Abstract
Background and Objectives
The aim of this study was to evaluate the efficacy of lacidipine in reducing blood pressure (BP) and to determine its effect on endothelial function in mild-to-moderate hypertensive patients with type 2 diabetes mellitus (DM).
Subjects and Methods
This was a prospective, multicenter, open-label, single-arm study, enrolling 290 patients with mild-to-moderate hypertension and type 2 DM. Patients were initially treated with 2 mg lacidipine orally once daily for 4 weeks, which was then increased as necessary every 4 weeks to a maximal dose of 6 mg daily. The primary endpoint was the mean change in systolic blood pressure (SBP) from baseline after 12 weeks of treatment. Secondary endpoints included mean changes in diastolic blood pressure (DBP), flow-mediated vasodilatation (FMD), and serum concentrations of biochemical markers such as high-sensitivity C-reactive protein (hs-CRP), monocyte chemo-attractant protein-1 (MCP-1), matrix metalloproteinase-9 (MMP-9), and plasminogen activator inhibitor-1 (PAI-1).
Results
Lacidipine treatment significantly reduced SBP by -13.4±13.0 mmHg (p<0.001) and DBP by -6.2±9.3 mmHg (p<0.001). Lacidipine treatment did not improve endothelial-dependent vasodilatation, despite significantly improved nitroglycerin-induced, endothelial-independent vasodilatation. MCP-1 levels significantly decreased from 283.66±110.08 pg/mL to 257.83±100.23 pg/mL (p<0.001); whereas there were no significant changes in the levels of hs-CRP, MMP-9, or PAI-1.
Conclusion
Twelve weeks of treatment with lacidipine was effective and well tolerated in mild-to-moderate hypertensive patients with type 2 DM. In spite of inducing a significant reduction in MCP-1 levels, lacidipine did not improve endothelial function.
Keywords: Lacidipine, Diabetes mellitus, Hypertension, Endothelium
Introduction
The prevalence of hypertension in patients with diabetes mellitus (DM) is extremely high (20-60%).1) Moreover, comorbid hypertension has been widely reported to increase the risk of cardiovascular events, which account for 86% of the deaths occurring among patients with DM; it is also known to increase the incidence of microvascular complications. However, it has been reported that only 28% of diabetic patients have adequately controlled blood pressure (BP) under 130/80 mmHg.2) This control rate is even lower (21.6%) among Korean patients.3) Although recent guidelines recommend angiotensin receptor antagonists or angiotensin-converting enzyme inhibitors as frontline antihypertensive agents in patients with DM, most patients eventually require more than 2 classes of antihypertensive agents to achieve target BP.4)
Lacidipine, a long-acting dihydropyridine calcium channel blocker, has been reported to reverse endothelial dysfunction,5) and to cause regression of atherosclerosis.6) The European Lacidipine Study on Atherosclerosis demonstrated that 4 years of lacidipine treatment retarded the progression of atherosclerosis more effectively than atenolol. Investigators suggested that this effect was due to restoration of endothelial function and reduction in oxidative stress.7)
However, the effects of lacidipine in type 2 DM patients have been only reported from small-scale, nonblinded studies.8) Furthermore, there is a paucity of data regarding the effects of lacidipine on endothelial dysfunction, which is observed in most patients with DM.
In this study, we evaluated the efficacy of lacidipine in reducing BP, as well as its effect on endothelial function, in mild-to-moderate hypertensive patients with type 2 DM.
Subjects and Methods
Study population
A subject was only eligible for inclusion in this study if all the following criteria applied: 1) male or female 35 to 75 years of age at screening, 2) newly diagnosed essential hypertension or essential hypertension untreated in the 2 months prior to screening, and 3) type 2 DM (ADA criteria 2004). Any subjects taking antihypertensive medications had to undergo a 2-week washout period before enrollment. The definition of hypertension was a sitting systolic blood pressure (SiSBP) of ≥130 mmHg, measured with a BP cuff, because the SBP goal is less than 130 mmHg in patients with diabetes. Exclusion criteria were as follows: severe hypertension with SiSBP >180 mmHg; or any serious disorder that could limit the ability of the patient to participate in the study, including severe coronary artery disease, uncontrolled DM (hemoglobin A1C >11%), or secondary hypertension.
Study design
This was a multicenter, open-label, single-arm study performed at 20 sites in the Republic of Korea. The study protocol was approved by the institutional review board at each site. Before entering the study, patients provided written, informed consent. A medical history was obtained during the screening phase, and a physical examination, 12-lead electrocardiogram, complete blood count, serum biochemistry, and routine urinalysis were performed at each center. During the 12-week treatment period, patients were initially treated with lacidipine 2 mg orally once daily for 4 weeks, and then were titrated with increasing doses every 4 weeks to a maximal dose of 6 mg daily if the SiSBP did not decrease to <130 mmHg.
Blood pressure measurement
At each visit, the SiSBP, sitting diastolic blood pressure (SiDBP), pulse rate, and body weight were recorded. To ensure consistency of trough values, these measurements were taken at the same time of day, before the next dose of lacidipine. At each center, a single investigator used the standard auscultatory technique for measuring BP in each patient's arm, using a mercury sphygmomanometer produced by one company (W. A. Baum Co. Inc, Copiague, NY, USA) and an appropriately-sized BP cuff.
Measurement of flow-mediated dilatation of the brachial artery
The examination was conducted at each center by the same examiner throughout the study, using a standard technique.9) Briefly, the diameter of the artery was measured using high-resolution, 2-dimensional images obtained by ultrasound with a 7.5-MHz linear-array transducer. Subjects were asked not to smoke or drink coffee or tea for at least 2 hours before the scan was performed. The right brachial artery was scanned over a longitudinal section 3 to 5 cm above the right medial elbow for the first resting image. The diameter of the brachial artery was measured from the anterior to the posterior interface between the media and adventitia ("m line") at a fixed distance.
The mean brachial artery diameter was calculated from 4 cardiac cycles synchronized with R-wave peaks on the electrocardiography. A pneumatic tourniquet placed around the forearm distal to the target artery was then inflated to a pressure of 250 mmHg, and inflation was maintained for 5 minutes. Increased flow was then induced by sudden cuff deflation. A second scan was performed continuously for 60 seconds before, and for 120 seconds after cuff deflation. Then, 15 minutes later, another resting image was recorded. A nitroglycerin tablet (0.4 mg) was then administered sublingually, and 3 minutes later the last scan was performed. All the images were stored in DICOM format for offline analysis, and then sent to the core laboratory. Two expert sonographers analyzed all the images. Interobserver coefficients of variation for measuring vessel diameters at baseline, after hyperemia, and after nitroglycerin were 3.3±0.25%, 2.9±0.28%, and 3.5±0.22%, respectively.
Blood tests
Blood samples were collected after at least 12 hours of fasting. Measurements of lipids, fasting glucose, and high-sensitivity C-reactive protein (hs-CRP) were performed in the local laboratories at each center. Other serum biochemical markers were analyzed in the central, core laboratory of GSK Korea using commercially available enzyme-linked immunosorbent assay kits from various vendors as follows: monocyte chemoattractant protein-1 {(MCP-1); Bio-Rad Laboratories, Hercules, CA, USA}, matrix metalloproteinase-9 {(MMP-9); Amersham Biosciences, Uppsala, Sweden}, and plasminogen activator inhibitor-1 {(PAI-1); IMUBIND, American Diagnostica, Stamford, CT, USA}.
Efficacy and safety variables
The primary efficacy variable in this study was the mean change in trough SiSBP from baseline after 12 weeks of treatment. Secondary efficacy variables included mean changes in SiDBP, flow-mediated dilatation (FMD), and biochemical markers such as hs-CRP, MCP-1, MMP-9, and PAI-1 after 12 weeks of treatment.
Safety variables included the incidence of all adverse events (AEs), drug-related AEs, and the number of discontinuations. AEs were evaluated at each visit by physical examination and direct questioning. The seriousness of AEs and their relation to the study drugs were determined by the chief investigator at each center. The results of all laboratory tests were assessed by the investigators for clinical significance and for their possible relationship to the study drug.
Statistical analysis
Effectiveness was defined as >4.5 mmHg decrease in the mean SiSBP from baseline after 12 weeks. We calculated that 321 patients would be required to fulfill the following parameters: standard deviation of 20 mmHg for SiSBP, overall 1-sided significance of 0.05, 90% power, and 20% exclusion rate. In terms of absolute benefit, data from the per-protocol (PP) population were used for the main analysis and those from the intention-to-treat (ITT) population were used as a supplement. The ITT population was used primarily to evaluate tolerability.
Continuous variables were analyzed using the t-test. Changes in BPs and serum markers were analyzed using a paired t-test. All statistical analyses were performed with Statistical Package for the Social Sciences (SPSS) 12.0 (SPSS Inc, Chicago, IL, USA), and p less than 0.05 were considered statistically significant.
Results
Baseline characteristics
A total of 333 patients were screened, and 290 patients (male : female=173 : 117) were enrolled in the study. Of them, 236 patients (81.4%) completed the study. The flow diagram of study patients is summarized in Fig. 1. Eighty subjects (33.9%) were treated with 2 mg of lacidipine throughout the study, 108 (45.8%) were titrated up to 4 mg, and 48 (20.3%) were titrated up to 6 mg.
Fig. 1.
Flow diagram of study patients. ITT: intention to treat, FMD: flow-mediated dilatation, PP: per-protocol.
The mean age in the study group was 56.6±9.2 (35-79) years, and 41% of the patients were over 60 years of age. The most common concomitant medications at baseline and during the study were oral hypoglycemic agents (75%), lipid-lowering agents (29%), antiplatelet agents (40%), and antacids (13%). The proportions of oral hypoglycemic agents were as follows: sulfonylureas 58%, biguanides 37%, alpha-glucosidase inhibitors 13%, thiazolidinediones 14%, combination of more than two drugs 4%.
Changes in blood pressure from baseline
In the PP population (n=236), SiSBP was significantly decreased by 13.4±13.0 mmHg (from 144.0±11.4 mmHg to 130.6±12.5 mmHg, p<0.0001) after 12 weeks of treatment. In the ITT population (n=277), SiSBP also significantly decreased by 13.0±13.3 mmHg (from 144.2±12.3 mmHg to 130.9±13.1 mmHg, p<0.0001) (Fig. 2).
Fig. 2.
Effect of lacidipine in mean sitting cuff- systolic blood pressure (SiSBP) in patients with mild-to-moderate hypertension after 4, 8, and 12 weeks of treatment. The mean change from baseline was significant in intention-to-treat (A) and per-protocol (B) groups (*p<0.001). LOCF: last observation carried forward.
SiDBP decreased by 6.2±9.3 mmHg (from 88.7±8.8 mmHg to 82.5±9.0 mmHg, p<0.001) in the PP population and by 6.1±9.1 mmHg (from 88.7±8.6 mmHg to 72.0±9.4 mmHg, p<0.001) in the ITT population.
The overall response rate (SiSBP <130 mmHg) after 12 weeks of treatment was 58.5%. In the subgroup with baseline SiSBP greater than 140 mmHg (PP population, n=132), the change in SiSBP was -18.1±13.3 mmHg (from 151.6±9.7 mmHg to 133.5±13.6 mmHg, p<0.001); whereas in the subgroup with baseline SiSBP less than 140 mmHg (n=104), the change in SiSBP was -7.5±9.8 mmHg (from 134.4±3.0 mmHg to 126.9±10.0 mmHg, p=0.03).
Changes in endothelial-dependent and endothelial-independent vasodilatation
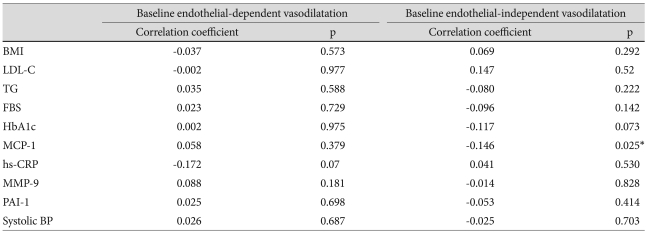
Changes in endothelial-dependent vasodilatation of the brachial artery were analyzed in the PP population. The mean baseline percentage of endothelial-dependent vasodilatation was not related to baseline body mass index, lipids, serum biomarkers, or SiSBP levels (Table 1). After 12 weeks of treatment with lacidipine, the percentage of endothelial-dependent vasodilatation changed from 5.22±4.91% to 5.52±4.92%, but this did not represent a statistically significant change (p=0.313).
Table 1.
Correlation coefficients between various baseline values and vasorelaxation
BMI: body mass index, LDL-C: low density lipoprotein-cholesterol, TG: triglyceride, FBS: fasting blood glucose, MCP-1: monocyte chemoattractant protein-1, MMP-9: matrix metalloproteinase-9, PAI-1: plasminogen activator inhibitor-1
In contrast, endothelial-independent vasodilatation after administration of sublingual nitroglycerin significantly increased from 13.94±7.21% to 14.97±7.42% (p=0.02). The baseline percentage of nitroglycerin-induced vasodilatation had a weak negative correlation with MCP-1 levels. All the subjects were divided into 2 subgroups. The FMD-responder group (n=130, 55.1%) included those patients who had increased endothelial-dependent vasodilatation, and the FMD-nonresponder group (n=106, 44.9%) included those patients who had no increase in endothelial-dependent vasodilatation. The FMD-responder group had significantly lower baseline triglyceride (TG) levels (150±97 mg/dL vs. 185±156 mg/dL, p=0.02) and higher baseline high density lipoprotein-cholesterol (HDL-C) levels (46±11 mg/dL vs. 49±12 mg/dL, p=0.01) (Table 2).
Table 2.
Baseline values and changes in the levels of glucose, lipids, serum markers, and SiSBP, stratified according to vasorelaxation
*p<0.05 between the increase group and the no increase group. TG: triglyceride, LDL-C: low density lipoprotein-cholesterol, hs-CRP: high-sensitivity CRP, MCP-1: monocyte chemoattractant protein-1, MMP-9: matrix metalloproteinase-9, PAI-1: plasminogen activator inhibitor-1, endothelial-independent vasodilatation group, SiSBP: sitting cuff-systolic blood pressure, ΔSiSBP: absolute amount of decrease in SiSBP
Changes in blood chemistry and biomarkers
HbA1C and lipid profiles, including TGs, low density lipoprotein-cholesterol, and HDL-C levels, did not change after 12 weeks of lacidipine treatment. Among the biomarkers evaluated, the plasma levels of MCP-1 significantly decreased from 283.66±110.08 pg/mL to 257.83±100.23 pg/mL (p<0.001). In contrast, hs-CRP did not show any significant change (from 0.51±1.07 mg/dL to 0.55±1.60 mg/dL, p=0.644). The results are presented in Table 2.
Tolerability
More than one AE event was reported in 40% of the patients in the ITT population (a total of 116 events in 290 patients). There were 105 events that were considered to be drug-related. The most common AEs thought to be drug-related were headache (n=16, 5.52%), palpitations (n=6, 2.07%), facial flushing (n=5, 1.72%), dizziness (n=4, 1.38%), and chest pain (n=4, 1.38%). There were 11 severe AEs reported in 9 patients (3.10%). All of the severe events required hospitalization, and included 2 cases of chest pain, and 1 case each of laryngeal cancer, bladder cancer, back pain, hypoglycemia, hemorrhoids, cerebral hemorrhage, depression, benign prostatic hyperplasia, and impaired glucose tolerance. All severe AEs were thought to be unrelated to drug therapy.
Discussion
This study was performed to evaluate the efficacy and tolerability of lacidipine in mild-to-moderate hypertensive patients with type 2 DM. To the best of our knowledge, this is the largest study evaluating the effect of calcium antagonists on endothelial function in hypertensive patients with DM.
Lacidipine treatment effectively reduced SBP and DBP. When the dose was titrated up to 6 mg once daily, the overall response rate after 12 weeks of treatment reached 58.5%. Although this study did not have a comparative design, the extent of lacidipine-induced SiSBP reduction in our Korean population was comparable to the effects of lacidipine seen in other races,10),11) as well as to the effects of other antihypertensive agents, such as nifedipine,12) atenolol,13) thiazide,14) isradipine,15) and amlodipine16) in Korean patients.
The most interesting findings in this study were related to the role of lacidipine in the improvement of endothelial dysfunction in hypertensive patients with DM. Although a few preclinical or small-scale clinical studies have suggested the beneficial effects of lacidipine on endothelial function in nondiabetic subjects,5) this is the first study evaluating the effects of lacidipine on endothelial function in diabetic patients with hypertension. Twelve weeks of lacidipine therapy did not improve endothelial-dependent vasodilatation, despite the fact that endothelial-independent vasodilatation was significantly improved following administration of sublingual nitroglycerin. The lack of improvement in endothelial-dependent vasodilatation stands in contrast to previous studies5) which have shown an improvement in overall endothelial-dependent vasodilatation in nondiabetic patients. A number of mechanisms may be invoked in understanding the discrepancy between our results and those in the nondiabetic population. Impairment of endothelial-dependent vasodilatation is more severe in diabetic patients than it is in nondiabetic patients. Thus, 12 weeks of treatment might not be sufficient for amelioration of this impairment. Impairment of endothelial-dependent vasodilatation has been widely observed in arteries from diabetic animals,17) as well as from normal animals exposed to hyperglycemia.18) In such studies, endothelial-dependent vasodilatation had already been impaired while endothelial-independent vasodilatation remained intact. Similarly, in a few animal studies,19) as well as in human studies,20) a diminished response to nitroglycerin was also observed in diabetes, which was preceded by a disturbed response to acetylcholine. Therefore, impaired vascular response to nitroglycerin is considered to occur at a more advanced stage of vascular dysfunction in diabetes. In this study, the baseline values of nitroglycerin-induced vasodilatation were more impaired than previously reported values of normal controls,21) which also suggests that the population in this study might have had more advanced vascular dysfunction. Similarly, HMG-CoA reductase inhibitors (statins) have been shown to have significant beneficial effects on endothelial dysfunction in nondiabetic subjects.22) However, many studies have also failed to show significant statin-induced improvement in endothelial-dependent vasodilatation in subjects with type 2 DM,23) and only one report21) has demonstrated statin-induced improvement in endothelial-independent vasodilatation, as was shown in this study.
In subgroup analysis, the group with increased endothelial-dependent vasodilatation had lower TG levels and higher HDL-C levels. An elevated TG concentration may increase the levels of intracellular adhesion molecule-1 and vascular cell adhesion molecule-1, which might result in increased inflammatory responses in endothelial cells.24) Additionally, improvement in endothelial dysfunction seems to be independent of BP-lowering effects, because there was no difference in the baseline BP or the absolute amount of decrease in SBP between those with FMD improvement and those without improvement.
Finally, we observed that the levels of MCP-1, a surrogate marker for inflammation,25) significantly decreased after lacidipine treatment. The levels of MCP-1 in hypertensive patients have been reported to correlate with the severity of carotid intima-media thickness (IMT).26) In this regard, decreased MCP-1 concentrations following lacidipine treatment might be consistent with the previously reported anti-atherosclerotic effect of lacidipine.7) In this study by contrast, the levels of hs-CRP did not change significantly. This discrepant result was consistent with that of a previous study in which patients with coronary artery disease had significantly reduced carotid IMT without a significant change in hs-CRP levels after 6 months of lacidipine treatment.27) This discrepancy between hs-CRP and MCP-1 has also been demonstrated in other studies of angiotensin receptor blockers.28)
Levels of PAI-1, a marker of fibrinolytic balance, did not decrease after lacidipine treatment. PAI-1 levels are elevated in subjects with risk factors for atherosclerosis and insulin resistance.29) PAI-1 expression is known to be regulated by nitiric oxide (NO) activity, and therefore NO synthase activity.30) In this regard, the lack of change in mean PAI-1 levels might be consistent with the insignificant endothelial-dependent vasodilatation changes observed in this study.
There are several limitations to this study. First, this is a single-arm, uncontrolled study. Owing to ethical concerns regarding not administering antihypertensive therapy to patients with confirmed hypertension, we were unable to create a control group. Second, the measurement and analysis of FMD could be observer-dependent with high variance. To reduce observer-dependent bias, two expert sonographers in the core lab analyzed all the images. Third, the measurements of lipids, fasting glucose, and hs-CRP were performed by local laboratories at each center, and the results could vary between laboratories. However, the serum biochemical markers, including MCP-1, MMP-9, and PAI-1, were all analyzed in a single central laboratory.
In summary, 12 weeks of lacidipine treatment was effective and well tolerated in diabetic patients with mild-to-moderate hypertension. Lacidipine partially improved vasorelaxation, which was associated with reduction in levels of inflammatory markers.
Acknowledgments
This study was sponsored by GlaxoSmithKline Korea.
References
- 1.Arauz-Pacheco C, Parrott MA, Raskin P. Treatment of hypertension in adults with diabetes. Diabetes Care. 2003;26(Suppl 1):S80–S82. doi: 10.2337/diacare.26.2007.s80. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Kim KI, Kim Y, Kim HJ, et al. Current status and characteristics of hypertension treatment by primary physicians in Korea: data from Korean Epidemiology Study on Hypertension (KEY Study) Am J Hypertens. 2008;21:884–889. doi: 10.1038/ajh.2008.191. [DOI] [PubMed] [Google Scholar]
- 4.Sowers JR, Haffner S. Treatment of cardiovascular and renal risk factors in the diabetic hypertensive. Hypertension. 2002;40:781–788. doi: 10.1161/01.hyp.0000042097.39655.b7. [DOI] [PubMed] [Google Scholar]
- 5.Taddei S, Virdis A, Ghiadoni L, Sudano I, Salvetti A. Effects of antihypertensive drugs on endothelial dysfunction: clinical implications. Drugs. 2002;62:265–284. doi: 10.2165/00003495-200262020-00003. [DOI] [PubMed] [Google Scholar]
- 6.Zanchetti A, Bond MG, Hennig M, et al. Absolute and relative changes in carotid intima-media thickness and atherosclerotic plaques during long-term antihypertensive treatment: further results of the European Lacidipine Study on Atherosclerosis (ELSA) J Hypertens. 2004;22:1201–1212. doi: 10.1097/00004872-200406000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Zanchetti A, Bond MG, Hennig M, et al. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation. 2002;106:2422–2427. doi: 10.1161/01.cir.0000039288.86470.dd. [DOI] [PubMed] [Google Scholar]
- 8.Frattola A, Parati G, Castiglioni P, et al. Lacidipine and blood pressure variability in diabetic hypertensive patients. Hypertension. 2000;36:622–628. doi: 10.1161/01.hyp.36.4.622. [DOI] [PubMed] [Google Scholar]
- 9.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 10.Salako BL, Kadiri S, Walker O, Fehintola FA. Evaluation of lacidipine (a calcium blocker) in the treatment of hypertension in black African people: a double-blind comparison with hydrochlorothiazide. Afr J Med Med Sci. 1998;27:73–75. [PubMed] [Google Scholar]
- 11.Tcherdakoff P. French large-scale study evaluating the tolerability and efficacy of lacidipine. J Cardiovasc Pharmacol. 1995;25(Suppl 3):S27–S32. [PubMed] [Google Scholar]
- 12.Kim DH, Oh SI, Kim YK, Choi SW, Yoo WS. Efficacy and safety of nifedipine gastrointestinal therapeutic system (Adalat OROS) in patients with mild to moderate essential hypertension. Korean Circ J. 1992;22:488–493. [Google Scholar]
- 13.Choi YJ, Lee MM, Choe SJ, et al. A randomized, double-blind clinical trial to determine the efficacy of carvedilol vs. atenolol in patients with stage 1 to 2 essential hypertension. Korean Circ J. 1998;28:359–365. [Google Scholar]
- 14.Jin SB, Rhee YW, Chang SW, Kim KC, Kim SP, Song CS. Effects of dihydrochlorothiazide, propranolol, and prazosin on serum lipids in patients with essential hypertension. Korean Circ J. 1985;15:329–336. [Google Scholar]
- 15.Cheong HJ, Kang HS, Choue CW, et al. Antihypertensive effects and safety of isradipine in patients with essential hypertension. Korean Circ J. 1993;23:741–749. [Google Scholar]
- 16.Park SW, Doo YC, Kim WH, et al. Amlodipine monotherapy in patients with mild to moderate essential hypertension. Korean Circ J. 1992;22:852–857. [Google Scholar]
- 17.Pieper GM, Gross GJ. Oxygen free radicals abolish endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1988;255:H825–H833. doi: 10.1152/ajpheart.1988.255.4.H825. [DOI] [PubMed] [Google Scholar]
- 18.Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992;263:H321–H326. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- 19.Dai FX, Diederich A, Skopec J, Diederich D. Diabetes-induced endothelial dysfunction in streptozotocin-treated rats: role of prostaglandin endoperoxides and free radicals. J Am Soc Nephrol. 1993;4:1327–1336. doi: 10.1681/ASN.V461327. [DOI] [PubMed] [Google Scholar]
- 20.Yugar-Toledo JC, Tanus-Santos JE, Sabha M, et al. Uncontrolled hypertension, uncompensated type II diabetes, and smoking have different patterns of vascular dysfunction. Chest. 2004;125:823–830. doi: 10.1378/chest.125.3.823. [DOI] [PubMed] [Google Scholar]
- 21.Balletshofer BM, Goebbel S, Rittig K, et al. Intense cholesterol lowering therapy with a HMG-CoA reductase inhibitor does not improve nitric oxide dependent endothelial function in type-2-diabetes: a multicenter, randomised, double-blind, three-arm placebo-controlled clinical trial. Exp Clin Endocrinol Diabetes. 2005;113:324–330. doi: 10.1055/s-2005-865642. [DOI] [PubMed] [Google Scholar]
- 22.Stroes ES, Koomans HA, de Bruin TW, Rabelink TJ. Vascular function in the forearm of hypercholesterolaemic patients off and on lipid-lowering medication. Lancet. 1995;346:467–471. doi: 10.1016/s0140-6736(95)91322-x. [DOI] [PubMed] [Google Scholar]
- 23.Economides PA, Caselli A, Tiani E, Khaodhiar L, Horton ES, Veves A. The effects of atorvastatin on endothelial function in diabetic patients and subjects at risk for type 2 diabetes. J Clin Endocrinol Metab. 2004;89:740–747. doi: 10.1210/jc.2003-031116. [DOI] [PubMed] [Google Scholar]
- 24.Guerci B, Bohme P, Kearney-Schwartz A, Zannad F, Drouin P. Endothelial dysfunction and type 2 diabetes: part 2. altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metab. 2001;27:436–447. [PubMed] [Google Scholar]
- 25.Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714–721. [PubMed] [Google Scholar]
- 26.Sardo MA, Campo S, Mandraffino G, et al. Tissue factor and monocyte chemoattractant protein-1 expression in hypertensive individuals with normal or increased carotid intima-media wall thickness. Clin Chem. 2008;54:814–823. doi: 10.1373/clinchem.2007.095547. [DOI] [PubMed] [Google Scholar]
- 27.Bae JH, Bassenge E, Lim DM, Synn YC, Kim KY, Schwemmer M. Effects of lacidipine on vascular responses in patients with coronary artery disease. Int J Cardiol. 2005;101:377–383. doi: 10.1016/j.ijcard.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 28.Koh KK, Han SH, Chung WJ, et al. Comparison of effects of losartan, irbesartan, and candesartan on flow-mediated brachial artery dilation and on inflammatory and thrombolytic markers in patients with systemic hypertension. Am J Cardiol. 2004;93:1432–1435. doi: 10.1016/j.amjcard.2004.02.050. A10. [DOI] [PubMed] [Google Scholar]
- 29.Juhan-Vague I, Alessi MC, Vague P. Thrombogenic and fibrinolytic factors and cardiovascular risk in non-insulin-dependent diabetes mellitus. Ann Med. 1996;28:371–380. doi: 10.3109/07853899608999095. [DOI] [PubMed] [Google Scholar]
- 30.Kaikita K, Fogo AB, Ma L, Schoenhard JA, Brown NJ, Vaughan DE. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation. 2001;104:839–844. doi: 10.1161/hc3301.092803. [DOI] [PubMed] [Google Scholar]