Abstract
Thyroid hormones (T3, T4) have a broad range of effects on bone, however, its role in determining the quality of bone matrix is poorly understood. In-vitro, the immortalized mouse osteoblast-like cell line MC3T3-E1 forms a tissue like structure, consisting of several cell layers, whose formation is affected by T3 significantly. In this culture system, we investigated the effects of T3 on cell multiplication, collagen synthesis, expression of genes related to the collagen cross-linking process and on the formation of cross-links.
T3 compared to controls modulated cell multiplication, up-regulated collagen synthesis time and dose dependently, and stimulated protein synthesis. T3 increased mRNA expressions of procollagen-lysine-1,2-oxoglutarate 5-dioxygenase 2 (Plod2) and of lysyloxidase (Lox), both genes involved in post-translational modification of collagen. Moreover, it stimulated mRNA expression of bone morphogenetic protein 1 (Bmp1), the processing enzyme of the lysyloxidase-precursor and of procollagen. An increase in the collagen cross-link-ratio Pyr/deDHLNL indicates, that T3 modulated cross-link maturation in the MC3T3-E1 culture system. These results demonstrate that T3 directly regulates collagen synthesis and collagen cross-linking by up-regulating gene expression of the specific cross-link related enzymes, and underlines the importance of a well-balanced concentration of thyroid hormones for maintenance of bone quality.
Keywords: Osteoblast, Thyroid hormones, Collagen cross-linking, Gene expression, Bone matrix quality
Research highlights
► We show that T3 dose dependently regulates cell multiplication. ► T3 regulates expression of collagen I, Lox, Plod2, Bmp1. ► T3 affects collagen cross-linking as shown by Fourier transformed infrared spectroscopy.
1. Introduction
Thyroid hormones (T3) and (T4) are critical regulators of skeletal development and maintenance. While hyperthyroidism could cause osteoporosis, hypothyroidism results in severe developmental disturbances of bone and brain [1,2]. Recently, it was demonstrated that depletion of the receptors for thyroid hormones, mimicking hypothyroidism, results in severe distortions of the growth plate and delayed bone development [3,4].
In-vitro, via thyroid hormone receptors, T3 regulates the differentiation of osteoblasts, by increasing the expression of many genes of the osteoblastic phenotype [5] like osteocalcin [6], osteoprotegerin [7] and MMP-13 [8,9]. In addition to hormones, the local environment regulates osteoblastic differentiation as well. Although, growth and differentiation factors are the primary determinants of the cell fate, interactions of the cells with the extracellular matrix (ECM) and other cells are important for the differentiation process [10,11]. The ECM can affect the behavior of the cells either by the proteins forming the ECM or by growth and differentiation factors with their binding proteins immobilised on it. Because ECM considerably influences the behavior of the cells, any event altering its composition or structure can have profound effects on the differentiation state [10]. Moreover, we have recently demonstrated that not only the biochemical structuring of ECM, but proper cross-linking of collagen (I) is a prerequisite for osteoblastic differentiation. Specifically, we recently demonstrated the diminution of osteoblastic differentiation on such a modified ECM [12] by using the lathyrogen β-aminopropionitrile (bAPN), an inhibitor of the enzyme lysyloxidase (Lox), which extracellularly processes collagen and initiates collagen cross-link formation [13]. Furthermore, homocysteine, an amino acid metabolite, which is suggested interfering with Lox action [14], negatively influences bone quality [15–18] and modulates gene expression and Runx2 activity in MC3T3-E1 osteoblastic cells [19].
Recently, in the osteoblastic MC3T3-E1 culture system it was demonstrated that 1,25D3 increases collagen quality by regulating some genes of the enzymatic apparatus, important for collagen cross-linking resulting in an increase of mature collagen cross-links [20], although, collagen mRNA levels were not influenced [20,21]. In rat osteosarcoma cells, however, 1,25D3 and thyroid hormones up-regulate collagen mRNA expression [22]. Interestingly, 1,25D3 up-regulated the collagen expression in the well-differentiated human osteosarcoma cells MG-63, unlike in the less differentiated SaOS-2 cell line [23].
Aware of the importance of thyroid hormones for bone development and maintenance, and the different effects of thyroid hormones and 1,25D3 on osteoblasts, especially in mice and humans [24,25], we investigated how T3 regulates collagen matrix formation and cross-linking in osteoblastic MC3T3-E1 cells.
2. Materials and methods
2.1. Cell culture
MC3T3-E1 cells (kindly donated by Dr. Kumegawa, Meikai University, Department of Oral Anatomy, Sakado, Japan) were cultured in alpha MEM (Sigma), supplemented with 4.5 g/l glucose, 5% FCS (Sigma) and 30 μg/ml Gentamycin (Sigma) at 37 °C under 5% CO2 in humidified air. They were subcultured twice a week using 0.001% pronase E (Roche) and 0.02% EDTA in Ca2+ and Mg2+ free phosphate-buffered saline (PBS). To prevent a potential phenotypic drift during repeated subcultures the cells were not used for more than four weeks after thawing.
2.2. Determination of the cell multiplication and total protein
To estimate the cell multiplication cells were seeded in 24 wells micro plates at a density of 5000 cells/cm2 and cultured for the indicated time in the culture medium described above with or without 10−7 M T3 for 1, 2, 3, 4, 8 and 12 days. Dose dependency of T3 on cell multiplication was investigated on days 4 and 8 at 10−9, 10−8, 10−7, 10−6 M T3. After the treatment period, the cell layer was washed with PBS and cells were detached from the culture plate by treatment with 0.002% pronase E (Roche) and 0.04% EDTA in PBS. All cells of a well were counted in a cell counter (Schärfe, Germany).
For the determination of cell number (DNA amount), cell layers were washed with PBS and frozen with 1 mM Tris–HCl buffer (pH 8.0) containing 0.1 mM EDTA. During thawing, Hoechst dye (Polysciences, Warrington, PA) was added (1 μg/ml in 150 mM NaCl) and, after an incubation of 15 min at room temperature, the fluorescence of the DNA was measured (excitation 360, emission 465 nm). Calf thymus DNA was used to prepare a standard curve.
To address the amount of proteins in the cultures, after washing with PBS, the cell layers were dissolved in 0.5 M sodium hydroxide solution and thereafter neutralized with 0.5 M Tris–HCl buffer (pH 7.4). The protein concentration of an aliquot was measured using bicirconic acid in 0.8% copper sulfate using bovine serum albumin as standard.
2.3. Estimation of collagenase digestible protein to address collagen synthesis
Collagen protein synthesis was assessed as collagenase digestible protein (CDS) by pulse-labeling of the cultures with [14]C-proline according to Petrokofsky and Diegelmann [26]. For this purpose the culture medium was replaced by serum free culture medium containing 0.1% bovine serum albumin, gentamycin, 50 μg/ml ascorbic acid and 100 μg/ml βAPN to prevent cross-linking. After addition of 10 μCi [14]C-proline the cultures were incubated for 6 h.
Because T3 strongly influences cell multiplication the estimated amount of collagen was normalized to the DNA-content of the culture.
2.4. RNA-isolation and expression analysis by northern hybridization
Cytoplasmic RNA was isolated using a mini-prep method at days 4, 8 and 12. The total amount of RNA was estimated by measuring the absorption at 260 nm with a Hitachi spectrophotometer. Northern hybridization was performed by fractionating 10 μg total RNA on a 1% agarose gel containing 2.2 M formaldehyde. After electrophoresis the gel was soaked in 0.1 M Tris–HCl and 0.15 M NaCl for 5 min and transferred to a nylon filter (NEN, Brussels) with 20xSSC (1xSSC is 0.15 M NaCl and 0.015 M sodium citrate). After baking the filter for 2 h at 80 °C, hybridization was done over night in 10% dextransulfate, 10 μg/ml shared salmon sperm DNA, 1 M NaCl and 1% sodium dodecylsulfate after 1 h prehybridization in the same solution. For estimation of the amount of mRNA, the filters were evaluated in an Instant Imager (Packard Instrument Company, Meriden, CT). As hybridization probe we used the 3′end of the mouse Col1a1 cDNA. As a control we hybridized the same northern blots using the Pst I fragment of rat glyceraldehyde-phosphate-dehydrogenase (Gapdh). Probe labeling was performed with [32]P-dCTP by multi prime labeling according to the suppliers suggestions (Roche).
2.5. Expression analysis by quantitative reverse transcription polymerase chain reaction (QRT-PCR)
mRNA was extracted using a mRNA Isolation Kit (Roche) and cDNA was synthesized from the mRNA using the 1st Strand cDNA Synthesis Kit (Roche). The obtained cDNA was subjected to PCR amplification with a real time cycler using TaqMan Gene Expression Master Mix (Applied Biosystems) and TaqMan primers (Applied Biosystems) that amplify lysyloxidase (Lox, Mm00495386_m1) bone morphogenetic protein 1 (Bmp1, Mm00802225_m1) and procollagen lysine, 2-oxoglutarate 5-dioxygenase 2 (Plod2, Mm00478767_m1). Gapdh was used as a housekeeping gene for normalization, amplified in the same tube. All QRT-PCR’s were performed in triplicate.
After 10 min of initial denaturation at 95 °C the PCR was performed with 60 cycles: 10 s denaturation at 95 °C; 30 s annealing and extension at 60 °C. Quantification using the 2^ [−δδC (T)] method [27].
2.6. Fourier transform infrared imaging (FTIRI)
For collagen cross-link analysis, the cells were fixed in alcohol, scraped off the culture dishes and transferred onto barium fluoride windows where they were air-dried. Following this, spectra were obtained in transmission with a Bruker Equinox 55 spectrometer coupled to a Bruker Hyperion 3000 FTIR microscope equipped with a motorized stage (±1 μm) and a 15× objective. The spectra were baseline-corrected in the amide I & II spectral area (∼1500–1700 cm−1), water vapor subtracted and then subjected to second derivative spectroscopy and curve fitting routines as published previously [28]. The ratio of the relative areas under the amide I peaks at 1660 and 1690 cm−1 corresponds to the two major coll-x pyridinoline (Pyr) and dehydro-dihydroxylysinonorleucine (deDHLNL). With maturation, the content of the divalent coll-x deDHLNL is diminished, whereas that of Pyr is increased, because the former matures into the latter with time. Therefore, the ratio (Pyr/deDHLNL) provides insight into the maturity of the ECM. The type and amount of collagen cross-links was determined as previously published [28].
2.7. Statistical analysis
Statistical analyses were performed by ANOVA (Turkey’s post hoc test) or Student’s t-test using Prism 4.03. (GraphPad Software Inc., CA, USA). P ⩽ 0.05 was considered to be significant and the data are presented as mean ± standard deviation (mean ± SD).
3. Results
Recently, we have demonstrated that T3 significantly influences formation of the ECM [29,30], and dramatically attenuates cell multiplication [31]. To assess specific effects of T3 on the synthesis of components of the ECM, it is important to relate the effects to cell number or its surrogate DNA-content of the cultures.
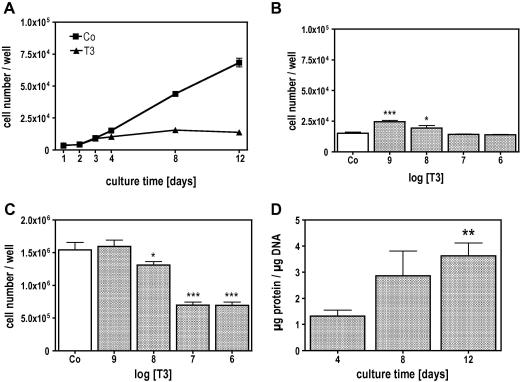
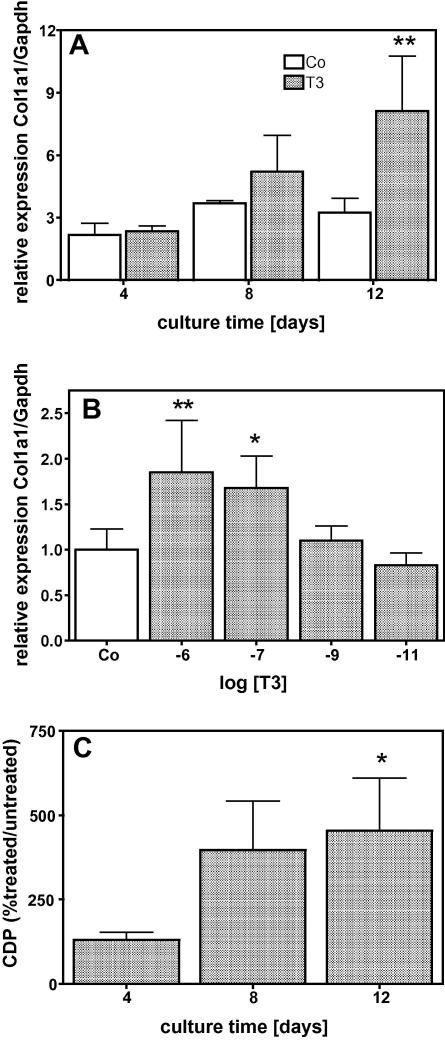
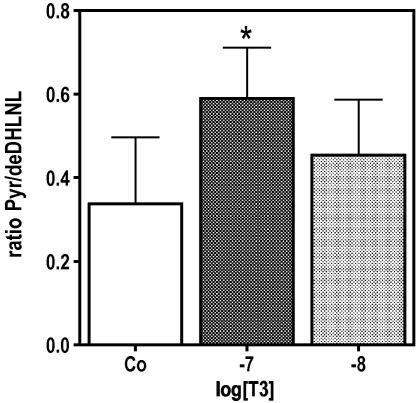
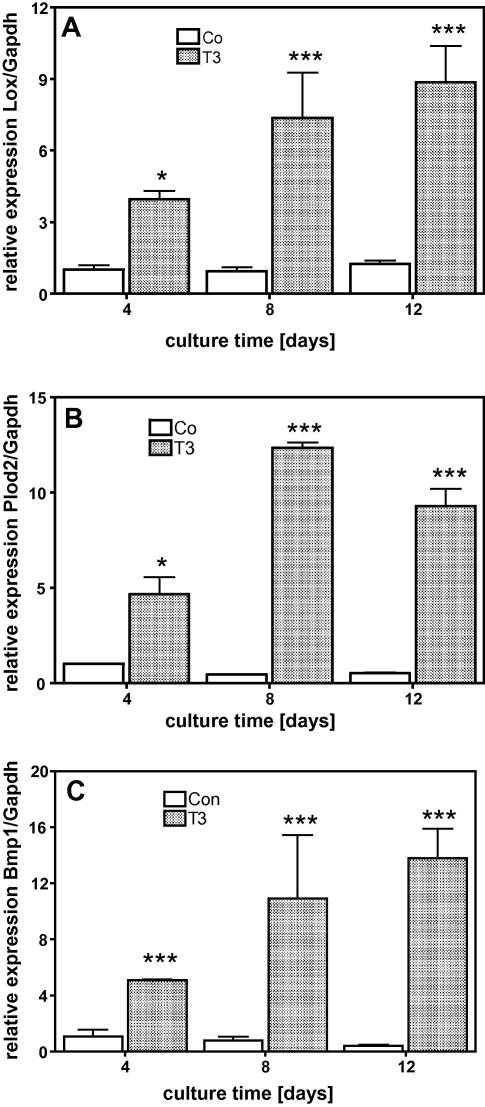
When MC3T3-E1 cells were treated with 10−7 M T3, there was a significant attenuation of cell number in the cultures compared to control. This effect was time and dose dependent as demonstrated in Fig. 1. In the early culture time (day 4), T3 at low concentrations increased cell multiplication significantly while at higher concentrations it had no significant stimulatory effect (Fig. 1B). On day 12, when untreated cells already formed several cell layers, T3 attenuated cell multiplication dose dependently (Fig. 1C). The protein synthesis, normalized to the DNA-content of the cultures, significantly increased during the culture time indicating that T3 up-regulated total protein synthesis in MC3T3-E1 cells (Fig. 1D). Osteoblasts are known to synthesize large amounts of collagen, thus, we analysed the expression of the collagen type (I) and found, that T3 increased the amount of Col1a1 mRNA levels, which were significantly different after 12 days of treatment (Fig. 2A). The T3 effect on Col1a1 mRNA levels was dose dependent (Fig. 2B). Measuring [14]C-proline incorporation into newly synthesized protein clearly demonstrated that the mRNA effect is translated into protein as demonstrated by collagenase digestible protein (CDP), a well-known surrogate for collagen synthesis (Fig. 2C). The influence of T3 on the cross-linking process was demonstrated by measuring collagen cross-links ratio by FTIR. This technique allows access to the specific types of cross-links that are abundant in mineralized tissues (Pyr and deDHLNL [28]) and gives information about the maturity and thus quality of the ECM. The collagen cross-link ratio was increased in T3 treated cultures and showed a dose-dependent effect, which reached significance at 10−7 M (Fig. 3). These findings led us to address the question whether T3, similar to 1,25D3 [20], regulates enzymes involved in post-translational collagen modification that are necessary to prepare collagen for the cross-linking process. mRNA of T3-treated and untreated cultures were isolated at different time points, reverse transcribed, and analysed for mRNA expression of Lox and Plod2 by QRT-PCR. Fig. 4 demonstrates that T3 stimulated the expression of both, Lox and Plod2. During the culture time the basal levels of Lox were not changed; stimulation by T3 was already established on day 4 (4-fold) and increased to day 12 were it was 8-fold (Fig. 4A). Plod2 was down-regulated during culture time by 50%. The stimulation by T3 was 4-fold at day 4, 12-fold at day 8 and about 9-fold on day 12 (Fig. 4B). Lox when exported to the extra-cellular space, must be activated by cleavage of the propeptide by Bmp1 a proteinase that also liberate the collagen type I chain from the C-terminal propeptide to enable collagen fibril formation. Fig. 4C demonstrates that during the culture time, Bmp1 mRNA expression was down-regulated in untreated cells to about 50% but T3 increased the mRNA levels about 5, 9 and 14-fold on day 4, 8 or 12, respectively.
Fig. 1.
T3 regulated cell multiplication and protein synthesis of MC3T3-E1 osteoblastic cells. Cells were cultured in absence (Co) or presence of T3 (10−7 M) for the indicated time. During culture T3 attenuated cell multiplication beginning with day 4 (A) significantly (P ⩽ 0.001). The effect of T3 was dose dependent. T3 significantly increased cell multiplication at 10−9 and 10−8 M on day 4 (B) while on day 12 (C) T3 attenuated cell multiplication at 10−8, 10−7 and 10−6 M, significantly. On day 12, T3 increased protein synthesis significantly when normalized to the DNA-amount. The bars indicate mean ± SD. ∗P ⩽ 0.05; ∗∗P ⩽ 0.01; ∗∗∗P ⩽ 0.001; Co vs. treatments (n = 4).
Fig. 2.
T3 stimulated collagen α1 (I) expression in MC3T3-E1 osteoblastic cells time and dose dependently. Cells were cultured in absence (Co) or presence of 10−7 M T3 for 4, 8 and 12 days (A) and on day 12 with increasing concentrations T3 (B). T3 time and dose dependently increased Col1a1 mRNA expression reaching significance on day 12 (A) at 10−7 and 10−6 M (B). (C) Up-regulation of Col1a1 mRNA was translated into protein as shown by collagenase digestible protein (CDP) that was estimated as a surrogate for collagen protein synthesis. The bars indicate mean ± SD. ∗P ⩽ 0.05; ∗∗P ⩽ 0.01; Co vs. treatments (n = 4).
Fig. 3.
T3 modify cross-link of the ECM produced by MC3T3-E1 cells. MC3T3-E1 cells were cultured in absence (Co) or presence of 10−8 and 10−7 M T3 for 12 days. Thereafter, cell layers were fixed with 70% Ethanol and specific cross-links were analysed by FTIR. T3 increased cross-link ratio (Pyr/deDHLNL) as a function of concentration, which showed significant difference at 10−7 M T3. Provided P-values indicate significantly different values vs. control cultures. The bars indicate mean ± SD. ∗P ⩽ 0.05 (n = 3).
Fig. 4.
T3 stimulated mRNA expression of genes related to collagen cross-linking process. Cells were cultured in absence (Co) or presence of 10−7 M T3 for 4, 8 and 12 days. Compared to untreated controls T3 increased expression of Lox (A), Plod2 (B) and Bmp1 (C) significantly. Total RNA was isolated and subjected to QRT-PCR, which was performed as triplicate. The bars indicate mean ± SD of a typical experiment. ∗P ⩽ 0.05; ∗∗∗P ⩽ 0.001 (n = 3).
4. Discussion
Osteoblastic differentiation is characterized by up-regulation of specific proteins. We and others have demonstrated that T3 up-regulates many proteins of the osteoblastic phenotype [5,6,8,9,32–36]. In vivo, the importance of thyroid hormones for the development and the maintenance of the skeleton is well established [37]. Those findings were supported by transgenic animal models, where it was demonstrated that mice lacking thyroid hormone receptors, are viable but exhibit disorders of the pituitary–thyroid axis, growth, and bone maturation [3,4,38,39]. Newborn mice show decreased femur and tibia length that persist into the adulthood, and the growth plates exhibit increased cartilage- and reduced bone area with delayed ossification. These facts clearly demonstrate the importance of thyroid hormones for the induction of hyperthrophy of chondroblasts [22], maturation of the growth plate, as well as for the differentiation of osteoblasts [6,32]. Another feature of thyroid hormone receptor depleted mice is a disorganized growth plate [3]. The fact that the growth plate consists mainly of collagen fibres points even more to the importance of thyroid hormones for proper collagen fibril organization; but a proper formation of collagen fibres requires the coordinated expression of the participating genes. Our findings that T3 up-regulated the expression of those genes affecting collagen cross-linking and collagen maturation, which is reflected in a change of the cross-linking pattern, suggest an improvement of collagen quality by thyroid hormones.
In this work we demonstrate that T3 up-regulated genes involved in bone matrix formation and collagen maturation. Nevertheless, it should be kept in mind that T3 not only up-regulates genes important for the bone formation and differentiation process [7], but also regulates genes of the resorption process, especially at higher “hyperthyroid” concentrations, as necessarily used in this study to demonstrate significant effects. Genes involved in bone resorption regulated by T3, are collagenases [8,9] and carbonic anhydrase, an enzyme important for the osteoclastic bone resorption [40]. Moreover, in a calvarial mouse model system for bone resorption, it was demonstrated that thyroid hormones strongly activate osteoclastic bone resorption [41–43].
Well regulated thyroid hormone concentrations in blood are of great importance: not only a hyper- or hypothyroid status is critical for the patient, but even tiny aberrations from the euthyroid status, as found in subclinical dysfunctions, can have repercussions on the cardiovascular system and bone, as well as on other organs and systems [44]. The in-vitro experiments presented in this and previous studies support the importance of well-balanced thyroid hormone concentrations; low to normal concentrations increase cell multiplication, while too high concentrations as found in the hyperthyroid status could induce apoptosis [31].
Recently, it was demonstrated that 1,25D3 also regulates collagen quality by increasing mRNA expression of Loxl2 as well as Plod1 and Plod2, especially the splicing variant Plod2b (LH2b). Although, we did not study the regulation of the Lox-like genes, we would like to emphasize the importance of the different iso-enzymes: cross-linking is a very tissue specific process, and different expression patterns of iso-enzymes of Plod and Lox and Lox-like proteins in the different tissues could be responsible for the formation of different types of collagen cross-links. Gene array analysis suggested that all three known Plod’s are expressed in the osteoblastic MC3T3-E1 cell line [19], while Plod2b seemed to be the key player because it is strongly regulated by the osteotropic hormones T3 (this work) and 1,25D3 [20,45]. Of the enzymes regulating cross-linking extracellularly, Loxl2 and Loxl4 are only marginally expressed in this cell line (not shown); 1,25D3 strongly up-regulates Loxl2 but not Lox [20] whereas T3 strongly up-regulated Lox. These findings may indicate that only a combined action of those hormones could guarantee a bone with high quality, but we are aware that other hormones and factors are also involved in this process.
Bmp1 a procollagen C-endopeptidase, processes both, procollagen and LOX; the propeptide of the LOX-precursor is splitted off and is suggested to be involved in regulation of cell differentiation as a tumor-suppressor [13] and modulator of signals from the ECM [46]. The demonstrated regulation of Bmp1 in this study underlines the overall influence of thyroid hormones on bone metabolism and cross-linking.
In summary, we demonstrated that T3 regulates the bone formation process by up-regulation of collagen and of enzymes important of collagen-fibre formation. Our results and recently published data emphasize the importance of fine-tuned thyroid hormone concentrations for proper bone maintenance.
Acknowledgments
This study was supported by the Fonds zur Foerderung der wissenschaftlichen Forschung (FWF; The Austrian Science Fund) Project P20646-B11, the WGKK (Social Health Insurance Vienna), and the AUVA (Austrian Social Insurance for Occupational Risks).
References
- 1.Murphy E., Williams G.R. The thyroid and the skeleton. Clin. Endocrinol. (Oxf.) 2004;61:285–298. doi: 10.1111/j.1365-2265.2004.02053.x. [DOI] [PubMed] [Google Scholar]
- 2.Allain T.J., McGregor A.M. Thyroid hormones and bone. J. Endocrinol. 1993;139:9–18. doi: 10.1677/joe.0.1390009. [DOI] [PubMed] [Google Scholar]
- 3.Gothe S., Wang Z., Ng L., Kindblom J.M., Barros A.C., Ohlsson C., Vennstrom B., Forrest D. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary–thyroid axis, growth, and bone maturation. Genes Dev. 1999;13:1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson O., Marino R., De Luca F., Phillip M., Baron J. Endocrine regulation of the growth plate. Horm. Res. 2005;64:157–165. doi: 10.1159/000088791. [DOI] [PubMed] [Google Scholar]
- 5.Harvey C.B., O’Shea P.J., Scott A.J., Robson H., Siebler T., Shalet S.M., Samarut J., Chassande O., Williams G.R. Molecular mechanisms of thyroid hormone effects on bone growth and function. Mol. Genet. Metab. 2002;75:17–30. doi: 10.1006/mgme.2001.3268. [DOI] [PubMed] [Google Scholar]
- 6.Varga F., Rumpler M., Luegmayr E., Fratzl Z.N., Glantschnig H., Klaushofer K. Triiodothyronine, a regulator of osteoblastic differentiation: depression of histone H4, attenuation of c-fos/c-jun, and induction of osteocalcin expression. Calcif. Tissue Int. 1997;61:404–411. doi: 10.1007/s002239900356. [DOI] [PubMed] [Google Scholar]
- 7.Varga F., Spitzer S., Klaushofer K. Triiodothyronine (T3) and 1,25-dihydroxyvitamin D3 (1,25D3) inversely regulate OPG gene expression in dependence of the osteoblastic phenotype. Calcif. Tissue Int. 2004;74:382–387. doi: 10.1007/s00223-003-0033-5. [DOI] [PubMed] [Google Scholar]
- 8.Fratzl-Zelman N., Glantschnig H., Rumpler M., Nader A., Ellinger A., Varga F. The expression of matrix metalloproteinase-13 and osteocalcin in mouse osteoblasts is related to osteoblastic differentiation and is modulated by 1,25-dihydroxyvitamin D3 and thyroid hormones. Cell Biol. Int. 2003;27:459–468. doi: 10.1016/s1065-6995(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 9.Pereira R.C., Jorgetti V., Canalis E. Triiodothyronine induces collagenase-3 and gelatinase B expression in murine osteoblasts. Am. J. Physiol. 1999;277:E496–E504. doi: 10.1152/ajpendo.1999.277.3.E496. [DOI] [PubMed] [Google Scholar]
- 10.Streuli C. Extracellular matrix remodelling and cellular differentiation. Curr. Opin. Cell Biol. 1999;11:634–640. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 11.Moursi A.M., Damsky C.H., Lull J., Zimmerman D., Doty S.B., Aota S., Globus R.K. Fibronectin regulates calvarial osteoblast differentiation. J. Cell Sci. 1996:1369–1380. doi: 10.1242/jcs.109.6.1369. [DOI] [PubMed] [Google Scholar]
- 12.Turecek C., Fratzl-Zelman N., Rumpler M., Buchinger B., Spitzer S., Zoehrer R., Durchschlag E., Klaushofer K., Paschalis E.P., Varga F. Collagen cross-linking influences osteoblastic differentiation. Calcif. Tissue Int. 2008;82:392–400. doi: 10.1007/s00223-008-9136-3. [DOI] [PubMed] [Google Scholar]
- 13.Kagan H.M., Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J. Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 14.Liu G., Nellaiappan K., Kagan H.M. Irreversible inhibition of lysyl oxidase by homocysteine thiolactone and its selenium and oxygen analogues. Implications for homocystinuria. J. Biol. Chem. 1997;272:32370–32377. doi: 10.1074/jbc.272.51.32370. [DOI] [PubMed] [Google Scholar]
- 15.Oxlund H., Mosekilde L., Ortoft G. Reduced concentration of collagen reducible cross links in human trabecular bone with respect to age and osteoporosis. Bone. 1996;19:479–484. doi: 10.1016/s8756-3282(96)00283-9. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann W. Significance of hyperhomocysteinemia. Clin. Lab. 2006;52:367–374. [PubMed] [Google Scholar]
- 17.Blouin S., Thaler H.W., Korninger C., Schmid R., Hofstaetter J.G., Zoehrer R., Phipps R., Klaushofer K., Roschger P., Paschalis E.P. Bone matrix quality and plasma homocysteine levels. Bone. 2009;44:959–964. doi: 10.1016/j.bone.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 18.van Meurs J.B., Dhonukshe-Rutten R.A., Pluijm S.M., van der Klift M., de Jonge R., Lindemans J., de Groot L.C., Hofman A., Witteman J.C., van Leeuwen J.P., Breteler M.M., Lips P., Pols H.A., Uitterlinden A.G. Homocysteine levels and the risk of osteoporotic fracture. N. Engl. J. Med. 2004;350:2033–2041. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 19.Thaler R., Spitzer S., Rumpler M., Fratzl-Zelman N., Klaushofer K., Paschalis E.P., Varga F. Differential effects of homocysteine and beta aminopropionitrile on preosteoblastic MC3T3-E1 cells. Bone. 2009;46:703–709. doi: 10.1016/j.bone.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 20.Nagaoka H., Mochida Y., Atsawasuwan P., Kaku M., Kondoh T., Yamauchi M. 1,25(OH)2D3 regulates collagen quality in an osteoblastic cell culture system. Biochem. Biophys. Res. Commun. 2008;377:674–678. doi: 10.1016/j.bbrc.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y.C., Worton L., Esteban L., Baldock P., Fong C., Eisman J.A., Gardiner E.M. Effects of continuous activation of vitamin D and Wnt response pathways on osteoblastic proliferation and differentiation. Bone. 2007;41:87–96. doi: 10.1016/j.bone.2007.04.174. [DOI] [PubMed] [Google Scholar]
- 22.Williams G.R., Robson H., Shalet S.M. Thyroid hormone actions on cartilage and bone: interactions with other hormones at the epiphyseal plate and effects on linear growth. J. Endocrinol. 1998;157:391–403. doi: 10.1677/joe.0.1570391. [DOI] [PubMed] [Google Scholar]
- 23.Mahonen A., Jukkola A., Risteli L., Risteli J., Maenpaa P.H. Type I procollagen synthesis is regulated by steroids and related hormones in human osteosarcoma cells. J. Cell Biochem. 1998;68:151–163. [PubMed] [Google Scholar]
- 24.Varga F., Spitzer S., Rumpler M., Klaushofer K. 1,25-Dihydroxyvitamin D3 inhibits thyroid hormone-induced osteocalcin expression in mouse osteoblast-like cells via a thyroid hormone response element. J. Mol. Endocrinol. 2003;30:49–57. doi: 10.1677/jme.0.0300049. [DOI] [PubMed] [Google Scholar]
- 25.Varga F., Rumpler M., Spitzer S., Karlic H., Klaushofer K. Osteocalcin attenuates T3- and increases vitamin D3-induced expression of MMP-13 in mouse osteoblasts. Endocr. J. 2009;56:441–450. doi: 10.1507/endocrj.k08e-192. [DOI] [PubMed] [Google Scholar]
- 26.Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971;10:988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- 27.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, CA) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Paschalis E.P., Verdelis K., Doty S.B., Boskey A.L., Mendelsohn R., Yamauchi M. Spectroscopic characterization of collagen cross-links in bone. J. Bone Miner. Res. 2001;16:1821–1828. doi: 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- 29.Luegmayr E., Varga F., Frank T., Roschger P., Klaushofer K. Effects of triiodothyronine on morphology, growth behavior, and the actin cytoskeleton in mouse osteoblastic cells (MC3T3-E1) Bone. 1996;18:591–599. doi: 10.1016/8756-3282(96)00068-3. [DOI] [PubMed] [Google Scholar]
- 30.Fratzl-Zelman N., Horandner H., Luegmayr E., Varga F., Ellinger A., Erlee M.P., Klaushofer K. Effects of triiodothyronine on the morphology of cells and matrix, the localization of alkaline phosphatase, and the frequency of apoptosis in long-term cultures of MC3T3-E1 cells. Bone. 1997;20:225–236. doi: 10.1016/s8756-3282(96)00367-5. [DOI] [PubMed] [Google Scholar]
- 31.Varga F., Luegmayr E., Fratzl-Zelman N., Glantschnig H., Ellinger A., Prinz D., Rumpler M., Klaushofer K. Tri-iodothyronine inhibits multilayer formation of the osteoblastic cell line, MC3T3-E1, by promoting apoptosis. J. Endocrinol. 1999;160:57–65. doi: 10.1677/joe.0.1600057. [DOI] [PubMed] [Google Scholar]
- 32.Kasono K., Sato K., Han D.C., Fujii Y., Tsushima T., Shizume K. Stimulation of alkaline phosphatase activity by thyroid hormone in mouse osteoblast-like cells (MC3T3-E1): a possible mechanism of hyperalkaline phosphatasia in hyperthyroidism. Bone Miner. 1988;4:355–363. [PubMed] [Google Scholar]
- 33.Sato K., Han D.C., Fujii Y., Tsushima T., Shizume K. Thyroid hormone stimulates alkaline phosphatase activity in cultured rat osteoblastic cells (ROS 17/2.8) through 3,5,3′-triiodo-l-thyronine nuclear receptors. Endocrinology. 1987;120:1873–1881. doi: 10.1210/endo-120-5-1873. [DOI] [PubMed] [Google Scholar]
- 34.Schmid C., Schlapfer I., Futo E., Waldvogel M., Schwander J., Zapf J., Froesch E.R. Triiodothyronine (T3) stimulates insulin-like growth factor (IGF)-1 and IGF binding protein (IGFBP)-2 production by rat osteoblasts in vitro. Acta Endocrinol. Copenh. 1992;126:467–473. doi: 10.1530/acta.0.1260467. [DOI] [PubMed] [Google Scholar]
- 35.Williams G.R., Bland R., Sheppard M.C. Retinoids modify regulation of endogenous gene expression by vitamin D3 and thyroid hormone in three osteosarcoma cell lines. Endocrinology. 1995;136:4304–4314. doi: 10.1210/endo.136.10.7664649. [DOI] [PubMed] [Google Scholar]
- 36.Klaushofer K., Varga F., Glantschnig H., Fratzl-Zelman N., Czerwenka E., Leis H.J., Koller K., Peterlik M. The regulatory role of thyroid hormones in bone cell growth and differentiation. J. Nutr. 1995;125:1996S–2003S. doi: 10.1093/jn/125.suppl_7.1996S. [DOI] [PubMed] [Google Scholar]
- 37.Greenspan S.L., Greenspan F.S. The effect of thyroid hormone on skeletal integrity. Ann. Intern. Med. 1999;130:750–758. doi: 10.7326/0003-4819-130-9-199905040-00016. [DOI] [PubMed] [Google Scholar]
- 38.Shao Y.Y., Wang L., Ballock R.T. Thyroid hormone and the growth plate. Rev. Endocr. Metab. Disord. 2006;7:265–271. doi: 10.1007/s11154-006-9012-2. [DOI] [PubMed] [Google Scholar]
- 39.O’Shea P.J., Williams G.R. Insight into the physiological actions of thyroid hormone receptors from genetically modified mice. J. Endocrinol. 2002;175:553–570. doi: 10.1677/joe.0.1750553. [DOI] [PubMed] [Google Scholar]
- 40.Margolis D.S., Szivek J.A., Lai L.W., Lien Y.H. Phenotypic characteristics of bone in carbonic anhydrase II-deficient mice. Calcif. Tissue Int. 2008;82:66–76. doi: 10.1007/s00223-007-9098-x. [DOI] [PubMed] [Google Scholar]
- 41.Britto J.M., Fenton A.J., Holloway W.R., Nicholson G.C. Osteoblasts mediate thyroid hormone stimulation of osteoclastic bone resorption. Endocrinology. 1994;134:169–176. doi: 10.1210/endo.134.1.8275930. [DOI] [PubMed] [Google Scholar]
- 42.Klaushofer K., Hoffmann O., Gleispach H., Leis H.J., Czerwenka E., Koller K., Peterlik M. Bone-resorbing activity of thyroid hormones is related to prostaglandin production in cultured neonatal mouse calvaria. J. Bone Miner. Res. 1989;4:305–312. doi: 10.1002/jbmr.5650040304. [DOI] [PubMed] [Google Scholar]
- 43.Conaway H.H., Ransjo M., Lerner U.H. Prostaglandin-independent stimulation of bone resorption in mouse calvariae and in isolated rat osteoclasts by thyroid hormones (T4 and T3) Proc. Soc. Exp. Biol. Med. 1998;217:153–161. doi: 10.3181/00379727-217-44217. [DOI] [PubMed] [Google Scholar]
- 44.Biondi B., Cooper D.S. The clinical significance of subclinical thyroid dysfunction. Endocr. Rev. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 45.Pornprasertsuk S., Duarte W.R., Mochida Y., Yamauchi M. Lysyl hydroxylase-2b directs collagen cross-linking pathways in MC3T3-E1 cells. J. Bone Miner. Res. 2004;19:1349–1355. doi: 10.1359/JBMR.040323. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y., Min C., Vora S.R., Trackman P.C., Sonenshein G.E., Kirsch K.H. The lysyl oxidase pro-peptide attenuates fibronectin-mediated activation of focal adhesion kinase and p130Cas in breast cancer cells. J. Biol. Chem. 2009;284:1385–1393. doi: 10.1074/jbc.M802612200. [DOI] [PMC free article] [PubMed] [Google Scholar]