Abstract
Background and Objectives
Although circadian variation in the onset of acute myocardial infarction (AMI) has been reported in a number of studies, not much is known about the impact of circadian variation on 12-month mortality. The aim of this study was to investigate the impact of circadian variation on 12-month mortality in patients with AMI.
Subjects and Methods
Eight hundred ninety two patients (mean age 67±12; 66.1% men) with AMI who visited Kyungpook National University Hospital from November 2005 to December 2007 were included in this study. Patients were divided into groups based on four 6-hours intervals: overnight (00:00-05:59); morning (06:00-11:59); afternoon (12:00-17:59) and evening (18:00-23:59).
Results
Kaplan-Meier survival curves showed 12-month mortality rates of 9.6%, 9.1%, 12.1%, and 16.7% in the overnight, morning, afternoon, evening-onset groups, respectively (p=0.012). Compared with the morning-onset AMI group, the serum creatinine levels (p=0.002), frequency of Killip class ≥3 (p=0.004), and prescription rate of diuretics (p=0.011) were significantly higher in the evening-onset AMI group, while the left ventricular ejection fraction (p=0.012) was significantly lower. The proportion of patients who arrived in the emergency room during routine duty hours was significantly lower in evening-onset groups irrespective of the presence or absence of ST-segment elevation (p<0.001). According to univariate analysis, the 12-month mortality rate in the evening group was significantly higher compared to the morning group (hazard ratio 1.998, 95% confidence interval 1.196 to 3.338, p=0.008).
Conclusion
Patients with evening-onset AMI had poorer baseline clinical characteristics, and this might affect the circadian impact on 12-month mortality. Further studies are needed to clarify the role of circadian variation on the long-term outcome of AMI.
Keywords: Myocardial infarction, Circadian rhythm
Introduction
A circadian variation in the frequency of onset of acute myocardial infarction (AMI) has been reported in a number of studies over the past several decades. A majority of large-scale reports have shown a peak incidence in morning hours, although a secondary peak incidence in the late evening has sometimes been reported.1-7) The higher incidence of ST-segment elevation myocardial infarction (STEMI) in early morning has been explained by changes in catecholamine levels, fibrinolytic activity, blood pressure, platelet aggregability, coronary tone, and endothelial function.4),5),8-10) Differences in the circadian variation of AMI in different regions of the world and in different ethnic groups have also been reported.4),5),7),11) Although associations between the time of onset of AMI and in-hospital or 30-day mortality were suggested in some previous studies,12),13) not much is known about the 12-month mortality of AMI, including STEMI and non-STEMI (NSTEMI). The aim of this study was to investigate the impact of circadian variation on 12-month mortality in patients with AMI.
Subjects and Methods
Study patients
We included 966 consecutive patients with AMI who were admitted to Kyungpook National University Hospital between November 2005 and December 2007. A diagnosis of AMI was made in patients having angina pain lasting more than 15 minutes that was associated with/without dyspnea or sweating, established/evolving ST-T wave changes on serial electrocardiograms, and an increase in levels of cardiac troponin I (cTnI). cTnI levels were measured on admission and every 6 hours to detect the presence of myocardial injury, using an enzyme immunoassay based on the sandwich principle (Dimension; Dade Behring, Deerfield, IL, USA); the lower detection limit of this assay was 0.04 ng/mL. Elevated cardiac biomarker levels were defined as having maximal values of cTnI that exceeded the cutoff (99th percentile of the values for a reference control group) on at least one occasion. The time of onset of AMI was determined by each patient's report of the chest pain that prompted hospital admission. Seventy-four patients with unclear onset of chest pain, including silent AMI, were excluded from further analysis. In total, 892 patients (mean age 67±12 years; 66.1% men) were included in this study. Informed consent was obtained from each patient.
Data analyses
Each day was divided into 12 equal parts of two hours each, and patients were grouped according to the time of symptom onset. For further analysis, a day was divided again into four equal 6-hour intervals: overnight (00:00 AM-05:59 AM); morning (06:00 AM-11:59 AM); afternoon (12:00 PM-17:59 PM) and evening (18:00 PM-23:59 PM).2),14) Routine duty hours were defined as Monday to Friday between 08:00 AM and 18:00 PM.13) Weekends were considered off-duty hours. We analyzed the baseline demographic characteristics, initial presentation, initial vital signs, results of laboratory tests, and discharge medications of the patients. Demographic and clinical characteristics were identified, including age, gender, body mass index (BMI), cardiovascular risk factors (hypertension, diabetes mellitus, hyperlipidemia, and current smoking), and co-morbidities, which indluded previous congestive heart failure and myocardial infarction (MI). Initial vital signs including the systolic pressure and Killip class were evaluated at admission. Initial blood samples collected at admission, except for those collected to determine peak cTnI, were used for baseline laboratory tests. The left ventricular ejection fraction (LVEF) was determined by 2-dimensional echocardiography during the index hospitalization. The mean symptom-to-door time (STD) and the proportion of patients with STD time ≥6 hours were assessed in patients with typical chest pain. In patients with STEMI, the median door-to-balloon (DTB) time and the proportion of patients with DTB time ≤90 minutes were also evaluated. Discharge medications assessed included aspirin, β-blockers, angiotensin converting enzyme inhibitors (ACE-I)/angiotensin receptor blockers (ARB), and diuretics. Mean follow-up duration was 357±141 days. The primary end-point was 12-month mortality, including both cardiac death and non-cardiac death. Cardiac death was defined as death from pump failure, arrhythmia, or mechanical complications, which included ventricular septal rupture and free wall rupture. During the follow-up period, data were obtained by reviewing medical records and by telephone interview with the patients.
Statistical analyses
Chi-squared goodness-of-fit was performed to test the uniformity of the distribution of patients among the time periods. Data are expressed as mean±SD for continuous variables and percentages for categorical variables. All comparisons between baseline variables were assessed with the analysis of variance for continuous variables, and with the Pearson's Chi-square test for categorical variables. A multivariate logistic regression model was used to determine independent predictors for evening-onset AMI. Twelve-month mortality according to the onset time of AMI was evaluated using Kaplan-Meier survival curve analysis. Univariate analyses were performed to determine the clinical predictors of 12-month mortality. The Cox proportional hazard model was used to calculate the risk of 12-month mortality related to the time of onset of AMI, and adjusted for baseline confounding characteristics. For all analyses, a two-sided p<0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Circadian variation in the onset of acute myocardial infarction
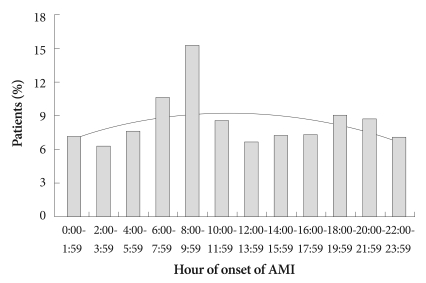
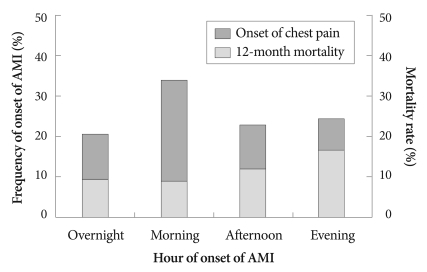
The frequency of the onset of AMI as determined by onset of chest pain for all 892 patients is shown in Fig. 1. The distribution is not uniform (p<0.001), and a peak was found between 08:00 and 09:59 hours (p<0.001). The distribution of AMI onset was obtained for each 6-hour interval, and a morning peak was found to be statistically significant (p<0.001) (Fig. 2). The numbers of patients grouped according to the AMI onset time were 184 (20.6%), 303 (34.0%), 187 (21.0%), and 218 (24.4%), corresponding to overnight, morning, afternoon and evening-onset groups, respectively. The incidence of morning-onset AMI was 54% higher than the average of the three other time periods. A morning peak of AMI was found irrespective of the presence or absence of ST-segment elevation (p=0.001 in STEMI and p<0.001 in NSTEMI).
Fig. 1.
The circadian variation of the onset of AMI for the total study population obtained at 2-hour intervals. The distribution is not uniform (p<0.001) and a peak occurring between 8:00 and 9:59 hours was statistically significant (p<0.001). AMI: acute myocardial infarction.
Fig. 2.
The circadian variation of the onset of AMI for the total study population obtained at 6-hour intervals. A morning peak was statistically significant (p<0.001). In contrast, the 12-month mortality rate was significantly higher in the evening-onset AMI group (p=0.012). AMI: acute myocardial infarction.
Clinical characteristics of patients according to acute myocardial infarction onset
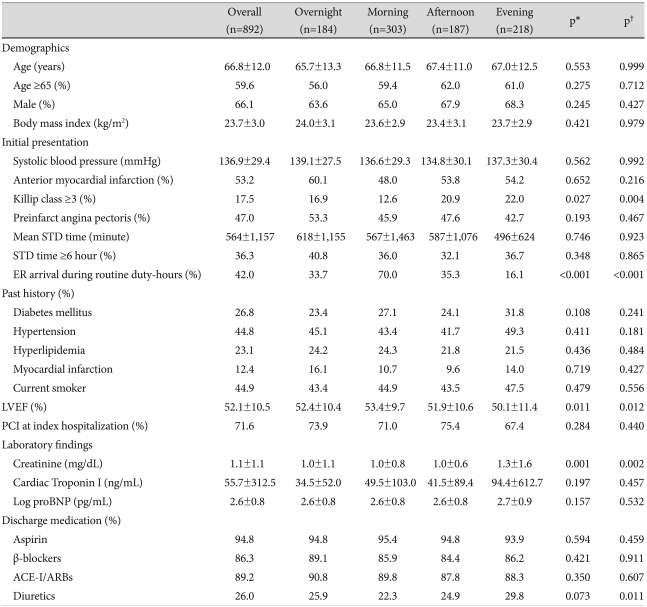
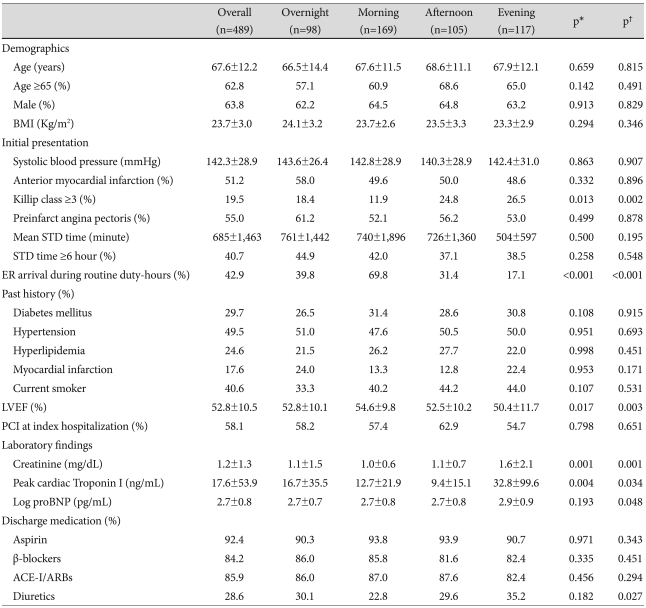
The clinical characteristics of the patients are shown in Table 1. The patients mean age was 67±12 years, and 590 (66.1%) were men. There were no significant differences in age, sex, BMI, systolic blood pressure, preinfarct angina pectoris, location of AMI, previous history of diabetes mellitus, hypertension, hyperlipidemia, MI, use of tobacco, percutaneous coronary intervention (PCI) at index hospitalization, peak cTnI, or pro-brain natriuretic peptide (proBNP) levels among the four groups. In the evening-onset AMI group serum creatinine levels (p=0.001) and the frequency of Killip class ≥3 (p=0.027) were significantly higher, while the LVEF (p=0.011) was significantly lower. The mean STD time was 564±1,157 minutes, with no significant differences in the mean STD time (p=0.746) or the proportion of patients with an STD time ≥6 hours (p=0.384) among the four groups. The proportion of patients who arrived in the emergency room (ER) during routine duty hours was significantly higher in the morning-onset AMI group (p<0.001). The discharge prescription rates of aspirin, β-receptor blockers, ACE-I/ARBs, and diuretics were not significantly different among the four groups.
Table 1.
Clinical characteristics of patients according to the hour of onset of AMI
*p for all comparisons, †p for the morning vs. evening group. AMI: acute myocardial infarction, STD: symptom-to-door, ER: emergency room, LVEF: left ventricular ejection fraction, PCI: percutaneous coronary intervention, proBNP: pro-brain natriuretic peptide, ACE-I/ARB: angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker
Compared with the morning-onset AMI group, serum creatinine levels (p=0.002),the frequency of Killip class ≥3 (p=0.004), and the prescription rate of diuretics (p=0.011) were significantly higher in the evening-onset AMI group, while LVEF (p=0.012) was significantly lower. The proportion of patients who arrived in the ER during routine duty hours was significantly lower in the evening-onset group compared to the morning-onset group (p<0.001).
Subgroup analysis
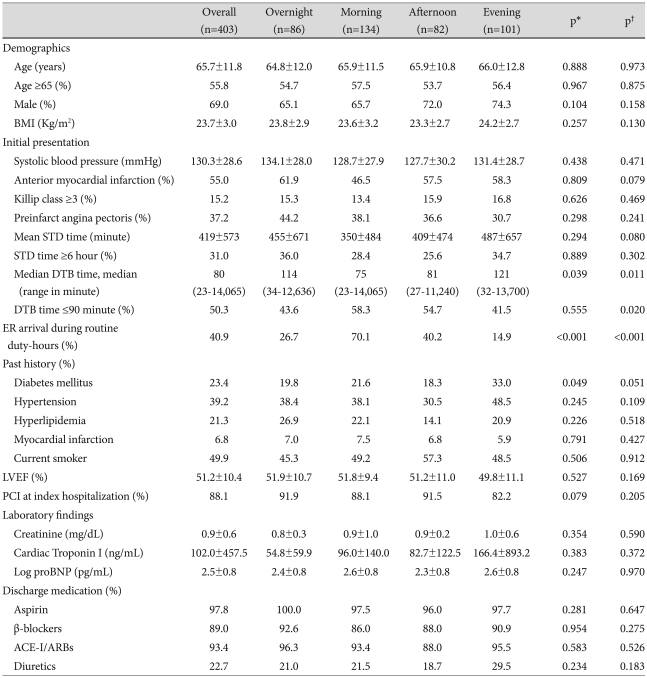
In the STEMI group (Table 2), the numbers of patients with a previous history of diabetes mellitus was significantly higher within the evening-onset group (p=0.049). The median DTB time was 80 minutes, and was significantly longer in the overnight-onset and evening-onset groups than in the morning-onset and afternoon-onset groups (p=0.039). The proportion of all patients with a DTB time ≤90 minutes was not significantly different among the four groups. However, compared with the morning-onset STEMI group, the proportion of patients with a DTB time ≤90 minutes was significantly lower for the evening-onset STEMI group (p=0.039). Serum creatinine levels, frequency of Killip class ≥3, and LVEF were not different among four STEMI groups.
Table 2.
Clinical characteristics of patients according to the hour of onset of STEMI
*p for all comparison, †p for the morning vs. evening group. STEMI: ST-segment elevation myocardial infarction, BMI: body mass index, STD: symptom-to-door, DTB: door-to-balloon, ER: emergency room, LVEF: left ventricular ejection fraction, PCI: percutaneous coronary intervention, proBNP: pro-brain natriuretic peptide, ACE-I/ARB: angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker
In the NSTEMI group (Table 3), the frequency of Killip class ≥3 (p=0.013), levels of serum creatinine (p=0.001), and peak cTnI (p=0.004) were significantly higher in the evening-onset NSTEMI group, while the LVEF (p=0.017) was significantly lower. Compared with the morning-onset NSTEMI group, the log proBNP level (p=0.048), prescription rate of diuretics (p=0.027), frequency of Killip class ≥3, levels of serum creatinine, and peak cTnI were significantly different in the evening-onset NSTEMI group. The proportion of patients who arrived in the ER during routine duty hours was significantly lower in the evening-onset group, irrespective of the presence or absence of ST-segment elevation (p<0.001).
Table 3.
Clinical characteristics of patients according to the hour of onset of NSTEMI
*p for all comparison, †p for the morning vs. evening group. NSTEMI: non-STEMI, BMI: body mass index, STD: symptom-to-door, ER: emergency room, LVEF: left ventricular ejection fraction, PCI: percutaneous coronary intervention, proBNP: pro-brain natriuretic peptide, ACE-I/ARB: angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker
Twelve-month mortality
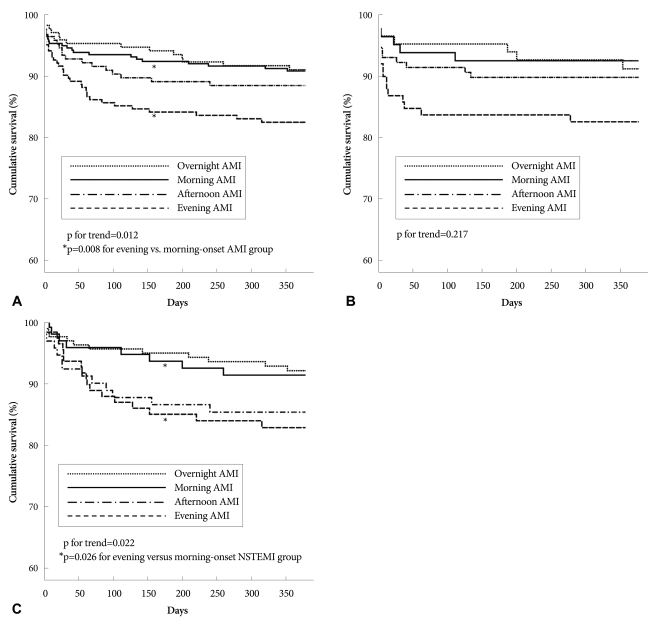
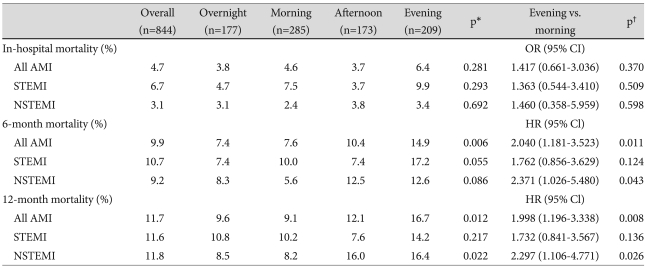
During the study period, 48 (5.4%) patients were lost during follow-up. The overall mortality rate was 11.7% (n=99), with 10.5% (n=89) from cardiac deaths and 1.2% (n=10) from non-cardiac deaths. No significant difference in the 12-month mortality rate was observed between STEMI and NSTEMI groups (11.6% vs. 11.8%, p=NS). Kaplan-Meier survival curves showed 12-month mortality rates of 9.6% (n=17), 9.1% (n= 26), 12.1% (n=21) and 16.7% (n=35) in the overnight, morning, afternoon and evening-onset groups, respectively (p= 0.012) (Figs. 2 and 3). Although there were no significant differences in inhospital mortality among the four groups according to univariate analysis, 6-month (p=0.006) and 12-month mortality rates (p=0.012) were significantly higher in the evening-onset group than for the three other groups. Compared with the morning onset-AMI group, 6-month {hazard ratio (HR) 2.040, 95% CI 1.181 to 3.523, p=0.011} and 12-month mortality rates [HR 1.998, 95% confidence interval (CI) 1.196 to 3.338, p= 0.008] were significantly higher in the evening onset-AMI group (Table 4). A similar result was obtained for 12-month mortality in patients with NSTEMI. However there were no significant differences between morning- and evening-onset STEMI groups in in-hospital, 6-month, and 12-month mortality rates. According to the multivariate Cox proportional-hazards model, evening-onset AMI (HR 0.518, 95% Cl 0.168 to 1.601, p=0.253) was not an independent predictor for 12-month mortality after adjusting for age, gender, ER arrival during off-duty hours, LVEF, Killip class ≥3, prescription of diuretics, levels of serum creatinine, peak cTnI, and log proBNP.
Fig. 3.
Kaplan-Meier survival curves for 12-month mortality according to onset time in patients with all AMI (A), STEMI (B), and NSTEMI (C). In patients with AMI and NSTEMI, 12-month mortality rates in the evening-onset groups were significantly higher than those in the morning-onset groups. AMI: acute myocardial infarction, STEMI: ST-segment elevation myocardial infarction, NSTEMI: non-STEMI.
Table 4.
In-hospital, 6-month, and 12-month mortality rates according to the hour of onset of AMI
*p for all comparisons, †p for the morning vs. evening group. AMI: acute myocardial infarction, OR: odds ratio, Cl: confidence interval, STEMI: ST-segment elevation myocardial infarction, NSTEMI: non-STEMI, HR: hazard ratio
Discussion
The main finding in this single center observational study is that 12-month mortality in patients with evening-onset AMI was significantly higher than that in the morning-onset AMI group. This was due to poorer baseline clinical characteristics for evening-onset AMI patients. In this study circadian variation of 12-month mortality might be secondary to differences in baseline characteristics at the onset time of AMI.
Killip class and serum creatinine levels have an impact on clinical outcome as surrogates for heart failure and acute renal failure after AMI.15-18) In this study, patients with Killip class ≥3, higher levels of serum creatinine, and lower LVEF were found significantly more frequent in evening-onset AMI group compared to the three other onset groups. In a study on the determinants of MI onset, Mukamal et al.14) showed that the risk of heart failure was highest in patients with infarctions that began between 6 PM and midnight and midnight to 6 AM. In another study, the peak between 6 PM and midnight was most pronounced, with patients with a history of congestive heart failure experiencing greater onset of MI.2) Although the mechanisms underlying these findings were not clear from their study, in this study newly developed heart failure following AMI, or underlying heart failure might affect the evening peak of 12-month mortality. Poor baseline clinical characteristics including Killip class ≥3, lower LVEF, higher levels of serum creatinine, and peak cTnI were also found in the evening-onset NSTEMI group. However, there were no significant differences in baseline clinical characteristics, except for diabetes mellitus, among the four STEMI groups. An evening peak of 12-month mortality was shown in the NSTEMI group, but was not demonstrated in the STEMI group. We hypothesized the differences in the baseline characteristics between the STEMI and NSTEMI groups might explain the difference in 12-month mortality between the two groups.
Outcomes after primary PCI have been reported to be related to the time delay from symptom onset to first-balloon inflation,19) and to hospital volume.20-22) In this study, the mean STD time was 564 minutes, and one third of patients had STD time ≥6 hours. There were no significant differences in STD time among the four groups. About 70% of patients in the morning-onset AMI group arrived at the ER during routine duty hours. However, only 16% of patients in the evening-onset AMI group arrived at the ER during routine duty hours. It is well known that patients presenting with AMI during off-duty hours have higher in-hospital mortality than patients presenting during routine duty hours.12),13),23),24) However, quality of care is difficult to measure, and it is unclear whether this affected the results of our study. Although the proportion of patients who arrived in the ER during routine duty hours was significantly higher in the morning-onset group, there were no significant differences in baseline characteristics between overnight-, morning-, and afternoon-onset AMI groups. Overall, there was no significant difference in the 12-month mortality rate among these three groups.
It is well known that longer DTB time is an independent predictor of short- and long-term outcomes in patients with STEMI.19),25) Although median DTB time in the STEMI subgroup was significantly longer among the evening-onset patients, the longer DTB time did not make a difference on 12-month mortality in the present study.
One possible explanation for the discrepancy between previous studies and our finding is the different study population. Previous studies generally included patients who presented acutely to the hospital within 12 or 24 hours of symptom onset.19),25) In this study, we included all consecutive patients with STEMI irrespective of the time from symptom onset to ER arrival. We thought the impact of DTB time on short- and long-term outcomes in patients with STEMI would be reduced because of the relatively long STD time.
There are several limitations in this study. First, this was a single-center observational study with a relatively small sample size. Second, multiple factors could affect the circadian variation and clinical outcome in this population. Finally, because timing of onset of AMI was self-reported by patients, they may have been assigned erroneously to particular groups.
In conclusion, there was a difference between the peak time of onset and 12-month mortality in patients with AMI. The twelve-month mortality rate was higher in the evening-onset AMI group compared to the morning-onset AMI group. Poorer baseline clinical characteristics included a higher Killip class, higher serum creatinine level, lower LVEF, and ER arrival during off-duty hours in patients with evening-onset AMI. This might affect the circadian impact on 12-month mortality. We conclude that the time of AMI onset must be considered in the management of patients with AMI. Further studies are needed to clarify the role of circadian variation on the long-term outcome of AMI.
Acknowledgments
The authors are deeply indebted to Roberto Patarca, MD, PhD and SungHee Kim, MD, PhD for their useful suggestions for the preparation of this manuscript.
References
- 1.Kinoshita N, Imai K, Kinjo K, Naka M. Longitudinal study of acute myocardial infarction in the southeast Osaka district from 1988 to 2002. Circ J. 2005;69:1170–1175. doi: 10.1253/circj.69.1170. [DOI] [PubMed] [Google Scholar]
- 2.Hjalmarson A, Gilpin EA, Nicod P, et al. Differing circadian patterns of symptom onset in subgroups of patients with acute myocardial infarction. Circulation. 1989;80:267–275. doi: 10.1161/01.cir.80.2.267. [DOI] [PubMed] [Google Scholar]
- 3.Kim KS, Song YS, Hur SH, et al. Circadian variation in acute myocardial infarction. Korean Circ J. 1993;23:173–183. [Google Scholar]
- 4.López F, Lee KW, Marín F, et al. Are there ethnic differences in the circadian variation in onset of acute myocardial infarction?: a comparison of 3 ethnic groups in Birmingham, UK and Alicante, Spain. Int J Cardiol. 2005;100:151–154. doi: 10.1016/j.ijcard.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 5.D'Negri CE, Nicola-Siri L, Vigo DE, Girotti LA, Cardinali DP. Circadian analysis of myocardial infarction incidence in an Argentine and Uruguayan population. BMC Cardiovasc Disord. 2006;6:1. doi: 10.1186/1471-2261-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rana JS, Mukamal KJ, Morgan JP, Muller JE, Mittleman MA. Circadian variation in the onset of myocardial infarction: effect of duration of diabetes. Diabetes. 2003;52:1464–1468. doi: 10.2337/diabetes.52.6.1464. [DOI] [PubMed] [Google Scholar]
- 7.Kinjo K, Sato H, Sato H, et al. Circadian variation of the onset of acute myocardial infarction in the Osaka area, 1998-1999: characterization of morning and nighttime peaks. Jpn Circ J. 2001;65:617–620. doi: 10.1253/jcj.65.617. [DOI] [PubMed] [Google Scholar]
- 8.Muller JE, Kaufmann PG, Luepker RV, Weisfeldt ML, Deedwania PC, Willerson JT. Mechanisms precipitating acute cardiac events: review and recommendations of an NHLBI workshop. National Heart, Lung, and Blood Institute. Circulation. 1997;96:3233–3239. doi: 10.1161/01.cir.96.9.3233. [DOI] [PubMed] [Google Scholar]
- 9.Bhalla A, Sachdev A, Lehl SS, Singh R, D'Cruz S. Ageing and circadian variation in cardiovascular events. Singapore Med J. 2006;47:305–308. [PubMed] [Google Scholar]
- 10.Willich SN. Circadian variation and triggering of cardiovascular events. Vasc Med. 1999;4:41–49. doi: 10.1177/1358836X9900400108. [DOI] [PubMed] [Google Scholar]
- 11.Sari I, Davutoglu V, Erer B, et al. Analysis of circadian variation of acute myocardial infarction: afternoon predominance in Turkish population. Int J Clin Pract. 2009;63:82–86. doi: 10.1111/j.1742-1241.2008.01717.x. [DOI] [PubMed] [Google Scholar]
- 12.Henriques JP, Haasdijk AP, Zijlstra F. Outcome of primary angioplasty for acute myocardial infarction during routine duty hours versus during off-hours. J Am Coll Cardiol. 2003;41:2138–2142. doi: 10.1016/s0735-1097(03)00461-3. [DOI] [PubMed] [Google Scholar]
- 13.Magid DJ, Wang Y, Herrin J, et al. Relationship between time of day, day of week, timeliness of reperfusion, and in-hospital mortality for patients with acute ST-segment elevation myocardial infarction. JAMA. 2005;294:803–812. doi: 10.1001/jama.294.7.803. [DOI] [PubMed] [Google Scholar]
- 14.Mukamal KJ, Muller JE, Maclure M, Sherwood JB, Mittleman MA. Increased risk of congestive heart failure among infarctions with nighttime onset. Am Heart J. 2000;140:438–442. doi: 10.1067/mhj.2000.108830. [DOI] [PubMed] [Google Scholar]
- 15.Park SR, Kang YR, Seo MK, et al. Clinical predictors of incomplete ST-segment resolution in the patients with acute ST segment elevation myocardial infarction. Korean Circ J. 2009;39:310–316. doi: 10.4070/kcj.2009.39.8.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CH, Joo SJ, Park DS, et al. Remodeling and changes of systolic and diastolic functions of left ventricle after acute myocardial infarction: comparison according to killip class at admission. Korean Circ J. 1998;28:1727–1739. [Google Scholar]
- 17.Al Suwaidi J, Reddan DN, Williams K, et al. Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation. 2002;106:974–980. doi: 10.1161/01.cir.0000027560.41358.b3. [DOI] [PubMed] [Google Scholar]
- 18.Naidu SS, Selzer F, Jacobs A, et al. Renal insufficiency is an independent predictor of mortality after percutaneous coronary intervention. Am J Cardiol. 2003;92:1160–1164. doi: 10.1016/j.amjcard.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Cannon CP, Gibson CM, Lambrew CT, et al. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA. 2000;283:2941–2947. doi: 10.1001/jama.283.22.2941. [DOI] [PubMed] [Google Scholar]
- 20.Magid DJ, Calonge BN, Rumsfeld JS, et al. Relation between hospital primary angioplasty volume and mortality for patients with acute MI treated with primary angioplasty vs thrombolytic therapy. JAMA. 2000;284:3131–3138. doi: 10.1001/jama.284.24.3131. [DOI] [PubMed] [Google Scholar]
- 21.Canto JG, Every NR, Magid DJ, et al. The volume of primary angioplasty procedures and survival after acute myocardial infarction. National Registry of Myocardial Infarction 2 Investigators. N Engl J Med. 2000;342:1573–1580. doi: 10.1056/NEJM200005253422106. [DOI] [PubMed] [Google Scholar]
- 22.Vakili BA, Kaplan R, Brown DL. Volume-outcome relation for physicians and hospitals performing angioplasty for acute myocardial infarction in New York state. Circulation. 2001;104:2171–2176. doi: 10.1161/hc3901.096668. [DOI] [PubMed] [Google Scholar]
- 23.Assali AR, Brosh D, Vaknin-Assa H, et al. The impact of circadian variation on outcomes in emergency acute anterior myocardial infarction percutaneous coronary intervention. Catheter Cardiovasc Interv. 2006;67:221–226. doi: 10.1002/ccd.20608. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez-Rodriguez A, Garcia-Gonzalez M, Abreu-Gonzalez P. Outcome of primary angioplasty for ST-segment elevation myocardial infarction during routine duty hours versus during off-hours: results of a single-center in Spain. Int J Cardiol. 2007;119:227–229. doi: 10.1016/j.ijcard.2006.07.110. [DOI] [PubMed] [Google Scholar]
- 25.Berger PB, Ellis SG, Holmes DR, Jr, et al. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction: results from the Global Use of Strategies to Open Occluded Arteries in Acute Coronary Syndromes (GUSTO-IIb) Trial. Circulation. 1999;100:14–20. doi: 10.1161/01.cir.100.1.14. [DOI] [PubMed] [Google Scholar]