Summary
The second messenger cAMP-dependent protein kinase A (PKA) plays an important role in the various cellular and physiological responses. On the sarcoplasmic reticulum (SR) in cardiomyocytes, PKA regulates the calcium cycling for exciting-contraction coupling, which is often dysfunctional in a variety of heart diseases including heart failure. Here, we have developed a novel FRET-based A-kinase activity biosensor (AKAR), termed SR-AKAR3, to visualize the PKA dynamics on the SR. Activation of adrenergic receptor induces a rapid and significant increase in SR-AKAR3 FRET ratio, which is dependent on agonist occupation of the receptor and inhibited by H-89, a PKA inhibitor. Interestingly, direct activation of adenylyl cyclases or application of a cAMP analog 8-br-cAMP induced much slower and smaller increases in SR-AKAR3 FRET ratio. These data indicate that the signaling induced by adrenergic stimulation displays a preferential access to the SR in comparison to those by direct activation of adenylyl cyclases. More, SR-AKAR3 mimics endogenous protein phospholamban on the SR for PKA-mediated phosphorylation and myocyte contraction response under adrenergic stimulation. Together, this new PKA activity biosensor provides a useful tool to directly visualize the dynamic regulation of PKA activity on the SR in cardiomyocytes under various physiological and clinical conditions.
1. Introduction
In cardiac muscle cells, sarcoplasmic reticulum (SR) represents one of the most important subcellular organelles for contraction. As the major site of calcium storage, it controls intracellular free calcium concentrations through ryanodine receptor (RyR)-mediated release and sarco/endoplasmic reticulum Ca2+-ATPase (SERCA)-mediated re-uptake. The activities of ion channels can be modulated by protein phosphorylation via hormone-induced signaling transduction. One of the major kinase systems involved in regulating calcium homeostasis in the SR is the cAMP-dependent PKA. Under stressing conditions, hormone-induced cAMP/PKA signaling promotes phosphorylation of channels and associated regulatory proteins, which increases channels activities to promote cardiac contraction. Such regulations are highly coordinated to ensure precise calcium handling for excitation-contraction (EC) coupling. Abnormal phosphorylation can lead to increased channel activities, disruption of calcium homeostasis and EC coupling. For example, increasing PKA phosphorylation of phospholamban (PLB), a binding protein of SERCA, releases its inhibitory effect on the pump and accelerates sequestration of calcium into the SR [1], which can lead to cardiac arrhythmias and potentially cardiac arrest [2]. Contrastingly, in the RyR 2 complex lacking phosphodiesterase 4D3, the increasing PKA phosphorylation of RyR2 leads to chronic leakage of calcium into the cytosol, which promotes arrhythmias, as well as heart failure by triggering apoptotic cell death pathways [2; 3; 4].
cAMP-dependent PKA is a heterotetramer composed of two regulatory and two catalytic subunits in its inactive state. It regulates signaling pathways involved in a broad range of cellular processes in cardiomyocytes [5]. cAMP activates PKA by binding to the regulatory subunits, causing release of the activated catalytic subunits and phosphorylation of serine and threonine residues on specific substrate proteins [5]. It’s now well accepted that the cAMP/PKA signaling machinery is organized into signaling complexes and distributed in the discrete compartments in cardiomyocytes [6; 7; 8]. These complexes are organized by A-kinase anchoring proteins (AKAPs) that tether inactive protein kinase A (PKA) holoenzymes and other signaling molecules, which work as cellular focal points for the spatial propagation of the specific cAMP signal to defined subcellular locations, including the SR in cardiomyocytes [7; 9].
Upon stimulation of G protein-coupled receptors by hormones, the activated receptors couple to Gs protein to stimulate adenylyl cyclases (ACs) on the plasma membrane to produce cAMP, which is a small and diffusible molecule. However, the temporal and spatial propagation of the cAMP signal are controlled by a family of phosphodiesterases (PDEs), which hydrolyze cAMP to attenuate the signal. In many cases, PDEs directly associate with PKA complexes, and regulate the action of the localized cAMP/PKA activities in cells [8]. In cardiomyocytes, the propagation of cAMP/PKA signals to the SR is critical for excitation-contraction coupling by promoting phosphorylation of substrates including PLB, RyR, and SERCA. Accumulating evidence points out that dysregulation of PKA activity in the SR is associated with different heart diseases [10]. The cAMP/PKA signaling and its targeted proteins on the SR thus become potential targets for treatment of the heart diseases. However, due to the lack of a convenient approach to directly access the SR, the mechanism governing the PKA activity for substrate phosphorylation on the SR is not well understood.
Recent progress with genetically encoded fluorescent biosensors based on fluorescence resonance energy transfer (FRET) has greatly facilitated our understanding of the dynamics of intracellular signaling properties. Biosensors have been developed to monitor the activities of cAMP [8] and PKA [11] in living cells. Here, we aim to develop a genetically encoded PKA biosensor to directly measure PKA activities on the SR in cardiomyocytes.
2. Materials and Methods
2.1. cDNA and Adenovirus Construction
The regular PKA activity biosensors AKAR3 [12] was used to fuse with the helical transmembrane domain (PQQARQKLQNLFINFCLILICLLLICIIVMLL) of PLB [13; 14] to generate a SR membrane-anchored AKAR3, termed SR-AKAR3. The construct in pcDNA3.1 (Invitrogen, CA) was verified by DNA sequencing for mammalian expression. The SR-AKAR3 cDNA was subcloned into the shuttle vector pAdTrack-CMV to generate recombinant adenovirus according to the manufactor’s instruction (Quantum Biotechnologies). The expression of SR-AKAR3 by viral infection was determined in HEK293 cells.
The FRET ratio of SR-AKAR3 could be affected by potential oligomerization of PLB transmembrane domain via oxidative crosslinking of cysteine residues [15; 16], which potentially increase intermolecular FRET between SR-AKAR3. We then introduced mutation on cysteine residues on PLB transmembrane domain SR-AFA-AKAR3 to reduce potential oligomerization and intermolecular FRET between SR-SKAR3. In addition, we have also added a flexible linker SGGGGGS between the cysteine-null transmembrane domain and AKAR3 to generate SR-AFA-SG-AKAR3 to increase the flexibility and mobility of CFP and YFP on the probe. Western blot confirmed no significant difference in protein composition when expressed in neonatal cardiac myocytes; and these three different versions of SR-AKAR3 biosensors displayed identical response to isoproterenol or forskolin stimulation (data not shown). Therefore, these data support that SR-AKAR3 is targeted on the SR and sensitive to adrenergic stimulation in cardiomyocytes.
2.2. Neonatal and Adult Cardiac Myocyte Culture and Adenovirus Infection
Neonatal and adult cardiac myocytes were isolated from new-born wild type FVB pups and 2 months old mice as previously described [17], and cultured in 35-mm dishes for protein phosphorylation and contraction assay or on glass coverslip chambers pre-coated with laminin for imaging. Adult myocytes were infected with adenovirus (moi 100) to express the PKA activity biosensor SR-AKAR3 right after cell plating. Neonatal myocytes were culture for 24 hrs before infection with adenoviruses (moi 100) in serum-free Dulbecco’s modified Eagle’s/F12 media. Relevant experiments were carried out after expression for 24h.
2.3. Immunofluorescence Microscopy
Cardiomyocytes expressing SR-AKAR3 PKA biosensor were fixed for immunofluorescence imaging. Cells were stained with the anti-α and β ryanodine receptors antibody (RyR2, obtained from the DSHB, the University of Iowa, IA), which was visualized with alexa 488-conjugated anti-mouse antibody (Molecular Probes). SR-AKAR3 was visualized with CFP and YFP channels. Myocyte images were obtained using a Zeiss Axioplan 2 microscope with Metamorph software (Universal Imaging).
2.4. Fluorescent Resonance Energy Transfer (FRET) Measurement
Myocytes expressing PKA biosensor were rinsed and maintained in PBS with calcium for FRET recording. Cells were imaged on a Zeiss Axiovert 200M microscope with a 40×/1.3NA oil-immersion objective lens and cooled CCD camera. Dual emission ratio imaging was acquired with a 420DF20 excitation filter, a 450DRLP dichroic mirror, and two emission filters (475DF40 for cyan and 535DF25 for yellow). The acquisition was set with 200 millisecond exposure in both channels and 20 second elapses. Images in both channels were subjected to background subtraction, and ratios of yellow-to-cyan color were calculated at different time points.
2.5. Contraction Rate Assay
Measurement of spontaneous neonatal myocyte contraction rate was carried out as previously described [17]. Responses in myocyte beating rate after drug treatments were recorded and analyzed by Metamorph software.
2.6. Drug treatments
Myocytes were stimulated with isoproterenol (ISO, 10 μM, Sigma), forskolin (FSK, 10 μM, Sigma) an adenylyl cyclase agonist, or 8-Bromo adenosine 3′, 5′-cyclic monophosphate (8-Br-cAMP, 10 μM, Sigma) a cell-permeable cAMP analog at indicated times. Cells were treated with selective inhibitor of PKA, N-[2-(p-Bromocinnamylamino) ethyl]-5-isoquinolinesulfonamide dihydrochloride (H-89, 10 μM), at indicated times.
2.7. Western blotting
Drug-treated myocytes were lysed in a buffer containing 25mM Hepes, pH 7.5, 2.5 mM EDTA, 50mM NaCl, 30 mM sodium pyrophosphate, 10% (v/v) glycerol, 1% (v/v) Triton X-100 and Complete protease inhibitor cocktail tablets (Pierce) after washing twice with ice cold PBS (phosphate buffered saline). Cell lysates were separated by SDS/PAGE for western blotting with antibodies against phosphorylated Serine 16 of phospholamban (phospho-PLB, Badrilla, UK), phospholamban, γ-tubulin, GFP, and PKA substrate phosphorylated (Santa Cruz). Primary antibodies were visualized with IRDye 680CW goat-anti mouse or with IRDye 800CW goat-anti rabbit secondary antibodies using an Odyssey scanner (Li-cor biosciences). Quantification of phospho-proteins was done using densitometry software Quantity One. Arbitrary phosphorylation units were normalized; and the results were plotted against controls. Plotted results represent the mean of at least three independent experiments with standard errors.
2.8. Statistical Analysis
One-way ANOVA and student t-test were performed using Prism (GraphPad Software, Inc. San Diego, CA).
3. Results
3.1 Development and characterization the novel biosensor for PKA activity on sarcoplasmic reticulum
We generated a SR-specific PKA biosensor (SR-AKAR3) by fusing the helical transmembrane domain of PLB [13; 14] to the C terminus of the FRET-based A-kinase activity biosensor AKAR3 [12] (Fig 1A). The expression of SR-AKAR3 by a recombinant virus displayed a dose-dependent fashion in HEK293 cells (Fig 1B). At 100 moi, the expression of SR-AKAR3 was saturated. When expressed in both neonatal and adult cardiomyocytes with viral infection, SR-AKAR3 displayed distinct patterns, and colocalization with RyR2, a SR marker in cardiomyocytes (Fig 1C). We then examined whether SR-AKAR3 expressed in cardiomyocytes can respond to PKA activation upon stimulation of beta adrenergic receptor. Stimulation of βARs with isoproterenol (10 μM) induced rapid changes in signals of both CFP and YFP channels in myocytes (Fig 1D), which indicates an increase in FRET upon PKA phosphorylation of the biosensor SR-AKAR3.
Fig.1.
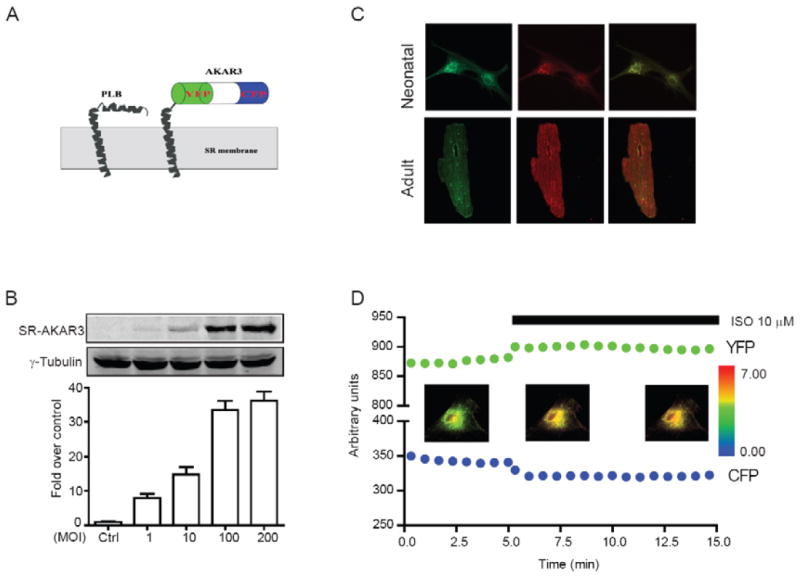
Characterization of SR-AKAR3 in cardiomyocytes. (A) Schematic cartoon representation of SR-AKAR3. (B) The expression levels of SR-AKAR3 in HEK293 after infection with different dose of recombinant adenovirus. (C) Distribution of SR-AKAR3 and RyR2 in both neonatal and adult cardiac myocytes. (D) Time profiles of individual CFP and YFP channels from SR-ARAR3 biosensor after stimulation of βAR with isoproterenol (10 μM) in myocytes. The inserted panels represent the SR-AKAR3 FRET ratio before and after stimulation with isoproterenol (10 μM) with pseudo-colored images.
3.2 Activation of βAR pathway induces increase in FRET ratio of PKA biosensor SR-AKAR3 on the Sarcoplasmic reticulum of cardiomyocytes
The changes in CFP and YFP signals yield a strong and sustained increase in FRET ratio (max = 0.31 ± 0.03, tmax = 55.0 ± 4.0 sec, n = 26, Fig 2A and 2D). Similarly forskolin induced a rapid but lower increase in SR-AKAR3 FRET ratio (max = 0.23 ± 0.02, tmax = 93.0 ± 2.8 sec, n = 35, Fig 2A and 2D), while vehicle control (DMSO) did not change the baseline level of SR-AKAR FRET ratio (Fig 2A and 2D). The saturated increase in SR-AKAR3 FRET ratio induced by forskolin was confirmed by a dose-dependent response curve (EC50 = 130 nM, n = 40, Fig 2B). Moreover, direct administration of a cAMP analogue 8-Br-cAMP (10 μM) induced an even smaller increase in SR-AKAR3 FRET ratio (max = 0.11 ± 0.02, tmax = 100.0 ± 11.7 sec, n = 44, Fig 2A and 2D) than those induced by isoproterenol. In adult myocytes, while isoproterenol (10 μM) induced a similar increase in SR-AKAR FRET ratio (max = 0.29 ± 0.03, t1/2 = 60.0 ± 3.4 sec, n = 13, Fig 2C and 2D) to those in neonatal myocytes, forskolin induced a even smaller increase in SR-AKAR3 FRET ratio (max = 0.1079 ± 0.025, tmax = 60.0 ± 9.7 sec, n = 8, Fig 2C and 2D) than those in neonatal myocytes. These data indicate that production of cAMP by adrenergic stimulation is preferentially targeted to the SR to promote PKA activities, and the signaling discrepancy in local domains is more tightly controlled in adult myocytes.
Fig.2.
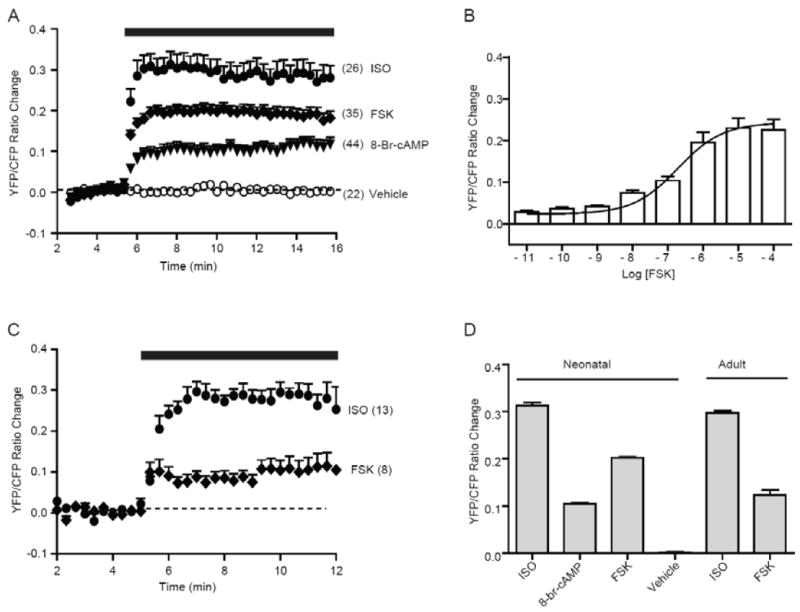
Activation of βAR and AC induce PKA activities on the SR in cardiomyocytes. (A) Neonatal myocytes with SR-AKAR3 expression were stimulated with vehicle (DMSO) control, adrenergic agonist isoproterenol (10 μM), adenylyl cyclase agonist forskolin (10 μM), or cAMP analog 8-Br-cAMP (10 μM). Time course of changes in SR-AKAR3 FRET ratio were recorded. (B) The maximal increases in SR-AKAR3 FRET displayed a forskolin dose-dependent response curve. (C) Adult myocytes with SR-AKAR3 expression were stimulated with adrenergic agonist isoproterenol (10 μM) and adenylyl cyclase agonist forskolin (10 μM). Time course of changes in SR-AKAR3 FRET ratio were recorded. (D) The maximal increases in SR-AKAR3 FRET ratio in (A and C) were plotted.
3.3 Detection of dynamic PKA activity on the Sarcoplasmic reticulum under activation of βAR and PKA in cardiomyocytes
We then used transient adrenergic stimulation to explore dynamic PKA activities in cardiomyocytes. While stimulation with isoproterenol induced a rapid and sustained increase in PKA SR-AKAR3 FRET ratio, the removal of agonist led to attenuation of PKA FRET ratio to the baseline level within 10 minutes (Fig 3A and 3F). The isoproterenol-induced response was absent in myocyte isolated from mice lacking both β1 and β2ARs, in which direct activation of adenylyl cyclases with forskolin (10 μM) induced significant increases in SR-AKAR3 FRET ratio similar to those induced by forskolin in wild type myocytes (Fig 3B and 3F). While administration of a PKA inhibitor H89 did not affect the baseline levels of SR-AKAR3 FRET ratio (Fig 3C and 3F), pretreated cells with H89 (10 μM) for 30 min significantly abolished the response induced by isoproterenol (0.07 ± 0.01, n = 30, Fig 3D and 3F). Moreover, the isoproterenol-induced and sustained increase in SR-AKAR3 FRET ratio underwent attenuation to the baseline level within 10 minutes upon addition of PKA inhibitor H89 (10 μM, Fig 3E and 3F).
Fig.3.
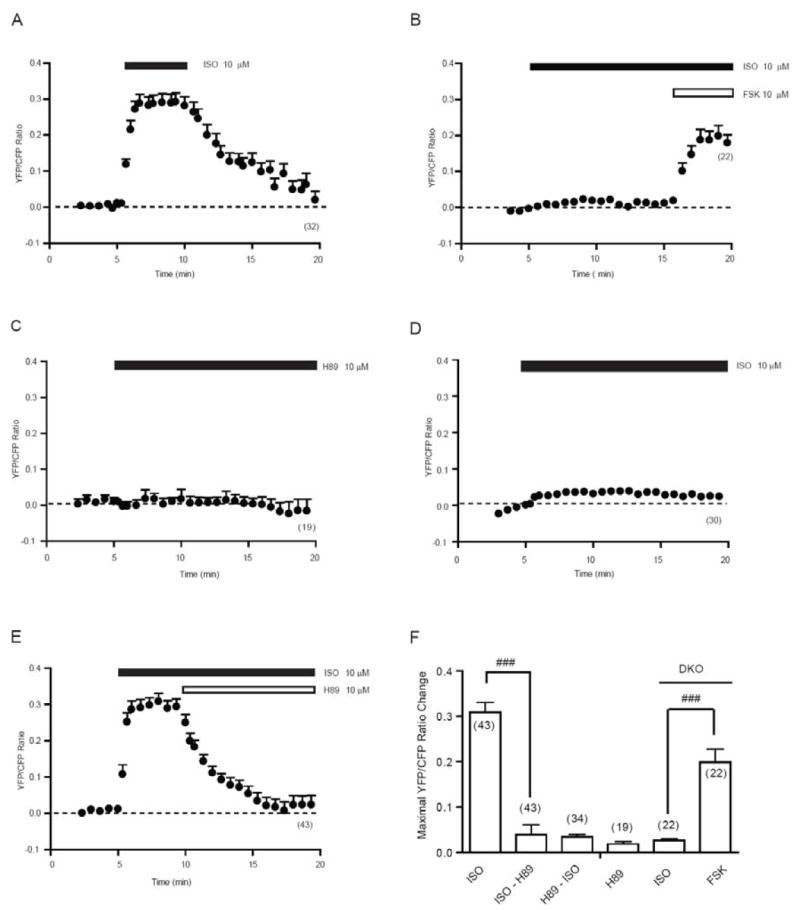
Adrenergic stimulation induced agonist-dependent dynamic PKA activities in the sarcoplasmic reticulum. Neonatal myocytes expressing SR-AKAR3 were stimulated with different drugs as indicated. (A) Time course of changes in SR-AKAR3 FRET ratio before and after a 5 minutes perfusion of isoproterenol (10 μM). (B) In myocytes lacking both β1 and β2 adrenergic receptors, the changes in SR-AKAR3 FRET ratio were recorded after stimulation with isoproterenol (10 μM) followed by forskolin stimulation (10 μM). (C) The effect of PKA inhibitor H-89 (10 μM) on the SR-AKAR3 FRET at resting state. (D) After pretreatment with PKA inhibitor H-89 (10 μM) for 30 min, the changes in SR-AKAR3 FRET ratio induced by isoproterenol (10 μM) were recorded. (E) The isoproterenol-induced changes in SR-AKAR3 FRET ratio were recorded after addition of PKA inhibitor H-89 (10 μM). Traces represent average of FRET ratios from number of cells as indicated. (F) The increases in SR-AKAR FRET ratio under different condition in (A-E) were plotted. ### p<0.001 when compared SR-AKAR FRET responses between indicated groups.
3.5 SR-AKAR3 mimics endogenous phospholamban for PKA-mediated phosphorylation and cardiac contraction upon adrenergic stimulation
We then further examine whether the changes in SR-AKAR3 FRET ratio reflects the protein phosphorylation that mimics the phosphorylation of endogenous proteins on the SR for myocyte contraction. While SR-AKAR3 displayed a basal level phosphorylation by PKA, stimulation with isoproterenol (10 μM, 10min) significantly the level of phosphorylation at the PKA site in neonatal cardiac myocytes (Fig 4A). Similarly, the PKA-mediated phosphorylation of endogenous PLB was also equally enhanced in both control and SR-AKAR3 expressing myocytes upon stimulation with isoproterenol (10 μM, 10min, Fig 4B). These data indicate that both SR-AKAR3 biosensor and PLB undergo equivalent PKA phosphorylation upon adrenergic stimulation, and the expression of biosensor did not affect the PKA phosphorylation of endogenous protein under physiological stimulation. In agreement with this notion, isoproterenol induced equivalent increases in contraction rate in both the control myocytes and myocytes with SR-AKAR3 expression (Fig. 4C).
Fig.4.
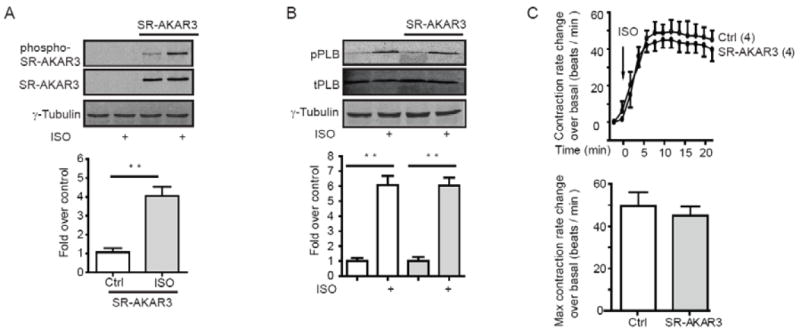
SR-AKAR3 mimics endogenous phospholamban for PKA phosphorylation under adrenergic stimulation. (A) Neonatal cardiac myocyte with or without overexpression of SR-AKAR3 were stimulated with 10 μM of isoproterenol for 10 min. Cells were lysed for Western blots (T = 10%) to detect total and phospho-SR-AKAR3. (B) Neonatal cardiac myocyte with or without overexpression of SR-AKAR3 were stimulated with 10 μM of isoproterenol for 10 min. Cells were lysed for Western blots (T = 10%) to detect total phospholamban (PLB) and phospho-PLB at Ser16 by PKA. (C) Neonatal cardiomyocytes with or without overexpression of SR-AKAR3 were stimulated with 10 μM of isoproterenol. The changes in contraction rate were recorded before and after stimulation, and the increase in contraction rate over the baseline levels were plotted. ** p<0.001 when compared to control by student t-test.
4. Discussion
In cardiomyocytes, the sarcoplasmic reticulum represents one of the most important subcellular compartments for muscle contraction. As the major calcium storage, it controls intracellular calcium cycling for EC coupling. These channels can be modulated by phosphorylation via hormone-induced cAMP/PKA signaling under stressing conditions, such as adrenergic signaling during exercise. Dysfunction in such regulations has been associated with numerous cardiac diseases, e.g. heart failure and arrhythmia [2; 4]. In this study, we have developed a novel FRET-based probe SR-AKAR3, and for the first time directly and specifically detected the dynamic PKA activity on the SR in myocytes. This probe is correctly targeted and sensitive to hormonal stimulation without affecting the adrenergic signaling-induced PKA phosphorylation of endogenous protein PLB and myocyte contraction rate responses (Fig 4).
As the major signaling mechanism for enhancing cardiac function, adrenergic stimulation potently enhances PKA activities on the SR for phosphorylation of PLB and for promoting myocyte contraction. In comparison, global stimulation of adenylyl cyclases with forskolin or directly applying the non-hydrolysable 8-Br-cAMP only partially enhances PKA activities on the SR (Fig 2). These data indicate a preferentially access of adrenergic stimulation to PKA on the SR in cardiomyocytes. Interestingly, the increase in SR-AKAR3 FRET ratio upon forskolin stimulation is also 2 fold slower than that of isoproterenol, indicating a possible diffusion of cAMP to the PKA anchored on the SR. These data support the notion that βARs are localized to T-tubular structures to be in the proximity of SR [18]. Together, these studies solidify the notion that cAMP/PKA activities are generated in specific compartment depending on the hormones applied, and that cAMP does not diffuse from one compartment to the others, allowing fidelity of the cellular response [19].
In summary, we have developed a novel FRET-based PKA biosensor to detect PKA activities in living myocytes. For the first time, we have revealed distinct profiles of PKA activities on the SR after stimulation of adrenergic signaling. The ability of this new PKA activity biosensor in revealing the dynamic of PKA activities on the SR will have significant implication in dissecting adrenergic signaling a broad range of physiological and pathological processes, such as heart failure. Therefore, our probe provides a great tool to understand the roles of hormone-induced PKA activities on this vital cellular organelle under various conditions.
Research Highlight in Liu SB et al.
Direct measurement of PKA activities on the SR with FRET biosensor
Adrenergic stimulation preferentially signals to the SR
PKA FRET biosensor mimics endogenous PLB phosphorylation in myocytes
Adrenergic stimulation induce dynamic PKA activity on the SR
Acknowledgments
This study was supported by NIH grants DK073368 to JZ and HL082646 to YKX. We thank the members of Xiang laboratory for critical reading and comments.
Abbreviations
- AR
adrenoceptor
- ISO
isoproterenol
- H-89
N-[2-(p-bromocinnamylamino) ethyl] - 5-isoquinolinesulfonamide dihydrochloride
- SR
sarcoplasmic reticulum
- AKAR
A kinase activity biosensor
- AKAP
A-kinase anchoring protein
- PKA
protein kinase A
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- FRET
fluorescence resonance energy transfer
- AC
adenylyl cyclase
- FSK
forskolin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacLennan DH, Kranias EG. Phospholamban: A crucial regulator of cardiac contractility. Nature Reviews Molecular Cell Biology. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 2.Venetucci LA, Trafford AW, O’Neill SC, Eisner DA. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovascular Research. 2008;77:285–292. doi: 10.1093/cvr/cvm009. [DOI] [PubMed] [Google Scholar]
- 3.Lehnart SE, Wehrens XHT, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SLC, Conti M, Marks AR. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mubagwa K. SARCOPLASMIC-RETICULUM FUNCTION DURING MYOCARDIAL-ISCHEMIA AND REPERFUSION. Cardiovascular Research. 1995;30:166–175. [PubMed] [Google Scholar]
- 5.Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–93. doi: 10.2741/skalhegg. [DOI] [PubMed] [Google Scholar]
- 6.Buxton ILO, Brunton LL. Compartments of Cyclic-Amp and Protein-Kinase in Mammalian Cardiomyocytes. Journal of Biological Chemistry. 1983;258:233–239. [PubMed] [Google Scholar]
- 7.Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annual Review of Pharmacology and Toxicology. 2001;41:751–773. doi: 10.1146/annurev.pharmtox.41.1.751. [DOI] [PubMed] [Google Scholar]
- 8.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 9.Dodge-Kafka KL, Langeberg L, Scott JD. Compartmentation of cyclic nucleotide signaling in the heart - The role of A-kinase anchoring proteins. Circulation Research. 2006;98:993–1001. doi: 10.1161/01.RES.0000218273.91741.30. [DOI] [PubMed] [Google Scholar]
- 10.George CH. Sarcoplasmic reticulum Ca2+ leak in heart failure: mere observation or functional relevance? Cardiovascular Research. 2008;77:302–314. doi: 10.1093/cvr/cvm006. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Ma YL, Taylor SS, Tsien RY. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14997–15002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen MD, Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochemical and Biophysical Research Communications. 2006;348:716–721. doi: 10.1016/j.bbrc.2006.07.136. [DOI] [PubMed] [Google Scholar]
- 13.Zamoon J, Mascioni A, Thomas DD, Veglia G. NMR solution structure and topological orientation of monomeric phospholamban in dodecylphosphocholine micelles. Biophysical Journal. 2003;85:2589–98. doi: 10.1016/s0006-3495(03)74681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oxenoid K, Chou JJ. The structure of phospholamban pentamer reveals a channel-like architecture in membranes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10870–5. doi: 10.1073/pnas.0504920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robia SL, Flohr NC, Thomas DD. Phospholamban pentamer quaternary conformation determined by in-gel fluorescence anisotropy. Biochemistry. 2005;44:4302–11. doi: 10.1021/bi0478446. [DOI] [PubMed] [Google Scholar]
- 16.Traaseth NJ, Verardi R, Torgersen KD, Karim CB, Thomas DD, Veglia G. Spectroscopic validation of the pentameric structure of phospholamban. Proc Natl Acad Sci U S A. 2007;104:14676–81. doi: 10.1073/pnas.0701016104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Molecular Pharmacology. 2001;60:577–83. [PubMed] [Google Scholar]
- 18.Caswell AH, Baker SP, Boyd H, Potter LT, Garcia M. beta-adrenergic receptor and adenylate cyclase in transverse tubules of skeletal muscle. Journal of Biological Chemistry. 1978;253:3049–54. [PubMed] [Google Scholar]
- 19.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, Baillie GS, Zaccolo M. Protein kinase a type I and type II define distinct intracellular signaling compartments. Circulation Research. 2008;103:836–844. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]


