Abstract
Background
Loss of cortical volume in frontotemporal regions has been reported in patients with schizophrenia and their relatives. Cortical area and thickness are determined by different genetic processes, and measuring these parameters separately may clarify disturbances in corticogenesis relevant to schizophrenia. Our study also explored clinical and cognitive correlates of these parameters.
Methods
Thirty-seven patients with first-episode psychosis (34 schizophrenia, 3 schizoaffective disorder) and 38 healthy control subjects matched for age and sex took part in the study. Imaging was performed on an magnetic resonance imaging 1.5-T scanner. Area and thickness of the frontotemporal cortex were measured using a surface-based morphometry method (Freesurfer). All subjects underwent neuropsychologic testing that included measures of premorbid and current IQ, working and verbal memory, and executive function.
Results
Reductions in cortical area, more marked in the temporal cortex, were present in patients. Overall frontotemporal cortical thickness did not differ between groups, although regional thinning of the right superior temporal region was observed in patients. There was a significant association of both premorbid IQ and IQ at disease onset with area, but not thickness, of the frontotemporal cortex, and working memory span was associated with area of the frontal cortex. These associations remained significant when only patients with schizophrenia were considered.
Conclusions
Our results suggest an early disruption of corticogenesis in schizophrenia, although the effect of subsequent environmental factors cannot be excluded. In addition, cortical abnormalities are subject to regional variations and differ from those present in neurodegenerative diseases.
Key Words: Cognitive impairment, cortical area and thickness, first-episode psychosis, frontotemporal cortex, magnetic resonance imaging, surface-based morphometry
Cortical volume is genetically determined with heritability (1) decreasing over time as environmental factors become relevant (2). The cortex is shaped in utero by the strength of interregional connectivity, and cortical changes are to be expected in schizophrenia, a disease with abnormal brain connectivity (3,4).
Meta-analyses of voxel-based morphometry studies identified gray matter loss in corticosubcortical networks involving frontotemporal and limbic cortex, thalamus, and striatum in chronic schizophrenia (5–7), and less extensive changes have also been reported in those with first-episode (8), schizotypal disorder, and high-risk individuals (9–12). Subtle cortical abnormalities, without volume loss, have been described using magnetization transfer imaging in first-episode psychosis (13,14).
Surface-based morphometry (SBM) methods allow the independent measurement of cortical area and thickness—indexes that share a high heritability but are determined by different genetic mechanisms (15). Freesurfer (16), an SBM method with realistic cortical reconstruction that allows for manual correction of topological errors (17), uses a segmentation procedure based on the identification of gray–white matter and gray matter–pial boundaries, as well as a surface-based registration that aligns cortical folding patterns. Freesurfer has an accuracy of .2 mm (18) compared with postmortem measures of cortical thickness and has been validated using different scanners and magnetic resonance imaging (MRI) protocols (16).
Studies using SBM have mainly measured regional or whole cortical thickness. Frontotemporal gyral and sulcal thinning has been reported in children and adolescents (19,20) and in young adults with first-episode schizophrenia by some (21,22) but not others (23). Cortical thinning, particularly prefrontal, followed a developmental trajectory different from that of control subjects (24,25). In chronic patients, thinning of the entorhinal (26) and frontotemporal cortex with relative sparing of somatosensory (27) and parietal cortex (28) has also been reported and changes in cortical thickness in the cingulate and temporal regions may represent the unexpressed genetic liability in relatives of schizophrenia patients (29).
Cortical area has been less frequently measured with contradictory results. Thus, although reduced area in paralimbic ventral frontal cortex in drug-naive patients (30) and in posterior cingulate in those with chronic schizophrenia and their relatives has been described (31), other studies (32) have reported area increases in the anterior cingulate. Early reports of decreased insular area in first-episode patients (30) have not been replicated (33). A study of patients with adolescent-onset schizophrenia (34) identified widespread, regionally variable cortical pathology in prefrontal and superior temporal gyrus, with variable reductions in area and/or thickness. Similar changes have been reported in unaffected relatives (35), and severity of positive symptoms has been associated with decrease entorhinal area (26).
We report here an exploratory study in patients with first-episode psychosis in whom cortical area and thickness were measured using SBM in frontal and temporal cortex, regions known to be implicated in schizophrenia. We aimed to clarify the pattern of cortical abnormalities, whether changes in area and cortical thickness occurred independently, and their possible associations with clinical and cognitive measures.
Methods and Materials
Subjects
Patients were part of a cohort recruited for the West London Longitudinal First-Episode Psychosis Study (36), aged between 16 and 49 years at recruitment, who had been receiving antipsychotic medication for less than 12 weeks. Diagnosis was ascertained using the diagnostic module of the Diagnostic Interview for Psychosis (DIP-DM) (37), which includes items from the Operational Criteria Checklist for Psychosis (38) and the World Health Organization Schedules for Clinical Assessment in Neuropsychiatry (39). Two nurses trained by an experienced psychiatrist (T.R.E.B.) conducted the interviews.
Forty-one patients who had MRI and neuropsychologic assessments participated. Four were excluded because of poor-quality MRI data. Thirty-seven patients (25 males) were included; 34 had a final diagnosis of schizophrenia and 3 had schizoaffective disorder (1 bipolar, 2 depressed subtypes). Mean age was 26.8 years (SD = 8.8; range, 16–49). The study was naturalistic with no restrictions on prescribed medication; all patients were prescribed antipsychotics (36 second-generation, 1 first-generation), and 8 were also prescribed antidepressants. At the time of scanning, the median duration of treatment was 102 days (range, 0−384), and 17 patients had received treatment for more than 12 weeks. Thirty-eight healthy subjects (22 males) who had neuropsychologic assessment and MRI served as control subjects. Their mean age was 25.0 years (SD = 5.4; range, 16–37). Exclusion criterion for all subjects, was the presence of a medical or neurological illness, including head injury leading to unconsciousness. Controls with psychiatric illness in themselves or their first-degree relatives were excluded.
Clinical Ratings
Mental state was assessed with the Scales for the Assessment of Positive and Negative Symptoms (SAPS and SANS) (40,41). Interrater agreement (linearly weighted kappa) was assessed using a standard set of videotaped interviews. Using global subscale items, linearly weighted kappas of .76 for SAPS and .74 for SANS were achieved (42). Scores for the three symptom-derived syndromes (negative, positive, and disorganization) were calculated (43). Affective symptoms were measured with the Young Mania Scale (44) and Hamilton Rating Scale for Depression (45). Age of onset and duration of untreated psychosis (DUP) were established using the Symptom Onset in Schizophrenia Inventory (46). Alcohol and drug use were assessed using the DIP (37), and criteria for abuse and dependence using the Alcohol and Drug Use Scales (47). None of the subjects fulfilled these criteria. Handedness was assessed using the Annett Scale (48).
Ethical permission was obtained from the local ethics committees. Participants gave written informed consent according to the Declaration of Helsinki and received an honorarium.
Neuropsychological Assessment
Premorbid IQ was estimated using the Revised National Adult Reading Test (49), validated in schizophrenia (50,51). Current IQ was measured using a short form of the Wechsler Adult Intelligence Scale—III (52) validated for schizophrenia (53), comprising the Information, Arithmetic, Block Design, and Digit Symbol subtests. Measures of executive function were derived from the Cambridge Neuropsychological Test Automated Battery (54): 1) Working memory span, from the Spatial Span Task, measures the ability to remember the order of sequences of colored squares presented in increasing numbers. The span was measured as the highest number recalled correctly. 2) Working memory manipulation, from the Spatial Working Memory Task, a self-ordered search task measures the ability to remember the location of previously found “tokens” while searching for new ones. An error occurs when a participant returns to the location where a token has already been found. Total errors were used as an index of working memory manipulation. 3) Planning, from the Stockings of Cambridge task (55), measures the ability to move colored “balls” in an arrangement displayed on the screen to match a goal arrangement. The number of problems solved in the minimum number of moves possible was the score.
Verbal memory was assessed with the Rey Auditory Verbal Learning Test (56). The participant is asked to recall nouns from a list of 15 immediately after each of five trials. The number of words recalled over the five trials was the final score.
MRI Data Acquisition
The MRIs were performed on a GE Signa 1.5-Tesla scanner (General Electric, Milwaukee, Wisconsin), using a standard quadrature head coil. T1-weighted volumetric images were obtained using an inversion recovery spoiled gradient-recalled echo sequence with an isotropic voxel size of 1.2 × 1.2 × 1.2 mm3. One hundred twenty-four axial contiguous slices were acquired. Other parameters were echo time (5.4 msec), repetition time (15 msec), inversion time (450 msec), field of view = 31 × 16 cm2, acquisition matrix 256 × 128, number of averages = 1, excitation flip angle = 15°, and receiver bandwidth = 15.63 kHz.
Image Processing
A rater (L.G.G.), blind to participant status, used Freesurfer 4.0.1 (http://surfer.nmr.mgh.harvard.edu) to generate maps of surface area and cortical thickness in standard Montreal Neurological Institute (MNI) space (57,58). After skull stripping and white matter segmentation, the cortical surface of each hemisphere was inflated to an average spherical surface to locate the pial surface and the gray–white matter boundary (57). The distance between the two at each vertex (i.e., surface point) across the cortex is considered a measure of cortical thickness. Cortical maps are smoothed with a 10-mm full-width at half-maximum Gaussian kernel and aligned to a common surface template using a high-resolution surface-based averaging technique, and 32 cortical parcellations are automatically generyated (59). The only manual step was the correction of topological errors when the above steps had been completed. Total brain volume was estimated using Freesurfer (60).
Analysis of Cortical Parameters
Two comparisons were made between patients and controls: 1) whole-brain cortical thickness using the “vertex-by-vertex” analysis; and 2) cortical thickness, surface area, and volume in frontal and temporal regions.
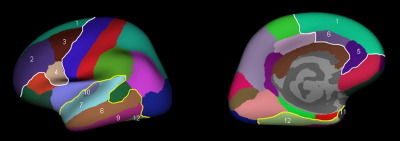
From the Desikan template (59), six frontal and six temporal parcellations in each hemisphere were selected. Superior frontal, pars opercularis, caudal middle frontal, rostral middle frontal, caudal anterior cingulate, and rostral anterior cingulate were selected in the frontal lobe; in the temporal lobe, the superior, middle, and inferior temporal, fusiform, temporal pole, and transverse temporal parcellations were selected (Figure 1). Average thickness, total surface area, and volume of the cortex for the frontal and temporal regions covered by these parcellations were calculated for each hemisphere and compared between the two groups.
Figure 1.
Lateral and midsagittal views of the frontal and temporal parcellations: 1, superior frontal; 2, rostral middle frontal; 3, caudal middle frontal; 4, pars opercularis; 5, rostral anterior cingulate; 6, caudal anterior cingulate; 7, superior temporal; 8, middle temporal; 9, inferior temporal; 10, transverse temporal; 11, temporal pole; 12, fusiform.
Statistical Analysis
Demographic and Cognitive Variables
Age, sex, total brain volume, and handedness were compared between groups using t and chi-square tests. Linear regression models adjusted by age and sex were used to compare cognitive scores.
Imaging Variables
Age and sex were used as covariates in all models.
For the comparison of whole brain cortical thickness, the vertex-by-vertex analysis was used, and means of cortical thickness were compared between groups using a two-tailed t test; cortical thickness was modeled as a function of group controlling for age and sex. Corrections for multiple comparisons were made using a false discovery rate (FDR), setting the level of significance at .05 (61).
Linear mixed models were used for the following comparisons of frontal and temporal cortical parameters between the groups: 1) differences due to diagnostic group, region, and side, with two-way interactions (diagnosis by region, diagnosis by side, and region by side); 2) differences due to sex; age; duration of treatment; DUP; and positive, negative, and disorganization syndrome scores with two-way interactions by region and side; and 3) associations between cognitive scores and cortical parameters with two- and three-way interactions. Region was entered as a within-subject effect and diagnosis, cognitive scores, sex, and age as between-subject effects. Three separate models were created for thickness, surface area, and cortical volume that allowed the inclusion of multiple measurements for each subject and the handling of missing data, thereby increasing statistical power (62). As in previous studies (27,34,63), we did not control for brain volume, a schizophrenia-related variable, because this would have obscured possible group differences.
We repeated the same model with two-way interaction (diagnosis by region) to identify cortical differences in each of the six frontal or temporal parcellations. When significant interactions were present, the linear mixed model was repeated with the corresponding indicators and interaction terms for the regional parcellations.
For these exploratory analyses significance was reported at the 5% level with no formal adjustment made for multiple comparisons, because this may be inappropriate (64,65) when no single null hypothesis covers the multiple tests. To highlight the strongest associations, we also report the linear regression results using FDR setting the level of significance at .05.
Results
Age, total brain volume, and sex did not differ between groups. Fewer patients (2 of 37) than control subjects (7 of 38) were left-handed, although this difference was not statistically significant. All cognitive scores were significantly worse in patients (at trend level for premorbid IQ; Table 1). Duration of treatment and DUP, positive, negative, and disorganization scores are shown on Table 1.
Table 1.
Demographic and Cognitive Measures in Patients and Controls
| Variable Measured | Patients (n = 37) | Control Subjects (n = 38) | Comparison |
|---|---|---|---|
| Age (years) | 26.8 (8.8) [16–49] | 25.0 (5.4) [16–37] | t(73) = −1.06, p = .291 |
| Male Sex, n (%) | 25 (67.6) | 22 (57.9) | χ2(1) = .75, p = .387 |
| Left-Handed, n (%) | 2 (5.4) | 7 (18.4) | χ2(1) = 3.01, p = .083 |
| Total Brain Volume (mm3) | 1543685 (198405.4) | 1571956 (188197.2) | t(73) = .63, p = .529 |
| Premorbid IQ | 99.8 (12.9) [73–120] | 105.3 (11.2) [74–120] | t(74) = −1.99, p = .051 |
| Current IQ | 89.6 (17.6) [61–135] | 110.6 (15.0) [81–140] | t(74) = −5.63, p < .001 |
| Working Memory Span | 5.5 (1.6) [2–9] | 6.5 (1.2) [4–9] | t(74) = −3.24, p = .002 |
| Planning | 7.2 (2.7) (0−12) | 8.8 (1.6) [6–12] | t(74) = −3.12, p = .003 |
| Working Memory Manipulation | 33.9 (16.7) (0−58) | 15.4 (14.3) (0−60) | t(74) = 5.26, p < .001 |
| Rey Auditory Verbal Learning Test | 38 (8.4) [14–52] | 49.4 (10.4) [18–64] | t(74) = −5.03, p < .001 |
| Duration of Treatment (days) | 80 (56) [9–186] | NA | NA |
| Duration of Untreated Psychosis (months) | 10.4 (22.8) (0−126) | NA | NA |
| Factor 1, Negative Syndrome | .29 (.25) (0−.8) | NA | NA |
| Factor 2, Positive Syndrome | .75 (.20) (0−1) | NA | NA |
| Factor 3, Disorganization Syndrome | .27 (.25) (0−.8) | NA | NA |
Values are means (SD) [range].
NA, not applicable.
Group Differences in Cortical Parameters
Analyses were performed with and without three patients with schizoaffective disorder. Results remained unchanged when the latter were excluded and findings for the whole group are reported (Table 2).
Table 2.
Cortical Parameters in Frontal and Temporal Regions in Patients and Controls Unadjusted by Age or Gender
| Region of Cortex | Thickness (mm)a |
Surface Area (mm2)b |
Volume (mm3)b |
|||
|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | Patients | Controls | |
| Frontal | ||||||
| Left | 2.71 (.15) | 2.70 (.13) | 15680.59 (2086.42) | 16048.87 (1989.18) | 47014.73 (6395.26) | 47862.50 (4818.93) |
| Right | 2.73 (.15) | 2.69 (.13) | 15567.30 (2252.66) | 15773.68 (1960.96) | 46597.27 (6766.11) | 46807.53 (4788.16) |
| Temporal | ||||||
| Left | 2.72 (.13) | 2.71 (.11) | 13782.35 (1557.68) | 14275.03 (1803.94) | 43358.57 (5253.97) | 44940.76 (4817.45) |
| Right | 2.73 (.15) | 2.75 (.12) | 13795.49 (1652.54) | 14183.13 (1731.98) | 43539.14 (5047.07) | 45196.03 (4879.04) |
Values are means (SD).
Values are means (SD) of the sums of six parcellations each.
Whole-brain cortical thickness (vertex by vertex) did not differ between groups.
Cortical parameters in frontal and temporal regions: 1) Cortical thickness did not vary by diagnosis or sex. Age was more closely associated with thinning in the frontal than in the temporal cortex (regional mean difference in thinning per year = −.0085 mm/year; 95% confidence interval [CI] −.0113 to −.0057; p < .001], with thinning of .0073 mm/year (95% CI −.0109 to −.0038; p < .001) in the frontal region. 2) Cortical area was not related to age or side in frontal or temporal regions but was larger in male subjects irrespective of diagnosis (regional mean difference between males and females = 416.13 mm2; 95% CI 153.16 to 679.09); p = .002) and more so for the frontal (sex mean difference = 2679.29 mm2; 95% CI 1995.44 to 3363.13; p < .001) than the temporal regions (sex mean difference = 2263.16 mm2; 95% CI 1579.32 to 2947.01; p < .001). In patients, temporal cortical area was smaller than in controls (regional means difference in patients = −724.12 mm2; 95% CI –1369.56 to –78.69; p = .028), explained by the smaller area in the superior (95% CI –326.42 to –67.32; p = .003), middle (95% CI −291.31 to −32.21; p = .014) and inferior (95% CI −271.43 to −12.33; p = .032) temporal parcellations. FDR-corrected analysis showed a reduction in right superior temporal area in patients (mean difference between patients and control subjects = −206.74 mm2; 95% CI −354.87 to −58.62); p = .028). In a post hoc multiple linear regressions of cortical thickness in these three parcellations, adjusted for age and sex, thickness was only reduced in the right superior temporal parcellation (mean difference between patients and control subjects = −.0751 mm; 95% CI −.1363 to −.0139; p = .017). There were no significant differences in frontal cortical area between groups. 3) Temporal cortical volume was smaller in patients (regional mean difference in patients = −2183.94 mm3; 95% CI −4104.17 to −263.70; p = .026), explained by reductions in the volume of the superior (95% CI −1052.96 to −216.31; p = .003), middle (95% CI −936.38 to −99.73; p = .015), inferior temporal (95% CI −938.57 to −101.92; p = .015), and fusiform (95% CI −939.37 to −102.72; p = .015) parcellations. Left frontal cortical volume was larger than right (mean difference between sides = 740.47 mm3; 95% CI 42.03 to 1438.91; p = .038). There were no group differences in the temporal cortical volume (mean difference between sides = −218.41 mm3; 95% CI −916.85 to 480.03; p = .540). 4) Cortical parameters were not associated with duration of treatment or DUP or syndrome scores. In a post hoc analysis of parcellations with reduced cortical area, using linear regressions adjusted for DUP, age, and sex, a trend level association with treatment duration (area increase of 1.46 mm2/day of untreated psychosis; 95% CI −.04 to 2.97; p = .056) was present for the superior temporal parcellation.
Cortical Parameters and Cognition
Whole group and the schizophrenia-only subgroup results are given for working memory because minor differences occurred when schizoaffective disorder patients were excluded.
Premorbid IQ
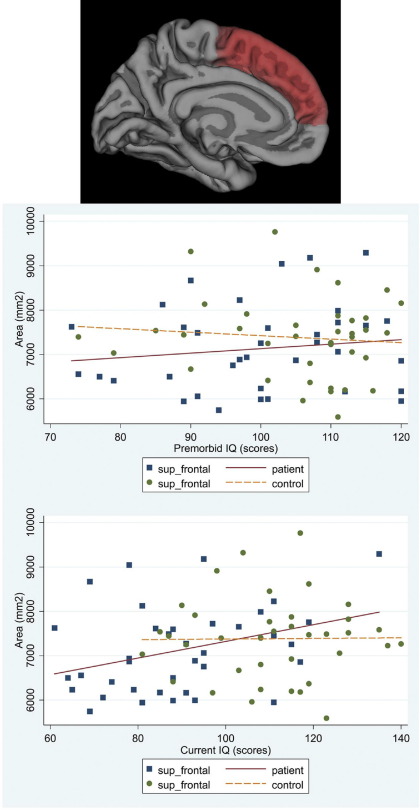
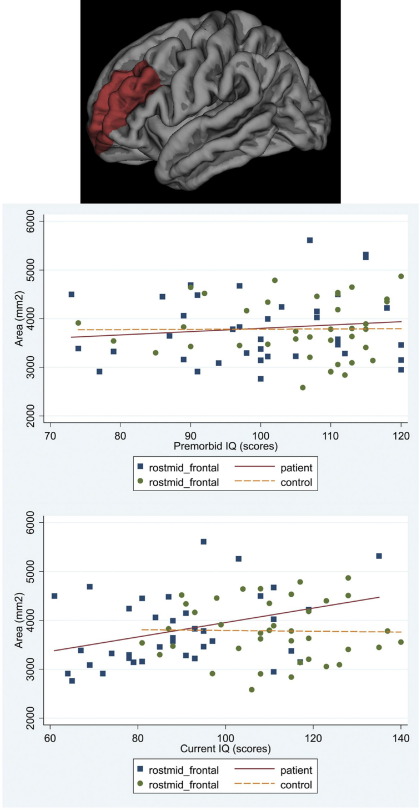
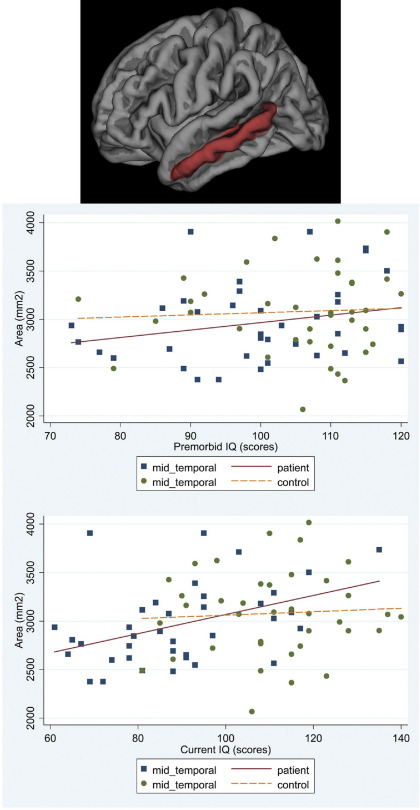
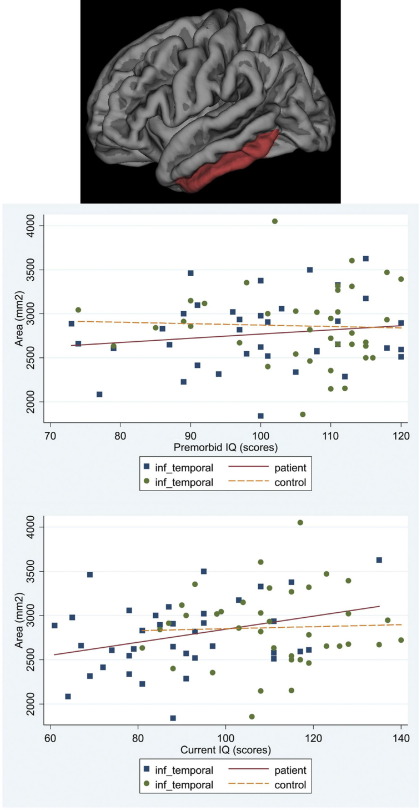
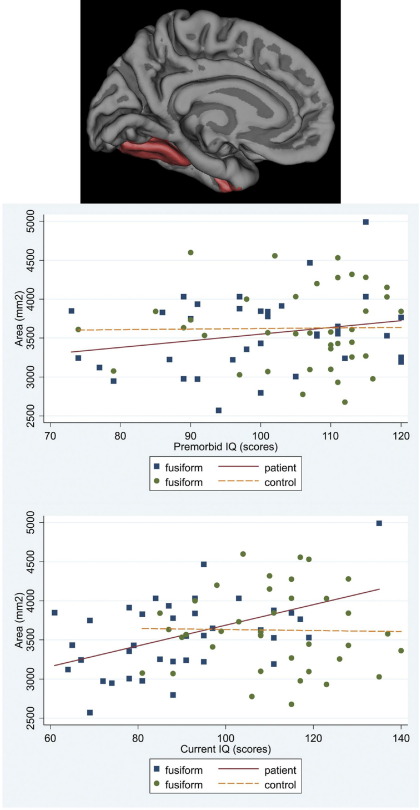
In patients there was a significant association between cortical area and premorbid IQ for frontal and temporal regions (mean difference of regional area increase per IQ point = 6.04 mm2; 95% CI −8.35 to 20.43; p = .411). Higher premorbid IQ was associated with larger frontal cortical area (increase of 46.01 mm2 per premorbid IQ point; 95% CI 11.13 to 80.88; p = .010) accounted for by superior frontal parcellation (95% CI 2.91 to 23.05; p = .012). Higher premorbid IQ was associated with larger temporal cortical area (increase of 39.97 mm2; 95% CI 5.09 to 74.84; per IQ point; p = .025). The middle (95% CI 3.28 to 17.48; p = .004), inferior temporal (95% CI .41 to 14.61; p = .038), and fusiform (95% CI 4.19 to 18.39; p = .002) parcellations accounted for this association. No such associations were present in control subjects (Figures 2–6). No significant associations survived FDR correction.
Figure 2.
Scatter plot of the associations between premorbid IQ and current IQ with the average cortical area for the right and left hemispheres in patients and controls: superior frontal.
Figure 3.
Scatter plot of the associations between premorbid IQ and current IQ with the average cortical area for the right and left hemispheres in patients and controls: rostral middle frontal.
Figure 4.
Scatter plot of the associations between premorbid IQ and current IQ with the average cortical area for the right and left hemispheres in patients and controls: middle temporal.
Figure 5.
Scatter plot of the associations between premorbid IQ and current IQ with the average cortical area for the right and left hemispheres in patients and controls: inferior temporal.
Figure 6.
Scatter plot of the associations between premorbid IQ and current IQ with the average cortical area for the right and left hemispheres in patients and controls: fusiform parcellations.
In patients, there was a significant association between cortical volume and premorbid IQ for both frontal and temporal regions (mean difference of regional volume increase per IQ point = 26.80 mm3; 95% CI −27.26 to 80.87; p = .311). Higher premorbid IQ was associated with larger frontal cortical volume (increase of 151.87 mm3 per IQ point; 95% CI 48.91 to 254.84; p = .004) accounted for by the volumes of the superior (95% CI 23.40 to 81.83; p < .001) and rostral middle (95% CI 2.85 to 61.28; p = .031) parcellations. Higher premorbid IQ was also associated with temporal cortical volume (increase of 125.07 mm3 per IQ point; 95% CI 22.11 to 228.03; p = .017). The volumes in superior (95% CI 5.69 to 51.32; p = .014), middle (95% CI 12.61 to 58.25; p = .002), inferior (95% CI .36 to 45.99; p = .047), and fusiform (95% CI 13.94 to 59.58; p = .002) parcellations accounted for the association. These associations were not present in controls.
Current IQ
In patients, current IQ had a stronger association with frontal than temporal cortical area (mean difference of regional area increase per IQ point = 14.56 mm2; 95% CI 4.13 to 24.98; p = .006), with an increase of 44.93 mm2 per IQ point (95% CI 19.89 to 69.97; p < .001). The superior (95% CI 10.35 to 24.85; p < .001), rostral middle (95% CI 6.38 to 20.87; p < .001), and caudal middle (95% CI .04 to 14.54; p = .049) parcellations accounted for the association. There was an increase of 30.37 mm2 per IQ point (95% CI 5.33 to 55.41; p = .017) in the temporal cortical area. The middle (95% CI 3.87 to 14.17; p = .001), inferior (95% CI 1.43 to 11.73; p = .012) and fusiform (95% CI 7.17 to 17.48; p < .001) parcellations accounted for this association. Associations remained significant after FDR correction for the right fusiform (95% CI 6.23 to 21.89; p = .012) and rostral middle frontal (95% CI 5.27 to 23.86; p = .016) parcellations. No significant associations were found in controls (Figures 2–6).
In patients there was a significant association between cortical volume and current IQ for frontal and temporal regions (mean difference of regional volume increase per IQ point = 33.72 mm3; 95% CI −5.84 to 73.28; p = .095), with an increase of 131.99 mm3 of frontal cortical area per IQ point (95% CI 57.62 to 206.35; p = .001). The superior (95% CI 38.65 to 80.88; p < .001), rostral middle (95% CI 10.51 to 52.75; p = .003), and caudal middle (95% CI .60 to 42.84; p = .044) parcellations accounted for this association. Temporal cortical volume increased by 98.27 mm3 per IQ point (95% CI 23.90 to 172.63; p = .010) and the superior (95% CI 2.87 to 36.04; p = .022), middle (95% CI 13.41 to 46.59; p < .001, inferior (95% CI 3.98 to 37.15; p = .015), and fusiform (95% CI 22.49 to 55.66; p < .001) parcellations accounted for this association. No significant associations were present for controls.
Working Memory Span
In patients, there was a trend level association with larger frontal cortical area (281.43 mm2 increase in area per score point; 95% CI −13.83 to 576.68; p = .062) that reached significance when schizoaffective disorder patients were excluded (95% CI 8.99 to 696.42; p = .044).
In patients, there was a stronger association with frontal than temporal cortical volume (mean difference of regional volume increase per score point = 569.12 mm3; 95% CI 123.13 to 1015.11; p = .012), with an increase of 962.94 mm3 per score point (95% CI 86.24 to 1839.64; p = .031); the superior frontal parcellation accounted for this association (95% CI 260.72 to 744.35; p < .001). No significant associations were present for control subjects.
Cortical thickness was not associated with IQ or working memory span. Scores of planning, working memory manipulation, and Rey Auditory Verbal Learning Test were not significantly associated with cortical parameters. There were no significant associations by side for any cognitive variable.
Discussion
Reduction in cortical volume, predominantly in temporal regions, due to smaller cortical area in patients with first-episode psychosis was our main finding, and area reductions were closely related to cognitive performance. Cortical thinning was only present in the right superior temporal region.
Our findings contrast with those of others reporting cortical thinning in patients with childhood (66,67), early-adulthood, or adult-onset (21,22) schizophrenia using SBM. The findings of Voets et al. (34), who reported reduced area and thickness in overlapping cortical regions, are closer to our own. These differences may be partly explained by the more severely compromised brain maturation trajectory in early onset schizophrenia (68,69). Methodologic variations may also be relevant. Thus, surface measurements in native space from the Desikan parcellations, used by Voets and colleagues (34) and ourselves, may be more sensitive than metric distortion used by others to estimate changes in cortical area.
The human brain is characterized by an expansion in the size and complexity of association areas in the neocortex (70), particularly prefrontal cortex (71), largely because of increased cortical area with little change in cortical thickness (72). In early fetal development, cortical area is determined by the migration of radial columns from the ventricular zone to the cortical plate (73,74), followed by the asymmetrical division of precursor cells in the ventricular zone and subsequent migration to the cortical plate increasing its thickness but not its area. Although neuronal migration is complete by the 25th week of gestation, glial migration and growth of cortical connections continue for longer with further increases in cortical surface, which is also influenced by differential expansion of cortical layers (75). Our finding of reduced area, without change in cortical thickness suggests a disruption of corticogenesis at a time of rapid cortical expansion, that is, late pregnancy and the perinatal period. Contemporary white matter abnormalities may have further reduced cortical area, as is thought to be the case in very low-birth-weight adolescents in whom area reduction is greater than cortical thinning (76,77). The reduction of brain volume over the first 2 decades of illness (78–80) suggests that mechanisms operating around disease onset may also be relevant. Regional gray matter changes may also be induced by atypical and typical antipsychotics (81), although we failed to find an association with treatment duration.
The pattern of cortical abnormalities reported here differs from that in degenerative conditions. Cortical thinning without area changes has been reported in early Huntington disease (18) and in Alzheimer's disease (85,86), validated at postmortem in the latter using stereologic methods (87).
We did not find significant correlations between cortical abnormalities and symptom severity, in keeping with other (82,83), but not all (84), SBM studies, but we found a strong association between IQ and frontotemporal cortical area. General intelligence depends on neural networks critically involving frontal and parietal cortex (88,89). It has high heritability and correlates strongly with gray matter volume (90,91) in normal twins (92) and singletons (93). These studies have mainly measured cortical thickness (94–96), which is modified by experience-dependent plasticity (97). Cognitive impairment, integral to schizophrenia (98), is best characterized by a generalized deficit (99,100). Those with schizophrenia have lower IQs than their childhood peers (101), and 40%–45% may decline further by illness onset (102–107). This is supported by the closer correlation between cortical area and current rather than premorbid IQ in our patients. We have previously reported that premorbid IQ and IQ at illness onset are prognostic indicators of clinical outcome 3 to 4 years later (106,108). The association between cortical area and IQ reported here suggests that cortical area changes may have prognostic relevance.
There are limitations to our study. Abnormalities in other than frontotemporal cortical areas cannot be excluded. It remains possible, although unlikely, that changes in cortical thickness could have been detected in a larger sample. However, Freesurfer reliability studies (109) suggest that differences in cortical thickness of less than .1 mm could have been detected with our sample size. Moreover, in a more detailed, regional analysis, cortical thinning was only present in one (the right superior temporal) of several temporal parcellations with reduced area suggesting that cortical abnormalities vary in different regions.
Acknowledgments
The study was funded by a program grant from the Wellcome Trust (Grant No. 064607) that also supported authors GP and EMC. Author LGG was supported by Spanish grants from the Instituto de Salud Carlos III (Formacion en Investigacion y Salud, Grant Nos. CM07/ 00048), and Caja de Ahorros de la Inmaculada. Author EMJ is supported by The Raymond Way Fund.
We thank Isobel Harrison and Stan Mutsatsa for recruiting patients for the West London study; Dr. Masuma Harrison for the neuropsychologic testing; and Dr. Ferran Molins for his help in preparing the figures. Our thanks are also due to Professor Miller and other members of the Nuclear Magnetic Resonance Unit of the University College London Institute of Neurology. We are grateful to all the subjects who participated in the study.
Author TREB has acted as a consultant for Servier, Johnson and Johnson, and Bristol-Myers Squibb. The remaining authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Bearden C.E., van Erp T.G., Thompson P.M., Toga A.W., Cannon T.D. Cortical mapping of genotype–phenotype relationships in schizophrenia. Hum Brain Mapp. 2007;28:519–532. doi: 10.1002/hbm.20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giedd J.N., Schmitt J.E., Neale M.C. Structural brain magnetic resonance imaging of pediatric twins. Hum Brain Mapp. 2007;28:474–481. doi: 10.1002/hbm.20403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friston K.J., Frith C.D. Schizophrenia: A disconnection syndrome. Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 4.Harrison P.J., Weinberger D.R. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 5.Honea R., Crow T.J., Passingham D., Mackay C.E. Regional deficits in brain volume in schizophrenia: A meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 6.Glahn D.C., Laird A.R., Ellison-Wright I., Thelen S.M., Robinson J.L., Lancaster J.L. Meta-analysis of gray matter anomalies in schizophrenia: Application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornito A., Yucel M., Patti J., Wood S.J., Pantelis C. Mapping grey matter reductions in schizophrenia: An anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–113. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Ellison-Wright I., Glahn D.C., Laird A.R., Thelen S.M., Bullmore E. The anatomy of first-episode and chronic schizophrenia: An anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Job D.E., Whalley H.C., Johnstone E.C., Lawrie S.M. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Borgwardt S.J., Riecher-Rossler A., Dazzan P., Chitnis X., Aston J., Drewe M. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Meisenzahl E.M., Koutsouleris N., Gaser C., Bottlender R., Schmitt G.J., McGuire P. Structural brain alterations in subjects at high-risk of psychosis: A voxel-based morphometric study. Schizophr Res. 2008;102:150–162. doi: 10.1016/j.schres.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Sun D., Phillips L., Velakoulis D., Yung A., McGorry P.D., Wood S.J. Progressive brain structural changes mapped as psychosis develops in “at risk” individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagary M.S., Symms M.R., Barker G.J., Mutsatsa S.H., Joyce E.M., Ron M.A. Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch Gen Psychiatry. 2003;60:779–788. doi: 10.1001/archpsyc.60.8.779. [DOI] [PubMed] [Google Scholar]
- 14.Price G., Cercignani M., Chu E.M., Barnes T.R., Barker G.J., Joyce EMRon M.A. Brain pathology in first-episode psychosis: Magnetization transfer imaging provides additional information to MRI measurements of volume loss. Neuroimage. 2010;49:185–192. doi: 10.1016/j.neuroimage.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panizzon M.S., Fennema-Notestine C., Eyler L.T., Jernigan T.L., Prom-Wormley E., Neale M. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.K., Lee J.M., Kim J.S., Kim I.Y., Evans A.C., Kim S.I. A novel quantitative cross-validation of different cortical surface reconstruction algorithms using MRI phantom. Neuroimage. 2006;31:572–584. doi: 10.1016/j.neuroimage.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 18.Rosas H.D., Liu A.K., Hersch S., Glessner M., Ferrante R.J., Salat D.H. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 19.White T., Andreasen N.C., Nopoulos P., Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003;54:418–426. doi: 10.1016/s0006-3223(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 20.Janssen J., Reig S., Aleman Y., Schnack H., Udias J.M., Parellada M. Gyral and sulcal cortical thinning in adolescents with first episode early-onset psychosis. Biol Psychiatry. 2009;66:1047–1054. doi: 10.1016/j.biopsych.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Narr K.L., Bilder R.M., Toga A.W., Woods R.P., Rex D.E., Szeszko P.R. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- 22.Schultz C.C., Koch K., Wagner G., Roebel M., Schachtzabel C., Gaser C. Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 2009;116:204–209. doi: 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Wiegand L.C., Warfield S.K., Levitt J.J., Hirayasu Y., Salisbury D.F., Heckers S. Prefrontal cortical thickness in first-episode psychosis: A magnetic resonance imaging study. Biol Psychiatry. 2004;55:131–140. doi: 10.1016/j.biopsych.2003.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenstein D., Lerch J., Shaw P., Clasen L., Giedd J., Gochman P. Childhood onset schizophrenia: Cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47:1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 25.Mattai A., Chavez A., Greenstein D., Clasen L., Bakalar J., Stidd R. Effects of clozapine and olanzapine on cortical thickness in childhood-onset schizophrenia. Schizophr Res. 2010;116:44–48. doi: 10.1016/j.schres.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultz C.C., Koch K., Wagner G., Roebel M., Schachtzabel C., Nenadic I. Psychopathological correlates of the entorhinal cortical shape in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2009 doi: 10.1007/s00406-009-0083-4. [published online ahead of print November 7] [DOI] [PubMed] [Google Scholar]
- 27.Kuperberg G.R., Broome M.R., McGuire P.K., David A.S., Eddy M., Ozawa F. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 28.Csernansky J.G., Gillespie S.K., Dierker D.L., Anticevic A., Wang L., Barch D.M., Van Essen D.C. Symmetric abnormalities in sulcal patterning in schizophrenia. Neuroimage. 2008;43:440–446. doi: 10.1016/j.neuroimage.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goghari V.M., Rehm K., Carter C.S., Macdonald A.W. Sulcal thickness as a vulnerability indicator for schizophrenia. Br J Psychiatry. 2007;191:229–233. doi: 10.1192/bjp.bp.106.034595. [DOI] [PubMed] [Google Scholar]
- 30.Crespo-Facorro B., Kim J., Andreasen N.C., O'Leary D.S., Magnotta V. Regional frontal abnormalities in schizophrenia: A quantitative gray matter volume and cortical surface size study. Biol Psychiatry. 2000;48:110–119. doi: 10.1016/s0006-2332(00)00238-9. [DOI] [PubMed] [Google Scholar]
- 31.Calabrese D.R., Wang L., Harms M.P., Ratnanather J.T., Barch D.M., Cloninger C.R. Cingulate gyrus neuroanatomy in schizophrenia subjects and their non-psychotic siblings. Schizophr Res. 2008;104:61–70. doi: 10.1016/j.schres.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fornito A., Yucel M., Wood S.J., Adamson C., Velakoulis D., Saling M.M. Surface-based morphometry of the anterior cingulate cortex in first episode schizophrenia. Hum Brain Mapp. 2008;29:478–489. doi: 10.1002/hbm.20412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crespo-Facorro B., Roiz-Santianez R., Quintero C., Perez-Iglesias R., Tordesillas-Gutierrez D., Mata I. Insular cortex morphometry in first-episode schizophrenia-spectrum patients: Diagnostic specificity and clinical correlations. J Psychiatr Res. 2009;44(Nos. 5):314–320. doi: 10.1016/j.jpsychires.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Voets N.L., Hough M.G., Douaud G., Matthews P.M., James A., Winmill L. Evidence for abnormalities of cortical development in adolescent-onset schizophrenia. Neuroimage. 2008;43:665–675. doi: 10.1016/j.neuroimage.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Goghari V.M., Rehm K., Carter C.S., Macdonald A.W. Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb Cortex. 2007;17:415–424. doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- 36.Huddy V.C., Hodgson T.L., Kapasi M., Mutsatsa S.H., Harrison I., Barnes T.R., Joyce E.M. Gaze strategies during planning in first-episode psychosis. J Abnorm Psychol. 2007;116:589–598. doi: 10.1037/0021-843X.116.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jablensky A., McGrath J., Herrman H., Castle D., Gureje O., Evans M. Psychotic disorders in urban areas: An overview of the Study on Low Prevalence Disorders. Aust N Z J Psychiatry. 2000;34:221–236. doi: 10.1080/j.1440-1614.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- 38.McGuffin P., Farmer A., Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness: Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48:764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- 39.Wing J.K., Babor T., Brugha T., Burke J., Cooper J.E., Giel R. SCAN: Schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 40.Andreasen N. The University of Iowa; Iowa City, IA: 1984. The Scale for the Assessment of Positive Symptoms (SAPS) [Google Scholar]
- 41.Andreasen N. The University of Iowa; Iowa City, IA: 1983. The Scale for the Assessment of Negative Symptoms (SANS) [Google Scholar]
- 42.Andreasen N.C. Methods for assessing positive and negative symptoms. Mod Probl Pharmacopsych. 1990;24:73–88. doi: 10.1159/000418013. [DOI] [PubMed] [Google Scholar]
- 43.Liddle P.F., Barnes T.R.E. Syndromes of chronic schizophrenia. Br J Psychiatry. 1990;157:558–561. doi: 10.1192/bjp.157.4.558. [DOI] [PubMed] [Google Scholar]
- 44.Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perkins D.O., Leserman J., Jarskog L.F., Graham K., Kazmer J., Lieberman J.A. Characterizing and dating the onset of symptoms in psychotic illness: The symptom onset in schizophrenia (SOS) inventory. Schizophr Res. 2000;44:1–10. doi: 10.1016/s0920-9964(99)00161-9. [DOI] [PubMed] [Google Scholar]
- 47.Drake R.E., Osher F.C., Noordsy D.L., Hurlbut S.C., Teague G.B., Beaudett M.S. Diagnosis of alcohol use disorders in schizophrenia. Schizophr Bull. 1990;16:57–67. doi: 10.1093/schbul/16.1.57. [DOI] [PubMed] [Google Scholar]
- 48.Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 49.Nelson H.E., Willison J. 2nd ed. NFER-Nelson; Windsor, United Kingdom: 1991. The Revised National Adult Reading Test (NART)—Test Manual. [Google Scholar]
- 50.Crawford J.R., Besson J.A., Bremner M., Ebmeier K.P., Cochrane R.H., Kirkwood K. Estimation of premorbid intelligence in schizophrenia. Br J Psychiatry. 1992;161:69–74. doi: 10.1192/bjp.161.1.69. [DOI] [PubMed] [Google Scholar]
- 51.O'Carroll R., Walker M., Dunan J., Murray C., Blackwood D., Ebmeier K.P. Selecting controls for schizophrenia research studies: The use of the national adult reading test (NART) is a measure of premorbid ability. Schizophr Res. 1992;8:137–141. doi: 10.1016/0920-9964(92)90030-9. [DOI] [PubMed] [Google Scholar]
- 52.Wechsler D. 3rd ed. Psychological Corporation; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- 53.Blyler C.R., Gold J.M., Iannone V.N., Buchanan R.W. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res. 2000;46:209–215. doi: 10.1016/s0920-9964(00)00017-7. [DOI] [PubMed] [Google Scholar]
- 54.Sahakian B.J., Owen A.M. Computerized assessment in neuropsychiatry using CANTAB: Discussion paper. J R Soc Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- 55.Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 56.Lezak M.D. 3rd ed. Oxford University Press; New York: 1995. Neuropsychological Assessment. [Google Scholar]
- 57.Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 58.Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 59.Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 60.Buckner R.L., Head D., Parker J., Fotenos A.F., Marcus D., Morris J.C., Snyder A.Z. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 61.Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 62.Diggle P., Liang K., Zeger S. Oxford University Press; Oxford: 1994. Analysis of Longitudinal Data. [Google Scholar]
- 63.Wisco J.J., Kuperberg G., Manoach D., Quinn B.T., Busa E., Fischl B. Abnormal cortical folding patterns within Broca's area in schizophrenia: Evidence from structural MRI. Schizophr Res. 2007;94:317–327. doi: 10.1016/j.schres.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rothman K.J. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 65.Perneger T.V. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thompson P.M., Vidal C., Giedd J.N., Gochman P., Blumenthal J., Nicolson R. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vidal C.N., Rapoport J.L., Hayashi K.M., Geaga J.A., Sui Y., McLemore L.E. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch Gen Psychiatry. 2006;63:25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- 68.Douaud G., Mackay C., Andersson J., James S., Quested D., Ray M.K. Schizophrenia delays and alters maturation of the brain in adolescence. Brain. 2009;132:2437–2448. doi: 10.1093/brain/awp126. [DOI] [PubMed] [Google Scholar]
- 69.Giorgio A., Watkins K.E., Chadwick M., James S., Winmill L., Douaud G. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toro R., Perron M., Pike B., Richer L., Veillette S., Pausova Z., Paus T. Brain size and folding of the human cerebral cortex. Cereb Cortex. 2008;18:2352–2357. doi: 10.1093/cercor/bhm261. [DOI] [PubMed] [Google Scholar]
- 72.Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rakic P. Defects of neuronal migration and the pathogenesis of cortical malformations. Prog Brain Res. 1988;73:15–37. doi: 10.1016/s0079-6123(08)60494-x. [DOI] [PubMed] [Google Scholar]
- 74.Rakic P., Hashimoto-Torii K., Sarkisian M.R. Genetic determinants of neuronal migration in the cerebral cortex. Novartis Found Symposium. 2007:45–53. doi: 10.1002/9780470994030.ch4. [DOI] [PubMed] [Google Scholar]
- 75.Medina L., Abellan A. Development and evolution of the pallium. Semin Cell Dev Biol. 2009;20:698–711. doi: 10.1016/j.semcdb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 76.Martinussen M., Fischl B., Larsson H.B., Skranes J., Kulseng S., Vangberg T.R. Cerebral cortex thickness in 15-year-old adolescents with low birthweight measured by an automated MRI-based method. Brain. 2005;128:2588–2596. doi: 10.1093/brain/awh610. [DOI] [PubMed] [Google Scholar]
- 77.Counsell S.J., Edwards A.D., Chew A.T., Anjari M., Dyet L.E., Srinivasan L. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 78.van Haren N.E., Hulshoff Pol H.E., Schnack H.G., Cahn W., Brans R., Carati I. Progressive brain volume loss in schizophrenia over the course of the illness: Evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63:106–113. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Sun D., Stuart G.W., Jenkinson M., Wood S.J., McGorry P.D., Velakoulis D. Brain surface contraction mapped in first-episode schizophrenia: A longitudinal magnetic resonance imaging study. Mol Psychiatry. 2008;14:976–986. doi: 10.1038/mp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cahn W., Rais M., Stigter F.P., van Haren N.E., Caspers E., Hulshoff Pol H.E. Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2009;19:147–151. doi: 10.1016/j.euroneuro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 81.Navari S., Dazzan P. Do antipsychotic drugs affect brain structure?: A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- 82.Hoff A.L., Sakuma M., Razi K., Heydebrand G., Csernansky J.G., DeLisi L.E. Lack of association between duration of untreated illness and severity of cognitive and structural brain deficits at the first episode of schizophrenia. Am J Psychiatry. 2000;157:1824–1828. doi: 10.1176/appi.ajp.157.11.1824. [DOI] [PubMed] [Google Scholar]
- 83.Ho B.C., Alicata D., Ward J., Moser D.J., O'Leary D.S., Arndt S. Untreated initial psychosis: Relation to cognitive deficits and brain morphology in first-episode schizophrenia. Am J Psychiatry. 2003;160:142–148. doi: 10.1176/appi.ajp.160.1.142. [DOI] [PubMed] [Google Scholar]
- 84.Lappin J.M., Morgan K., Morgan C., Hutchison G., Chitnis X., Suckling J. Gray matter abnormalities associated with duration of untreated psychosis. Schizophr Res. 2006;83:145–153. doi: 10.1016/j.schres.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 85.Du A.T., Schuff N., Kramer J.H., Rosen H.J., Gorno-Tempini M.L., Rankin K. Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain. 2007;130:1159–1166. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dickerson B.C., Feczko E., Augustinack J.C., Pacheco J., Morris J.C., Fischl B., Buckner R.L. Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30:432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Regeur L. Increasing loss of brain tissue with increasing dementia: A stereological study of post-mortem brains from elderly females. Eur J Neurol. 2000;7:47–54. doi: 10.1046/j.1468-1331.2000.00017.x. [DOI] [PubMed] [Google Scholar]
- 88.Duncan J., Seitz R.J., Kolodny J., Bor D., Herzog H., Ahmed A. A neural basis for general intelligence. Science. 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- 89.Gray J.R., Chabris C.F., Braver T.S. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- 90.Posthuma D., de Geus E.J., Baare W.F., Hulshoff Pol H.E., Kahn R.S., Boomsma D.I. The association between brain volume and intelligence is of genetic origin. Nat Neurosci. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- 91.Hulshoff Pol H.E., Schnack H.G., Posthuma D., Mandl R.C., Baare W.F., van O.C. Genetic contributions to human brain morphology and intelligence. J Neurosci. 2006;26:10235–10242. doi: 10.1523/JNEUROSCI.1312-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thompson P.M., Cannon T.D., Narr K.L., van E.T., Poutanen V.P., Huttunen M. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 93.Colom R., Jung R.E., Haier R.J. Distributed brain sites for the g-factor of intelligence. Neuroimage. 2006;31:1359–1365. doi: 10.1016/j.neuroimage.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 94.Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 95.Narr K.L., Woods R.P., Thompson P.M., Szeszko P., Robinson D., Dimtcheva T. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- 96.Choi Y.Y., Shamosh N.A., Cho S.H., DeYoung C.G., Lee M.J., Lee J.M. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J Neurosci. 2008;28:10323–10329. doi: 10.1523/JNEUROSCI.3259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Draganski B., Gaser C., Busch V., Schuierer G., Bogdahn U., May A. Neuroplasticity: Changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 98.Joyce E., Huddy V. Defining the cognitive impairment in schizophrenia. Psychol Med. 2004;34:1151–1155. doi: 10.1017/s0033291704003472. [DOI] [PubMed] [Google Scholar]
- 99.Dickinson D., Iannone V.N., Wilk C.M., Gold J.M. General and specific cognitive deficits in schizophrenia. Biol Psychiatry. 2004;55:826–833. doi: 10.1016/j.biopsych.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 100.Dickinson D., Ragland J.D., Gold J.M., Gur R.C. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64:823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Woodberry K.A., Giuliano A.J., Seidman L.J. Premorbid IQ in schizophrenia: A meta-analytic review. Am J Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 102.Rabinowitz J., Reichenberg A., Weiser M., Mark M., Kaplan Z., Davidson M. Cognitive and behavioural functioning in men with schizophrenia both before and shortly after first admission to hospital: Cross-sectional analysis. Br J Psychiatry. 2000;177:26–32. doi: 10.1192/bjp.177.1.26. [DOI] [PubMed] [Google Scholar]
- 103.Cosway R., Byrne M., Clafferty R., Hodges A., Grant E., Abukmeil S.S. Neuropsychological change in young people at high risk for schizophrenia: Results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychol Med. 2000;30:1111–1121. doi: 10.1017/s0033291799002585. [DOI] [PubMed] [Google Scholar]
- 104.Caspi A., Reichenberg A., Weiser M., Rabinowitz J., Kaplan Z., Knobler H. Cognitive performance in schizophrenia patients assessed before and following the first psychotic episode. Schizophr Res. 2003;65:87–94. doi: 10.1016/s0920-9964(03)00056-2. [DOI] [PubMed] [Google Scholar]
- 105.Lencz T., Smith C.W., McLaughlin D., Auther A., Nakayama E., Hovey L., Cornblatt B.A. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 106.Leeson V.C., Sharma P., Harrison M., Ron M.A., Barnes T.R.E., Joyce E. IQ trajectory, cognitive reserve and clinical outcome following a first-episode of psychosis: A three year longitudinal study. Schizophr Bull. 2010 doi: 10.1093/schbul/sbp143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joyce E.M., Hutton S.B., Mutsatsa S.H., Barnes T.R.E. Cognitive heterogeneity in first-episode schizophrenia. Br J Psychiatry. 2005;187:516–522. doi: 10.1192/bjp.187.6.516. [DOI] [PubMed] [Google Scholar]
- 108.Leeson V.C., Barnes T.R., Hutton S.B., Ron M.A., Joyce E.M. IQ as a predictor of functional outcome in schizophrenia: A longitudinal, four-year study of first-episode psychosis. Schizophr Res. 2009;107:55–60. doi: 10.1016/j.schres.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]