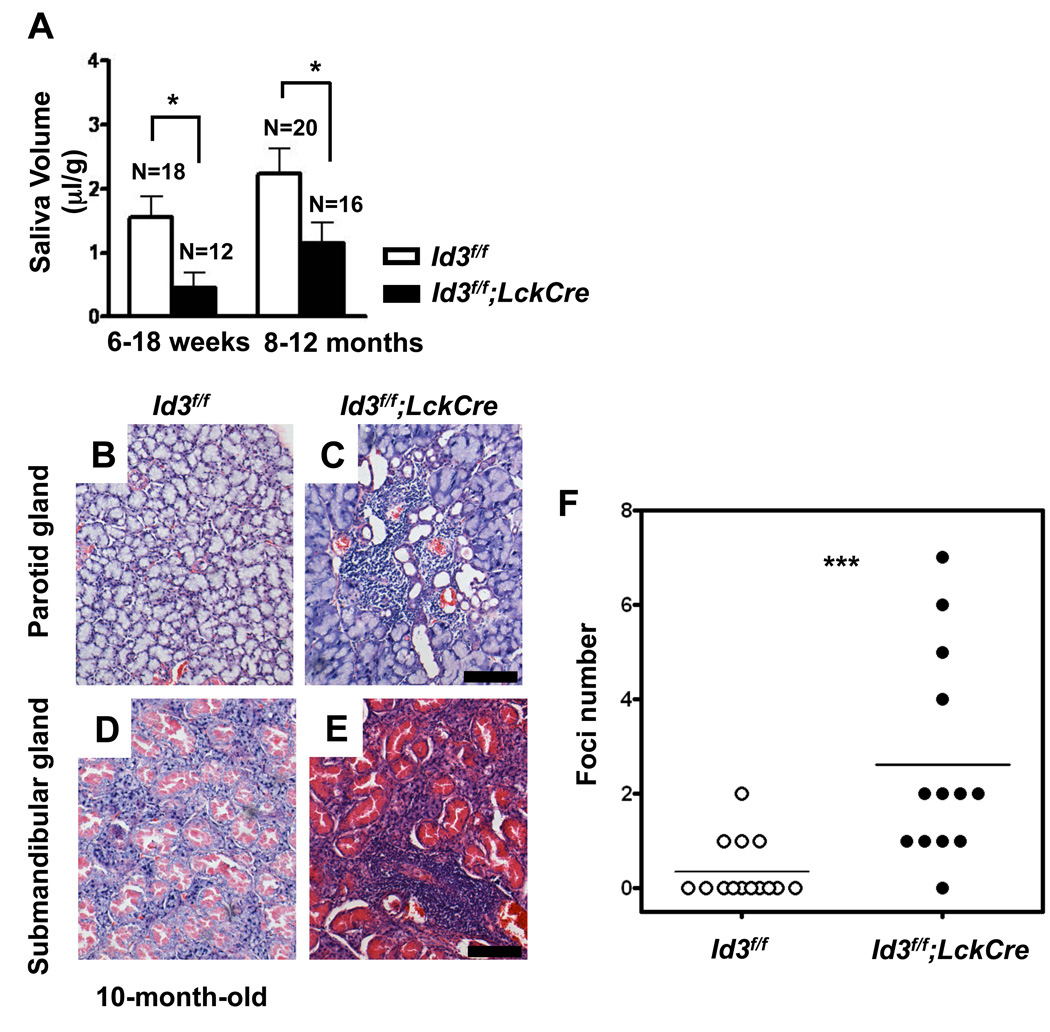
The Id3f/f;LckCre mouse model allowed us to explicitly evaluate the role of Id3 deficient T cells in disease development without any genetic or experimental perturbation of non-T cells [11]. Similar to the phenotypes observed in Id3−/− mice, a reduction of saliva secretion after pilocarpine stimulation was observed in both young and aged Id3f/f;LckCre mice (Figure 3 A). Salivary glands from aged mice (8–12 months old) were examined for the presence of infiltrates with H&E staining. Lymphocyte infiltrates were observed in parotid gland and submandibular gland from Id3f/f;LckCre mice at a frequency significantly higher than those of age matched controls (Figure 3 B–F). Meanwhile, no significant lymphocyte infiltration was observed in other organs such as kidney and lung among the same experimental groups (data not shown). Serum antibody analysis among a group of 8–13 month old mice (7 Id3f/f and 7 Id3f/f;LckCre mice) did not detect autoantibodies such as anti-Ro and anti-La (data not shown). These results suggested that deletion of Id3 in T cells is sufficient to induce most but not all clinical symptoms resembling primary Sjögren’s syndrome.
Figure 3. Sjögren’s syndrome-like symptoms in Id3f/f;LckCre mice.
(A) Decreased saliva secretion in Id3f/f;LckCre mice after pilocarpine stimulation. Id3f/f;LckCre mice were divided into young (6–18 weeks) and old (8–12 months) groups. The volume of saliva was determined after pilocarpine stimulation. Values were normalized by the body weight. *P=0.02 (young) and 0.046 (old). The graphed results are means with SEM. (B–E) H&E staining of parotid (B&C) and submandibular (D&E) salivary glands from 10-month-old mice. All the pictures were taken with the original magnification of 10×10. The scale bar equals 100µm. (F) Focus score of lymphocyte infiltrates in parotid and submandibular salivary glands of Id3f/f;LckCre (n=13) and Id3f/f mice (n=14) with age ranging from 8–12 month according to the method described previously [11]. **P=0.009. The significance was analyzed by two-tail unpaired student’s t test.
Absence of Id3 deficient T cells in gland tissues
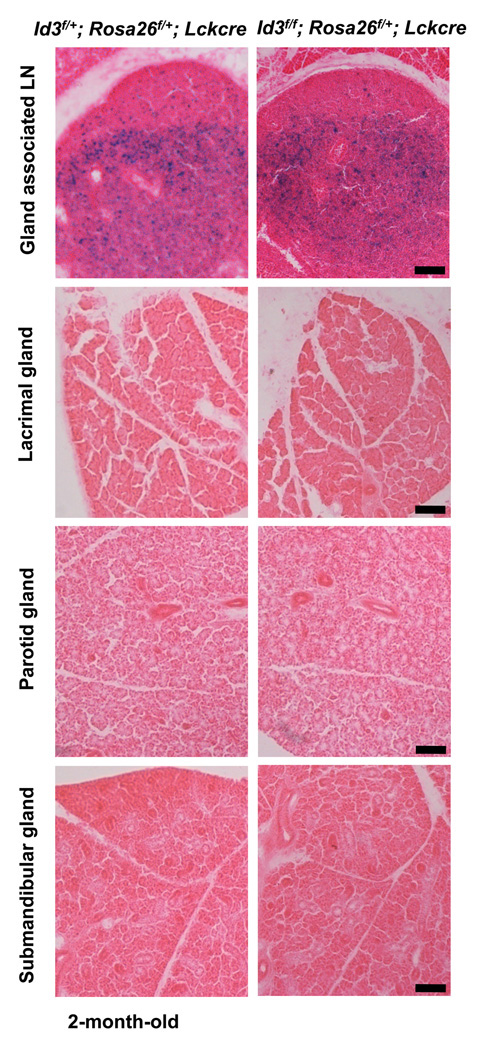
We have shown in previous studies that the impairment of gland function at the early age is not due to direct lymphocyte infiltration. However, we cannot rule out the possibility that gland function may be regulated by a small number of Id3 deficient T cells, which are undetectable by H&E staining. To further examine the possible interaction between Id3 deficient T cells and the gland tissues, we introduced the Rosa26-floxstop-LacZ allele (Rosa26f) as a lineage tracer to track Id3 deficient T cells in our conditional knockout mice [21]. Because LckCre mediated deletion of the Id3 gene is coupled with the activation of the LacZ marker, Id3 deficient T cells in the periphery tissues can be detected by LacZ expression. Frozen sections of gland-associated lymph node, parotid glands, submandibular gland, and lachrymal gland from two-month old mice were stained with the LacZ substrate X-gal. While LacZ positive T cells were clearly seen in gland-associated lymph node, these labeled cells were absent in parotid glands, submandibular glands, and lachrymal glands (Figure 4). We thus conclude that Id3 deficient T cells may affect exocrine function without focal infiltration into the gland tissues.
Figure 4. Absence of Id3-deficient T cells in gland tissues of young adult Id3f/f;LckCre mice.
Id3 deficient T cells were traced with a Cre reporter in 2 month old Id3f/f;Rosa26f/+;LckCre and Id3f/+;Rosa26f/+;LckCre control mice. Tissue section (20 µm) of gland-associated lymph node, parotid salivary gland, submandibular gland, and lachrymal gland were stained with x-gal. The slides were counterstained by eosin. The scale bar equals 100 µm.
Accumulation of activated/memory T cells occurs in aged Id3f/f;LckCre mice
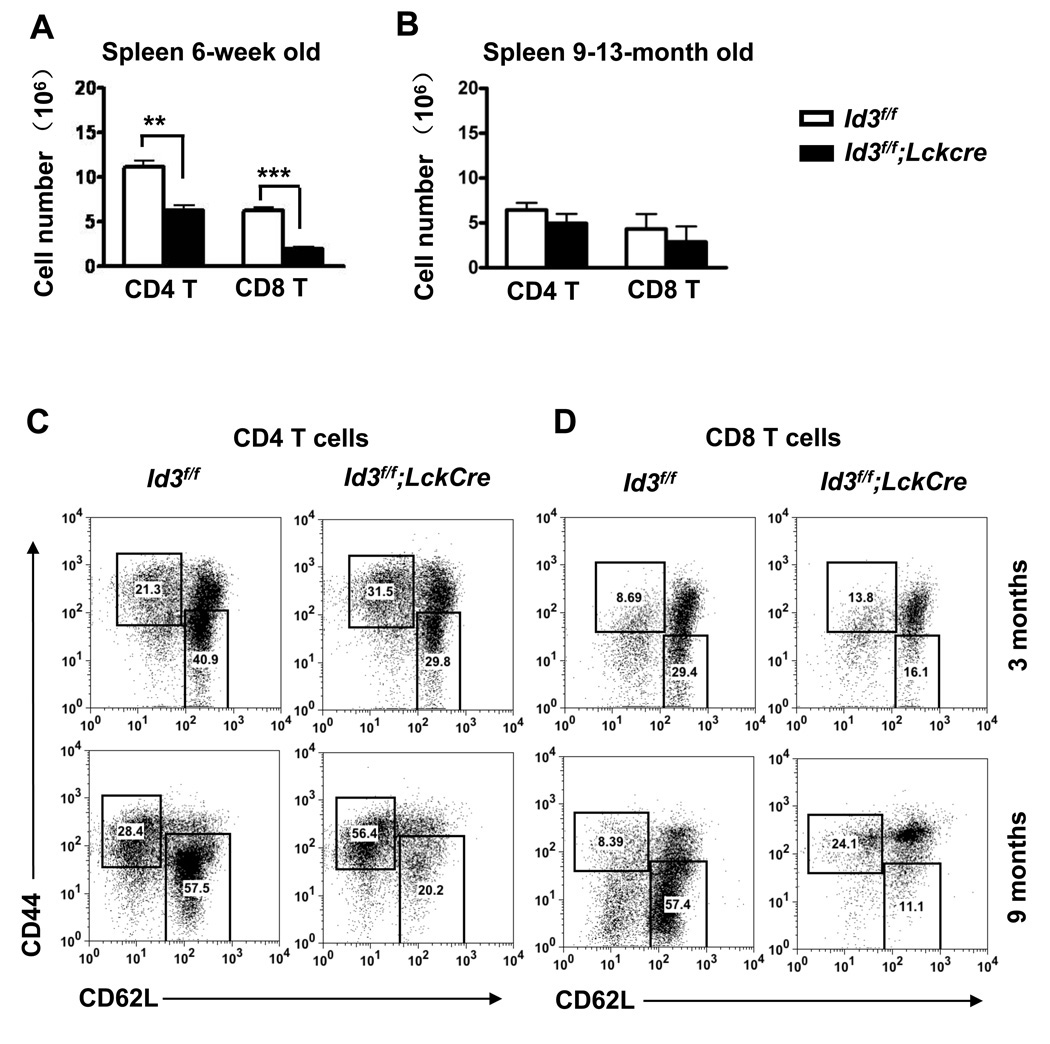
Chronic autoimmune conditions are often accompanied with an accumulation of activated and/or memory T cells [22,23]. We examined T cell populations in the spleen and lymph nodes of young adult Id3f/f;LckCre mice (6-week old). The percentage and absolute number of CD4 and CD8 T cells were significantly decreased in the spleen (Figure 5 A). However, no difference was found in the cell numbers of CD4 and CD8 T cells in lymph node (data not shown). Among aged mice numbers of T cells in the spleen were similar between Id3f/f;LckCre and Id3f/f control groups (Figure 5 B). We next examined the activation/memory status of T cells using CD44 and CD62L markers. Most T cells in young adult mice are in naïve state expressing low level of CD44 (CD44low) and high level of CD62L (CD62Lhigh). Memory T cells expressing high level of CD44 (CD44high) and low level of CD62L (CD62Llow) may accumulate with age and especially under chronic stimulation of the immune system. The relative percentage of CD4 and CD8 CD44high CD62Llow memory T cells in Id3f/f controls were only slightly increased and unchanged, respectively, with age (Figure 5 C and D). In contrast, Id3f/f;LckCre mice exhibited a greater shift from naïve to memory phenotype for both CD4 and CD8 T cells from 3 months to 9 months of age (Figure 5 C and D). This age dependent shift in T cell phenotype correlates with the development of lymphocyte infiltrates in gland tissues.
Figure 5. Accumulation of activated/memory T cells in aged Id3f/f;LckCre mice.
(A) Numbers of CD4 and CD8 T cells in the spleen from 6-week old Id3f/f;LckCre (n=3) and Id3f/f mice (n=4). The graphed results were means with SEM. The significance was analyzed by two-tail unpaired student’s t test. **P=0.002. ***P=0.0003. (B) Numbers of CD4 and CD8 T cells in the spleen of 9–13 month old Id3f/f;LckCre (n=4) and Id3f/f mice (n=3). Analysis was performed as in (A). No significant difference was observed between the genotype groups. (C,D) FACS plots of activated/memory T cells (CD44hiCD62Llow, the upper left rectangle gate) and naïve T cells (CD44lowCD62Lhi, the lower right rectangle gate) among CD4 T cells (C) and CD8 T cells (D). Gated CD4 or CD8 lymphocytes are from spleen of 3-month old (upper panel of C and D) and 9-month old mice (bottom panel of C and D). Results are representative of at least three mice for each age and genotype group.
Discussion
Sjögren’s syndrome is an autoimmune disease with limited knowledge of genetic basis [24]. Id3 deficient mice represent one of few animal models to recapitulate the disease symptoms [11]. T cells have been implicated to play important roles in disease development in this model. It has been shown that Id3 deficient T cells can act dominantly to suppress gland function when adoptively transferred to the wild type hosts. However, this suppressive role only lasted a couple of weeks and the long-term impact of adoptive transfer has not been evaluated due to the complexity of the adoptive transfer system. The importance of T cells in this disease model is further supported by the fact that Id3 deficient mice do not develop the disease symptoms when T cell development is blocked by deletion of the LAT gene, which encodes a signaling adaptor for pre-TCR and TCR. However, neither of these experiments is sufficient to demonstrate that loss function of Id3 in T cells alone is sufficient to account for the disease symptoms reported in Id3 deficient mice. Here, our T lineage conditional knockout system provides direct evidence that Id3 deficient T cells play a primary role in promoting the disease symptoms in Id3 deficient mice.
A leading hypothesis for the disease mechanism of Id3 deficient mice is that loss function of Id3 in developing T cells breaks the central tolerance. Id3 may directly regulate positive selection, negative selection, and thymocyte migration. First, Id3 is known to play particularly important roles downstream of the TCR signal during the transition from DP to SP stage of thymoycte development [10]. TCR signals activate Id3 via the Erk signaling pathway [3]. The primary role for Id3 is to inhibit E-protein function. Downregulation of E-protein activity and then expression lead to thymocyte maturation into SP cells [25]. In our conditional Id3 knockout model, this maturation process is significantly impaired leading to inefficient positive selection, confirming what has been observed in germline Id3 knockout mice. It is conceivable that Id3 deletion may affect positive selection by shifting the TCR repertoire toward higher affinity. Consequently, T cells with higher affinity to certain self antigens may be positively instead of negatively selected. Second, the development of autoreative T cells may also be due to a requirement for Id3 in negative selection. It has been shown that negative selection against the male specific antigen HY is altered in HY specific TCR transgenic mice on the Id3 deficient background [10]. Although the exact mechanism for Id3 function in negative selection is not known, Id3 has been implicated to play a role in activation of several caspase genes in other cell types [26–28]. It remains to be determined whether Id3 plays any role in regulating apoptosis during negative selection. Another way Id3 may perturb negative selection is through regulation of chemokine receptor expression and therefore thymocyte migration from cortex to medulla where negative selection takes place. Indeed, the level of CCR7 expression was found prematurely enhanced in DP thymocytes after removal of E-proteins in developing thymocytes [25] and moderately reduced in SP thymocytes from Id3 deficient mice (data not shown). CCR7 deficient mice have been shown to develop autoimmune symptoms similar to Sjögren’s syndrome [29]. However, thymic architecture of Id3f/f;LckCre mice was found grossly normal, implying that the moderate change in CCR7 expression is not sufficient to block the migration of most SP cells from cortex to medulla. At present, our study is not sufficient to rule out the possibility of inappropriate migration involving a small subset of thymocytes.
It is possible that Id3 also plays important roles in T cell homeostasis, activation, effector function, or development and function of T regulatory (Treg) cells. We found Id3f/f;LckCre mice exhibit a reduced number of T cells in the spleen. However, no significant phenotype change can be detected for these Id3 mutant T cells at the young age when the mice begin to show impaired secretory function. This result is reminiscent of the study of Id3 germline knockout mice. Our study also revealed a gradual change in T cell phenotypes as Id3f/f;LckCre mice became older. T cell mediated chronic conditions may result in an elevation of inflammatory cytokines. However, an analysis of serum cytokine profile from a group of 8–12 month old mice (10 Id3f/f and 11 Id3f/f;LckCre mice) failed to detect any obvious change in the level of interferon γ, IL2, and TNF α(data not shown). Finally, we found normal number of Treg cells present in the aged Id3f/f;LckCre mice, indicating that Id3 is not absolutely essential in the development of Treg cells (Supplemental figure 2). Our studies thus far are not sufficient to rule out the possibility that Id3 is involved in the effector function of conventional and/or regulatory T cells. The disease mechanism would require additional analysis of function of Id3 deficient T cells and other relevant effector cells. However, functional study of Id3 deficient T cells must consider the fact that developmental defect in the thymus may impact T function later in life. The conditional knockout model with the LckCre transgene cannot separate the role of Id3 in early development vs. later function in mature T cells. Therefore, the exact role of Id3 in mature T cells would require further investigation with additional Cre system, which allows for removal of Id3 specifically in the mature T cells.
Although the phenotypic analysis of Id3 conditional knockout revealed many developmental and disease features similar to that of Id3 germline knockout, a discrepancy has also been noticed. An assessment of autoantibodies with 8–13 month old mice did not detect any anti-Ro or anti-La antibodies. In contrast, anti-Ro and anti-La antibodies have been detected in 67% Id3 germline knockout mice at age 12 months or older [11]. Because the appearance of autoantibodies is associated with the older age of Id3 knockout mice, we do not know whether T lineage specific Id3 conditional knockout mice do not produce or are slow to produce autoantibodies. The role of Id3 in humoral immunity may be further investigated after removal of Id3 specifically in B cells or simultaneously in both T and B cells using the conditional allele.
In conclusion, our results demonstrated a T cell intrinsic role for Id3 in regulating thymocyte development and maintaining tolerance. Our study also suggested that Id3 deficient T cells could modulate gland secretory function without a massive infiltration into gland tissues. The experimental system established in our study provides a valuable mouse model for further investigating the molecular and cellular mechanism of primary Sjögren’s syndrome.
Supplementary Material
Acknowledgements
We thank Yanling Yang, Yanfeng Tan, Chunyan Gao, and Xiaoping Huang for technical assistance in generating and maintaining Id3 conditional knockout mice; Juan Liu and Xuetao Cao of Second Military Medical University for assistance in measuring serum cytokine; Wufan Tao, Kejing Deng, Ling Sun, and Beibei Ying for advice and assistance. This study was supported by National Basic Research Program of China (973) Grant 2006CB806700, National Hi-tech Research and Development Program of China (863) Grant 2007AA022101, National Natural Science Foundation of China Grants 30228014 and 30671109, Shanghai Rising-Star Program Grant 06QA14006, and the 211 and 985 projects of the Chinese Ministry of Education. This study was partially supported by Arthritis Foundation and NIH funds to Y.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.von Boehmer H, Kisielow P. Negative selection of the T-cell repertoire: where and when does it occur? Immunol Rev. 2006;209:284–289. doi: 10.1111/j.0105-2896.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 3.Bain G, Cravatt CB, Loomans C, Alberola-Ila J, Hedrick SM, Murre C. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat Immunol. 2001;2:165–171. doi: 10.1038/84273. [DOI] [PubMed] [Google Scholar]
- 4.Quong MW, Romanow WJ, Murre C. E protein function in lymphocyte development. Annu Rev Immunol. 2002;20:301–322. doi: 10.1146/annurev.immunol.20.092501.162048. [DOI] [PubMed] [Google Scholar]
- 5.Lazorchak A, Jones ME, Zhuang Y. New insights into E-protein function in lymphocyte development. Trends Immunol. 2005;26:334–338. doi: 10.1016/j.it.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Rivera R, Murre C. The regulation and function of the Id proteins in lymphocyte development. Oncogene. 2001;20:8308–8316. doi: 10.1038/sj.onc.1205091. [DOI] [PubMed] [Google Scholar]
- 7.Christy BA, Sanders LK, Lau LF, Copeland NG, Jenkins NA, Nathans D. An Id-related helix-loop-helix protein encoded by a growth factor-inducible gene. Proc Natl Acad Sci U S A. 1991;88:1815–1819. doi: 10.1073/pnas.88.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan L, Sato S, Frederick JP, Sun XH, Zhuang Y. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol. 1999;19:5969–5980. doi: 10.1128/mcb.19.9.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda-Hayakawa I, Mahlios J, Zhuang Y. Id3 restricts the developmental potential of gamma delta lineage during thymopoiesis. J Immunol. 2009;182:5306–5316. doi: 10.4049/jimmunol.0804249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivera RR, Johns CP, Quan J, Johnson RS, Murre C. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity. 2000;12:17–26. doi: 10.1016/s1074-7613(00)80155-7. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Dai M, Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren's syndrome. Immunity. 2004;21:551–560. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Fox RI. Sjogren's syndrome: immunobiology of exocrine gland dysfunction. Adv Dent Res. 1996;10:35–40. doi: 10.1177/08959374960100010601. [DOI] [PubMed] [Google Scholar]
- 13.Fox RI, Stern M, Michelson P. Update in Sjogren syndrome. Curr Opin Rheumatol. 2000;12:391–398. doi: 10.1097/00002281-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Fox RI, Tornwall J, Michelson P. Current issues in the diagnosis and treatment of Sjogren's syndrome. Curr Opin Rheumatol. 1999;11:364–371. doi: 10.1097/00002281-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Hayakawa I, Tedder TF, Zhuang Y. B-lymphocyte depletion ameliorates Sjogren's syndrome in Id3 knockout mice. Immunology. 2007;122:73–79. doi: 10.1111/j.1365-2567.2007.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sellam J, Miceli-Richard C, Gottenberg JE, Proust A, Ittah M, Lavie F, Loiseau P, Mariette X. Is Inhibitor of differentiation 3 involved in human primary Sjogren's syndrome? Rheumatology (Oxford) 2008;47:437–441. doi: 10.1093/rheumatology/ken013. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 18.Pan L, Hanrahan J, Li J, Hale LP, Zhuang Y. An analysis of T cell intrinsic roles of E2A by conditional gene disruption in the thymus. J Immunol. 2002;168:3923–3932. doi: 10.4049/jimmunol.168.8.3923. [DOI] [PubMed] [Google Scholar]
- 19.Yan W, Young AZ, Soares VC, Kelley R, Benezra R, Zhuang Y. High incidence of T-cell tumors in E2A-null mice and E2A/Id1 double-knockout mice. Molecular and Cellular Biology. 1997;17:7317–7327. doi: 10.1128/mcb.17.12.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennet T, Hagen FK, Tabak LA, Marth JD. T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc Natl Acad Sci U S A. 1995;92:12070–12074. doi: 10.1073/pnas.92.26.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 22.Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? The Journal of experimental medicine. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theofilopoulos AN, Dummer W, Kono DH. T cell homeostasis and systemic autoimmunity. J Clin Invest. 2001;108:335–340. doi: 10.1172/JCI12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cobb BL, Lessard CJ, Harley JB, Moser KL. Genes and Sjogren's syndrome. Rheum Dis Clin North Am. 2008;34:847–868. doi: 10.1016/j.rdc.2008.08.003. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones ME, Zhuang Y. Acquisition of a functional T cell receptor during T lymphocyte development is enforced by HEB and E2A transcription factors. Immunity. 2007;27:860–870. doi: 10.1016/j.immuni.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trabosh VA, Daher A, Divito KA, Amin K, Simbulan-Rosenthal CM, Rosenthal DS. UVB upregulates the bax promoter in immortalized human keratinocytes via ROS induction of Id3. Exp Dermatol. 2009;18:387–395. doi: 10.1111/j.1600-0625.2008.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kee BL. Id3 induces growth arrest and caspase-2-dependent apoptosis in B lymphocyte progenitors. J Immunol. 2005;175:4518–4527. doi: 10.4049/jimmunol.175.7.4518. [DOI] [PubMed] [Google Scholar]
- 28.Simbulan-Rosenthal CM, Daher A, Trabosh V, Chen WC, Gerstel D, Soeda E, Rosenthal DS. Id3 induces a caspase-3- and -9-dependent apoptosis and mediates UVB sensitization of HPV16 E6/7 immortalized human keratinocytes. Oncogene. 2006;25:3649–3660. doi: 10.1038/sj.onc.1209407. [DOI] [PubMed] [Google Scholar]
- 29.Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, Arakaki R, Hayashi Y, Kitagawa T, Lipp M, Boyd RL, Takahama Y. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24:165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.